Adenovirus 36 and Obesity: An Overview
Abstract
:1. Introduction
2. Association of Viral Infections with Obesity in Animal Models
3. Adenoviruses

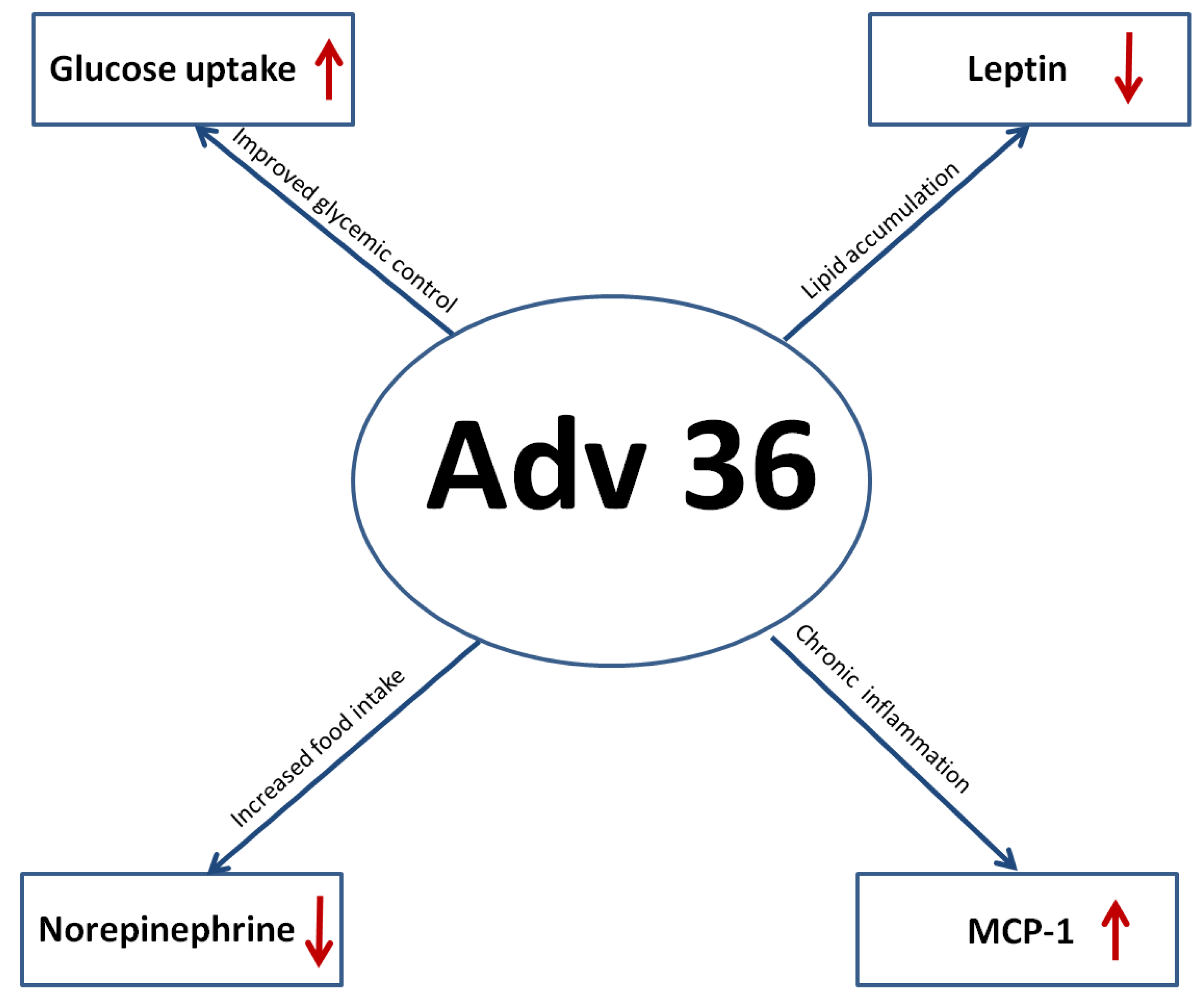
4. Adv36 and Obesity
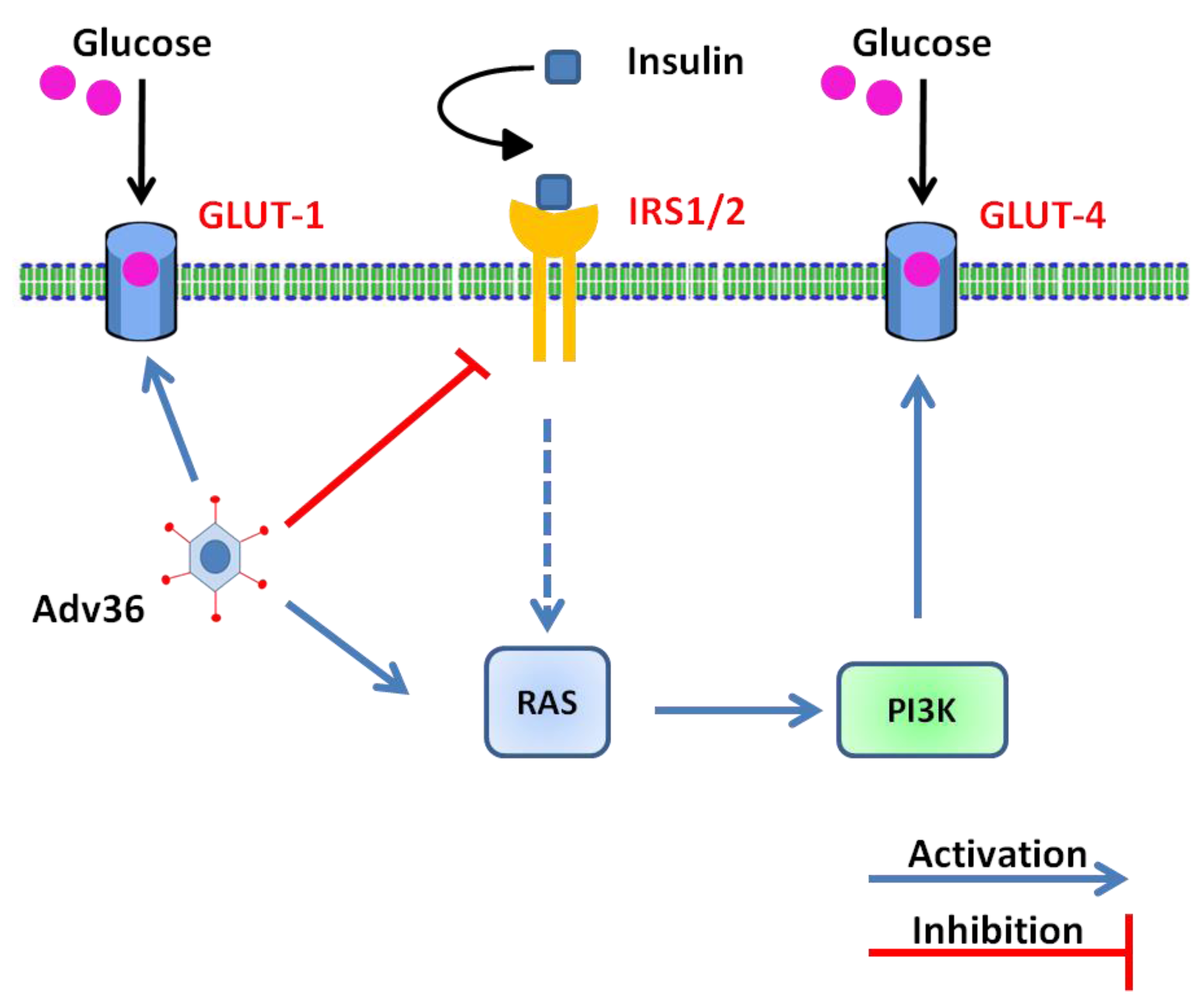
4.1. In Vivo Studies
4.2. In Vitro Studies
5. Prevalence of Adv36 in Human Obesity
| First Author | Country | Parameters | BMI | Subjects | Prevalence of Adv36 | Method |
|---|---|---|---|---|---|---|
| Atkinson, 2005 [49] | USA | Obesity, BMI, TG, TC | BMI ≥ 30 | 360 obese and 142 non-obese adults | Obese 30% Non-obese 11% | SNA |
| Atkinson, 2005 [49] | USA | BMI, TG, TC | NA | 28 sets of twins | Overall 22% | SNA |
| Trovato, 2009 [95] | ITALY | Obesity, BMI, TG, TC, LDL,HDL, SBP | BMI ≥ 30 | 68 obese and 135non-obese adults | Obese 65% Non-obese 33% | SNA |
| Atkinson, 2010 [56] | South Korea | TC, WC, SBP, BG | NA | 83 obese or overweightchildren and one nonobese child | Overall 30% | SNA |
| Broderick, 2010 [50] | USA | Obesity | BMI ≥ 29 | 146 obese and 147 non-obese adults | Obese 34% Non-obese 39% | SNA |
| Gabbert, 2010 [58] | USA | Obesity, BMI, WC | BMI.95 thpercentile | 67 obese and 57 non obese children | Obese 22% Non-obese 7% | SNA |
| Na, 2010 [53] | South Corea | Obesity, BMI, TG, TC, WC,LDL, HDL, SBP, BG | BMI ≥ 30 | 259 obese and 59 nonobese children | Obese 29% Non-obese 14% | SNA |
| Trovato, 2010 [96] | ITALY | BMI, TG, TC, LDL, HDL, BG | NA | 65 NAFLD and 114 non-NAFLD adults | NAFLD 32% Non-NAFLD 46% | SNA |
| Krishnapuram, 2011 [97] | USA | Fasting insulin, Fasting glucose, Insulin sensitivity, HOMA, | NA | (1) HERITAGE Family Study (n 671) | (1) HERITAGE Family Study 13% | SNA |
| (2) PBRC Study (n 206) | (2) PBRC Study 18% | |||||
| (3) MET Study (n 45) | (3) MET Study 22% | |||||
| (4) VIVA LA FAMILIA Study (n 585) | (4) VIVA LA FAMILIA Study 7.1% | |||||
| Goossens, 2011 [51] | Netherlands | Obesity, BMI | NA | 136 obese, 281 nonobese, and 92 BMI-unknown adults | 5.5% were positive for Adv36 antibodies, No adenoviral DNA | SNA, PCR |
| Na, 2012 [98] | South Korea | Obesity, BMI, TG, TC, WC, HDL, SBP, BG | BMI ≥ 25 | 180 obese and 360 non-obese adults | Obese 30% Non-obese36% | |
| Trovato, 2012 [99] | ITALY | BMI, TG, TC, LDL, HDL, BG | NA | 62 NAFLD adults | Overall 40% | SNA |
| Almgren, 2012 [54] | Sweden | Obesity, BMI, TG, TC, LDL, HDL, BG | BMI ≥ 35; 28 ≥ BMI ≤ 25; BMI < 25 | 424 children and 1522 nondiabetic adults, and 89 anonymous blood donors | 7% in 1992–1998 to 15%–20% in 2002–2009, increase in obesity prevalence | SNA and ELISA |
| Aldhoon-Hainerova, 2014 [55] | Czech Republic | anthropometric (body weight, height, BMI, WC, fat mass), blood pressure, biochemical and hormonal (lipid profile, glucose, insulin, liver enzymes, adiponectin) | NA | 1179 Czech adolescents (85 underweight, 506 normal weight, 160 overweight and 428 obese) | 26.5% were positive for Adv36 antibodies (underweight: 22.3%; normal weight: 21.5%; overweight: 40.0% and obese: 28.0%) | ELISA |
| Vander Wal, 2013 [61] | USA | BMI, TC, HDL, LDL, TG | Mean BMI 33.77 | 73 youth aged 10–17 years | 17 youth (23.3%; 2 boys, 15 girls) tested Ad-36 AB+ and 56 youth (76.6%; 14 boys, 42 girls) tested Ad-36 AB−. | SNA |
| Lin, 2013 [100] | MEXICO | Age, sex, Body FAT, BMI, Fasting glucose, Fasting insulin | Mean BMI 29.15 | 1,400 enrolled in the San Antonio Family Heart Study | Seropositive subjects (14.5%) had greater adiposity at baseline, compared with seronegative subjects. | SNA |
| Laing, 2013 [101] | USA | DXA | 21 ≥ BMI ≤ 24 | 115 females aged 18 to 19 years | 52% and 64% in normal-fat and high-fat groups | ELISA |
| Vander Wal, 2013 [61] | USA | TC, HDL, LDL, TG | Mean BMI 37.77 | 73 youth aged 10-17 years | 17 youth (23.3%; 2 boys, 15 girls) tested Ad-36 AB+ and 56 youth (76.6%; 14 boys, 42 girls) tested Ad-36 AB– | SNA |
| Parra-Rojas, 2013 [59] | MEXICO | LDL, HDL, TG, Insulin, Fasting glucose, HOMA | NA | 75 children with normal-weight and 82 with obesity | Seroprevalence was 73.9%. Ad-36 seropositivity had a higher prevalence in obese children than in normal weight group 58.6 versus 41.4% | ELISA |
| Berger, 2014 [102] | USA | TNF-α, IL-6, VEGF, MCP-1, DXA. | 20 ≥ BMI ≤ 21 | 291 children aged 9-13 years (50% female, 49% black) | seropositivity [Ad36(+)] was 42% | ELISA |
| Voss, 2014 [103] | USA | NA | 20–30 kg/m(2) | 500 young, 18–22 years | seropositivity [Ad36(+)] was 20.8% | ELISA |
| Karamese, 2015 [104] | Turkey | TG, TC, LDL, TNF-α, IL-6, leptin | NA | 146 children and 130 adults | 27.1% and 6% in obese and non-obese children and 17.5% and 4% in obese and non-obese adults | ELISA |
| Ergin, 2015 [105] | Turkey | TC, TG, leptin | Obese BMI > 30; non-obese adults with BMI < 25 | 49 obese adults and 49 non-obese adults | seroprevalence was 12.2%, DNA was not detected | SNA, ELISA, PCR |
6. Molecular and Cellular Mechanisms Involved in Ad-36-Induced Glucose Uptake
7. Adenovirus 36 and Immune Response
7.1. Inflammation: MCP-1 (Macrophage Chemoattractant Protein I) and Adv36
7.2. Effect of Adv36 on Leptin
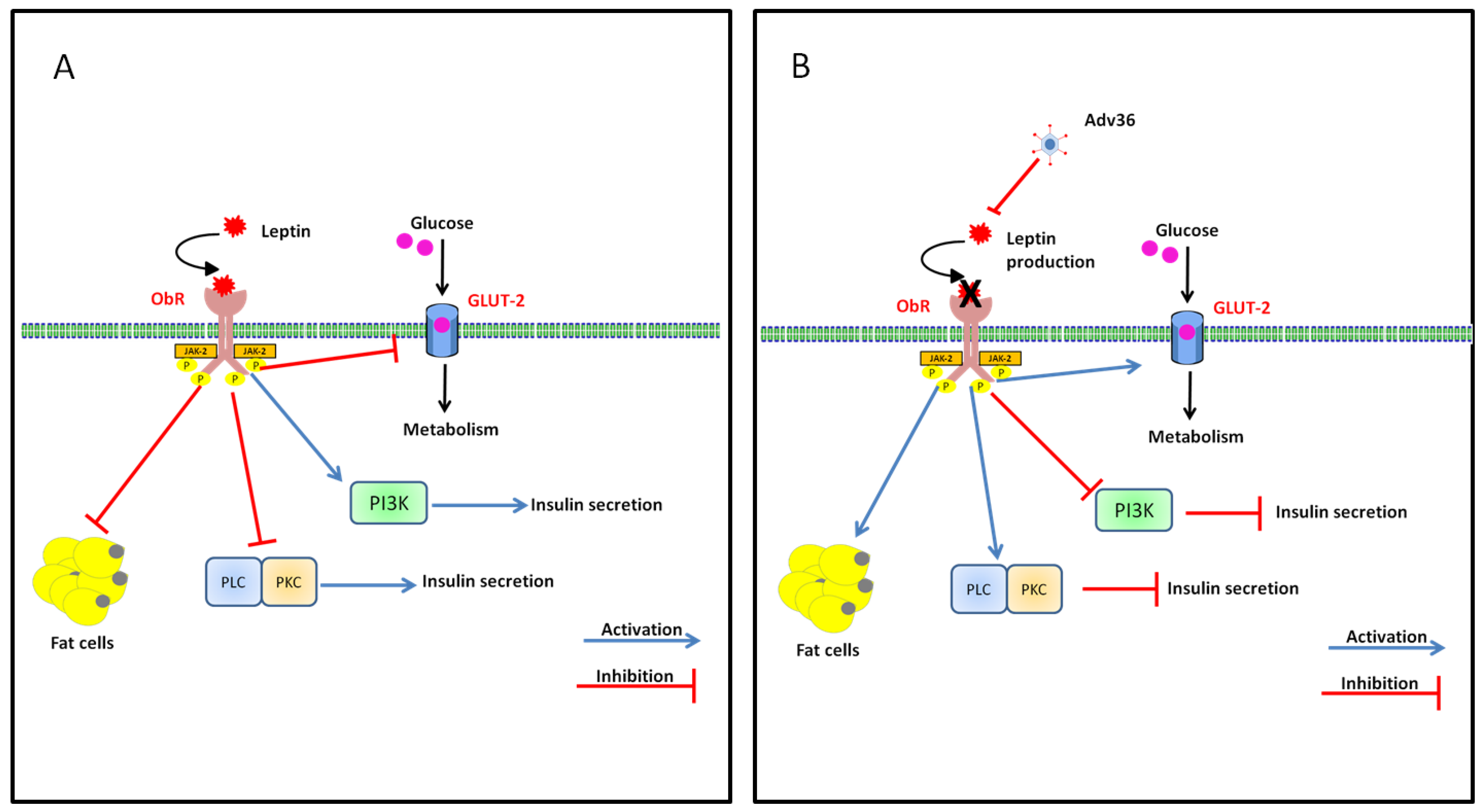
8. Proof-of-Concept of a Vaccine Using UV Inactivated Adv36
9. Conclusions
Acknowledgments
Author contributions
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- WHO. Obesity and overweight. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 5 June 2015).
- Bray, G.A.; Bellanger, T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine 2006, 29, 109–117. [Google Scholar] [CrossRef]
- Hill, J.O.; Peters, J.C. Environmental contributions to the obesity epidemic. Science 1998, 280, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, E.A.; Beck, M.A. The burden of obesity on infectious disease. Exp. Biol. Med. 2010, 235, 1412–1424. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.M. Pulmonary complications of obesity. Am. J. Med. Sci. 2001, 321, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Jubber, A.S. Respiratory complications of obesity. Int. J. Clin. Pract. 2004, 58, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Campitelli, M.A.; Rosella, L.C.; Kwong, J.C. The association between obesity and outpatient visits for acute respiratory infections in ontario, Canada. Int. J. Obes. 2014, 38, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mullen, J.T.; Moorman, D.W.; Davenport, D.L. The obesity paradox: Body mass index and outcomes in patients undergoing nonbariatric general surgery. Ann. Surg. 2009, 250, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Thun, M.J.; Petrelli, J.M.; Rodriguez, C.; Heath, C.W., Jr. Body-mass index and mortality in a prospective cohort of U.S. Adults. N. Engl. J. Med. 1999, 341, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Choban, P.S.; Flancbaum, L. The impact of obesity on surgical outcomes: A review. J. Am. Coll. Surg. 1997, 185, 593–603. [Google Scholar] [PubMed]
- Arslan, E.; Atilgan, H.; Yavasoglu, I. The prevalence of helicobacter pylori in obese subjects. Eur. J. Intern. Med. 2009, 20, 695–697. [Google Scholar] [CrossRef] [PubMed]
- Uberos, J.; Molina-Carballo, A.; Fernandez-Puentes, V.; Rodriguez-Belmonte, R.; Munoz-Hoyos, A. Overweight and obesity as risk factors for the asymptomatic carrier state of neisseria meningitidis among a paediatric population. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Morgan, O.W.; Bramley, A.; Fowlkes, A.; Freedman, D.S.; Taylor, T.H.; Gargiullo, P.; Belay, B.; Jain, S.; Cox, C.; Kamimoto, L.; et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza a(h1n1) disease. PLoS ONE 2010, 5, e9694. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.; Dhurandhar, N.V. Microbes and obesity—Interrelationship between infection, adipose tissue and the immune system. Clin. Microbiol. Infect. 2013, 19, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, R.; Syrjanen, J. Obesity and the risk and outcome of infection. Int. J. Obes. 2013, 37, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Verlaeten, O.; Griffond, B.; Khuth, S.T.; Giraudon, P.; Akaoka, H.; Belin, M.F.; Fellmann, D.; Bernard, A. Down regulation of melanin concentrating hormone in virally induced obesity. Mol. Cell. Endocrinol. 2001, 181, 207–219. [Google Scholar] [CrossRef]
- Pasarica, M.; Dhurandhar, N.V. Infectobesity: Obesity of infectious origin. Adv. Food Nutr. Res. 2007, 52, 61–102. [Google Scholar] [PubMed]
- Mitra, A.K.; Clarke, K. Viral obesity: Fact or fiction? Obes. Rev. 2010, 11, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.J.; Faust, I.M.; Hemmes, R.B.; Buskirk, D.R.; Hirsch, J.; Zabriskie, J.B. A virally induced obesity syndrome in mice. Science 1982, 216, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.K.; Ow, C.L.; Smith, R.E. Rous-associated virus type 7 induces a syndrome in chickens characterized by stunting and obesity. Infect. Immun. 1983, 39, 410–422. [Google Scholar] [PubMed]
- Dhurandhar, N.V. Is obesity caused by an adenovirus? Expert Rev. Anti-Infect. Ther. 2012, 10, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Swenson, P.D.; Lowens, M.S.; Celum, C.L.; Hierholzer, J.C. Adenovirus types 2, 8, and 37 associated with genital infections in patients attending a sexually transmitted disease clinic. J. Clin. Microbiol. 1995, 33, 2728–2731. [Google Scholar] [PubMed]
- Dhurandhar, N.V. A framework for identification of infections that contribute to human obesity. Lancet Infect. Dis. 2011, 11, 963–969. [Google Scholar] [CrossRef]
- Dhurandhar, N.V. Insulin sparing action of adenovirus 36 and its e4orf1 protein. J. Diabetes Complicat. 2013, 27, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, E.J.; Dubuisson, O.; Mashtalir, N.; Krishnapuram, R.; Hegde, V.; Dhurandhar, N.V. E4orf1: A novel ligand that improves glucose disposal in cell culture. PLoS ONE 2011, 6, e23394. [Google Scholar] [CrossRef] [PubMed]
- Rathod, M.A.; Rogers, P.M.; Vangipuram, S.D.; McAllister, E.J.; Dhurandhar, N.V. Adipogenic cascade can be induced without adipogenic media by a human adenovirus. Obesity 2009, 17, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, N.V.; Kulkarni, P.; Ajinkya, S.M.; Sherikar, A. Effect of adenovirus infection on adiposity in chicken. Vet. Microbiol. 1992, 31, 101–107. [Google Scholar] [CrossRef]
- Dhurandhar, N.V.; Kulkarni, P.R.; Ajinkya, S.M.; Sherikar, A.A.; Atkinson, R.L. Association of adenovirus infection with human obesity. Obes. Res. 1997, 5, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, N.V.; Israel, B.A.; Kolesar, J.M.; Mayhew, G.F.; Cook, M.E.; Atkinson, R.L. Increased adiposity in animals due to a human virus. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Whigham, L.D.; Israel, B.A.; Atkinson, R.L. Adipogenic potential of multiple human adenoviruses in vivo and in vitro in animals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R190–R194. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, N.V. Infectobesity: Obesity of infectious origin. J. Nutr. 2001, 131, 2794S–2797S. [Google Scholar] [PubMed]
- Dhurandhar, N.V.; Whigham, L.D.; Abbott, D.H.; Schultz-Darken, N.J.; Israel, B.A.; Bradley, S.M.; Kemnitz, J.W.; Allison, D.B.; Atkinson, R.L. Human adenovirus ad-36 promotes weight gain in male rhesus and marmoset monkeys. J. Nutr. 2002, 132, 3155–3160. [Google Scholar] [PubMed]
- Pasarica, M.; Shin, A.C.; Yu, M.; Ou Yang, H.M.; Rathod, M.; Jen, K.L.; MohanKumar, S.; MohanKumar, P.S.; Markward, N.; Dhurandhar, N.V. Human adenovirus 36 induces adiposity, increases insulin sensitivity, and alters hypothalamic monoamines in rats. Obesity 2006, 14, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, N.V.; Israel, B.A.; Kolesar, J.M.; Mayhew, G.; Cook, M.E.; Atkinson, R.L. Transmissibility of adenovirus-induced adiposity in a chicken model. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Kapila, M.; Khosla, P.; Dhurandhar, N.V. Novel short-term effects of adenovirus ad-36 on hamster lipoproteins. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Pasarica, M.; Loiler, S.; Dhurandhar, N.V. Acute effect of infection by adipogenic human adenovirus ad36. Arch. Virol. 2008, 153, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Vangipuram, S.D.; Sheele, J.; Atkinson, R.L.; Holland, T.C.; Dhurandhar, N.V. A human adenovirus enhances preadipocyte differentiation. Obes. Res. 2004, 12, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Rathod, M.; Vangipuram, S.D.; Krishnan, B.; Heydari, A.R.; Holland, T.C.; Dhurandhar, N.V. Viral mrna expression but not DNA replication is required for lipogenic effect of human adenovirus ad-36 in preadipocytes. Int. J. Obes. 2007, 31, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.; Janoska, M.; Kajon, A.E.; Metzgar, D.; Hudson, N.R.; Torres, S.; Harrach, B.; Seto, D.; Chodosh, J.; Jones, M.S. Genomic characterization of human adenovirus 36, a putative obesity agent. Virus Res. 2010, 149, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [PubMed]
- Rogers, P.M.; Fusinski, K.A.; Rathod, M.A.; Loiler, S.A.; Pasarica, M.; Shaw, M.K.; Kilroy, G.; Sutton, G.M.; McAllister, E.J.; Mashtalir, N.; et al. Human adenovirus ad-36 induces adipogenesis via its e4 orf-1 gene. Int. J. Obes. 2008, 32, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Vangipuram, S.D.; Yu, M.; Tian, J.; Stanhope, K.L.; Pasarica, M.; Havel, P.J.; Heydari, A.R.; Dhurandhar, N.V. Adipogenic human adenovirus-36 reduces leptin expression and secretion and increases glucose uptake by fat cells. Int. J. Obes. 2007, 31, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Pasarica, M.; Mashtalir, N.; McAllister, E.J.; Kilroy, G.E.; Koska, J.; Permana, P.; de Courten, B.; Yu, M.; Ravussin, E.; Gimble, J.M.; et al. Adipogenic human adenovirus ad-36 induces commitment, differentiation, and lipid accumulation in human adipose-derived stem cells. Stem Cells 2008, 26, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Yu, Y.; Zhang, X.H.; Floyd, E.Z.; Cefalu, W.T. Human adenovirus 36 decreases fatty acid oxidation and increases de novo lipogenesis in primary cultured human skeletal muscle cells by promoting cidec/fsp27 expression. Int. J. Obes. 2010, 34, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H. Why is not there a match between the serological and genomic prevalence of adenovirus 36? J. Clin. Virol. 2013, 56, 370–371. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Na, H.N.; Atkinson, R.L.; Dhurandhar, N.V. Genomic stability of adipogenic human adenovirus 36. Int. J. Obes. 2014, 38, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Salehian, B.; Forman, S.J.; Kandeel, F.R.; Bruner, D.E.; He, J.; Atkinson, R.L. Adenovirus 36 DNA in adipose tissue of patient with unusual visceral obesity. Emerg. Infect. Dis. 2010, 16, 850–852. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.L.; Dhurandhar, N.V.; Allison, D.B.; Bowen, R.L.; Israel, B.A.; Albu, J.B.; Augustus, A.S. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int. J. Obes. 2005, 29, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Broderick, M.P.; Hansen, C.J.; Irvine, M.; Metzgar, D.; Campbell, K.; Baker, C.; Russell, K.L. Adenovirus 36 seropositivity is strongly associated with race and gender, but not obesity, among us military personnel. Int. J. Obes. 2010, 34, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Goossens, V.J.; deJager, S.A.; Grauls, G.E.; Gielen, M.; Vlietinck, R.F.; Derom, C.A.; Loos, R.J.; Rensen, S.S.; Buurman, W.A.; Greve, J.W.; et al. Lack of evidence for the role of human adenovirus-36 in obesity in a european cohort. Obesity 2011, 19, 220–221. [Google Scholar] [CrossRef] [PubMed]
- Bil-Lula, I.; Plonek, T.; Wozniak, M. Lack of adenovirus DNA in mediastinal adipose tissue of obese/overweight adults with cardiovascular disorders? J. Med. Virol. 2014, 86, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Na, H.N.; Hong, Y.M.; Kim, J.; Kim, H.K.; Jo, I.; Nam, J.H. Association between human adenovirus-36 and lipid disorders in korean schoolchildren. Int. J. Obes. 2010, 34, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Almgren, M.; Atkinson, R.; He, J.; Hilding, A.; Hagman, E.; Wolk, A.; Thorell, A.; Marcus, C.; Naslund, E.; Ostenson, C.G.; et al. Adenovirus-36 is associated with obesity in children and adults in sweden as determined by rapid elisa. PLoS ONE 2012, 7, e41652. [Google Scholar] [CrossRef] [PubMed]
- Aldhoon-Hainerova, I.; Zamrazilova, H.; Atkinson, R.L.; Dusatkova, L.; Sedlackova, B.; Hlavaty, P.; Lee, Z.P.; Kunesova, M.; Hainer, V. Clinical and laboratory characteristics of 1179 czech adolescents evaluated for antibodies to human adenovirus 36. Int. J. Obes. 2014, 38, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.L.; Lee, I.; Shin, H.J.; He, J. Human adenovirus-36 antibody status is associated with obesity in children. Int. J. Pediatr. Obes. 2010, 5, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Cakmakliogullari, E.K.; Sanlidag, T.; Ersoy, B.; Akcali, S.; Var, A.; Cicek, C. Are human adenovirus-5 and 36 associated with obesity in children? J. Investig. Med. 2014, 62, 821–824. [Google Scholar] [PubMed]
- Gabbert, C.; Donohue, M.; Arnold, J.; Schwimmer, J.B. Adenovirus 36 and obesity in children and adolescents. Pediatrics 2010, 126, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Parra-Rojas, I.; del Moral-Hernandez, O.; Salgado-Bernabe, A.B.; Guzman-Guzman, I.P.; Salgado-Goytia, L.; Munoz-Valle, J.F. Adenovirus-36 seropositivity and its relation with obesity and metabolic profile in children. Int. J. Endocrinol. 2013, 2013, e463194. [Google Scholar] [CrossRef] [PubMed]
- Tosh, A.K.; Broy-Aschenbrenner, A.; El Khatib, J.; Ge, B. Adenovirus-36 antibody status & bmi comparison among obese missouri adolescents. Mo. Med. 2012, 109, 402–403. [Google Scholar] [PubMed]
- Vander Wal, J.S.; Huelsing, J.; Dubuisson, O.; Dhurandhar, N.V. An observational study of the association between adenovirus 36 antibody status and weight loss among youth. Obes. Facts 2013, 6, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Hara, K.; Kadowaki, T. Association of adenovirus 36 infection with obesity and metabolic markers in humans: A meta-analysis of observational studies. PLoS ONE 2012, 7, e42031. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Wang, H.; Song, Y.; Wei, L.; Lavebratt, C.; Zhang, F.; Gu, H. Serological data analyses show that adenovirus 36 infection is associated with obesity: A meta-analysis involving 5739 subjects. Obesity 2014, 22, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Cefalu, W.T.; Zhang, X.H.; Yu, Y.; Qin, J.; Son, L.; Rogers, P.M.; Mashtalir, N.; Bordelon, J.R.; Ye, J.; et al. Human adenovirus type 36 enhances glucose uptake in diabetic and nondiabetic human skeletal muscle cells independent of insulin signaling. Diabetes 2008, 57, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Krishnapuram, R.; Dhurandhar, E.J.; Dubuisson, O.; Hegde, V.; Dhurandhar, N.V. Doxycycline-regulated 3t3-l1 preadipocyte cell line with inducible, stable expression of adenoviral e4orf1 gene: A cell model to study insulin-independent glucose disposal. PLoS ONE 2013, 8, e60651. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, E.J.; Krishnapuram, R.; Hegde, V.; Dubuisson, O.; Tao, R.; Dong, X.C.; Ye, J.; Dhurandhar, N.V. E4orf1 improves lipid and glucose metabolism in hepatocytes: A template to improve steatosis & hyperglycemia. PLoS ONE 2012, 7, e47813. [Google Scholar] [PubMed]
- Lynch, L.A.; O’Connell, J.M.; Kwasnik, A.K.; Cawood, T.J.; O’Farrelly, C.; O’Shea, D.B. Are natural killer cells protecting the metabolically healthy obese patient? Obesity 2009, 17, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Youm, Y.H.; Vandanmagsar, B.; Rood, J.; Kumar, K.G.; Butler, A.A.; Dixit, V.D. Obesity accelerates thymic aging. Blood 2009, 114, 3803–3812. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Nehlsen-Cannarella, S.I.; Henson, D.A.; Butterworth, D.E.; Fagoaga, O.R.; Warren, B.J.; Rainwater, M.K. Immune response to obesity and moderate weight loss. Int. J. Obes. Relat. Metab. Disord. 1996, 20, 353–360. [Google Scholar] [PubMed]
- O'Rourke, R.W.; Kay, T.; Scholz, M.H.; Diggs, B.; Jobe, B.A.; Lewinsohn, D.M.; Bakke, A.C. Alterations in T-cell subset frequency in peripheral blood in obesity. Obes. Surg. 2005, 15, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, D.; Corrigan, M.; Dunne, M.R.; Jackson, R.; Woods, C.; Gaoatswe, G.; Moynagh, P.N.; O’Connell, J.; Hogan, A.E. Changes in human dendritic cell number and function in severe obesity may contribute to increased susceptibility to viral infection. Int. J. Obes. 2013, 37, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Na, H.N.; Nam, J.H. Adenovirus 36 as an obesity agent maintains the obesity state by increasing mcp-1 and inducing inflammation. J. Infect. Dis. 2012, 205, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Stienstra, R.; Duval, C.; Muller, M.; Kersten, S. Ppars, obesity, and inflammation. PPAR Res. 2007, 2007, e95974. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, J.; Guerre-Millo, M.; Clement, K. Adipose tissue inflammation and liver pathology in human obesity. Diabetes Metab. 2008, 34, 658–663. [Google Scholar] [CrossRef]
- Bastard, J.P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar] [PubMed]
- Vendrell, J.; Broch, M.; Vilarrasa, N.; Molina, A.; Gomez, J.M.; Gutierrez, C.; Simon, I.; Soler, J.; Richart, C. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: Relationships in obesity. Obes. Res. 2004, 12, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Na, H.N.; Kim, H.; Nam, J.H. Novel genes and cellular pathways related to infection with adenovirus-36 as an obesity agent in human mesenchymal stem cells. Int. J. Obes. 2012, 36, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. Mcp-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Kirk, E.A.; Sagawa, Z.K.; McDonald, T.O.; O’Brien, K.D.; Heinecke, J.W. Monocyte chemoattractant protein deficiency fails to restrain macrophage infiltration into adipose tissue [corrected]. Diabetes 2008, 57, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Fujisaka, S.; Usui, I.; Bukhari, A.; Ikutani, M.; Oya, T.; Kanatani, Y.; Tsuneyama, K.; Nagai, Y.; Takatsu, K.; Urakaze, M.; et al. Regulatory mechanisms for adipose tissue m1 and m2 macrophages in diet-induced obese mice. Diabetes 2009, 58, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Tordjman, J.; Poitou, C.; Darakhshan, F.; Hugol, D.; Basdevant, A.; Aissat, A.; Guerre-Millo, M.; Clement, K. Human adipose tissue macrophages: M1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J. Clin. Endocrinol. Metab. 2009, 94, 4619–4623. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, S.E.; Gee, L.L.; Wachtel, M.S.; Frezza, E.E. Adipose tissue: The new endocrine organ? A review article. Dig. Dis. Sci. 2009, 54, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, J.; Heimburger, O.; Lindholm, B.; Stenvinkel, P. Adipose tissue and its relation to inflammation: The role of adipokines. J. Ren. Nutr. 2005, 15, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M. Adipose tissue dysfunction in obesity. Exp. Clin. Endocrinol. Diabetes 2009, 117, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Gale, S.M.; Castracane, V.D.; Mantzoros, C.S. Energy homeostasis, obesity and eating disorders: Recent advances in endocrinology. J. Nutr. 2004, 134, 295–298. [Google Scholar] [PubMed]
- Mancuso, P. Obesity and respiratory infections: Does excess adiposity weigh down host defense? Pulm. Pharmacol. Ther. 2013, 26, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.G.; Cowley, M.A.; Munzberg, H. Mechanisms of leptin action and leptin resistance. Annu. Rev. Physiol. 2008, 70, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.D. Leptin resistance and the response to positive energy balance. Physiol. Behav. 2008, 94, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Martin-Romero, C.; Santos-Alvarez, J.; Goberna, R.; Sanchez-Margalet, V. Human leptin enhances activation and proliferation of human circulating t lymphocytes. Cell. Immunol. 2000, 199, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Iikuni, N.; Lam, Q.L.; Lu, L.; Matarese, G.; La Cava, A. Leptin and inflammation. Curr. Immunol. Rev. 2008, 4, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Kohm, A.P.; Sanders, V.M. Norepinephrine and beta 2-adrenergic receptor stimulation regulate cd4+ t and b lymphocyte function in vitro and in vivo. Pharmacol. Rev. 2001, 53, 487–525. [Google Scholar] [PubMed]
- Na, H.N.; Nam, J.H. Proof-of-concept for a virus-induced obesity vaccine; vaccination against the obesity agent adenovirus 36. Int. J. Obes. 2014, 38, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
- Na, H.N.; Kim, H.; Nam, J.H. Prophylactic and therapeutic vaccines for obesity. Clin. Exp. Vaccine Res. 2014, 3, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Trovato, G.M.; Castro, A.; Tonzuso, A.; Garozzo, A.; Martines, G.F.; Pirri, C.; Trovato, F.; Catalano, D. Human obesity relationship with ad36 adenovirus and insulin resistance. Int. J. Obes. 2009, 33, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Trovato, G.M.; Martines, G.F.; Garozzo, A.; Tonzuso, A.; Timpanaro, R.; Pirri, C.; Trovato, F.M.; Catalano, D. Ad36 adipogenic adenovirus in human non-alcoholic fatty liver disease. Liver Int. 2010, 30, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Krishnapuram, R.; Dhurandhar, E.J.; Dubuisson, O.; Kirk-Ballard, H.; Bajpeyi, S.; Butte, N.; Sothern, M.S.; Larsen-Meyer, E.; Chalew, S.; Bennett, B.; et al. Template to improve glycemic control without reducing adiposity or dietary fat. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E779–E789. [Google Scholar] [CrossRef] [PubMed]
- Na, H.N.; Kim, J.; Lee, H.S.; Shim, K.W.; Kimm, H.; Jee, S.H.; Jo, I.; Nam, J.H. Association of human adenovirus-36 in overweight korean adults. Int. J. Obes. 2012, 36, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Trovato, G.M.; Martines, G.F.; Trovato, F.M.; Pirri, C.; Pace, P.; Garozzo, A.; Castro, A.; Catalano, D. Adenovirus-36 seropositivity enhances effects of nutritional intervention on obesity, bright liver, and insulin resistance. Dig. Dis. Sci. 2012, 57, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Dubuisson, O.; Rubicz, R.; Liu, N.; Allison, D.B.; Curran, J.E.; Comuzzie, A.G.; Blangero, J.; Leach, C.T.; Goring, H.; et al. Long-term changes in adiposity and glycemic control are associated with past adenovirus infection. Diabetes Care 2013, 36, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Laing, E.M.; Tripp, R.A.; Pollock, N.K.; Baile, C.A.; Della-Fera, M.A.; Rayalam, S.; Tompkins, S.M.; Keys, D.A.; Lewis, R.D. Adenovirus 36, adiposity, and bone strength in late-adolescent females. J. Bone Miner. Res. 2013, 28, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.K.; Pollock, N.K.; Laing, E.M.; Warden, S.J.; Hill Gallant, K.M.; Hausman, D.B.; Tripp, R.A.; McCabe, L.D.; McCabe, G.P.; Weaver, C.M.; et al. Association of adenovirus 36 infection with adiposity and inflammatory-related markers in children. J. Clin. Endocrinol. Metab. 2014, 99, 3240–3246. [Google Scholar] [CrossRef] [PubMed]
- Voss, J.D.; Burnett, D.G.; Olsen, C.H.; Haverkos, H.W.; Atkinson, R.L. Adenovirus 36 antibodies associated with clinical diagnosis of overweight/obesity but not bmi gain: A military cohort study. J. Clin. Endocrinol. Metab. 2014, 99, E1708–E1712. [Google Scholar] [CrossRef] [PubMed]
- Karamese, M.; Altoparlak, U.; Turgut, A.; Aydogdu, S.; Karamese, S.A. The relationship between adenovirus-36 seropositivity, obesity and metabolic profile in turkish children and adults. Epidemiol. Infect. 2015, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ergin, S.; Altan, E.; Pilanci, O.; Sirekbasan, S.; Cortuk, O.; Cizmecigil, U.; Ersin, I.; Elbey, H.; Dinc, H.O.; Habip, Z.; et al. The role of adenovirus 36 as a risk factor in obesity: The first clinical study made in the fatty tissues of adults in turkey. Microb. Pathog. 2015, 80, 57–62. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponterio, E.; Gnessi, L. Adenovirus 36 and Obesity: An Overview. Viruses 2015, 7, 3719-3740. https://doi.org/10.3390/v7072787
Ponterio E, Gnessi L. Adenovirus 36 and Obesity: An Overview. Viruses. 2015; 7(7):3719-3740. https://doi.org/10.3390/v7072787
Chicago/Turabian StylePonterio, Eleonora, and Lucio Gnessi. 2015. "Adenovirus 36 and Obesity: An Overview" Viruses 7, no. 7: 3719-3740. https://doi.org/10.3390/v7072787
APA StylePonterio, E., & Gnessi, L. (2015). Adenovirus 36 and Obesity: An Overview. Viruses, 7(7), 3719-3740. https://doi.org/10.3390/v7072787





