BST2/Tetherin Inhibition of Alphavirus Exit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses and Plasmids
2.2. Antibodies
2.3. Cell Lines
2.4. siRNA Mediated Depletion of Tetherin
2.5. Analysis of Tetherin Expression
2.6. Virus and VLP Release Assays
2.7. Quantitation of Release Efficiency
2.8. Immunofluorescence Analysis
2.9. Statistics
3. Results
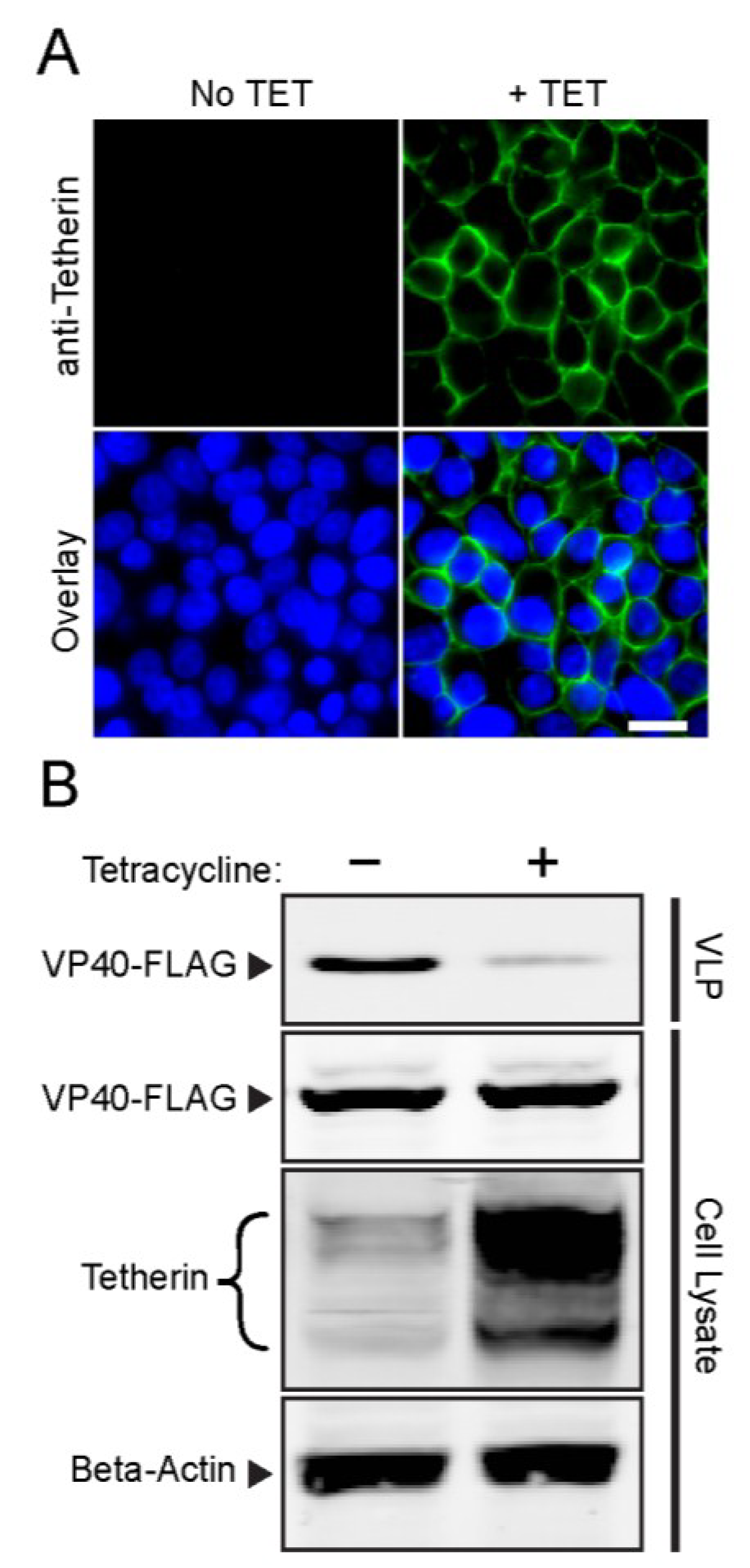
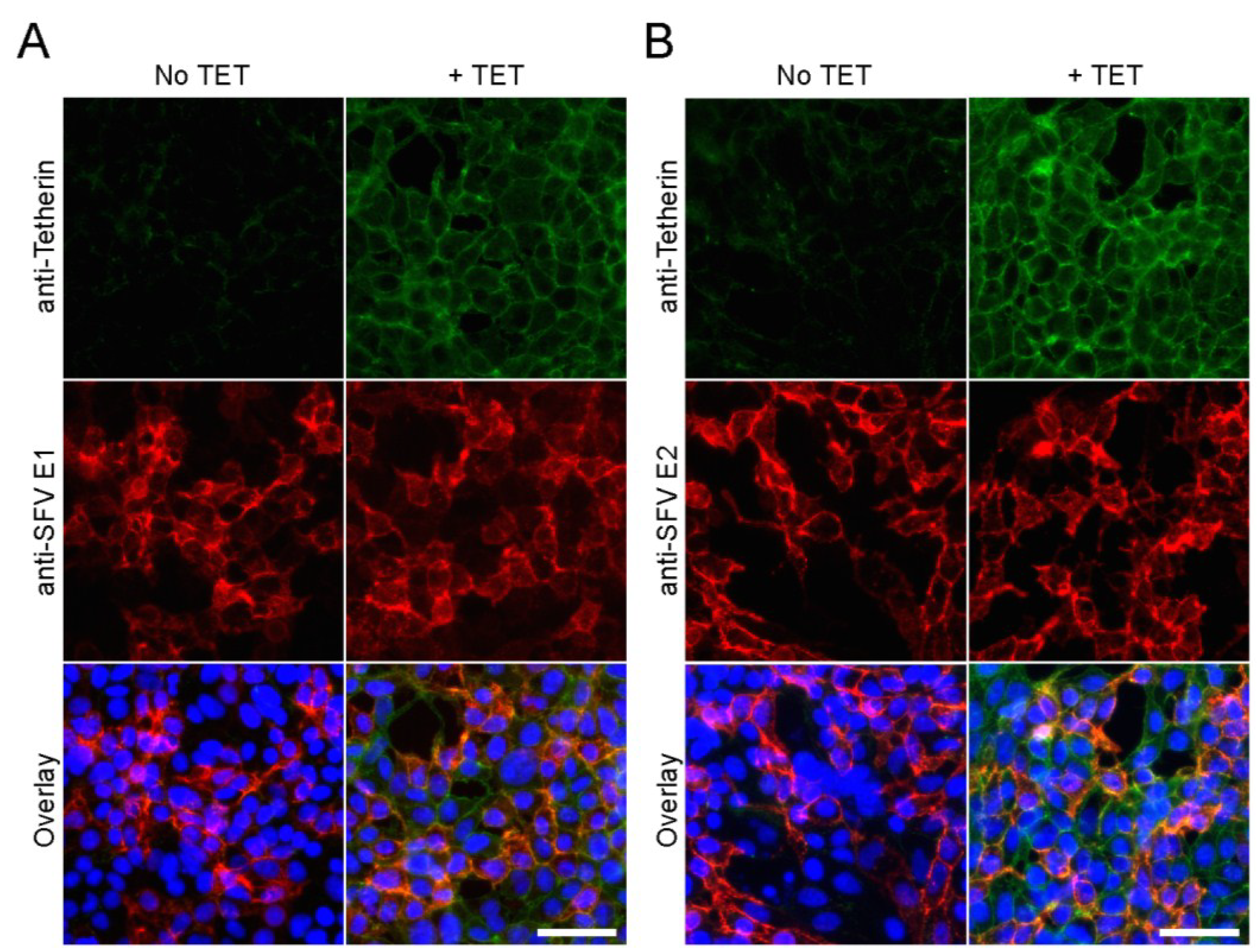
3.1. Development and Validation of an Inducible Tetherin-Expressing Cell Line
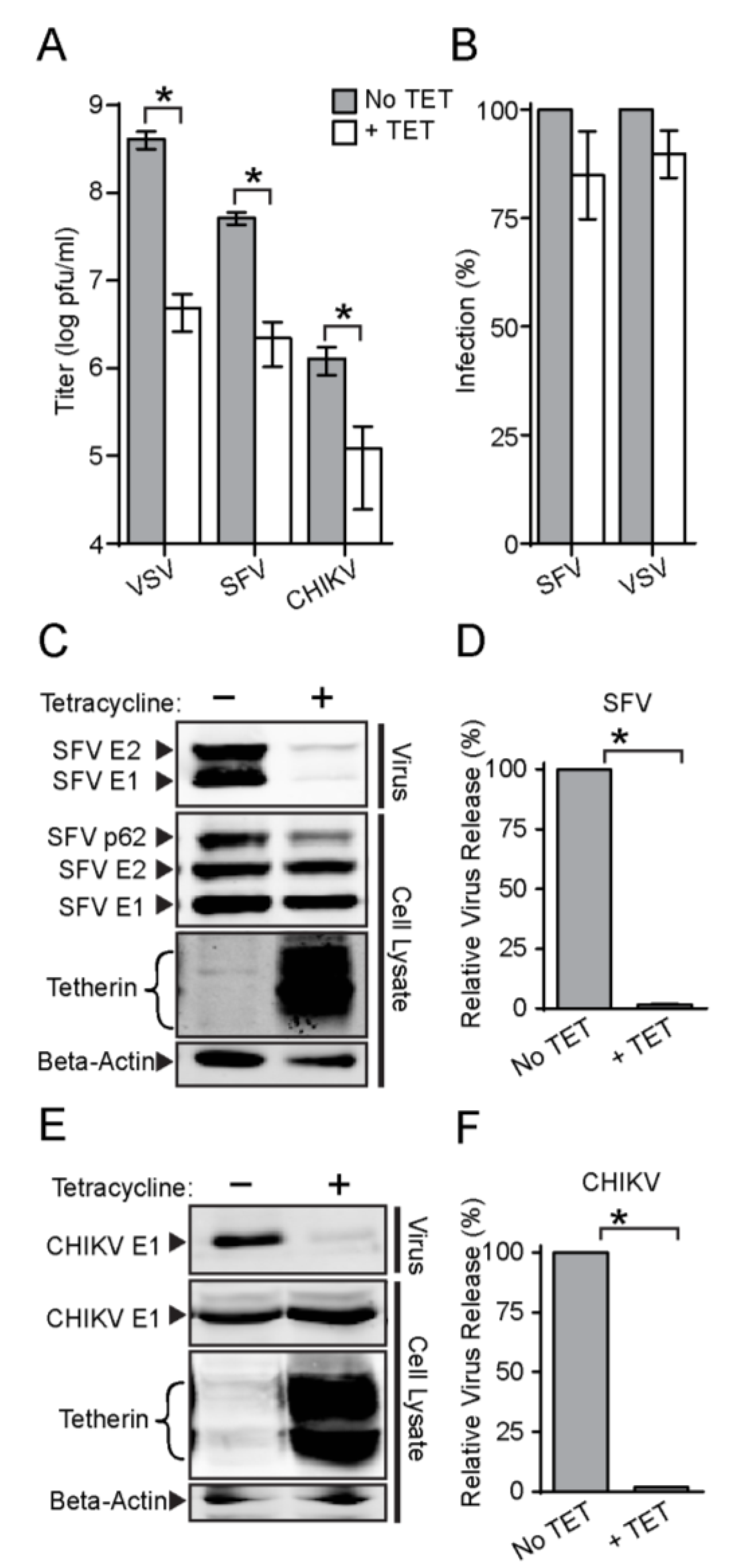
3.2. Tetherin Inhibits Release of SFV and CHIKV
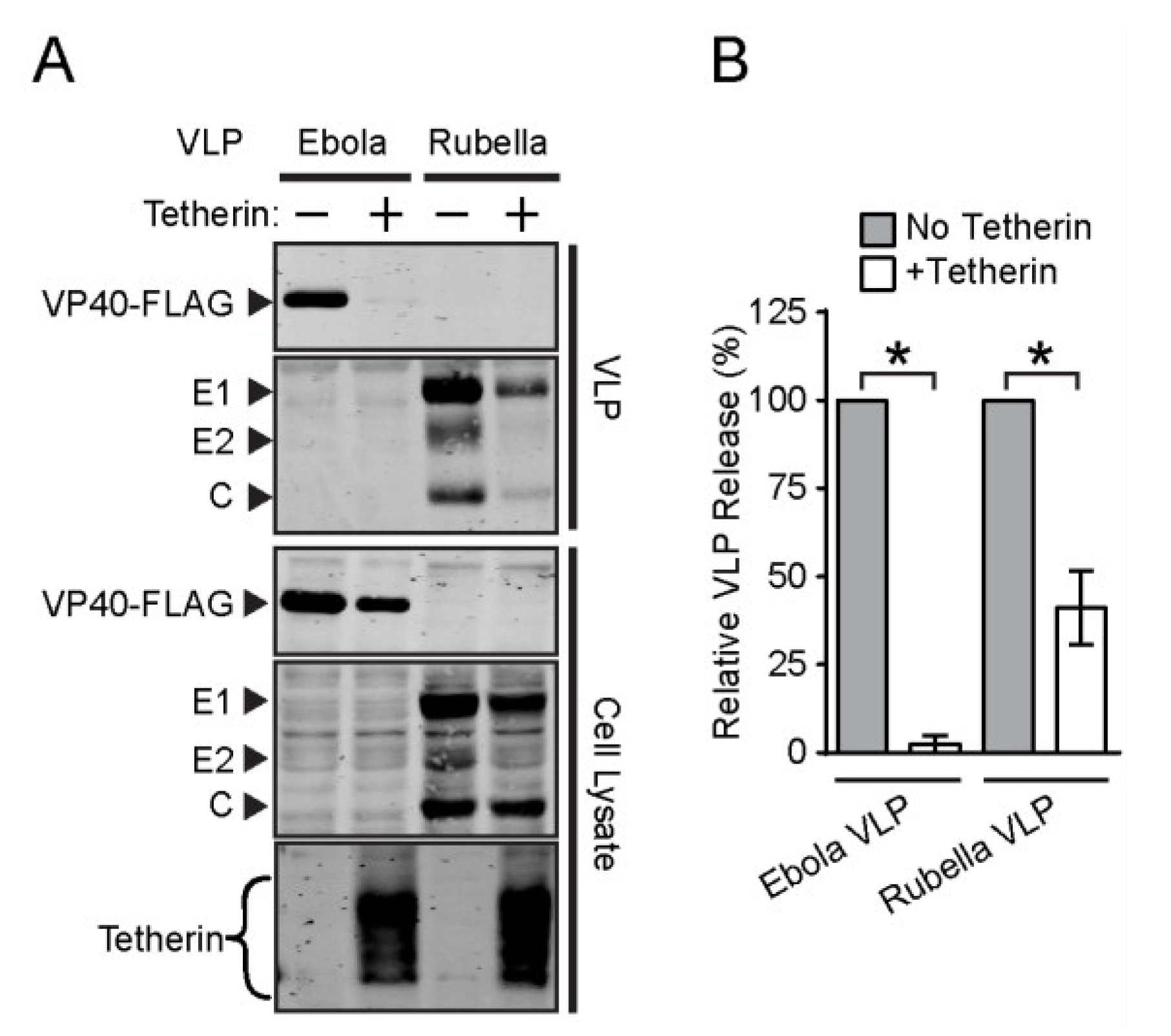
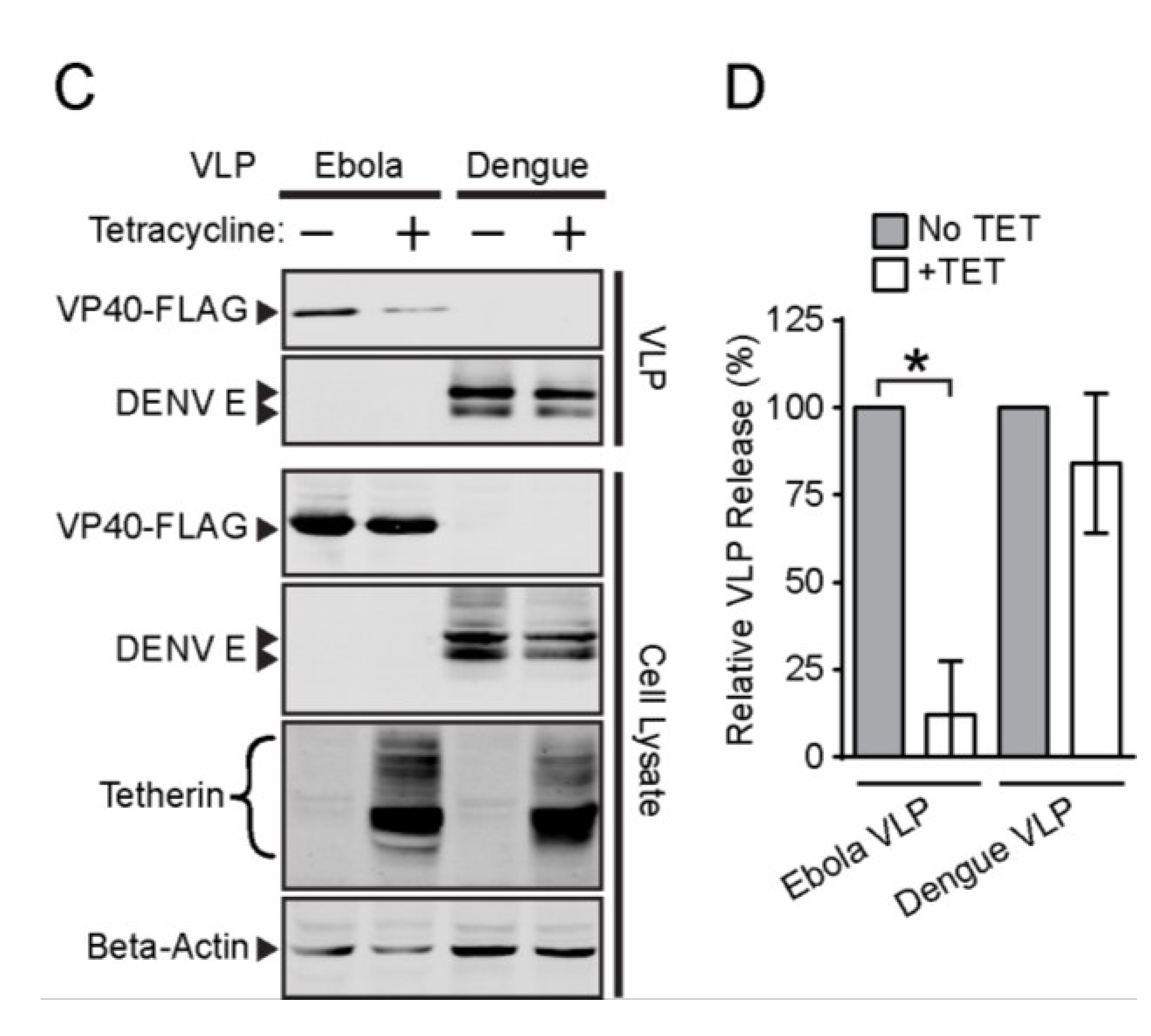
3.3. Effect of Tetherin on the Release of Rubella and Dengue Virus
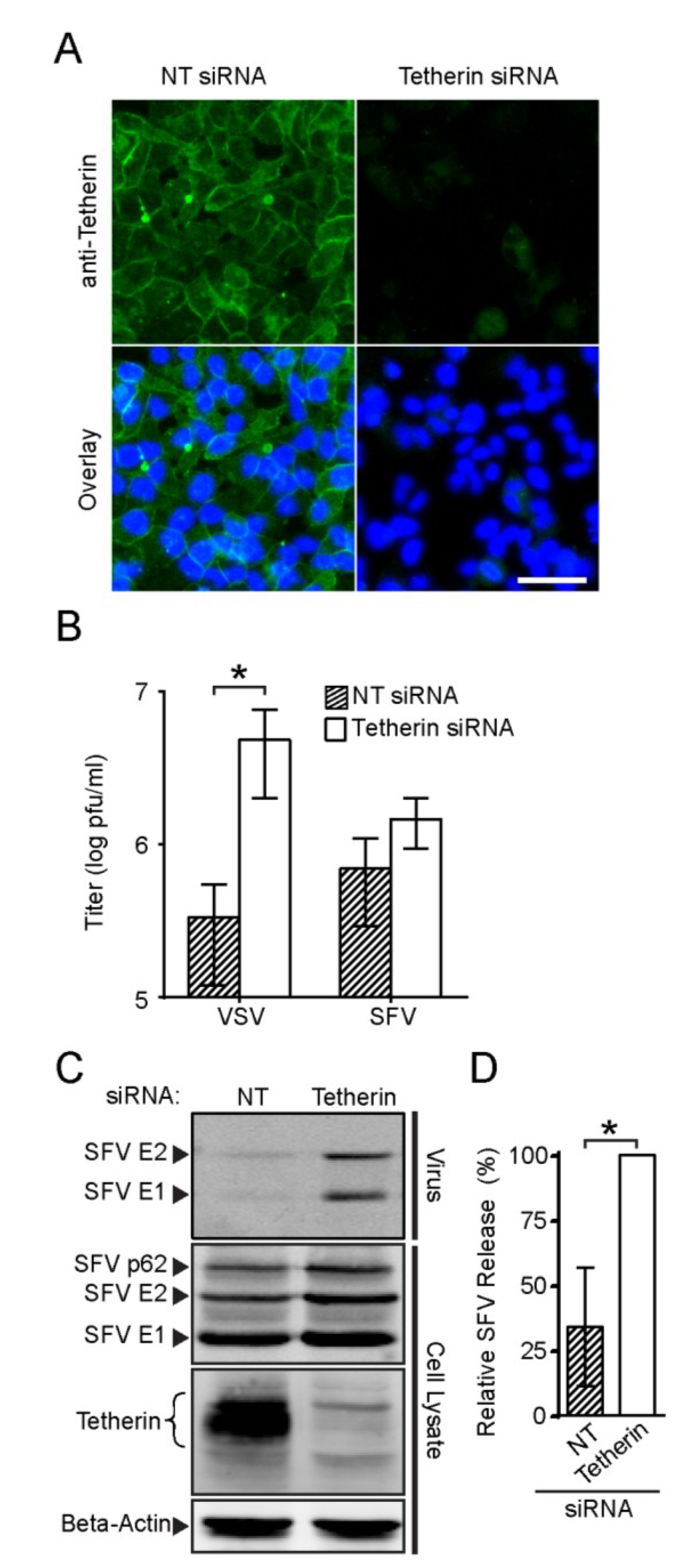
3.4. Endogenous Tetherin Inhibits SFV Release
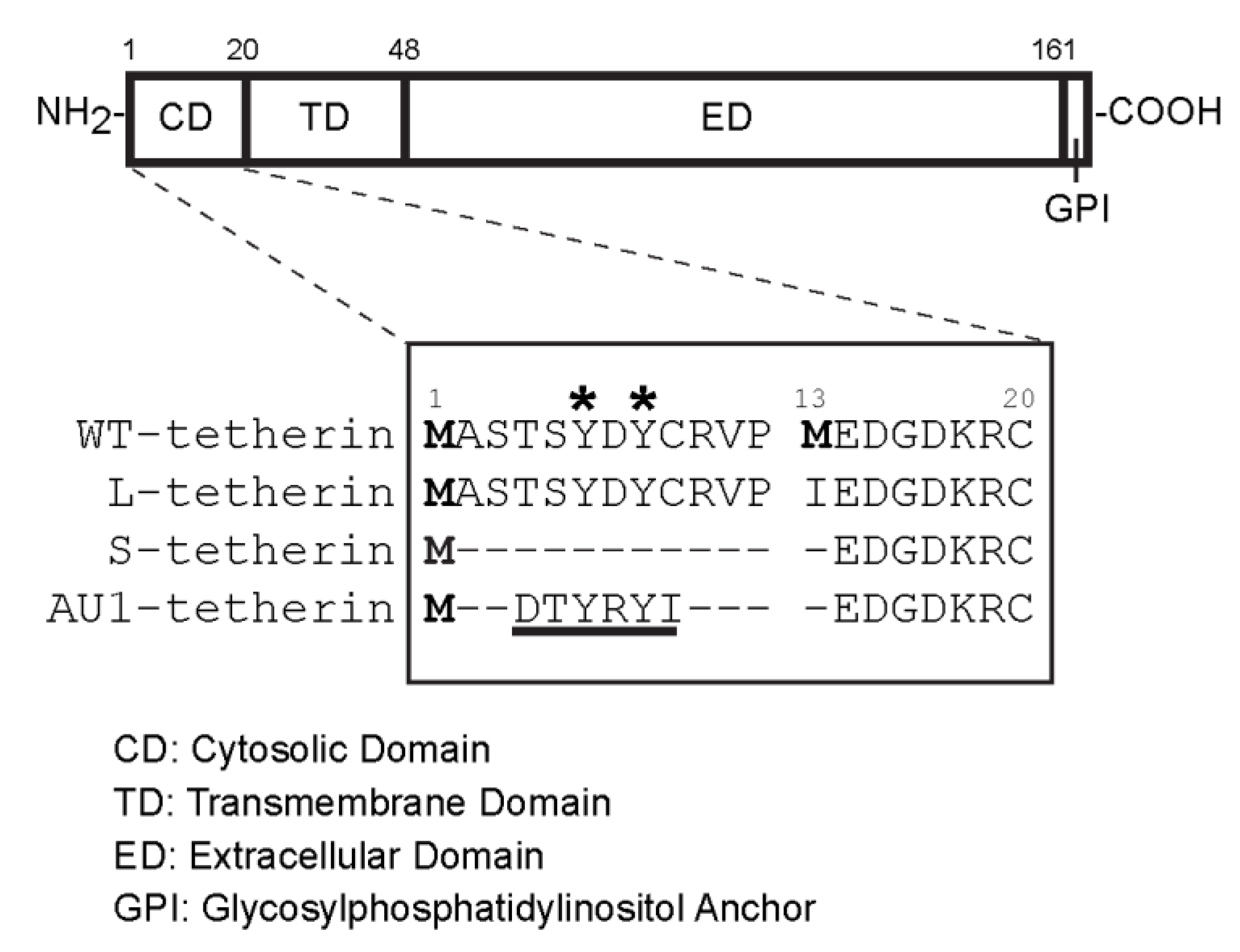
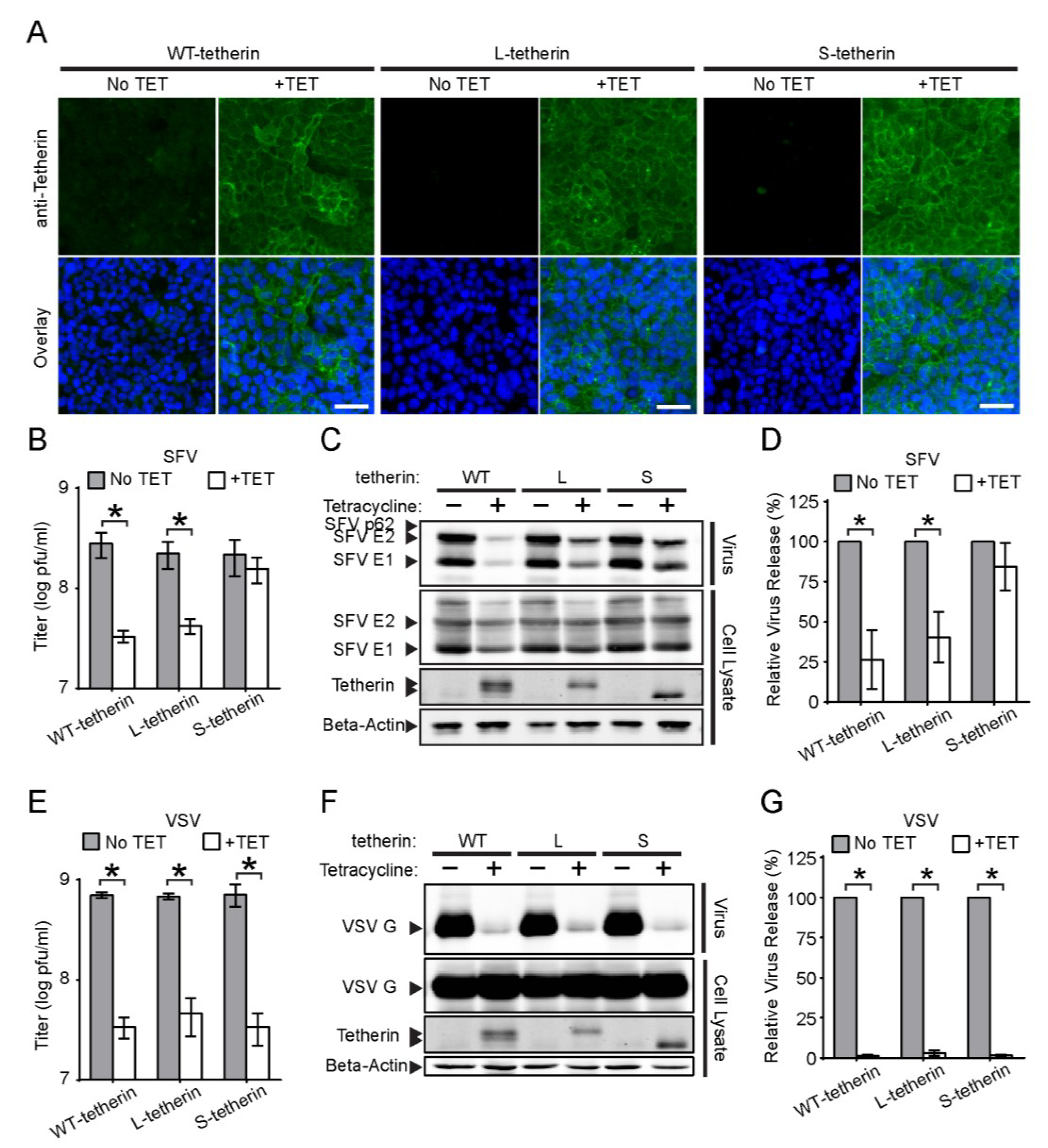
3.5. Effects of Different Tetherin Isoforms on the Release of SFV and VSV
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuhn, R.J. Togaviridae. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2013; Volume 1, pp. 629–650. [Google Scholar]
- Weaver, S.C.; Winegar, R.; Manger, I.D.; Forrester, N.L. Alphaviruses: Population genetics and determinants of emergence. Antivir. Res. 2012, 94, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Paessler, S.; Weaver, S.C. Vaccines for venezuelan equine encephalitis. Vaccine 2009, 27 (Suppl. 4), D80–D85. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Snyder, J.E.; Kuhn, R.J. A structural and functional perspective of alphavirus replication and assembly. Future Microbiol. 2009, 4, 837–856. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Zhang, W.; Gabler, S.; Chipman, P.R.; Strauss, E.G.; Strauss, J.H.; Baker, T.S.; Kuhn, R.J.; Rossmann, M.G. Mapping the structure and function of the e1 and e2 glycoproteins in alphaviruses. Structure 2006, 14, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Voss, J.E.; Vaney, M.C.; Duquerroy, S.; Vonrhein, C.; Girard-Blanc, C.; Crublet, E.; Thompson, A.; Bricogne, G.; Rey, F.A. Glycoprotein organization of chikungunya virus particles revealed by x-ray crystallography. Nature 2010, 468, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jose, J.; Xiang, Y.; Kuhn, R.J.; Rossmann, M.G. Structural changes of envelope proteins during alphavirus fusion. Nature 2010, 468, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Kielian, M.; Chanel-Vos, C.; Liao, M. Alphavirus entry and membrane fusion. Viruses 2010, 2, 796–825. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Schelhaas, M.; Helenius, A. Virus entry by endocytosis. Annu. Rev. Biochem. 2010, 79, 803–833. [Google Scholar] [CrossRef] [PubMed]
- Kielian, M. Mechanisms of virus membrane fusion proteins. Annu. Rev. Virol. 2014, 1, 171–189. [Google Scholar] [CrossRef]
- Martinez, M.G.; Snapp, E.L.; Perumal, G.S.; Macaluso, F.P.; Kielian, M. Imaging the alphavirus exit pathway. J. Virol. 2014, 88, 6922–6933. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H. The ESCRT complexes. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.M.; Hanson, P.I.; Kielian, M. Ubiquitin depletion and dominant-negative vps4 inhibit rhabdovirus budding without affecting alphavirus budding. J. Virol. 2007, 81, 13631–13639. [Google Scholar] [CrossRef] [PubMed]
- Erikson, E.; Adam, T.; Schmidt, S.; Lehmann-Koch, J.; Over, B.; Goffinet, C.; Harter, C.; Bekeredjian-Ding, I.; Sertel, S.; Lasitschka, F.; et al. In vivo expression profile of the antiviral restriction factor and tumor-targeting antigen cd317/bst-2/hm1.24/tetherin in humans. Proc. Natl. Acad. Sci. USA 2011, 108, 13688–13693. [Google Scholar] [CrossRef] [PubMed]
- Neil, S.J.; Zang, T.; Bieniasz, P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 vpu. Nature 2008, 451, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Le Tortorec, A.; Neil, S.J. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 2009, 83, 11966–11978. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.; Viswanathan, K.; Douglas, J.L.; Hines, J.; Gustin, J.; Moses, A.V.; Fruh, K. Molecular mechanism of bst2/tetherin downregulation by k5/mir2 of kaposi's sarcoma-associated herpesvirus. J. Virol. 2009, 83, 9672–9681. [Google Scholar] [CrossRef] [PubMed]
- Kaletsky, R.L.; Francica, J.R.; Agrawal-Gamse, C.; Bates, P. Tetherin-mediated restriction of filovirus budding is antagonized by the ebola glycoprotein. Proc. Natl. Acad. Sci. USA 2009, 106, 2886–2891. [Google Scholar] [CrossRef] [PubMed]
- Weidner, J.M.; Jiang, D.; Pan, X.B.; Chang, J.; Block, T.M.; Guo, J.T. Interferon-induced cell membrane proteins, ifitm3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J. Virol. 2010, 84, 12646–12657. [Google Scholar] [CrossRef] [PubMed]
- Le Tortorec, A.; Willey, S.; Neil, S.J. Antiviral inhibition of enveloped virus release by tetherin/bst-2: Action and counteraction. Viruses 2011, 3, 520–540. [Google Scholar] [CrossRef] [PubMed]
- Neil, S.J. The antiviral activities of tetherin. Curr. Top. Microbiol. Immunol. 2013, 371, 67–104. [Google Scholar] [PubMed]
- Swiecki, M.; Omattage, N.S.; Brett, T.J. Bst-2/tetherin: Structural biology, viral antagonism, and immunobiology of a potent host antiviral factor. Mol. Immunol. 2013, 54, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Hinz, A.; Miguet, N.; Natrajan, G.; Usami, Y.; Yamanaka, H.; Renesto, P.; Hartlieb, B.; McCarthy, A.A.; Simorre, J.P.; Gottlinger, H.; et al. Structural basis of HIV-1 tethering to membranes by the bst-2/tetherin ectodomain. Cell Host Microbe 2010, 7, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Kupzig, S.; Korolchuk, V.; Rollason, R.; Sugden, A.; Wilde, A.; Banting, G. Bst-2/hm1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 2003, 4, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Rollason, R.; Korolchuk, V.; Hamilton, C.; Schu, P.; Banting, G. Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif. J. Cell Sci. 2007, 120, 3850–3858. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, N.; Kuronita, T.; Tanaka, R.; Muto, T.; Hirota, Y.; Takigawa, A.; Fujita, H.; Aso, Y.; Amano, J.; Tanaka, Y. Hm1.24 is internalized from lipid rafts by clathrin-mediated endocytosis through interaction with alpha-adaptin. J. Biol. Chem. 2009, 284, 15927–15941. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, S.; Bieniasz, P.D. Mechanism of HIV-1 virion entrapment by tetherin. PLOS Pathog. 2013, 9, e1003483. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, K.; Ryo, A.; Murakami, T.; Ohba, K.; Yamaoka, S.; Fukuda, M.; Guatelli, J.; Yamamoto, N. Bca2/rabring7 promotes tetherin-dependent HIV-1 restriction. PLOS Pathog. 2009, 5, e1000700. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, N.; Guatelli, J. HIV-1 vpu inhibits accumulation of the envelope glycoprotein within clathrin-coated, gag-containing endosomes. Cell. Microbiol. 2008, 10, 1040–1057. [Google Scholar] [CrossRef] [PubMed]
- Cocka, L.J.; Bates, P. Identification of alternatively translated tetherin isoforms with differing antiviral and signaling activities. PLOS Pathog. 2012, 8, e1002931. [Google Scholar] [CrossRef] [PubMed]
- Tokarev, A.; Suarez, M.; Kwan, W.; Fitzpatrick, K.; Singh, R.; Guatelli, J. Stimulation of nf-kappab activity by the HIV restriction factor bst2. J. Virol. 2013, 87, 2046–2057. [Google Scholar] [CrossRef] [PubMed]
- Galao, R.P.; Le Tortorec, A.; Pickering, S.; Kueck, T.; Neil, S.J. Innate sensing of HIV-1 assembly by tetherin induces nfkappab-dependent proinflammatory responses. Cell Host Microbe 2012, 12, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Glomb-Reinmund, S.; Kielian, M. Fus-1, a ph-shift mutant of semliki forest virus, acts by altering spike subunit interactions via a mutation in the e2 subunit. J. Virol. 1998, 72, 4281–4287. [Google Scholar] [PubMed]
- Wang, P.G.; Kudelko, M.; Lo, J.; Siu, L.Y.; Kwok, K.T.; Sachse, M.; Nicholls, J.M.; Bruzzone, R.; Altmeyer, R.M.; Nal, B. Efficient assembly and secretion of recombinant subviral particles of the four dengue serotypes using native prm and e proteins. PLOS ONE 2009, 4, e8325. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, E.; Andrew, A.J.; Kao, S.; Strebel, K. Vpu enhances HIV-1 virus release in the absence of bst-2 cell surface down-modulation and intracellular depletion. Proc. Natl. Acad. Sci. USA 2009, 106, 2868–2873. [Google Scholar] [CrossRef] [PubMed]
- Vashishtha, M.; Phalen, T.; Marquardt, M.T.; Ryu, J.S.; Ng, A.C.; Kielian, M. A single point mutation controls the cholesterol dependence of semliki forest virus entry and exit. J. Cell Biol. 1998, 140, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Lefrancois, L.; Lyles, D.S. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology 1982, 121, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Crill, W.D.; Chang, G.J. Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes. J. Virol. 2004, 78, 13975–13986. [Google Scholar] [CrossRef] [PubMed]
- Phalen, T.; Kielian, M. Cholesterol is required for infection by semliki forest virus. J. Cell Biol. 1991, 112, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Kielian, M.C.; Keränen, S.; Kääriäinen, L.; Helenius, A. Membrane fusion mutants of semliki forest virus. J. Cell Biol. 1984, 98, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Umashankar, M.; Kielian, M. In vitro and in vivo studies identify important features of dengue virus pr-E protein interactions. PLOS Pathog. 2010, 6, e1001157. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [PubMed]
- Uhlen, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; et al. Towards a knowledge-based human protein atlas. Nat. Biotechnol. 2010, 28, 1248–1250. [Google Scholar] [CrossRef] [PubMed]
- Neil, S.J.; Sandrin, V.; Sundquist, W.I.; Bieniasz, P.D. An interferon-alpha-induced tethering mechanism inhibits HIV-1 and ebola virus particle release but is counteracted by the HIV-1 vpu protein. Cell Host Microbe 2007, 2, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, K.; Smith, M.S.; Malouli, D.; Mansouri, M.; Nelson, J.A.; Fruh, K. Bst2/tetherin enhances entry of human cytomegalovirus. PLOS Pathog. 2011, 7, e1002332. [Google Scholar] [CrossRef] [PubMed]
- Helenius, A.; Marsh, M.; White, J. Inhibition of semliki forest virus penetration by lysosomotropic weak bases. J. Gen. Virol. 1982, 58, 47–61. [Google Scholar] [CrossRef] [PubMed]
- DuBois, R.M.; Vaney, M.C.; Tortorici, M.A.; Kurdi, R.A.; Barba-Spaeth, G.; Krey, T.; Rey, F.A. Functional and evolutionary insight from the crystal structure of rubella virus protein e1. Nature 2013, 493, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.J.; Zhang, W.; Rossman, M.G.; Pletnev, S.V.; Corver, J.; Lenches, E.; Jones, C.T.; Mukhopadhyay, S.; Chipman, P.R.; Strauss, E.G.; et al. Structure of dengue virus: Implications for flavivirus organization, maturation, and fusion. Cell 2002, 108, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Risco, C.; Carrascosa, J.L.; Frey, T.K. Structural maturation of rubella virus in the golgi complex. Virology 2003, 312, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Hobman, T.C.; Lundstrom, M.L.; Mauracher, C.A.; Woodward, L.; Gillam, S.; Farquhar, M.G. Assembly of rubella virus structural proteins into virus-like particles in transfected cells. Virology 1994, 202, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.B.; Han, J.C.; Cong, X.; Wei, L. Bst2/tetherin inhibits dengue virus release from human hepatoma cells. PLOS ONE 2012, 7, e51033. [Google Scholar] [CrossRef] [PubMed]
- Atasheva, S.; Akhrymuk, M.; Frolova, E.I.; Frolov, I. New parp gene with an anti-alphavirus function. J. Virol. 2012, 86, 8147–8160. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.H.; Maric, M.; Madison, M.N.; Maury, W.; Roller, R.J.; Okeoma, C.M. Bst-2/tetherin-mediated restriction of chikungunya (chikv) vlp budding is counteracted by chikv non-structural protein 1 (nsp1). Virology 2013, 438, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Mahauad-Fernandez, W.D.; Jones, P.H.; Okeoma, C.M. Critical role for bst-2 in acute chikungunya virus infection. J. Gen. Virol. 2014, 22, 068643–068640. [Google Scholar]
- Sauter, D. Counteraction of the multifunctional restriction factor tetherin. Front. Microbiol. 2014, 5, e163. [Google Scholar] [CrossRef]
- Billcliff, P.G.; Gorleku, O.A.; Chamberlain, L.H.; Banting, G. The cytosolic n-terminus of cd317/tetherin is a membrane microdomain exclusion motif. Biol. Open 2013, 2, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Galao, R.P.; Pickering, S.; Curnock, R.; Neil, S.J. Retroviral retention activates a syk-dependent hemitam in human tetherin. Cell Host Microbe 2014, 16, 291–303. [Google Scholar] [CrossRef]
- Perez-Caballero, D.; Zang, T.; Ebrahimi, A.; McNatt, M.W.; Gregory, D.A.; Johnson, M.C.; Bieniasz, P.D. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 2009, 139, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, J.; Jia, X.; McNatt, M.W.; Zang, T.; Pan, B.; Meng, W.; Wang, H.W.; Bieniasz, P.D.; Xiong, Y. Structural insight into the mechanisms of enveloped virus tethering by tetherin. Proc. Natl. Acad. Sci. USA 2010, 107, 18428–18432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mukhopadhyay, S.; Pletnev, S.V.; Baker, T.S.; Kuhn, R.J.; Rossmann, M.G. Placement of the structural proteins in sindbis virus. J. Virol. 2002, 76, 11645–11658. [Google Scholar] [CrossRef] [PubMed]
- Battisti, A.J.; Yoder, J.D.; Plevka, P.; Winkler, D.C.; Prasad, V.M.; Kuhn, R.J.; Frey, T.K.; Steven, A.C.; Rossmann, M.G. Cryo-electron tomography of rubella virus. J. Virol. 2012, 86, 11078–11085. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.C.; Baines, J.D. Herpesviruses remodel host membranes for virus egress. Nat. Rev. Microbiol. 2011, 9, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Zenner, H.L.; Mauricio, R.; Banting, G.; Crump, C.M. Herpes simplex virus 1 counteracts tetherin restriction via its virion host shutoff activity. J. Virol. 2013, 87, 13115–13123. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, C.; Pelchen-Matthews, A.; Mlcochova, P.; Marsh, M.; Milne, R.S.; Towers, G.J. Tetherin restricts herpes simplex virus 1 and is antagonized by glycoprotein m. J. Virol. 2013, 87, 13124–13133. [Google Scholar] [CrossRef] [PubMed]
- Hurtley, S.M.; Helenius, A. Protein oligomerization in the endoplasmic reticulum. Annu. Rev. Cell Biol. 1989, 5, 277–307. [Google Scholar] [CrossRef] [PubMed]
- Andrew, A.J.; Miyagi, E.; Kao, S.; Strebel, K. The formation of cysteine-linked dimers of bst-2/tetherin is important for inhibition of HIV-1 virus release but not for sensitivity to vpu. Retrovirology 2009, 6, e80. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ooi, Y.S.; Dubé, M.; Kielian, M. BST2/Tetherin Inhibition of Alphavirus Exit. Viruses 2015, 7, 2147-2167. https://doi.org/10.3390/v7042147
Ooi YS, Dubé M, Kielian M. BST2/Tetherin Inhibition of Alphavirus Exit. Viruses. 2015; 7(4):2147-2167. https://doi.org/10.3390/v7042147
Chicago/Turabian StyleOoi, Yaw Shin, Mathieu Dubé, and Margaret Kielian. 2015. "BST2/Tetherin Inhibition of Alphavirus Exit" Viruses 7, no. 4: 2147-2167. https://doi.org/10.3390/v7042147
APA StyleOoi, Y. S., Dubé, M., & Kielian, M. (2015). BST2/Tetherin Inhibition of Alphavirus Exit. Viruses, 7(4), 2147-2167. https://doi.org/10.3390/v7042147





