Abstract
Polyomaviruses are non-enveloped, dsDNA viruses that are common in mammals, including humans. All polyomaviruses encode the large T-antigen and small t-antigen proteins that share conserved functional domains, comprising binding motifs for the tumor suppressors pRb and p53, and for protein phosphatase 2A, respectively. At present, 13 different human polyomaviruses are known, and for some of them their large T-antigen and small t-antigen have been shown to possess oncogenic properties in cell culture and animal models, while similar functions are assumed for the large T- and small t-antigen of other human polyomaviruses. However, so far the Merkel cell polyomavirus seems to be the only human polyomavirus associated with cancer. The large T- and small t-antigen exert their tumorigenic effects through classical hallmarks of cancer: inhibiting tumor suppressors, activating tumor promoters, preventing apoptosis, inducing angiogenesis and stimulating metastasis. This review elaborates on the putative roles of human polyomaviruses in some of the emerging hallmarks of cancer. The reciprocal interactions between human polyomaviruses and the immune system response are discussed, a plausible role of polyomavirus-encoded and polyomavirus-induced microRNA in cancer is described, and the effect of polyomaviruses on energy homeostasis and exosomes is explored. Therapeutic strategies against these emerging hallmarks of cancer are also suggested.
1. Introduction
Polyomaviruses are naked, circular double-stranded DNA viruses that infect birds and mammals, and recently the first fish-associated polyomavirus was described [1,2]. The genome of most polyomaviruses is approximately 5000 base-pairs and encodes regulatory proteins and structural proteins. The major regulatory proteins are the large tumor antigen (LT-ag) and the small tumor antigen (st-ag), while at least two structural proteins (VP1 and VP2) form the capsid. The regulatory proteins are expressed early during infection and participate in viral replication and viral transcription, while the structural proteins are expressed later in the infection cycle [3]. Many polyomaviruses encode additional regulatory and structural proteins (e.g., ALTO, VP3, VP4, agnoprotein) [4,5,6].
Studies with mice in the 1950s initiated by Ludwik Gross, and extended by Sarah Stewart and Bernice Eddy led to the identification of the first polyomavirus. They showed that a filtrate from a mouse leukaemia could cause multiple tumors in new-born mice and later it was demonstrated that these multiple tumors were virus-indeed. Hence the virus was referred to as polyomavirus from the Greek πολύσ for many and ωµα for tumors (reviewed in [7]). The first primate polyomavirus was isolated in 1960 [8]. This virus, Simian virus 40 (SV40), was shown to transform cells, including human cells, to induce tumors in animal models, and to be present in human cancers. The oncogenic potential of SV40 primarily depends on its LT-ag, which can bind the tumor suppressor proteins p53 and pRb, interfere with DNA repair, apoptosis, cellular transcription, protein degradation, telomerase activity, immune- and inflammatory responses, and stimulate angiogenesis and cell migration. SV40 st-ag can contribute to transformation by inactivating protein phosphatase 2A [9,10]. Besides SV40 and murine polyomavirus, other non-human polyomavirus such as hamster polyomavirus, lymphotropic polyomavirus, and simian agent 12 were shown to possess oncogenic properties in cell cultures or animal models [11,12,13]. However, the oncogenic role of these viruses in their natural host is unclear. In fact, only one mammalian polyomavirus seems to be firmly associated with cancer in its genuine host. Raccoon polyomavirus (RacPyV) was first identified in tumors of frontal lobes and olfactory tracts from raccoons. Ten out of 52 (19%) raccoons had brain tumors within the cranial portion of their frontal lobe(s), and all tumors contained RacPyV DNA, though not tissues from 20 unaffected animals. RacPyV genome was episomal in all tumors tested [14]. One case of hamster polyomavirus-induced lymphoma in a hamster outside of the laboratory environment has been described [15], while two novel mammalian polyomaviruses have been isolated from benign tumors. A polyomavirus was isolated from fibropapilloma on the tongue of a sea lion, and the complete genome of another polyomavirus was amplified in a biopsy from a fibroma on the trunk of an African elephant [16,17]. Further studies are required to assess whether these mammals are the genuine host, and whether these polyomaviruses are the causal infectious agent of such hyperplastic fibrous tissue in their natural host.
In contrast to mammalian polyomaviruses, bird polyomaviruses do not seem to induce tumors. Despite a similar genetic organization to that of mammalian polyomavirus, their LT-ag lacks homologies to the p53 binding sequences of mammalian polyomavirus and not all avian polyomavirus LT-ag possess the consensus sequence LXCXE required for pRb binding [18].
2. Human Polyomaviruses and Cancer
The first two human polyomavirus viruses were isolated in 1971, and were named after the initials of the patient in which the virus was found: the BK virus (BKPyV) and the JC virus (JCPyV) [19,20]. Both BKPyV and JCPyV possess a genomic organization that resembles SV40 more than the murine polyomavirus. The former three viruses lack the middle T-antigen that is encoded by the murine polyomavirus, but have an additional late gene referred to as the agnogene [3]. Because the genomic organization of SV40 displays a higher functional and sequence similarity with the BKPyV and JCPyV, SV40 became the polyomavirus model system for unveiling the oncogenic mechanisms of this family [21,22]. Since 2007, 11 novel human polyomaviruses have been described: KIPyV, WUPyV, Merkel cell PyV (MCPyV), HPyV6, HPyV7, Trichodysplasia spinulosa-associated PyV (TSPyV), HPyV9, HPyV10 (and the isolates MW and MX), STLPyV, HPyV12, and NJPyV-2013 [23,24,25,26,27,28,29,30,31,32,33,34,35]. The seroprevalence of the different human polyomavirus ranges from ~25% to ~100% depending on the virus. The high seropositivity therefore demonstrates that these viruses are common in the adult human population [36,37,38].
Whereas the oncogenic properties of BKPyV, JCPyV and MCPyV in cell culture and animal models are well-documented [39,40,41,42], only MCPyV seems to be associated with cancer in its natural host. Approximately 80% of Merkel cell carcinoma tumors are positive for the MCPyV genome, which is typically integrated and encodes a truncated form of LT-ag [43]. BKPyV and JCPyV DNA, RNA and proteins have been detected in several tumor tissues, but are also often present in control non-malignant tissues [44,45,46]. Hence, a causal role for these viruses in human cancers remains controversial, although the presence of BKPyV may increase the risk of the development of renal and prostate cancer, while JCPyV may be associated with colorectal cancer and CNS tumors [47,48,49,50]. Polyomavirus-associated colorectal cancer may be due to other polyomaviruses present in meat as suggested by Harald zur Hausen [51]. Recent analyses of beef samples have identified several bovine polyomaviruses related to the human polyomaviruses MCPyV, HPyV 6, HPyV7 or other animal polyomaviruses including fruit bat polyomavirus, RacPyV and chimpanzee polyomavirus [52,53]. It remains to be established whether these viruses can be detected in human colorectal biopsies. The possible association of the other human polyomaviruses with cancer has been scarcely examined, and in only few cases was viral DNA or protein detected in tumor tissue (Table 1). Based on our present knowledge, convincing proof of their role in these cancers is lacking.

Table 1.
Prevalence of the novel human polyomaviruses in human cancers. BK virus (BKPyV), JC virus (JCPyV), Merkel cell PyV (MCPyV) are not included.
| Number of samples | Method | Number of positive samples | Comments | Reference | |
|---|---|---|---|---|---|
| Melanoma (st-age IV) | 18 | PCR and IHC (HPyV6 VP1moAb) | HPyV6: 18 HPyV7: 17 TSPyV: 4 HPyV9: 1 HPyV10: 12 | Low viral DNA loads, but higher for HPyV6 | [54] |
| Mucosal melanoma | 37 | PCR | KIPyV: 0 WUPyV: 0 HPyV6:0 HPyV7:0 TSPyV: 0 HPyV9:0 MWPyV: 0 | [55] | |
| Squamous cell carcinoma | 63 | PCR | HPyV6: 2 HPyV7: 1 | Low viral DNA loads | [56] |
| Basal cell carcinoma | 50 | PCR | HPyV6: 1 HPyV7: 2 | Low viral DNA loads | [56] |
| Melanoma | 47 | PCR | HPyV6: 2 HPyV7: 2 | Low viral DNA loads | [56] |
| Basal cell carcinoma | 41 | PCR | HPyV6:3 HPyV7:0 TSPyV: 0 HPyV9:0 | [57] | |
| Squamous cell carcinoma | 52 | PCR | HPyV6:2 HPyV7:0 TSPyV: 0 HPyV9:0 | [57] | |
| SCC in situ | 8 | PCR | HPyV6:1 HPyV7:0 TSPyV: 0 HPyV9:0 | [57] | |
| Keratoacanthoma | 42 | PCR | HPyV6:2 HPyV7:0 TSPyV: 0 HPyV9:0 | [57] | |
| Microcystic adnexal carcinoma | 5 | PCR | HPyV6:0 HPyV7:0 TSPyV: 0 HPyV9:0 | [57] | |
| Atypical fibroxanthoma | 14 | PCR | HPyV6:0 HPyV7:0 TSPyV: 0 HPyV9:0 | [57] | |
| Actinic keratosis | 31 | PCR | HPyV6:1 HPyV7:0 TSPyV: 0 HPyV9:0 | [57] | |
| Breast cancer | 54 | PCR | HPyV6: 1 HPyV7:1 | [58] | |
| Merkel cell carcinoma | deep sequencing | HPyV6: 1 HPyV7:1 HPyV9:1 | [59] | ||
| Extracutaneous melanoma | 38 | PCR | KIPyV: 0 WUPyV: 0 | [60] | |
| SCC+AK | 142 | deep sequencing | HPyV6: 1 | [61] | |
| Chronic lymphocytic leukaemia | 27 | PCR | HPyV9: 0 | [62] | |
| Primary cutaneous B-cell lymphomas (CBCLs) or cutaneous T-cell lymphomas (CTCLs) | 130 | PCR | HPyV6: 6 HPyV7: 1 TSPyV: 0 | [63] | |
| MCC | 28 | PCR | HPyV6: 0 HPyV7:0 | [64] | |
| Pilomatricomas (benign skin tumor associated with hair follicles | ? | ? | TSPyV: 0 | [65] | |
| Lung cancer | 20 | PCR | KIPyV:9 | [66] | |
| CNS tumors | 25 | PCR | KIPyV: 0 WUPyV: 0 | [67] | |
| Neuroblastoma | 31 | PCR | KIPyV: 0 WUPyV: 0 | [67] | |
| Acute lymphoblastic leukaemia | 50 | PCR | KIPyV: 0 WUPyV: 0 | [68] | |
| Lung cancer | 30 32 | PCR PCR | KIPyV: 0 WUPyV: 0 KIPyV: 0 WUPyV: 0 | [69] [70] | |
| Neuroendocrine tumors | 50 | PCR | KIPyV: 0 WUPyV: 0 HPyV6:0 HPyV7:0 TSPyV: 0 | [71] | |
| Skin lesions from CTCL patients | 39 | PCR | HPyV6:11 HPyV7:5 TSPyV: 0 HPyV9:0 | [72] | |
| Blood from CTCL patients | 39 | PCR | HPyV6:0 HPyV7:0 TSPyV: 0 HPyV9:0 | [72] | |
| Glioblastoma multiforme | 39 | PCR | HPyV6:0 HPyV7:0 HPyV9:0 | [73] | |
Thymic epithelial tumors Thymic hyperplasias Foetal thymus tissue | 37 20 20 | PCR, FISH, IHC | PCR FISH IHC HPyV7: 20 23 17 HPyV6: 0 HPyV7: 8 14 6 HPyV7: 0 | [74] |
The cancer biology of BKPyV, JCPyV and MCPyV has been extensively reviewed by others [39,43,45,46,75,76,77] and is also discussed by others in this special issue on Tumor Viruses. This review will focus on novel strategies that human polyomaviruses may use to transform cells. Figure 1 summarizes the novel mechanisms by which HPyV may contribute to cancer.
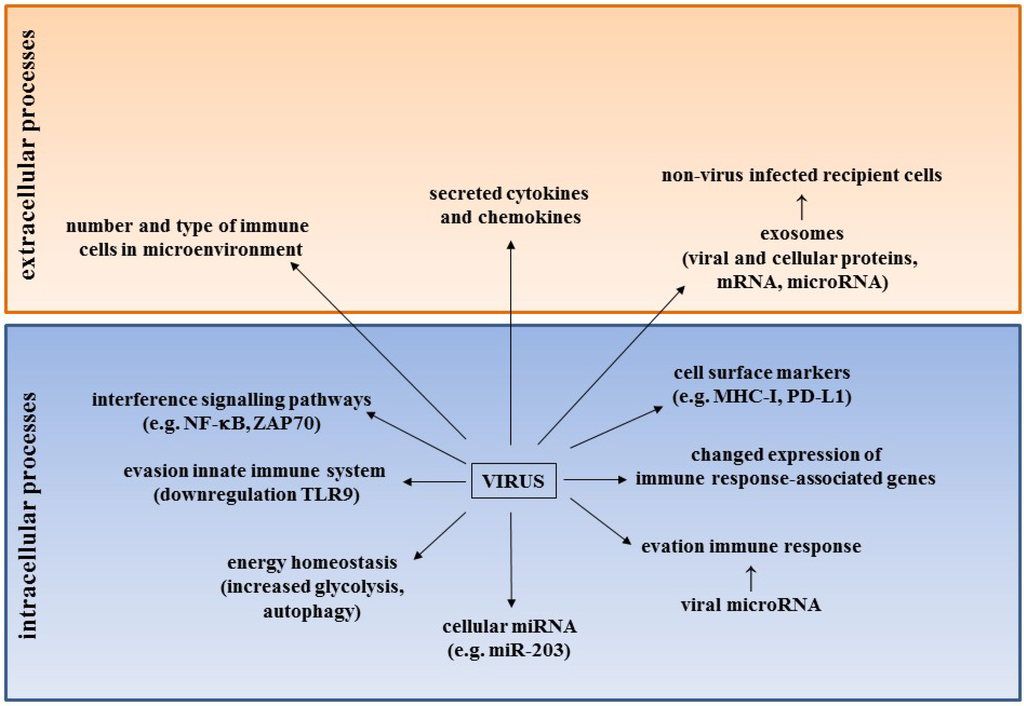
Figure 1.
Novel mechanisms by which HPyV may contribute to cancer. See text and Table 2 for details.
3. HPyV and Emerging Hallmarks of Cancer
3.1. The Immune System and HPyV in Cancer
Individuals with a dysfunctional immune system are more disposed to diseases, infections and (viral-induced) cancers. Moreover, oncoviruses can induce inflammation, which may predispose host cells to acquire carcinogenic mutations [78]. In accordance with the cancer immunoediting hypothesis, tumor cells need to proficiently traverse separate phases in a sequential order to attain cancer manifestation and progression. These phases constitute interactions between the immune system and the cancer cell, and include the elimination of newly transformed cells, an equilibrium in which the immune system restrains the outgrowth of tumors, and an escape in which the tumor cells are able to circumvent the host immune response phases [79,80,81]. For a virus to induce tumors, they need to circumvent elimination by the immune system and to induce alternations in the tumor microenvironment, including in the infected cell allowing the virus-transformed cell to progress [82,83]. Because MCPyV is the only HPyV associated with cancer, the main focus will be on MCPyV’s interaction with the immune system.
Epidemiologic data show that patients with T cell dysfunction are at a 5- to 50-fold increased risk of developing MCC, thereby indicating the importance of the immune system (reviewed in [83]). However, immunocompetent individuals may also develop MCPyV-positive MCC, suggesting that the virus and virus-infected cells can avoid elimination by the immune system.
3.1.1. HPyV and Evasion of the Innate Immune System
One mechanism by which MCPyV circumvents the immune system is to abate the innate defence mechanism. MCPyV LT-ag and st-ag downregulate the Toll-like receptor 9 (TLR9), an important receptor of the host innate immune system that senses viral dsDNA in epithelial and MCC cells [84]. LT-ag inhibits TLR9 expression by decreasing the mRNA levels of the transcription factor C/EBPβ. LT-ag of BKPyV, but not JCPyV, KIPyV and WUPyV, is also able to repress TLR9 expression. Interestingly, C/EBPβ has a vital role in regulating IL-6, IL-8, and TNF-α cytokine transcription [85]. Moreover, it is also suggested that C/EBPβ has a tumor-suppressive activity by down-regulating CDK2, CDK4, and E2F complex activity [86,87]. Thus MCPyV LT-ag mediated suppression of C/EBPβ expression may perturb immune responses and provoke cell proliferation.
3.1.2. Immune Cells in the Microenvironment of MCC
To investigate inflammatory modulators in MCC required for escaping of the tumor from immune surveillance, and to deduce a possible contribution of MCPyV in oncogenesis, several groups have examined immune cells and inflammatory mediators virus-positive and virus-negative MCC. Differences in immune and inflammatory cells, markers, and gene expression in MCPyV-positive and MCPyV-negative MCC tumors are summarized in Table 2. Compared to virus-negative tumors, a higher number of infiltrating CD8+ T-cells in MCPyV-positive MCC has been observed [89,90,91], while others group have not detected a relationship with virus status and the number of intratumoral CD8+ T-cells [92,93]. Other differences in the microenvironment of virus-positive and virus-negative MCC include a higher number of CD3+ T-cells, CD20+ B cells, CD16+ natural killer cells, and CD68+, CD69+, CD163+ macrophages [88,89,90,93,94,95]. FoxP3+ regulatory T-cells were present in 4/4 LT-ag positive MCC, whereas 3/6 LT-ag negative tumors did not contain FoxP3+ regulatory T-cells [93].

Table 2.
Immune cells and inflammatory mediators in MCPyV-positive and MCPyV-negative Merkel cell carcinoma (MCC).
| Component | MCPyV-positive versus MCPyV-negative MCC | Reference |
|---|---|---|
| Cells in tumor microenvironment | ||
| -CD3+ T-cells | ||
| higher number in MCPyV-positive MCC | [88,89,90] | |
| -CD4+ T-cells | ||
| high number associated with high LT-ag expression | [90] | |
| -CD8+ T-cells | higher number in MCPyV-positive MCC | |
| [89,91,92] | ||
| - CD16+ natural killer cell | higher number in MCPyV-positive MCC | |
| [88,90] | ||
| -CD20+ B cells | more common in MCPyV-positive MCC; | |
| no significant difference between MCPyV-positive and –negative MCC | [93] [89] | |
| -CD68+ macrophages | higher number in MCPyV-positive MCC | |
| [88,90,94,95] | ||
| -CD69+ macrophages | higher number in MCPyV-positive MCC | |
| [90,94,95] | ||
| -FoxP3+ regulatory T-cells | more common in MCPyV-positive MCC | |
| [93] | ||
| Cell surface markers: | ||
| -CD3D | enrichment of transcripts in MCPyV-positive MCC | [89] |
| enrichment of transcripts in MCPyV-positive MCC | ||
| -CD3G | lacking in CD8+ T-cells | [89] |
| -CXCR3 | lower levels in MCPyV-positive MCC | [93] |
| -MHC-I | higher in MCPyV-positive MCC | [96] |
| -PD1 | higher in MCPyV-positive MCC | [95,97,98] |
| -Tim-3 | [97] | |
| Signal transduction proteins | ||
| -NFκB levels | lower in MCPyV-positive MCC | [99] |
| -IκB levels | lower in MCPyV-positive MCC | [99] |
| -TANK | ||
| reduction in MCPyV st-ag expressing cells MCC13 cells | [99] | |
| - ZAP70 | compared to virus-negative cells | |
| enrichment of transcripts in MCPyV-positive MCC | [89] | |
| Cytokines/chemokines | ||
| -CCL20 | reduction in MCPyV st-ag expressing cells MCC13 cells | [99] |
| compared to virus-negative cells | ||
| -CXCL-9 | reduction in MCPyV st-ag expressing cells MCC13 cells | [99] |
| compared to virus-negative cells | ||
| -IL-2 | reduction in MCPyV st-ag expressing cells MCC13 cells | [99] |
| compared to virus-negative cells | ||
| -IL-8 | reduction in MCPyV st-ag expressing cells MCC13 cells | [99] |
| compared to virus-negative cells | ||
| -Prokineticin 1 mRNA | higher in MCPyV-negative MCC | [90] |
| -Prokineticin 2 mRNA | higher in MCPyV-positive MCC | [90] |
| Other differentially expressed proteins | ||
| -granzyme B (role in apoptosis) | ||
| Expression was rare in CD8+ cells | [93] |
Afanasieve et al. proposed that MCC tumors may prevent the invasion of lymphocytes by a reduction of E-selectin-positive vessels within the tumors because the downregulation of E-selectin in human squamous cell carcinomas was associated with a restricted entry of T-cells into tumors [100,101]. Of 56 tested MCC biopsies, approximately half displayed a reduction of E-selectin-positive vessels within the tumors compared with vessels in peritumoral areas [102]. However, the association between the presence of virus and E-selectin levels was not investigated.
3.1.3. Changes in Expression of Cell Surface Markers on MCC Cells
Expression of cell surface markers was performed to determine the functionality of the immune cells. These analyses revealed that the expression of MHC-I in MCPyV-positive MCC was significantly lower than in virus-negative MCC [96]. Cell-surface MHC-I expression was down-regulated in 84% (n = 114) of MCC, and approximately half of the tumors had poor or undetectable MHC-I levels. The downregulation of MHC-I expression has been identified as a vital immune evasion strategy used by several viruses, including oncoviruses [103,104,105,106,107,108]. An identical mechanism can be employed by MCPyV, but it remains to be determined as to whether viral proteins are implicated in MHC-I down-regulation. Tumors that undergo a significant downregulation of MHC-I should become a target of natural killer cells. MCC can avoid this by e.g., reducing the expression of NK-activating receptors such as natural killer group 2, member D (NKG2D) [109]. Interestingly, BKPyV and JCPyV microRNA target ULBP3, which is the ligand of NKG2D (see further), though it is not known whether MCPyV microRNA targets ULBP3 or NKG2D. Another surface marker that was differentially expressed on MCPyV-positive and negative tumors is the immune-inhibitory ligand programmed death ligand-1 (PD-L1) [95,97,98]. The major receptor for PD-L1, PD-1 is expressed by activated T lymphocytes, and when this receptor is engaged by its ligands PD-L1 it serves to inhibit the T-cell response. PD-L1 may be aberrantly expressed by tumor cells and protect against immune attack [110]. The number of intra-tumor T-cells is commonly higher in virus-positive MCC than virus-negative MCC, and PD-1 was expressed on a high percentage of MCPyV-positive tumors [95,97,98]. Moreover, approximately 50% of MCPyV-positive MCC express PD-L1 on tumor cells, while no expression was detected in MCPyV-negative MCC. Hence, the association between PD-1-positive cells and PD-L1 expression in the tumor microenvironment seems to create immune resistance by the tumor, thereby allowing the tumor to progress [97,98]. The mechanism by which MCPyV provokes the expression of PD-L1 remains to be determined, but Lipson and co-workers anticipated that IFN-γ may drive PD-L1 expression, but other interleukins such as IL-6, IL-10, IL-17 and IL-21 cannot be excluded. A role for PD-1 positive cells in protecting PD-L1-expressing MCC cells is buttressed by observations in a complete or partial regression of MCC. The exact mechanism for spontaneous regression is not known, although T-cell-mediated response and apoptosis by T-cells has been suggested [111]. The rate of regression of MCPyV-positive versus MCPyV-negative MCC has not been evaluated, but complete regression has been reported in a 76-year old Japanese man with virus-positive MCC [112]. In this patient, only ~3% of the tumor-infiltrating T-cells were PD-1 positive, while in three other patients (females, mean age 81.3 years) with MCPyV-positive MCC who did not show any regression of the tumor, 18.2%–23.0% of the T-cells were of PD-1 positive. This suggests that a reduction of PD-1-positive T-cells may be associated with spontaneous tumor regression [112]. Another surface protein that was aberrantly expressed on immune cells in the tumor microenvironment was CXCR3 [97]. All CD8+ cells lacked CXCR3, thus indicating that these T-cells were functionally compromised. CXCL12 or stromal cell-derived factor 1, a chemokine with pleiotropic functions, including the attraction of inflammatory cells [113], was expressed outside malignant nodules, but its receptor CXCR4 was expressed by tumor cells, though not on infiltrating CD8+ cells. Finally, the cell-surface protein T-cell immunoglobulin and mucin domain-3 (Tim-3), which also functions to inhibit T-cell responses, was also upregulated on infiltrating T-cells in MCPyV-positive MCC [97].
3.1.4. Expression Profile of Genes Associated with the Immune Response in MCC
Gene expression profile analysis has been applied to identify differentially expressed genes in MCPyV-positive and MCPyV-negative MCC. Microarray technology, using >54,000 probes, identified 1593 genes that were differently (≥2-fold) expressed comparing virus-positive and virus-negative MCCs [89]. An enrichment of genes associated with the immune response included genes encoding the δ and γ chains of CD3, the tyrosine kinase ZAP70, which plays an important role in the T-cell response, and the C-region of the µ heavy chain. Another approach compared the transcriptome from cells with an inducible expression of MCPyV st-ag with that of control cells, and revealed that the induction of st-ag expression resulted in ≥2-fold reduced transcript levγels of genes associated with the immune response such as CCL20, CXCL-9, IL-2, IL-8 and TANK, a negative regulator of TLR signaling. Less CCL20 and IL-8 were secreted by MCC13 cells expressing MCPyV st-ag compared with virus-negative MCC13 cells after TNFα stimulation [99]. mRNA profiling of 35 MCC tumors (both MCPyV-positive and negative) with favorable prognoses overexpressed genes such as components of cytotoxic granules (granzymes A, B, H and K), chemokine CCL19 and chemokine receptor 2, MHC-II and NKG2D [92]. The contribution of MCPyV on the expression of these genes cannot be appreciated because the data originate from both MCPyV-positive and MCPyV-negative tumors. Gene expression profiling of MCC tumor cells showed the lack of expression of IL-2 and IFN-γ, whereas IL-12 was expressed [113]. However, this study was performed before MCPyV was identified, so therefore a role of the virus in altered gene expression cannot be deduced. Another study monitored the transcript levels of the chemokine-like proteins prokineticin-1 and prokineticin-2, which are involved in angiogenesis, inflammation and cancer. MCPyV-positive MCCs had a higher than median prokineticin-2 mRNA levels, while virus-negative tumors had a higher than median prokineticin-1 transcript levels [90]. A high tumor prokineticin-2 mRNA content was associated with the expression of MCPyV LT-ag. The biological relevance of this observation for virus-induced MCC remains to be established. Wheat and co-workers observed that the expression of granzyme B, a mediator of apoptosis [114], was rare in MCC infiltrating CD8+ cells, hence suggesting that these cytotoxic T cells were functionally compromised [93].
3.1.5. Effect of st-ag on the NF-κB Pathway
The molecular mechanism by which MCPyV may perturb gene expression in virus-positive MCC tumor cells is not known, but several of the genes listed in Table 2 (e.g., CXCL9, IL-2, IL-8, MHC-I, IκB) are known to be a target for NF-κB [115,116]. Interestingly, MCPyV st-ag was shown to downregulate NF-κB-mediated transcription [99]. St-ag-mediated inhibition of the NF-κB pathway seems to require an interaction of st-ag with NF-κB essential modulator (NEMO) adaptor protein and protein phosphatases 2A and 4C. This will prevent IKKα/IIKβ-mediated phosphorylation of IκB, thus leading to a reduced nuclear translocation of NF-κB. MCPyV interference with the NF-κB pathway is further sustained by the observations that IκB levels were 60% lower in the MCPyV-positive MCC cell line MKL-1 compared with MCPyV-negative MCC13 cells, and by a declined expression of NF-κB and NF-κB-associated genes in virus-positive MCC compared to virus-negative MCC [99,117]. All these findings indicate that MCPyV interferes with the NF-κB pathway, and that MCPyV st-ag may help the virus to evade the host antiviral defence and to persist in the infected cell [99]. It is not known whether the st-ag of other HPyV has the same property, but residues 95 to 111, which are crucial for the interaction between MCPyV st-ag, NEMO and PP2A and PP4C are not conserved [118]. Interestingly, ultraviolet (UV) exposure, a risk factor for MCC [119], was shown to stimulate mutations in LT-ag and increase the expression of st-ag in the tumor cells [120]. Hence, UV exposure may be a virus-dependent mechanism that promotes MCPyV-induced MCC through the aforementioned st-ag:NF-κB interaction.
3.1.6. Viral Microrna and Evation of the Immune Response
Another mechanism by which HPyV may affect gene expression is by microRNA. MicroRNAs (miRNAs) are small RNAs that can down-regulate protein production by either degrading transcripts or inhibiting the translation of mRNA. SV40 miRNA, the first PyV miRNA to be described, was shown to reduce cytotoxic T lymphocyte-mediated lysis and IFN-γ release [121], whereas other HPyV seem to apply different strategies to escape the immune system. BKPyV, JCPyV and MCPyV miRNA were unable to inhibit IFN-induced transcription of the luciferase reporter gene [122], but BKPyV and JCPyV miRNAs inhibited the translation of UL16-binding protein 3 (ULBP3) mRNA [123]. ULBP3 is a ligand recognized by natural killer group 2, member D (NKG2D) receptor. NKG2D is expressed by NK and CD8+ T-cells and binding to ULBP3 triggers killing of the target cell [124]. Consequently, BKPyV- and JCPyV-infected cells may escape from NKG2D-mediated killing and circumvent the immune system. The proteins PSME3 and PIK3CD/p110δ, which are implicated in immune functions, were predicted to be putative targets for MCPyV miRNA [125]. PSME3 is a subunit of a proteasome responsible for the generation of peptides loaded onto MHC I, and PI3KCD plays a unique role in antigen receptor signaling by activating T-cells and B-cell proliferation [126,127,128]. The depletion of these proteins may prevent MCPyV infection to be cleared by the immune system, thereby allowing the viral infection to sustain. One of the SV40 strain RI257 miRNA targets is α-actinin 4 (ACTN4), a protein that activates the NFκB pathway [129]. Stable knockdown of ACTN4 reduces TNFα-mediated induction of NFκB and expression of e.g., IL-1β [130]. SV40-RI257I miRNA may therefore interfere with inflammatory responses. The 3p, and the 5p miRNAs of BKPyV and JCPyV share sequence identity (16 out of 22 nucleotides) with SV40-RI257I miRNA [6], but it is not known whether they also target ACTN4. SV40 strain 776 microRNA was shown to diminish the expression of the Serine/Threonine kinase MST4 in the African green monkey kidney epithelial cell line BSC-40, though not in human embryonal kidney 293T cells [129]. Interestingly, knockdown of MST4 in mice resulted in an exacerbated inflammation upon septic shock [131]. It is not known whether any of the HPyV encodes a miRNA that targets MST4, but if so, the following scenario can be imagined: A persistent HPyV infection may result in the depletion of MST4, thus causing the aggravation of inflammatory responses and a contribution to malignancy.
3.2. The Role of HPyV microRNA and HPyV-induced microRNA in Cancer
Some polyomaviruses have been shown to express viral miRNA, while others may encode a putative miRNA [6,132,133,134]. Although several viral miRNAs have been suggested to play a role in cancer [135], a direct implication of HPyV miRNA in cancer is lacking. Because RacPyV and MCPyV are the only PyV to so far be associated with cancer in their natural host, the expression of their miRNAs was examined in tumors. RacPyV miRNA was among the most abundant miRNAs detectable in RacPyV-associated tumors, but was not observed in RacPyV-negative non-tumor raccoon tissue [134]. This stands in contrast to MCPyV-positive MCC tumors, in which viral miRNA is only detectable in less than half of the tumors tested, and when present, MCPyV miRNA levels were <0.025% of total miRNAs in MCPyV-positive MCC [125,136]. This observation suggests that MCPyV miRNA is not involved in MCC.
PyV miRNA can modulate biological activities that can contribute to malignancy such as evading the immune system, apoptosis and perturbing cellular gene expression [137,138]. The role of HPyV-encoded miRNA in immune evasion was discussed above. PyV miRNAs may also prevent apoptosis. MCPyV miRNA targets the host cell protein AMBRA1, which is involved in autophagy and apoptosis [139], while mouse PyV miRNA downregulates the pro-apoptotic factor Smad2, resulting in a suppression of apoptosis in vivo [140]. Aberrant cellular gene expression may promote neoplastic progression, and PyV miRNAs may perturb cellular gene expression by interfering with splicing, thereby targeting transcription factors or proteins controlling the activity of transcription factors, or by inducing the expression of cellular miRNAs. SV40 miRNA is predicted to target the dual-specificity protein phosphatase DUSP8, a negative regulator of the JNK and p38 mitogen-activated protein kinases, whereas MCPyV may downregulate the expression of transcription factor RUNX1, the splicing factor RBM9/FOX2, as well as the repressor MECP2 [125,129]. Viral infection can induce a unique signature of host cell miRNAs, which may contribute to viral pathogenic processes [141]. MiRNAs are initially transcribed by RNA polymerase II, and SV40 LT-ag has been shown to interfere with RNA polymerase II-dependent transcription [142,143]. Hence, PyV infection may alter the pattern of cellular miRNA expression. However, a common feature shared by all known PyV miRNA is the silencing expression of LT-ag so that no effect on cellular miRNA expression is expected [121,122,132,134,144,145]. On the other hand, interference with cellular miRNA expression is plausible in MCPyV-positive tumors because MCC do express LT-ag [146], and RacPyV-positive tumors also express LT-ag [147]. The effect of LT-ag on cellular miRNA expression has not been investigated, but the proteins of the oncovirus HBV, EBV, KSHV and HCV help regulate the levels of cellular miRNAs, including oncogenic miRNAs [148,149,150,151,152,153,154].
Xie and co-workers compared miRNA profile in MCPyV-positive and negative MCC. One miRNA that was significantly lower expressed in MCPyV-positive MCC compared to MCPyV-negative MCC was miR-203. The overexpression of miR-203 in MCPyV-negative MCC inhibited cell growth and induced cell cycle arrest [155]. This finding suggests that MCPyV may cause cell proliferation by repressing the expression of miR-203, but the exact mechanism by which MCPyV may regulate this miRNA remains to be elucidated.
3.3. Effect of HPyV on Energy Homeostasis
In healthy cells, glycolysis initiates in the cytoplasm where glucose is metabolized into pyruvate, which then enters the mitochondria where it is converted into acetyl-CoA and enters the Krebs’ cycle to generate ATP. In cancer cells, a metabolic switch occurs: the suppression of mitochondrial glucose oxidation and the upregulation of aerobic breakdown of glucose. This phenomenon was first described by Otto Heinrich Warburg, and is known as the Warburg effect [156]. While mitochondrial glucose oxidation generates 36 molecules of ATP per molecule of glucose, only two molecules of ATP are produced per molecule of glucose by aerobic breakdown. Cancer cells compensate for this by increasing the uptake of glucose and by stimulating the transcription of almost all the glycolytic enzymes in the cytoplasm [79,157,158]. Metabolism in Merkel cells and (MCPyV-positive) MCC has not been studied, but a Positron Emission Tomography (PET) scan of MCC with glucose analogues suggests a high rate of glycolysis in these tumors [159]. However, a possible role for MCPyV in enhanced glucose metabolism in MCC remains to be determined. Several studies with polyomavirus-transformed cells indicate that these viruses may affect glucose metabolism. Additionally, a redistribution of membrane glucose transporters, increased aerobic glycolysis and an increased activity of glycolytic enzymes were observed in SV40-transformed cells compared to non-transformed cells [160,161,162]. A role for JCPyV LT-ag in regulating the metabolic utilization of glucose in brain tumors has been recently suggested [163]. Another study showed that JCPyV LT-ag expressing medulloblastoma cells had a significantly lower mitochondrial respiration and glycolysis, but a three-fold higher consumption of glutamine compared to non-LT-ag expressing cells [164]. Oxygen consumption and glucose uptake were compared in fibroblast transduced with the telomerase catalytic subunit, or in combination with SV40 LT-ag or LT-ag plus st-ag. A progressive increase in both metabolic markers was measured, as cell lines expressed more oncogenes. This observation underscores a role for LT-ag and st-ag in decreasing the cell’s dependence on mitochondrial energy production [165]. SV40 st-ag can activate Akt, while Akt can stimulate the expression of glycolytic enzymes and aerobic glycolysis [166,167,168]. SV40 st-ag can also activate the MEK/ERK mitogen-activated protein kinase pathway, which can then increase glucose transport [169,170]. These findings suggest that st-ag may stimulate glucose uptake and aerobic glycolysis. The tumor suppressor p53 acts as an anti-Warburg molecule because it acts as a potent inhibitor of glycolysis [171]. The LT-ag of BKPyV and JCPyV has been shown to bind and inactivate p53 [172,173]. Although the LT-ag-mediated inactivation of p53 may promote glucose uptake and stimulate the glycolytic pathway, it may not be operational in MCPyV-positive MCC because the truncated form of LT-ag expressed in Merkel cell carcinomas does not bind p53 [40]. The interaction between p53 and LT-ag of other HPyV has not been investigated, but they all encompass a putative p53-binding motif [6].
Autophagy is another mechanism that allows cancer cells to maintain the levels of nutrients and energy in nutrient-limited environments, which helps to facilitate the survival of tumor cells. Moreover, autophagy regulates cellular invasion and metastasis [173]. The human tumor viruses EBV, KSHV, HBV, and HCV can modulate the autophagy pathway to favor viral infection by enhancing viral replication, prevent apoptosis or maintain a persistent and life-long infection [174]. Hence, viral interference with the autophagy pathway may contribute to tumorigenesis by these viruses, though less is known about the effect of HPyV on autophagy and the biological consequences. Bouley et al. could establish a supporting role for autophagy in BKPyV infection [175]. Using transformed human foreskin fibroblasts and HEK cells expressing or lacking the SV40 st-ag, it was demonstrated that st-ag helps to maintain energy homeostasis in glucose-deprivation cancer cells by activating AMP-activated protein kinase (AMPK), thereby inhibiting the mammalian target of rapamycin (mTOR) to shut down protein translation, and inducing autophagy as an alternate energy source. This protective role of st-ag under conditions of glucose deprivation depends on its ability to interact with protein phosphatase 2A [176]. It is not known whether the st-ag of other HPyV may exert similar functions, but BKPyV, JCPyV, MCPyV and MWPyV (HPyV10) st-ag have also been shown to interact with PP2A [99,177,178,179,180,181]. Other effects of HPyV on autophagy came from a study by Khalili et al., who found that JCPyV LT-ag suppressed the expression of Bcl-2-associated athanogene Bag3, a protein implicated in apoptosis and autophagy [182]. On the other hand, overexpression of Bag3 induces autophagy-mediated degradation of JCPyV LT-ag. Bag3 interacts with the C-terminal half region of LT-ag which encompasses a zinc finger structure and partially overlaps with the p53 binding domain [183]. Consequently, LT-ag may repress the expression of Bag3 and protect itself from being degraded by Bag3-mediated autophagy. MCPyV-positive MCCs express C-terminal truncated LT-ag, which may impede interaction with Bag3. Under stress conditions, primary neuroglial cells immortalized with SV40 LT-ag had increased levels of the autophagy marker LC3B compared to non-LT-ag expressing cells [184]. However, the biological implication was not investigated.
3.4. HPyV and Exosomes
Exosomes are endosome-derived membrane vesicles of approximately 50 nm–100 nm in diameter that are shed by cells and act as a communication tool between cells. Exosomes contain cellular proteins, carbohydrates, lipids, DNA, rRNA, mRNA, siRNA and other non-coding RNAs (for recent reviews, see [185,186,187]). Exosomes secreted by tumor cells participate in the modulation of angiogenesis, cell proliferation, cell invasion, gene regulation and immune evasion, thereby creating advantages for malignant growth [186]. Exosomes released by virus-infected cells can also contain viral-derived components and are implicated in the pathogenesis of viruses. Indeed, the human oncoviruses Epstein-Barr virus, Kaposi sarcoma-associated herpes virus, hepatitis B virus, hepatitis C virus and human T-lymphotropic virus type 1 all utilize exosomes to transfer viral (onco)proteins, mRNA and miRNAs to non-infected cells [188,189,190,191,192,193,194]. Exosomes captured by target cells may facilitate the spread of (onco)viral proteins and nucleic acids, thereby promoting malignancy in the recipient cells in the absence of an infection by virions. Exosomes can provoke immune alterations that may play a role to create an immunotolerogenic microenvironment during the carcinogenesis process. They can promote host immune and inflammatory responses by activating T- and B-cells, and by releasing exosome-trapped inflammatory molecules such as TNFα and IL1β in the recipient cells. Even so, exosomes have also been shown to inhibit immune responses by preventing CD4+ T-cell proliferation, CD8+ CTL response or transporting anti-inflammatory molecules (reviewed in [187]).
The generation of exosomes by HPyV-infected cells has scarcely been investigated. Studies with mouse primitive glioblastoma-like brain tumor cell lines harbouring integrated SV40 large T-antigen DNA revealed the presence of SV40 large T-antigen sequences in exosomes produced by these cells [195]. Recently, JCPyV microRNA was detected in exosomes derived from human plasma and urine [196], although studies on the possible roles of exosomes released by HPyV-infected hosts cells are lacking.
4. Therapeutic Strategies against Emerging Hallmarks of Cancer
Specific inhibitors against HPyV are lacking, and the development of vaccines and vaccination are still in a very preliminary phase [197,198,199,200,201]. Therapeutic strategies directed against emerging features of cancer such as inflammation, immune evasion, exosomes, microRNA and energy homeostasis may offer alternatives to help combat HPyV-positive tumors. In vitro studies and MCC xenograft mouse models suggested a beneficial effect of IFN. Intratumoral administering of a mixture of different IFNα subtypes and IFNβ resulted in a regression of MCPyV-positive, but not MCPyV-negative xenografts of MCC cells, while IFNα-2b, IFNβ-1b, and IFNγ-1b challenge resulted in an increased cell-surface expression of MHC-I on MCC cell lines [96,202]. However, studies in patients with MCPyV-positive MCC have been proven to exhibit variable effects. Subcutaneous administration of IFN-β resulted in a complete regression of MCPyV-positive MCC tumors in a Japanese patient, but IFN-α-2b treatment of an 84-year-old man and an 81-year-old woman had no effect [203,204]. IFNβ stimulated MHC-I expression on tumor cells of 3/3 MCPyV-positive MCC patients [96]. Intralesional treatment of a 67-year old MCPyV-positive MCC patient with INFβ-Ib, followed by re-infusion of expanded MCPyV LT-ag-specific CD8+ T-cells resulted in a complete response in two of three metastatic lesions and a delayed appearance of new metastasis compared to controls [205]. Tumor necrosis factor (TNF) can be considered as an alternative for treating MCC because this cytokine displayed a high efficacy in three patients [206,207], and in one patient out of three treated with IFNγ plus TNFα a complete response was noticed, while a partial and no response was observed in two others [208]. Because the three aforementioned TNFα studies were performed before the discovery of MCPyV, the presence of virus in the tumors was not known. Interestingly, patients who have been treated with TNFα inhibitors show an increased risk of developing MCC [209,210]. Another immunotherapy approach for the treatment of MCC could be PD-1 ligand and Tim-3, with these receptors highly expressed on MCPyV-specific CD+ T-cells. Drugs targeting the PD-1/PDL-1 pathway such as nivolumab (a blocking antibody against PD-1), pembrolizumab (anti-PD-1 antibody) and BMS-936559 (anti-PDL-1 antibody) have been used in other cancers and may be used to treat MCC [211].
Therapeutic strategies aimed at other emerging hallmarks of cancer have been little explored. Intratumoral delivery of anti-microRNA may help in silencing viral microRNA or viral-induced cellular microRNA, while RNA interference may turn off the expression of viral oncoproteins. RNA interference targeting LT-ag has been shown to abrogate HPyV replication in vitro and suppress tumor growth in vitro and in an animal model [180,212,213,214,215,216,217]. The use of exosomes as vaccines against cancer and infectious diseases has been suggested and exosomes pulsed with MCPyV LT-ag could be considered to treat MCPyV-positive MCC patients [187,218]. Lastly, therapeutic strategies directed against the metabolic changes in tumor cells (anti-glycolysis therapy) may be considered. The hexokinase inhibitor 2-deoxyglucose inhibited growth of fibroblasts transformed by the telomerase catalytic subunit plus SV40 LT-ag, and by the telomerase catalytic subunit plus SV40 LT-ag plus st-ag [165]. To our best knowledge, the effect of 2-deoxyglucose on the growth of MCPyV-positive MCC cells has not been investigated.
5. Conclusion and Future Perspectives
Seroprevalence studies demonstrate that HPyV viruses are common in the human population [36]. Although HPyV LT-ag and st-ag possess proven or putative transforming properties, only MCPyV seems to be associated with human cancer. This virus encodes additional early proteins (ALTO protein and 57 kD protein), whose functions are not completely understood [4,219]. Proper immune surveillance may explain why HPyVs establish a harmless life-long infection in most individuals, while immune deficiencies may lead to viral-associated pathologies, including malignancy. The role of HPyVs in the emerging hallmarks of cancer has been little investigated and further investigations are required to elucidate the mechanisms by which HPyV-positive tumors can evade the antiviral responses of the host and affect energy homeostasis. A better understanding of the tumor microenvironment is required to comprehend the development of MCC. A possible involvement of exosomes in HPyV-induced cancer and modulation of the immune system have not been addressed, and the role of viral microRNA or HPyV-induced microRNA in tumorigenesis is incompletely understood. Unveiling the mechanisms by which these viruses participate in emerging hallmarks of cancer may therefore enable the development of novel therapeutic strategies.
Author Contributions
U.M, K.R., I.A. and B.S. were all involved in gathering information, reading articles and writing and critically revising the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Johne, R.; Buck, C.B.; Allander, T.; Atwood, W.J.; Garcea, R.L.; Imperiale, M.J.; Major, E.O.; Ramqvist, T.; Norkin, L.C. Taxonomical developments in the family Polyomaviridae. Arch. Virol. 2011, 156, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Peretti, A.; FitzGerald, P.C.; Bliskovsky, V.; Pastrana, D.V.; Buck, C.B. Genome Sequence of a Fish-Associated Polyomavirus, Black Sea Bass (Centropristis striata) Polyomavirus 1. Genome Announc. 2015, 3, e01476–14. [Google Scholar] [CrossRef] [PubMed]
- DeCaprio, J.A.; Imperiale, M.J.; Major, E.O. Polyomaviruses. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins, and Wolters Kluwer: Philadelphia, PA, USA, 2013; Volume 2, pp. 1633–1661. [Google Scholar]
- Carter, J.J.; Daugherty, M.D.; Qi, X.; Bheda-Malge, A.; Wipf, G.C.; Robinson, K.; Roman, A.; Malik, H.S.; Galloway, D.A. Identification of an overprinting gene in Merkel cell polyomavirus provides evolutionary insight into the birth of viral genes. Proc. Natl. Acad. Sci. USA 2013, 110, 12744–12749. [Google Scholar] [CrossRef] [PubMed]
- Schowalter, R.W.; Buck, C.B. The merkel cell polyomavirus minor capsid protein. PLOS Pathog. 2013, 9, e1003558. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, B.; Moens, U. Genome analysis of non-human primate polyomaviruses. Infect. Genet. Evol. 2014, 26, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Fulghieri, C.; Bloom, S. SarahElizabeth Stewart. Emerg. Infect. Dis. 2014, 20, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Sweet, B.H.; Hilleman, M.R. The vacuolating virus, SV40. Proc. Soc. Exp. Biol. Med. 1960, 105, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; DeCaprio, J.A.; Fluck, M.M.; Schaffhausen, B.S. Cellular transformation by Simian Virus 40 and Murine Polyomavirus T antigens. Semin. Cancer Biol. 2009, 19, 218–228. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Sáenz Robles, M.T.; Pipas, J.M. Large T antigens of polyomaviruses: Amazing molecular machines. Annu. Rev. Microbiol. 2012, 66, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Valis, J.D.; Strandberg, J.D.; Shah, K.V. Transformation of hamster kidney cells by simian papovavirus SA12. Proc. Soc. Exp. Biol. Med. 1979, 160, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Bastien, C.; Feunteun, J. The hamster polyomavirus transforming properties. Oncogene 1988, 2, 129–135. [Google Scholar] [PubMed]
- Chen, J.D.; Neilson, K.; van Dyke, T. Lymphotropic papovavirus early region is specifically regulated in transgenic mice and efficiently induces neoplasia. J. Virol. 1989, 63, 2204–2214. [Google Scholar] [PubMed]
- Dela Cruz, F.N., Jr.; Giannitti, F.; Li, L.; Woods, L.W.; del Valle, L.; Delwart, E.; Pesavento, P.A. Novel polyomavirus associated with brain tumors in free-ranging raccoons, western United States. Emerg. Infect Dis. 2013, 19, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.H.; Riley, L.K.; Franklin, C.L.; Besch-Williford, C.L. Hamster polyomavirus infection in a pet Syrian hamster (Mesocricetus auratus). Vet. Pathol. 2001, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Colegrove, K.M.; Wellehan, J.F., Jr.; Rivera, R.; Moore, P.F.; Gulland, F.M.; Lowenstine, L.J.; Nordhausen, R.W.; Nollens, H.H. Polyomavirus infection in a free-ranging California sea lion (Zalophus californianus) with intestinal T-cell lymphoma. J. Vet. Diagn. Invest. 2010, 22, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Stevens, H.; Bertelsen, M.F.; Sijmons, S.; van Ranst, M.; Maes, P. Characterization of a novel polyomavirus isolated from a fibroma on the trunk of an African elephant (Loxodonta africana). PLOS ONE 2013, 8, e77884. [Google Scholar] [CrossRef] [PubMed]
- Johne, R.; Müller, H. Polyomaviruses of birds: Etiological agents of inflammatory diseases in a tumor virus family. J. Virol. 2007, 81, 11554–11559. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.D.; Field, A.M.; Coleman, D.V.; Humle, B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet 1971, 1, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Padgett, B.L.; Walker, D.L.; ZuRhein, G.M.; Eckroade, R.J.; Dessel, B.H. Cultivation of papova-like virus from human brain with progressive multifocal leukoencephalopathy. Lancet 1971, 1, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Atkin, S.J.; Griffin, B.E.; Dilworth, S.M. Polyoma virus and simian virus 40 as cancer models: History and perspectives. Semin. Cancer Biol. 2009, 19, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Pipas, J.M. SV40: Cell transformation and tumorigenesis. Virology 2009, 384, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Allander, T.; Andreasson, K.; Gupta, S.; Bjerkner, A.; Bogdanovic, G.; Persson, M.A.; Dalialis, T.; Ramqvist, T.; Andersson, B. Identification of a third human polyomavirus. J. Virol. 2007, 81, 4130–4136. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, A.; Nissen, M.D.; Whiley, D.M.; Mackay, I.M.; Lambert, S.B.; Wu, G.; Brennan, D.C.; Storch, G.A.; Wang, D. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLOS Pathog. 2007, 3, 595–604. [Google Scholar] [CrossRef]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Schowalter, R.M.; Pastrana, D.V.; Pumphrey, K.A.; Moyer, A.L.; Buck, C.B. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe 2010, 7, 509–515. [Google Scholar] [CrossRef] [PubMed]
- van der Meijden, E.; Janssens, R.W.; Lauber, C.; Bouwens Bavinck, J.N.; Gorbalenya, A.E.; Feltkamp, M.C. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLOS Pathog. 2010, 6, e1001024. [Google Scholar] [CrossRef] [PubMed]
- Scuda, N.; Hofmann, J.; Calvignac-Spencer, S.; Ruprecht, K.; Liman, P.; Kühn, J.; Hengel, H.; Ehlers, B. A novel human polyomavirus closely related to the African green monkey-derived lymphotropic polyomavirus. J. Virol. 2011, 85, 4586–4590. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, V.; Foulongne, V.; Cheval, J.; Pariente, K.; Le Gouil, M.; Burguiere, A.M.; Manuguerra, J.C.; Caro, V.; Eloit, M. Human polyomavirus related to the green monkey lymphotropic polyomavirus. Emerg. Infect. Dis. 2011, 17, 1364–1370. [Google Scholar] [PubMed]
- Buck, C.B.; Phan, G.Q.; Raiji, M.T.; Murphy, P.M.; McDermott, D.H.; McBride, A.A. Complete genome sequence of a tenth human polyomavirus. J. Virol. 2012, 86, 10887. [Google Scholar] [CrossRef] [PubMed]
- Siebrasse, E.A.; Reyes, A.; Lim, E.S.; Zhao, G.; Mkakosya, R.S.; Manary, M.J.; Gordon, J.I.; Wang, D. Identification of MW polyomavirus, a novel polyomavirus in human stool. J. Virol. 2012, 86, 10321–10326. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Greninger, A.L.; Isa, P.; Phan, T.G.; Martinez, M.A.; de la Luz Sanchez, M.; Contreras, J.F.; Santos-Preciado, J.I.; Parsonnet, J.; Miller, S.; et al. Discovery of a novel polyomavirus in acute diarrheal samples from children. PLOS ONE 2012, 7, e49449. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.S.; Reyes, A.; Antonio, M.; Saha, D.; Ikumapayi, U.N.; Adeyemi, M.; Stine, O.C.; Skelton, R.; Brennan, D.C.; Mkakosya, R.S.; et al. Discovery of STL polyomavirus, a polyomavirus of ancestral recombinant origin that encodes a unique T antigen by alternative splicing. Virology 2013, 439, 163–164, Erratum in: Virology 2013, 439, 163–164. [Google Scholar]
- Korup, S.; Rietscher, J.; Calvignac-Spencer, S.; Trusch, F.; Hofmann, J.; Moens, U.; Sauer, I.; Voight, S.; Schmuck, R.; Ehlers, B. Identification of a novel human polyomavirus in organs of the gastrointestinal tract. PLOS ONE 2013, 8, e58021. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Pereira, M.; Rhodes, R.H.; An, P.; Pipas, J.M.; Jain, K.; Kapoor, A.; Briese, T.; Faust, P.L.; Lipkin, W.I. Identification of a novel polyomavirus in a pancreatic transplant recipient with retinal blindness and vascular myopathy. J. Infect. Dis. 2014, 210, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Moens, U.; van Ghelue, M.; Song, X.; Ehlers, B. Serological cross-reactivity between human polyomaviruses. Rev. Med. Virol. 2013, 23, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.S.; Meinerz, N.M.; Primi, B.; Wang, D.; Garcea, R.L. Common exposure to STL polyomavirus during childhood. Emerg. Infect. Dis. 2014, 20, 1559–1561. [Google Scholar] [CrossRef] [PubMed]
- Nicol, J.T.; Leblond, V.; Arnold, F.; Guerra, G.; Mazzoni, E.; Tognon, M.; Coursaget, P.; Touzé, A. Seroprevalence of human Malawi polyomavirus. J. Clin. Microbiol. 2014, 52, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Moens, U.; van Ghelue, M.; Johannessen, M. Oncogenic potentials of the human polyomavirus regulatory proteins. Cell Mol. Life Sci. 2007, 64, 1656–1678. [Google Scholar] [CrossRef] [PubMed]
- Borchert, S.; Czech-Sioli, M.; Neumann, F.; Schmidt, C.; Wimmer, P.; Dobner, T.; Grundhoff, A.; Fischer, N. High-affinity Rb binding, p53 inhibition, subcellular localization, and transformation by wild-type or tumor-derived shortened Merkel cell polyomavirus large T antigens. J. Virol. 2014, 88, 3144–3160. [Google Scholar] [CrossRef] [PubMed]
- Verhaegen, M.E.; Mangelberger, D.; Harms, P.W.; Vozheiko, T.D.; Weick, J.W.; Wilbert, D.M.; Saunders, T.L.; Ermilov, A.N.; Bichakjian, C.K.; Johnson, T.M.; Imperiale, M.J.; Dlugosz, A.A. Merkel cell polyomavirus small t antigen is oncogenic in transgenic mice. J. Invest. Dermatol. 2014. [Google Scholar] [CrossRef]
- Spurgeon, M.E.; Cheng, J.; Bronson, R.T.; Lambert, P.F.; DeCaprio, J.A. Tumorigenic activity of Merkel cell polyomavirus T antigens expressed in the stratified epithelium of mice. Cancer Res. 2015. [Google Scholar] [CrossRef]
- Chang, Y.; Moore, P.S. Merkel cell carcinoma: A virus-induced human cancer. Annu. Rev. Pathol. 2012, 7, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Moens, U.; Johannessen, M. Human polyomaviruses and cancer: Expanding repertoire. J. Dtsch. Dermatol. Ges. 2008, 6, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Abend, J.R.; Jiang, M.; Imperiale, M.J. BK virus and cancer: Innocent until proven guilty. Semin. Cancer Biol. 2009, 19, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Maginnis, M.S.; Atwood, W.J. JC virus: An oncogenic virus in animals and humans? Semin. Cancer Biol. 2009, 19, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Enam, S.; Del Valle, L.; Lara, C.; Gan, D.D.; Ortiz-Hidalgo, C.; Palazzo, J.P.; Khalili, K. Association of human polyomavirus JCV with colon cancer: Evidence for interaction of viral T-antigen and beta-catenin. Cancer Res. 2002, 62, 7093–7101. [Google Scholar] [PubMed]
- Bulut, Y.; Ozdemir, E.; Ozercan, H.I.; Etem, E.O.; Aker, F.; Toraman, Z.A.; Seyrek, A.; Firdolas, F. Potential relationship between BK virus and renal cell carcinoma. J. Med. Virol. 2013, 85, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Delbue, S.; Ferrante, P.; Provenzano, M. Polyomavirus BK and prostate cancer: An unworthy scientific effort? Oncoscience 2014, 1, 296–303. [Google Scholar] [PubMed]
- Carluccio, S.; Signorini, L.; Elia, F.; Villani, S.; Delbue, S.; Ferrante, P. A potential linkage between the JC and BK polyomaviruses and brain and urinary tract tumors: A review of the literature. Adv. Tumor. Virol. 2014, 4, 17–24. [Google Scholar]
- zur Hausen, H. Red meat consumption and cancer: Reasons to suspect involvement of bovine infectious factors in colorectal cancer. Int. J. Cancer 2012, 130, 2475–2483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, L.; Deng, X.; Kapusinszky, B.; Delwart, E. What is for dinner? Viral metagenomics of US store bought beef, pork and chicken. Virology 2014, 468–470, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Peretti, A.; FitzGerald, P.C.; Bliskovsky, V.; Buck, C.B.; Pastrana, D.V. Hamburger polyomaviruses. J. Gen. Virol. 2015. [Google Scholar] [CrossRef]
- Schrama, D.; Groesser, L.; Ugurel, S.; Hafner, C.; Pastrana, D.V.; Buck, C.B.; Cerroni, L.; Theiler, A.; Becker, J.C. Presence of human polyomavirus 6 in mutation-specific BRAF inhibitor-induced epithelial proliferation. JAMA Dermatol. 2014, 150, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Ramqvist, T.; Nordfors, C.; Dalianis, T.; Ragnarsson-Olding, B. DNA from human polyomaviruses, TSPyV, MWPyV, HPyV6, 7, and 9 was not detected in primary mucosal melanomas. Anticancer Res. 2014, 34, 639–643. [Google Scholar] [PubMed]
- Imajoh, M.; Hashida, Y.; Nakajima, H.; Sano, S.; Daibata, M. Prevalence and viral DNA loads of three novel human polyomaviruses in skin cancers from Japanese patients. J. Dermatol. 2013, 40, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Scola, N.; Wieland, U.; Silling, S.; Altmeyer, P.; Stücker, M.; Kreuter, A. Prevalence of human polyomaviruses in common and rare types of non-Merkel cell carcinoma skin cancer. Br. J. Dermatol. 2012, 167, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Antonsson, A.; Bialasiewicz, S.; Rockett, R.J.; Jacob, K.; Bennett, I.C.; SLoots, T.P. Exploring the Prevalence of Ten Polyomaviruses and Two Herpes Viruses in Breast Cancer. PLOS ONE 2012, 7, e39842. [Google Scholar] [CrossRef] [PubMed]
- Foulongne, V.; Sauvage, V.; Hebert, C.; Dereure, O.; Cheval, J.; Gouilh, M.A.; Pariente, K.; Segondy, M.; Burguière, A.; Manuguerra, J.C.; Caro, V.; Eloit, M. Human skin microbiota: High diversity of DNA viruses identified on the human skin by high throughput sequencing. PLOS ONE 2012, 7, e38499. [Google Scholar] [CrossRef] [PubMed]
- Giraud, G.; Ramqvist, T.; Ragnarsson-Olding, B.; Dalialis, T. DNA from BK virus and JC virus and from KI, WU, and MC polyomaviruses as well as from simian virus 40 is not detected in non-UV-light-associated primary malignant melanomas of mucous membranes. J. Clin. Microbiol. 2008, 46, 3595–3598. [Google Scholar] [CrossRef] [PubMed]
- Bzhalava, D.; Johansson, H.; Ekström, J.; Faust, H.; Möller, B.; Eklund, C.; Nordin, P.; Stenquist, B.; Paoli, J.; Persson, B.; Forslund, O.; Dillner, J. Unbiased approach for virus detection in skin lesions. PLOS ONE 2013, 8, e655953. [Google Scholar] [CrossRef]
- Imajoh, M.; Hashida, Y.; Taniguchi, A.; Kamioka, M.; Daibata, M. Novel human polyomaviruses, Merkel cell polyomavirus and human polyomavirus 9, in Japanese chronic lymphocytic leukemia cases. J. Hematol. Oncol. 2012, 5, e25. [Google Scholar] [CrossRef]
- Kreuter, A.; Silling, S.; Dewan, M.; Stücker, M.; Wieland, U. Evaluation of 4 recently discovered human polyomaviruses in primary cutaneous B-cell and T-cell lymphoma. Arch. Dermatol. 2011, 147, 1449–1451. [Google Scholar] [CrossRef] [PubMed]
- Duncavage, E.J.; Pfeifer, J.D. Human polyomaviruses 6 and 7 are not detectable in Merkel cell polyomavirus-negative Merkel cell carcinoma. J. Cutan. Pathol. 2011, 38, 790–796. [Google Scholar] [PubMed]
- Kanitakis, J.; Kazem, S.; van Der Meijden, E.; Feltkamp, M. Absence of the trichodysplasia spinulosa-associated polyomavirus in human pilomatricomas. Eur. J. Dermatol. 2011, 21, 453–454. [Google Scholar] [PubMed]
- Babakir-Mina, M.; Ciccozzi, M.; Campitelli, L.; Aquaro, S.; Lo Coco, A.; Perno, C.F.; Ciotti, M. Identification of the novel KI Polyomavirus in paranasal and lung tissues. J. Med. Virol. 2009, 81, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Giraud, G.; Ramqvist, T.; Pastrana, D.V.; Pavot, V.; Lindau, C.; Kogner, P.; Orrego, A.; Buck, C.B.; Allander, T.; Holm, S.; Gustavsson, B.; Dalianis, T. DNA from KI, WU and Merkel cell polyomavirus is not detected in childhood central nervous system tumors or neuroblastomas. PLOS ONE 2009, 4, e8239. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, B.; Honkaniemi, E.; Goh, S.; Giraud, G.; Forestier, E.; von Döbeln, U.; Allander, T.; Dalianis, T.; Bogdanovic, G. KI, WU, and Merkel cell polyomavirus DNA was not detected in guthrie cards of children who later developed acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 2012, 34, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, S.; Kaiho, M.; Takano, Y.; Endo, R.; Kikuta, H.; Sawa, H.; Ariga, T.; Ishiguro, N. Detection of KI polyomavirus and WU polyomavirus DNA by real-time polymerase chain reaction in nasopharyngeal swabs and in normal lung and lung adenocarcinoma tissues. Microbiol. Immunol. 2011, 55, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Duncavage, E.J.; Le, B.M.; Wang, D.; Pfeifer, J.D. Merkel cell polyomavirus: A specific marker for Merkel cell carcinoma in histologically similar tumors. Am. J. Surg. Pathol. 2009, 33, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Höfler, D.; Koleganova, N.; Pawlita, M. Human polyomaviruses and other human viruses in neuroendocrine tumors. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 1558–1561. [Google Scholar] [CrossRef] [PubMed]
- Du-Thanh, A.; Foulongne, V.; Guillot, B.; Dereure, O. Recently discovered human polyomaviruses in lesional and non-lesional skin of patients with primary cutaneous T-cell lymphomas. J. Dermatol. Sci. 2013, 71, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Hashida, Y.; Taniguchi, A.; Yawata, T.; Hosokawa, S.; Murakami, M.; Hiroi, M.; Ueba, T.; Daibata, M. Prevalence of human cytomegalovirus, polyomaviruses, and oncogenic viruses in glioblastoma among Japanese subjects. Infect Agent Cancer 2015, 10, e3. [Google Scholar]
- Rennspiess, D.; Pujari, S.; Keijzers, M.; Abdul-Hamid, M.A.; Hochstenbag, M.; Dingemans, A.; Kurz, A.K.; Speel, E.; Haugg, A.; Pastrana, D.V.; et al. Detection of Human Polyomavirus 7 in human thymic epithelial tumors. J. Thorac. Oncol. 2015, 10, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Gjoerup, O.; Chang, Y. Update on human polyomaviruses and cancer. Adv. Cancer Res. 2010, 106, 1–51. [Google Scholar] [PubMed]
- DeCaprio, J.A.; Garcea, R.L. A cornucopia of human polyomaviruses. Nat. Rev. Microbiol. 2013, 11, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Spurgeon, M.E.; Lambert, P.F. Merkel cell polyomavirus: A newly discovered human virus with oncogenic potential. Virology 2013, 435, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.S.; Chang, Y. Why do viruses cause cancer? Highlights of the first century of human tumor virology. Nat. Rev. Cancer 2010, 10, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Vasanthakumar, A.; Grigoriadis, G. Modulating T regulatory cells in cancer: How close are we? Immunol. Cell Biol. 2013, 91, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Vossen, M.T.; Westerhout, E.M.; Söderberg, N.C.; Wiertz, E.J. Viral immune evasion: A masterpiece of evolution. Immunogenetics 2002, 54, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Afanasiev, O.; Nghiem, P. Immunobiology of Merkel cell carcinoma: Implications for immunotherapy of a polyomavirus-associated cancer. Curr. Oncol. Rep. 2011, 13, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, N.; Shuda, M.; Gheit, T.; Kwun, H.J.; Cornet, I.; Saidj, D.; Zannetti, C.; Hasan, U.; Chang, Y.; Moore, P.S.; et al. The T Antigen Locus of Merkel Cell Polyomavirus downregulates Human Toll-Like Receptor 9 Expression. J. Virol. 2013, 87, 13009–13019. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gade, P.; Xiao, W.; Kalvakolanu, D.V. The interferon signaling network and transcription factor C/EBP-beta. Cell Mol. Immunol. 2007, 4, 407–418. [Google Scholar] [PubMed]
- Johnson, P.F. Molecular stop signs: Regulation of cell-cycle arrest by C/EBP transcription factors. J. Cell Sci. 2005, 118, 2545–2555. [Google Scholar] [CrossRef] [PubMed]
- Nerlov, C. The C/EBP family of transcription factors: A paradigm for interaction between gene expression and proliferation control. Trends Cell Biol. 2007, 17, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Sihto, H.; Joensuu, H. Tumor-infiltrating lymphocytes and outcome in Merkel cell carcinoma, a virus-associated cancer. Oncoimmunology 2012, 1, 1420–1421. [Google Scholar] [CrossRef] [PubMed]
- Harms, P.W.; Patel, R.M.; Verhaegen, M.E.; Giordano, T.J.; Nash, K.T.; Johnson, C.N.; Daignault, S.; Thomas, D.G.; Gudjonsson, J.E.; Elder, J.T.; et al. Distinct gene expression profiles of viral- and nonviral-associated merkel cell carcinoma revealed by transcriptome analysis. J. Invest. Dermatol. 2013, 133, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Lauttia, S.; Sihto, H.; Kavola, H.; Koljonen, V.; Böhling, T.; Joensuu, H. Prokineticins and Merkel cell polyomavirus infection in Merkel cell carcinoma. Br. J. Cancer 2014, 110, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Andea, A.A.; Coit, D.G.; Amin, B.; Busam, K.J. Merkel cell carcinoma: Histologic features and prognosis. Cancer 2008, 113, 2549–2558. [Google Scholar] [CrossRef] [PubMed]
- Paulson, K.G.; Iyer, J.G.; Tegeder, A.R.; Thibodeau, R.; Schelter, J.; Koba, S.; Schrama, D.; Simonson, W.T.; Lemos, B.D.; Byrd, D.R.; et al. Transcriptome-wide studies of Merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J. Clin. Oncol. 2011, 29, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Wheat, R.; Roberts, C.; Waterboer, T.; Steele, J.; Marsden, J.; Steven, N.M.; Blackbourn, D.J. Inflammatory cell distribution in primary merkel cell carcinoma. Cancers 2014, 6, 1047–1064. [Google Scholar] [CrossRef] [PubMed]
- Sihto, H.; Böhling, T.; Kavola, H.; Koljonen, V.; Salmi, M.; Jalkanen, S.; Joensuu, H. Tumor infiltrating immune cells and outcome of Merkel cell carcinoma: A population-based study. Clin. Cancer Res. 2012, 18, 2872–2881. [Google Scholar] [CrossRef] [PubMed]
- Triozzi, P.L.; Fernandez, A.P. The role of the immune response in merkel cell carcinoma. Cancers (Basel) 2013, 5, 234–254. [Google Scholar] [CrossRef]
- Paulson, K.G.; Tegeder, A.; Willmes, C.; Iyer, J.G.; Afanasiev, O.K.; Schrama, D.; Koba, S.; Thibodeau, R.; Nagase, K.; Simonson, W.T.; et al. Downregulation of MHC-I expression is prevalent but reversible in Merkel cell carcinoma. Cancer Immunol. Res. 2014, 2, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Afanasiev, O.K.; Yelistratova, L.; Miller, N.; Nagase, K.; Paulson, K.; Iyer, J.G.; Ibrani, D.; Koelle, D.M.; Nghiem, P. Merkel polyomavirus-specific T cells fluctuate with merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markers. Clin. Cancer Res. 2013, 19, 5351–5360. [Google Scholar] [CrossRef] [PubMed]
- Lipson, E.J.; Vincent, J.G.; Loyo, M.; Kagohara, L.T.; Luber, B.S.; Wang, H.; Xu, H.; Nayar, S.K.; Wang, T.S.; Sidransky, D.; et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: Association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol. Res. 2013, 1, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.A.; Abdul-Sada, H.; Knight, L.M.; Jackson, B.R.; Richards, K.; Prescott, E.L.; Peach, A.H.; Blair, G.E.; Macdonald, A.; Whitehouse, A. Merkel cell polyomavirus small T antigen targets the NEMO adaptor protein to disrupt inflammatory signaling. J. Virol. 2013, 87, 13853–13867. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Huang, S.J.; Murphy, G.F.; Mollet, I.G.; Hijnen, D.; Muthukuru, M.; Schanbacher, C.F.; Edwards, V.; Miller, D.M.; Kim, J.E.; et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J. Exp. Med. 2008, 205, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- Gehad, A.E.; Lichtman, M.K.; Schmults, C.D.; Teaque, J.E.; Calarese, A.W.; Jiang, Y.; Watanabe, R.; Clark, R.A. Nitric oxide-producing myeloid-derived suppressor cells inhibit vascular E-selectin expression in human squamous cell carcinomas. J. Invest Dermatol. 2012, 132, 2642–2651. [Google Scholar] [CrossRef] [PubMed]
- Afanasiev, O.K.; Nagase, K.; Simonson, W.; Vandeven, N.; Blom., A.; Koelle, D.M.; Clark, R.; Nghiem, P. Vascular E-selectin expression correlates with CD8 lymphocyte infiltration and improved outcome in Merkel cell carcinoma. J. Invest. Dermatol. 2013, 133, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Tanaka, K.; Jay, F.; Khoury, G.; Jay, G. Modulation of the tumorigenicity of human adenovirus-12-transformed cells by interferon. Cell 1985, 43, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Cromme, F.V.; van Bommel, P.F.; Walboomers, J.M.; Gallee, M.P.; Stern, P.L.; Kenemans, P.; Helmerhorst, T.J.; Stukart, M.J.; Meijer, C.J. Differences in MHC and TAP-1 expression in cervical cancer lymph node metastases as compared with the primary tumors. Br. J. Cancer 1994, 69, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Jugovic, P.; York, I.; Russ, G.; Bennink, J.; Yewdell, J.; Ploeggh, H.; Johnson, D. Herpes simplex virus turns off the TAP to evade host immunity. Nature 1995, 375, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Koopman, L.A.; van Der Slik, A.R.; Giphart, M.J.; Fleuren, G.J. Human leukocyte antigen class I gene mutations in cervical cancer. J. Natl. Cancer Inst. 1999, 91, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Ueda, K.; Nakano, K.; Hirata, Y.; Parravicini, C.; Corbellino, M.; Yamanishi, K. Major histocompatibility complex class I molecules are down-regulated at the cell surface by the K5 protein encoded by Kaposi’s sarcoma-associated herpesvirus/human herpesvirus-8. J. Gen. Virol. 2001, 82, 1175–1180. [Google Scholar] [PubMed]
- Hansen, T.H.; Bouvier, M. MHC class I antigen presentation: Learning from viral evasion strategies. Nat. Rev. Immunol. 2009, 9, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Chretien, A.S.; Le Roy, A.; Vey, N.; Prebet, T.; Blaise, D.; Fauriat, C.; Olive, D. Cancer-Induced Alterations of NK-Mediated Target Recognition: Current and Investigational Pharmacological Strategies Aiming at Restoring NK-Mediated Anti-Tumor Activity. Front. Immunol. 2014, 5, e122. [Google Scholar] [CrossRef]
- Riella, L.V.; Paterson, A.M.; Sharpe, A.H.; Chandraker, A. Role of the PD-1 pathway in the immune response. Am. J. Transplant. 2012, 12, 2575–2587. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, H.; Kishimoto, S.; Shibagaki, T.; Nagata, M.; Yasuno, H. Merkel cell carcinoma with spontaneous regression: An immunohistochemical, ultrastructural, and TUNEL labeling study. Am. J. Dermatopathol. 1997, 19, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, N.; Nakanishi, G.; Kabuto, M.; Nakano, T.; Eto, H.; Nakajima, H.; Sano, S.; Tanaka, T. Merkel cell carcinoma showing regression after biopsy: Evaluation of programmed cell death 1-positive cells. J. Dermatol. 2015. [Google Scholar] [CrossRef]
- Arany, I.; Tyring, S.K. Status of cytokine and antigen presentation genes in Merkel cell carcinoma of the skin. J. Cutan. Med. Surg. 1998, 2, 138–141. [Google Scholar] [PubMed]
- Lord, S.J.; Rajotte, R.V.; Korbutt, G.S.; Bleackley, R.C. Granzyme B: A natural born killer. Immunol. Rev. 2003, 193, 31–38. [Google Scholar] [CrossRef]
- Pahl, H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, M.; Ohmori, Y. Constitutive nuclear factor kappaB activity is required to elicit interferon-gamma-induced expression of chemokine CXC ligand 9 (CXCL9) and CXCL10 in human tumor cell lines. Biochem. J. 2003, 376, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Shuda, M.; Guastafierro, A.; Feng, H.; Toptan, T.; Tolstov, Y.; Normolle, D.; Vollmer, L.L.; Vogt, A.; Dömling, A.; et al. Survivin is a therapeutic target in Merkel cell carcinoma. Sci. Transl. Med. 2012, 4, 133ra56. [Google Scholar] [CrossRef] [PubMed]
- Van Ghelue, M.; Khan, M.T.; Ehlers, B.; Moens, U. Genome analysis of the new human polyomaviruses. Rev. Med. Virol. 2012, 22, 354–377. [Google Scholar] [CrossRef] [PubMed]
- Youlden, D.R.; Youl, P.H.; Peter Soyer, H.; Fritschi, L.; Baade, P.D. Multiple primary cancers associated with Merkel cell carcinoma in Queensland, Australia, 1982–2011. J. Invest. Dermatol. 2014, 134, 2883–2889. [Google Scholar] [CrossRef] [PubMed]
- Mogha, A.; Fautrel, A.; Mouchet, N.; Guo, N.; Corre, S.; Adamski, H.; Watier, E.; Misery, L.; Galibert, M.D. Merkel cell polyomavirus small T-antigen mRNA level is increased following in vivo UV-radiation. PLOS ONE 2010, 5, e11423. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.S.; Grundhoff, A.T.; Tevethia, S.; Pipas, J.M.; Ganem, D. SV40-encoded microRNAs regulate viral gene expression and reduce susceptibility to cytotoxic T cells. Nature 2005, 435, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.E.; McClure, L.V.; Goga, A.; Sullivan, C.S. Pan-viral-microRNA screening identifies interferon inhibition as a common function of diverse viruses. Proc. Natl. Acad. Sci. USA 2015, 112, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Bauman, Y.; Mandelboim, O. MicroRNA based immunoevasion mechanism of human polyomaviruses. RNA Biol. 2011, 8, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Bauman, Y.; Nachmani, D.; Vitenshtein, A.; Tsukerman, P.; Drayman, N.; Stern-Ginossar, N.; Lankry, D.; Gruda, R.; Mandelboim, O. An identical miRNA of the human JC and BK polyoma viruses targets the stress-induced ligand ULBP3 to escape immune elimination. Cell Host Microbe 2011, 9, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Paulson, K.G.; Murchison, E.P.; Afanasiev, O.K.; Alkan, C.; Leonard, J.H.; Byrd, D.R.; Hannon, G.J.; Nghiem, P. Identification and validation of a novel mature microRNA encoded by the Merkel cell polyomavirus in human Merkel cell carcinomas. J. Clin. Virol. 2011, 52, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Groettrup, M.; Soza, A.; Eggers, M.; Kuehn, L.; Dick, T.P.; Schild, H. A role for the proteasome regulator PA28alpha in antigen presentation. Nature 1996, 381, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Okkenhaug, K.; Bilancio, A.; Farjot, G.; Priddle, H.; Sancho, S.; Peskett, E.; Pearce, W.; Meek, S.E.; Salpekar, A.; Waterfield, M.D.; Smith, A.J.; Vanhaesevroeck, B. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science 2002, 297, 1031–1034. [Google Scholar] [PubMed]
- Yan, Q.; Sharma-Kuinkel, B.K.; Deshmukh, H.; Tsalik, E.L.; Cyr, D.D.; Lucas, J.; Woods, C.W.; Scott, W.K.; Sempowski, G.D.; Thaden, J.T.; et al. Dusp3 and Psme3 Are Associated with Murine Susceptibility to Staphylococcus aureus Infection and Human Sepsis. PLOS Pathog. 2014, 10, e1004149. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Cox, J.E.; Kincaid, R.P.; Martinez, A.; Sullivan, C.S. Divergent MicroRNA targetomes of closely related circulating strains of a polyomavirus. J. Virol. 2013, 87, 11135–11147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hsu, K.S.; Lim, J.H.; Bruggeman, L.A.; Kao, H.Y. α-Actinin 4 Potentiates Nuclear Factor κ-Light-chain-enhancer of Activated B-cell (NF-κB) Activity in Podocytes Independent of Its Cytoplasmic Actin Binding Function. J. Biol. Chem. 2015, 290, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Zhang, Z.; Li, C.; Huang, M.; Shi, Z.; Wang, Y.; Song, X.; Liu, H.; Li, C.; Chen, M.; et al. The kinase MST4 limits inflammatory responses through direct phosphorylation of the adaptor TRAF6. Nat. Immunol. 2015, 16, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Kincaid, R.P.; Sullivan, C.S. Virus-Encoded microRNAs: An Overview and a Look to the Future. PLOS Pathog. 2012, 8, e1003018. [Google Scholar] [CrossRef] [PubMed]
- Lagatie, O.; Tritsmans, L.; Stuyver, L.J. The miRNA world of polyomaviruses. Virol. J. 2013, 10, e268. [Google Scholar] [CrossRef]
- Chen, C.J.; Cox, J.E.; Azarm, K.D.; Wylie, K.N.; Woolard, K.D.; Pesavento, P.A.; Sullivan, C.S. Identification of a polyomavirus microRNA highly expressed in tumors. Virology 2015, 476, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Haecker, I.; Yang, Y.; Gao, S.J.; Renne, R. γ-Herpesvirus-encoded miRNAs and their roles in viral biology and pathogenesis. Curr. Opin. Virol. 2013, 3, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Renwick, N.; Cekan, P.; Masry, P.A.; McGeary, S.E.; Miller, J.B.; Hafner, M.; Li, Z.; Mihailovic, A.; Morozov, P.; Brown, M.; et al. Multicolor microRNA FISH effectively differentiates tumor types. J. Clin. Invest. 2013, 123, 2694–2702. [Google Scholar] [CrossRef] [PubMed]
- Grundhoff, A.; Sullivan, C.S. Virus-encoded microRNAs. Virology 2011, 411, 325–343. [Google Scholar] [CrossRef] [PubMed]
- Cullen, B.R. MicroRNAs as mediators of viral evasion of the immune system. Nat. Immunol. 2013, 14, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Fimia, G.M.; Corazzari, M.; Antonioli, M.; Piacentini, M. Ambra1 at the crossroad between autophagy and cell death. Oncogene 2013, 32, 3311–3318. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.K.; Yim, H.; Andrews, E.; Benjamin, T.L. A mouse polyomavirus-encoded microRNA targets the cellular apoptosis pathway through Smad2 inhibition. Virology 2014, 468–470, 57–62. [Google Scholar]
- Skalsky, R.L.; Cullen, B.R. Viruses, microRNAs, and host interactions. Annu. Rev. Microbiol. 2010, 64, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Moens, U.; Seternes, O.M.; Johansen, B.; Rekvig, O.P. Mechanisms of transcriptional regulation of cellular genes by SV40 large T- and small t-antigens. Virus Genes 1997, 15, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Cullen, B.R. Transcription and processing of human microRNA precursors. Mol. Cell 2004, 16, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.J.; Fink, L.H.; O’Hara, B.; Atwood, W.J.; Sullivan, C.S. Evolutionary conserved function of a viral microRNA. J. Virol. 2008, 82, 9823–9828. [Google Scholar] [CrossRef] [PubMed]
- Broekema, N.M.; Imperiale, M.J. miRNA regulation of BK polyomavirus replication during early infection. Proc. Natl. Acad. Sci. USA 2013, 110, 8200–8205. [Google Scholar] [CrossRef] [PubMed]
- Shuda, M.; Arora, R.; Kwun, H.J.; Feng, H.; Sarid, R.; Fernández-Figueras, M.T.; Tolstov, Y.; Gjoerup, O.; Mansukhani, M.M.; Swerdlow, S.H.; et al. Human Merkel cell polyomavirus infection I. MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues and lymphoid tumors. Int. J. Cancer 2009, 125, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Brostoff, T.; Dela Cruz, F.N., Jr.; Church, M.E.; Woolard, K.D.; Pesavento, P.A. The raccoon polyomavirus genome and tumor antigen transcription are stable and abundant in neuroglial tumors. J. Virol. 2014, 88, 12816–12824. [Google Scholar] [CrossRef] [PubMed]
- Braconi, C.; Valeri, N.; Gasparini, P.; Huang, N.; Tacciolo, C.; Nuovo, G.; Suzuki, T.; Croce, C.M.; Patel, T. Hepatitis C virus proteins modulate microRNA expression and chemosensitivity in malignant hepatocytes. Clin. Cancer Res. 2010, 16, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Hu, T.F.; Chen, Y.C.; Tsai, Y.N.; Tsai, Y.H.; Cheng, C.C.; Wang, H.W. The manipulation of miRNA-gene regulatory networks by KSHV induces endothelial cell motility. Blood 2011, 118, 2896–2905. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Tilahun, Y.; Taha, O.; Alao, H.; Kodys, K.; Catalano, D.; Szabo, G. Increased microRNA-155 expression in the serum and peripheral monocytes in chronic HCV infection. J. Transl. Med. 2012, 10, e151. [Google Scholar] [CrossRef]
- Lin, L.; Yin, X.; Hu, X.; Wang, Q.; Zheng, L. The impact of hepatitis B virus x protein and microRNAs in hepatocellular carcinoma: A comprehensive analysis. Tumor. Biol. 2014, 35, 11695–11700. [Google Scholar] [CrossRef]
- Ma, L.; Deng, X.; Wu, M.; Zhang, G.; Huang, J. Down-regulationof miRNA204 by LMP-1 enhances CDC2 activity and facilitates invasion of EBV-associated nasopharyngeal carcinoma cells. FEBS Lett. 2014, 588, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.; Pan, Q.; Blencowe, B.J.; Claycomb, J.M.; Frappier, L. Epstein-Barr virus EBNA1 protein regulates viral latency through effects on let-7 microRNA and dicer. J. Virol. 2014, 88, 11166–11177. [Google Scholar] [CrossRef] [PubMed]
- Vernin, C.; Thenoz, M.; Pinatel, C.; Gessain, A.; Gout, O.; Delfau-Larue, M.H.; Nazaret, N.; Legras-Lachuer, C.; Wattel, E.; Mortreux, F. HTLV-1 bZIP factor HBZ promotes cell proliferation and genetic instability by activating OncomiRs. Cancer Res. 2014, 74, 6082–6093. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Lee, L.; Caramuta, S.; Höög, A.; Browaldh, N.; Björnhagen, V.; Larsson, C.; Lui, W.O. MicroRNA expression patterns related to merkel cell polyomavirus infection in human merkel cell carcinoma. J. Invest. Dermatol. 2014, 134, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar] [PubMed]
- Zhao, Y.; Liu, H.; Riker, A.I.; Fodstad, O.; Ledoux, S.P.; Wilson, G.L.; Tan, M. Emerging metabolic targets in cancer therapy. Front. Biosci. 2011, 16, 1844–1860. [Google Scholar] [CrossRef]
- Kinnaird, A.; Michelakis, E.D. Metabolic modulation of cancer: A new frontier with great translational potential. J. Mol. Med. 2015, 93, 127–142. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Girault, S.; Testard, A.; Delva, R.; Soulié, P.; Couturier, O.F.; Morel, O. The impact of (18)F-FDG-PET/CT on Merkel cell carcinoma management: A retrospective study of 66 scans from a single institution. Nucl. Med. Commun. 2014, 35, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, K.; Nishino, H.; Iwashima, A. Analysis of hexose transport in untransformed and sarcoma virus-transformed mouse 3T3 cells by photoaffinity binding of cytochalasin B. Biochim. Biophys Acta 1985, 821, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Bravard, A.; Beaumatin, J.; Luccioni, C.; Fritsch, P.; Lefrançois, D.; Thenet, S.; Adolphe, M.; Dutrillaux, B. Chromosomal, mitochondrial and metabolic alterations in SV40-transformed rabbit chondrocytes. Carcinogenesis 1992, 13, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Lachaise, F.; Martin, G.; Drougard, C.; Perl, A.; Vuillaume, M.; Wegnez, M.; Sarasin, A.; Daya-Grosjean, L. Relationship between posttranslational modification of transaldolase and catalase deficiency in UV-sensitive repair-deficient xeroderma pigmentosum fibroblasts and SV40-transformed human cells. Free Radic. Biol. Med. 2001, 30, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Noch, E.; Sariyer, I.K.; Gordon, J.; Khalili, K. JC virus T-antigen regulates glucose metabolic pathways in brain tumor cells. PLOS ONE 2012, 7, e35054. [Google Scholar] [CrossRef] [PubMed]
- Duracher, D.; Wyczechowska, D.; Wilk, A.; Lassak, A.; Zea, A.; Estrada, J.; Reis, K. The Effects of JCV T-antigen on Tumor Cell Metabolism. Available online: http://www.medschool.lsuhsc.edu/cancer_center/docs/Dustin%20Duracher%20Poster%2036x60.pdf (accessed on 10 March 2015).
- Ramanathan, A.; Wang, C.; Schreiber, S.L. Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc. Natl. Acad. Sci. USA 2005, 102, 5992–5997. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Veldman, T.; Rundell, K.; Schlegel, R. Simian virus 40 small tumor antigen activates AKT and telomerase and induces anchorage-independent growth of human epithelial cells. J. Virol. 2002, 76, 10685–10691. [Google Scholar] [CrossRef] [PubMed]
- Elstrom, R.L.; Bauer, D.E.; Buzzai, M.; Karnauskas, R.; Harris, M.H.; Plas, D.R.; Zhuang, H.; Cinalli, R.M.; Alavi, A.; Rudin, C.M.; Thompson, C.B. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004, 64, 3892–3899. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Viciana, P.; Collins, C.; Fried, M. Polyoma and SV40 proteins differentially regulate PP2A to activate distinct cellular signaling pathways involved in growth control. Proc. Natl. Acad. Sci. USA 2006, 103, 19290–19295. [Google Scholar] [CrossRef] [PubMed]
- Sontag, E.; Fedorov, S.; Kamibayashi, C.; Robbins, D.; Cobb, M.; Mumby, M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell 1993, 75, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Agnetti, G.; Maraldi, T.; Giorentini, D.; Giordano, E.; Prata, C.; Hakim, G.; Muscari, C.; Guarnieri, C.; Caldarera, C.M. Activation of glucose transport during simulated ischemia in H9c2 cardiac myoblasts is mediated by protein kinase C isoforms. Life Sci. 2005, 78, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Madan, E.; Gogna, R.; Bhatt, M.; Pati, U.; Kuppusamy, P.; Mahdi, A.A. Regulation of glucose metabolism by p53: Emerging new roles for the tumor suppressor. Oncotarget 2011, 2, 948–957. [Google Scholar] [PubMed]
- Shivakumar, C.V.; Das, G.C. Interaction of human polyomavirus BK with the tumor-suppressor protein p53. Oncogene 1996, 13, 323–332. [Google Scholar] [PubMed]
- Staib, C.; Pesch, J.; Gerwig, R.; Gerber, J.K.; Brehm, U.; Stangl, A.; Grummt, F. p53 inhibits JC virus DNA replication in vivo and interacts with JC virus large T-antigen. Virology 1996, 219, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Kenific, C.M.; Debnath, J. Cellular and metabolic functions for autophagy in cancer cells. Trends Cell Biol. 2015, 25, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.; Jung, J.U. Modulation of the Autophagy Pathway by Human Tumor Viruses. Semin. Cancer Biol. 2013, 23, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Bouley, S.J.; Maginnis, M.S.; Derdowski, A.; Gee, G.V.; O’Hara, B.A.; Nelson, C.D.; Bara, A.M.; Atwood, W.J.; Dugan, A.S. Host cell autophagy promotes BK virus infection. Virology 2014, 456–457, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.H.; Rangarajan, A. Simian virus 40 small T antigen activates AMPK and triggers autophagy to protect cancer cells from nutrient deprivation. J. Virol. 2009, 83, 8565–8574. [Google Scholar] [CrossRef] [PubMed]
- Rundell, K.; Major, E.O.; Lampert, M. Association of cellular 56,000- and 32,000-molecular-weight protein with BK virus and polyoma virus t-antigens. J. Virol. 1981, 37, 1090–1093. [Google Scholar] [PubMed]
- Bollag, B.; Hofstetter, C.A.; Reviriego-Mendoza, M.M.; Frisque, R.J. JC virus small T antigen binds phosphatase PP2A and Rb family proteins and is required for efficient viral DNA replication activity. PLOS ONE 2010, 5, e10606. [Google Scholar] [CrossRef] [PubMed]
- Shuda, M.; Kwun, H.J.; Feng, H.; Chang, Y.; Moore, P.S. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J. Clin. Invest. 2011, 121, 3623–3634. [Google Scholar] [CrossRef] [PubMed]
- Berrios, C.; Jung, J.; Primi, B.; Wang, M.; Pedamallu, C.; Duke, F.; Marcelus, C.; Cheng, J.; Garcea, R.L.; Meyerson, M.; DeCaprio, J.A. Malawi polyomavirus is a prevalent human virus that interacts with known tumor suppressors. J. Virol. 2015, 89, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Kwun, H.J.; Shuda, M.; Camacho, C.J.; Gamper, A.M.; Thant, M.; Chang, Y.; Moore, P.S. Restricted Protein Phosphatase 2A Targeting by Merkel Cell Polyomavirus Small T Antigen. J. Virol. 2015, 89, 4191–4200. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Darbinian, N.; Kaminski, R.; White, M.K.; Gentilella, A.; Turco, M.C.; Khalili, K. Evidence for modulation of BAG3 by polyomavirus JC early protein. J. Gen. Virol. 1999, 90, 1629–1640. [Google Scholar] [CrossRef]
- Sariyer, I.K.; Merabova, N.; Patel, P.K.; Knezevic, T.; Rosati, A.; Turco, M.C.; Khalili, K. Bag3-induced autophagy is associated with degradation of JCV oncoprotein, T-Ag. PLOS ONE 2012, 7, e45000. [Google Scholar] [CrossRef] [PubMed]
- Ribbens, J.J.; Moser, A.B.; Hubbard, W.C.; Bongarzone, E.R.; Maegawa, G.H. Characterization and application of a disease-cell model for a neurodegenerative lysosomal disease. Mol. Genet. Metab. 2014, 111, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Meckes, D.G., Jr.; Raab-Traub, N. Microvesicles and viral infection. J. Virol. 2011, 85, 12844–12854. [Google Scholar] [CrossRef] [PubMed]
- Brinton, L.T.; Sloane, H.S.; Kester, M.; Kelly, K.A. Formation and role of exosomes in cancer. Cell Mol. Life Sci. 2015, 72, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Schorey, J.S.; Cheng, Y.; Singh, P.P.; Smith, V.L. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015, 16, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnaiah, V.; Thumann, C.; Fofana, I.; Habersetzer, F.; Pan, Q.; de Ruiter, P.E.; Willemsen, R.; Demmers, J.A.; Stalin Raj, V.; Jenster, G.; et al. Exosome-mediated transmission of hepatitis C virus between hepatoma Huh7.5 cells. Proc. Natl. Acad. Sci. USA 2013, 110, 13109–13113. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, E.; Narayanan, A.; van Duyne, R.; Shabbeer-Meyering, S.; Iordanskiy, S.; Saifuddin, M.; Das, R.; Afonso, P.V.; Sampey, G.C.; Chung, M.; et al. Human T-lymphotropic virus type 1-infected cells secrete exosomes that contain Tax protein. J. Biol. Chem. 2014, 289, 22284–22305. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, Y.; Duan, J.; Ma, Y.; Shen, Z.; Wei, L.; Cui, X.; Zhang, J.; Xie, Y.; Liu, J. Quantitative proteomic analysis of exosome protein content changes induced by hepatitis B virus in Huh-7 cells using SILAC labeling and LC-MS/MS. J. Proteome Res. 2014, 13, 5391–5402. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Chennakrishnaiah, S.; Audemard, E.; Montermini, L.; Meehan, B.; Rak, J. Oncogenic ras-driven cancer cell vesiculation leads to emission of double-stranded DNA capable of interacting with target cells. Biochem. Biophys. Res. Commun. 2014, 451, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, I.; Martelli, F.; Repice, A.; Massacesi, L.; Azzi, A.; Giannecchini, S. Detection of JCPyV microRNA in blood and urine samples of multiple sclerosis patients under natalizumab therapy. J. Neurovirol. 2015. [Google Scholar] [CrossRef]
- Meckes, D.G., Jr.; Shair, K.H.; Marquitz, A.R.; Kung, C.P.; Edwards, R.H.; Raab-Traub, N. Human tumor virus utilizes exosomes for intercellular communication. Proc. Natl. Acad. Sci. USA 2010, 107, 20370–20375. [Google Scholar] [CrossRef] [PubMed]
- Dreux, M.; Garaigorta, U.; Boyd, B.; Décembre, E.; Chung, J.; Whitten-Bauer, C.; Wieland, S.; Chisari, F.V. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe 2012, 12, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Chugh, P.E.; Sin, S.H.; Ozgur, S.; Henry, D.H.; Menezes, P.; Griffith, J.; Eron, J.J.; Damania, B.; Dittmer, D.P. Systemically circulating viral and tumor-derived microRNAs in KSHV-associated malignancies. PLOS Pathog. 2013, 9, e1003484. [Google Scholar] [CrossRef] [PubMed]
- Meckes, D.G., Jr.; Gunawardena, H.P.; Dekroon, R.M.; Heaton, P.R.; Edwards, R.H.; Ozgur, S.; Griffith, J.D.; Damania, B.; Raab-Traub, N. Modulation of B-cell exosome proteins by gamma herpesvirus infection. Proc. Natl. Acad. Sci. USA 2013, 110, E2925–E2933. [Google Scholar] [CrossRef] [PubMed]
- Velders, M.P.; Macedo, M.F.; Provenzano, M.; Elmishad, A.G.; Holzhütter, H.G.; Carbone, M.; Kwast, W.M. Human T cell responses to endogenously presented HLA-A*0201 restricted peptides of Simian virus 40 large T antigen. J. Cell Biochem. 2001, 82, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Vlastos, A.; Andreasson, K.; Tegerstedt, K.; Holländerová, D.; Heidari, S.; Forstová, J.; Ramqvist, T.; Dalianis, T. VP1 pseudocapsids, but not a glutathione-S-transferase VP1 fusion protein, prevent polyomavirus infection in a T-cell immune deficient experimental mouse model. J. Med. Virol. 2003, 70, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.B.; Shearer, M.H.; Tarbox, J.A.; Kang, H.S.; Jumper, C.A.; Bright, R.K.; Kennedy, R.C. In vitro simian virus 40 large tumor antigen expression correlates with differential immune responses following DNA immunization. Virology 2005, 332, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Gomez, B.P.; Viscidi, R.P.; Peng, S.; He, L.; Ma, B.; Wu, T.C.; Hung, C.F. Development of a DNA vaccine targeting Merkel cell polyomavirus. Vaccine 2012, 30, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Sospedra, M.; Schippling, S.; Yousef, S.; Jelcic, I.; Bofill-Mas, S.; Planas, R.; Stellmann, J.P.; Demina, V.; Cinque, P.; Garcea, R.; et al. Treating progressive multifocal leukoencephalopathy with interleukin 7 and vaccination with JC virus capsid protein VP1. Clin. Infect Dis. 2014, 59, 1588–1592. [Google Scholar] [CrossRef] [PubMed]
- Willmes, C.; Adam, C.; Alb, M.; Völkert, L.; Houben, R.; Becker, J.C.; Schrama, D. Type I and II IFNs inhibit Merkel cell carcinoma via modulation of the Merkel cell polyomavirus T antigens. Cancer Res. 2012, 72, 2120–2128. [Google Scholar] [CrossRef] [PubMed]
- Nakajima., H.; Takaishi, M.; Yamamoto, M.; Kamijima, R.; Kodama, H.; Tarutani, M.; Sano, S. Screening of the specific polyoma virus as diagnostic and prognostic tools for Merkel cell carcinoma. J. Dermatol. Sci. 2009, 56, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Biver-Dalle, C.; Nguyen, T.; Touzé, A.; Saccomani, C.; Penz, S.; Cunat-Peultier, S.; Riou-Gotta, M.O.; Humbert, P.; Coursaget, P.; Aubin, F. Use of interferon-alpha in two patients with Merkel cell carcinoma positive for Merkel cell polyomavirus. Acta Oncol. 2011, 50, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, A.G.; Afanasiev, O.K.; Iyer, J.G.; Paulson, K.G.; Parvathaneni, U.; Hwang, J.H.; Lai, I.; Roberts, I.M.; Sloan, H.L.; Bhatia, S.; et al. Regression of metastatic Merkel cell carcinoma following transfer of polyomavirus-specific T cells and therapies capable of re-inducing HLA class-I. Cancer Immunol. Res. 2014, 2, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Hata, Y.; Matsuka, K.; Ito, O.; Matsuda, H.; Furuichi, H.; Konstantinos, A.; Nuri, B. Two cases of Merkel cell carcinoma cured by intratumor injection of natural human tumor necrosis factor. Plast Reconstr. Surg. 1997, 99, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Lampreave, J.L.; Bénard, F.; Alavi, A.; Jimeneze-Hoyuela, J.; Fraker, D. PET evaluation of therapeutic limb perfusion in Merkel’s cell carcinoma. J. Nucl. Med. 1998, 39, 2087–2090. [Google Scholar] [PubMed]
- Olieman, A.F.; Liénard, D.; Eggermont, A.M.; Kroon, B.B.; Lejeune, F.J.; Hoekstra, H.J.; Koops, H.S. Hyperthermic isolated limb perfusion with tumor necrosis factor alpha, interferon gamma, and melphalan for locally advanced nonmelanoma skin tumors of the extremities: A multicenter study. Arch. Surg. 1999, 134, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.M.; Kim, C.N. Merkel cell carcinoma in a patient treated with adalimumab: Case report. Cutis 2011, 87, 81–84, Erratum in Cutis 2011, 87, 185. [Google Scholar]
- Linn-Rasker, S.P.; van Albada-Kuipers, G.A.; Dubois, S.V.; Janssen, K.; Zweers, P.G. Merkel cell carcinoma during treatment with TNF-alpha inhibitors: Coincidence or warning? Ned. Tijdschr. Geneeskd. 2012, 156, A44–A64. [Google Scholar]
- Reiss, K.A.; Forde, P.M.; Brahmer, J.R. Harnessing the power of the immune system via blockade of PD-1 and PD-L1: A promising new anticancer strategy. Immunotherapy 2014, 6, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Orba, Y.; Sawa, H.; Iwata, H.; Tanaka, S.; Nagashima, K. Inhibition of virus production in JC virus-infected cells by postinfection RNA interference. J. Virol. 2004, 78, 7270–7273. [Google Scholar] [CrossRef] [PubMed]
- Sabbioni, S.; Callegari, E.; Spizzo, R.; Veronese, A.; Altavilla, G.; Corallini, A.; Negrini, M. Anticancer activity of an adenoviral vector expressing short hairpin RNA against BK virus T-ag. Cancer Gene Ther. 2007, 14, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Houben, R.; Shuda, M.; Weinkam, R.; Schrama, D.; Feng, H.; Chang, Y.; Moore, P.S.; Becker, J.C. Merkel cell polyoma virus-infected Merkel cell carcinoma cells require expression of viral T antigens. J. Virol. 2010, 84, 7064–7072. [Google Scholar] [CrossRef] [PubMed]
- Angermeyer, S.; Hesbacher, S.; Becker, J.C.; Schrama, D.; Houben, R. Merkel cell polyomavirus-positive Merkel cell carcinoma cells do not require expression of the viral small T antigen. J. Invest Dermatol. 2013, 133, 2059–2064. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Wang, M.; Fang, C.Y.; Chen, P.L.; Shen, C.H.; Chang, D. Inhibition of BK virus replication in human kidney cells by BK virus large tumor antigen-specific shRNA delivered by JC virus-like particles. Antiviral Res. 2014, 103, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Hartman, Z.C.; Wei, J.; Glass, O.K.; Guo, H.; Lei, G.; Yang, X.Y.; Osada, T.; Hobeika, A.; Delcayre, A.; Le Pecq, J.B.; Morse, M.A.; Clay, T.M.; Lyerly, H.K. Increasing vaccine potency through exosome antigen targeting. Vaccine 2011, 29, 9361–9367. [Google Scholar] [CrossRef] [PubMed]
- Shuda, M.; Feng, H.; Kwun, H.J.; Rosen, S.T.; Gjoerup, O.; Moore, P.S.; Chang, Y. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc. Natl. Acad. Sci. USA 2008, 105, 16272–16277. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).