Abstract
Human immunodeficiency virus type 1 (HIV-1) encodes four accessory genes: vif, vpu, vpr, and nef. Recent investigations using in vitro cell culture systems have shed light on the roles of these HIV-1 accessory proteins, Vif, Vpr, Vpu, and Nef, in counteracting, modulating, and evading various cellular factors that are responsible for anti-HIV-1 intrinsic immunity. However, since humans are the exclusive target for HIV-1 infection, conventional animal models are incapable of mimicking the dynamics of HIV-1 infection in vivo. Moreover, the effects of HIV-1 accessory proteins on viral infection in vivo remain unclear. To elucidate the roles of HIV-1 accessory proteins in the dynamics of viral infection in vivo, humanized mouse models, in which the mice are xenotransplanted with human hematopoietic stem cells, has been utilized. This review describes the current knowledge of the roles of HIV-1 accessory proteins in viral infection, replication, and pathogenicity in vivo, which are revealed by the studies using humanized mouse models.
1. Introduction
Human immunodeficiency virus type 1 (HIV-1), a virus belonging to the genus Retroviridae, was identified in 1983 as the causative agent of acquired immunodeficiency syndrome (AIDS) [1,2]. HIV-1 genome consists of nine genes (Figure 1, top), and five out of the nine genes, gag, pol, env, tat, and rev, are essential for viral replication [3]. On the other hand, the remaining four genes, vif, vpu, vpr, and nef, are not always required for viral replication in in vitro studies using cell culture system [3,4]. Recent investigations have shed light on the roles of these viral accessory proteins in counteracting, modulating, and evading various host restriction factors responsible for anti-HIV-1 cellular intrinsic immunity [4,5].
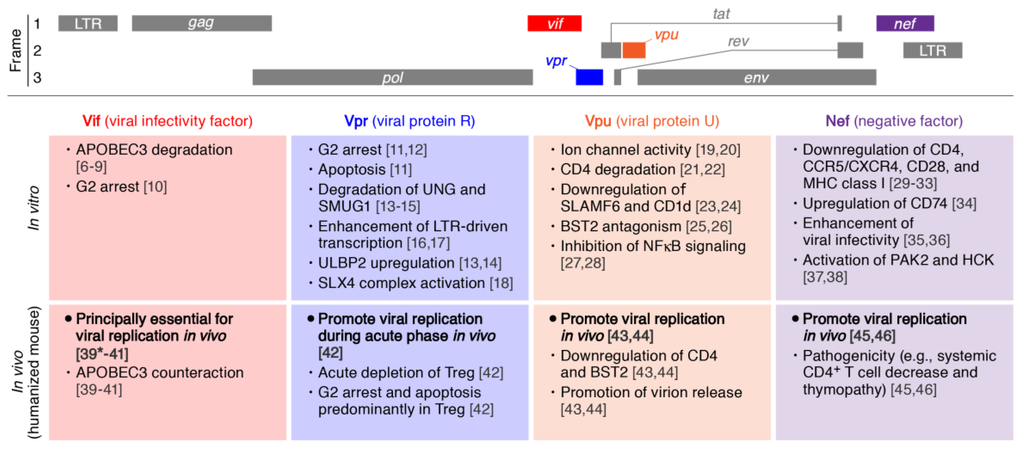
Figure 1.
Roles of Human immunodeficiency virus type 1 (HIV-1) accessory proteins in vitro and in vivo. (Top) The scheme of HIV-1 genome. Three reading frames are respectively indicated. (Middle) Major roles of HIV-1 accessory proteins reported from the experiments using cell cultures. (Bottom) The roles of HIV-1 accessory proteins elucidated from the experiments using humanized mouse models. The numbers in parentheses indicate the references. *, as an exception; Vif is dispensable if a vif-deficient CXCR4-tropic HIV-1 (strain LAI) is intravenously inoculated into BLT humanized mice [39]. APOBEC3, apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3; UNG, Uracil-DNA glycosylase; SMUG1, single-strand-selective monofunctional uracil-DNA glycosylase 1; LTR, long terminal repeat; ULBP2, UL16 binding protein 2; SLX4, SLX4 structure-specific endonuclease subunit; BST2, bone marrow stromal cell antigen 2; SLAMF6, signaling lymphocyte activation molecule family member 6; PAK2, p21 protein (Cdc42/Rac)-activated kinase 2; HCK, hematopoietic cell kinase.
For the basic research of HIV-1 infection, in vitro cell culture systems including cell lines and primary human CD4+ T cells have been extensively utilized (Figure 2). However, the cell lines are transformed and abnormal. Primary human CD4+ T cells are artificially activated by mitogens (e.g., phytohemaggluttinin and anti-CD3/CD28 antibodies) to allow efficient HIV-1 replication. On the other hand, only a small portion of cell subsets in CD4+ T cells are activated in vivo, especially after antigen stimulation [47]. Therefore, the expression patterns of cellular genes, which are positively or negatively associated with HIV-1 replication, may be quite different in in vitro and in vivo, and it is important to investigate the dynamic interplay between cellular factors and HIV-1 accessory proteins in vivo. However, the observations on the roles of HIV-1 accessory proteins, which are summarized in Figure 1, are principally based on the investigations using cell culture system in vitro.
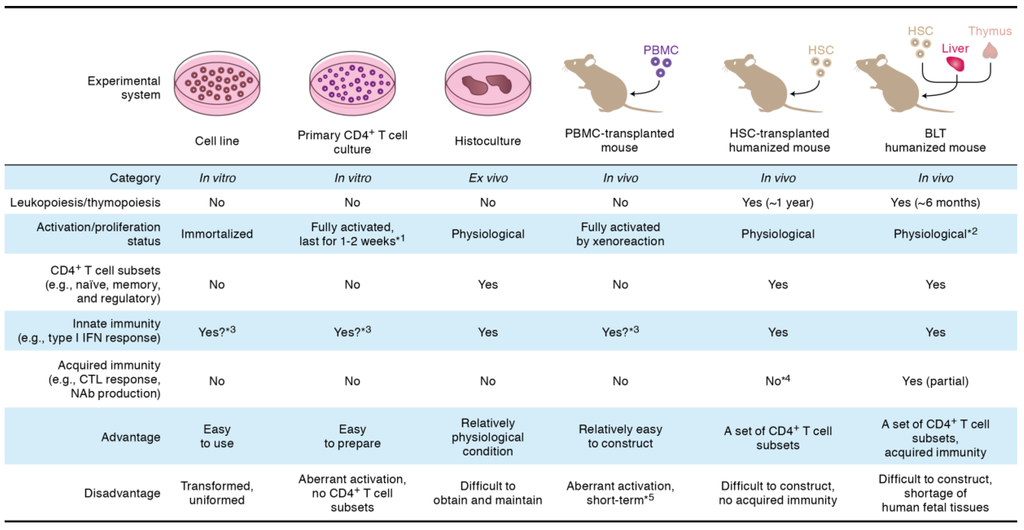
Figure 2.
Experimental system for HIV-1 infection. The detailed explanation of each experimental system is described in the text. *1, to perform HIV-1 replication assays, primary CD4+ T cells should be artificially activated by mitogens (e.g., phytohemaggluttinin and anti-CD3/CD28 antibodies); *2, because human thymocytes are efficiently educated in human thymic transplant (i.e., human MHC), the human T cells differentiated in BLT humanized mice may recognize the tissues of recipient mouse as foreign antigen, which can lead to the onset of graft-versus-host reaction; *3, these systems are capable of responding type I interferon stimulation, which can lead to the expression of interferon-stimulating genes. However, these systems are incapable of triggering innate immune sensing because of the absence of dendritic cells and macrophages; *4, because human thymocytes are educated in the thymus of recipient mouse (i.e., murine MHC), the human T cells differentiated in HSC-transplanted humanized mice are unable to efficiently receive the antigen stimulation from human antigen presenting cells; *5, the transplanted human PBMCs recognize the tissues of recipient mouse as foreign antigen and cause graft-versus-host reaction, which results in the aberrant xenoactivation. BLT, bone marrow/liver/thymus; CTL, cytotoxic T lymphocyte; HSC, hematopoietic stem cell; IFN, interferon; NAb, neutralizing antibody; PBMC, peripheral blood mononuclear cell.
To closely mimic HIV-1 infection in in vivo conditions, human histoculture systems such as the tissue explants from tonsil [48], cervix [49,50], vagina [49,51], and thymus [52], have been used (Figure 2). Compared to the cell cultures in vitro, these ex vivo histoculture systems reflect physiological conditions more closely because of the intact tissue architecture with multiple human leukocyte lineages including human CD4+ T cell subsets (e.g., naïve, memory, and regulatory cells (Tregs)), monocytes/macrophages, dendritic cells, and stromal cells. However, because of its surgical technique and human donors are needed, it appears to be difficult to routinely use this system for basic HIV-1 research. Moreover, the organ culture can only study HIV-1 infection in the isolated small tissue pieces that might not be ideal for many other experimental purposes.
To reconstruct human immunity in vivo, mouse models xenotransplanted with human cells have been developed. One of the classical small animal models is the severe combined immunodeficient (SCID) mouse xenotransplanted with human peripheral blood mononuclear cells (PBMCs) (Figure 2). This PBMC-transplanted mouse model is relatively easy to construct and efficiently allows HIV-1 replication [53]. Also, this model has been used for multiple purposes on HIV-1 research such as the efficacy evaluation of passive immunization of anti-HIV-1 antibodies [54]. However, since the human lymphocytes in this mouse model are aberrantly activated because of xenoreactions against murine antigens [55], the condition of human CD4+ T cells are not physiological.
In order to reproduce more physiological condition of human immunity in vivo, a new generation mouse model, called “humanized mouse” has been developed [56,57,58,59]. One is the mouse xenotransplanted with human CD34+ hematopoietic stem cells (HSCs), while the other, which is called bone marrow/liver/thymus (BLT) mouse, is xenotransplanted with the tissue sections of human fetal thymus and liver as well as human HSCs (Figure 2) [60,61]. Notably, both HSC-transplanted and BLT humanized mouse models are capable of supporting human lymphopoiesis and thymopoiesis for 6–12 months [60,61]. In addition, the human lymphocytes including human CD4+ T cell subsets, which are reconstituted in these humanized mouse models, are maintained in a physiological condition [42]. In HSC-transplanted humanized mouse, human acquired immune response (e.g., antibody production and cytotoxic T cell responses) is poorly elicited because human T cells/thymocytes are educated in murine thymus (i.e., the mismatching of major histocompatibility complex [MHC]) (Figure 2) [62,63]. On the other hand, BLT humanized mouse can potently elicit human acquired immunity because human T cells/thymocytes are educated in transplanted human thymic organoid [64,65]. However, it should be noted that BLT humanized mouse may suffer from aberrant immune activation which is triggered by the highly educated human T cells (Figure 2).
In terms of the condition of human CD4+ T cells, we can say that these two humanized mouse models are the best choices to reproduce and investigate the dynamics of HIV-1 infection in vivo (i.e., under the physiological condition) at present. However, special facilities (e.g., specific pathogen-free condition), surgical technique, and appropriate recipient mice (e.g., NOG and NSG mice; [62,66]) are required for the construction of humanized mouse models. Furthermore, due to the ethical issues in the use and acquisition of the tissues from abortive fetuses for basic investigations, it is difficult to construct BLT humanized mouse model in many countries except for the United States. To circumvent this problem and for better understanding the mechanisms of the development of human immunity, lines of studies to improve the genetic background of the recipient mouse for the establishment of more efficient and appropriate humanized models have been pursued [67,68,69,70].
Because HIV-1 infects and causes disorders only in humans and chimpanzees, there is no perfect animal model to investigate the roles of HIV-1 accessory proteins in viral infection, replication, and pathogenesis in vivo so far. In this review, we describe the current state-of-the-art of novel findings on the roles of HIV-1 accessory proteins in vivo, which are obtained from the investigations using HSC-transplanted and BLT humanized mouse models.
2. Viral Infectivity Factor (Vif)
Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3 (APOBEC3) proteins are cellular cytidine deaminases that convert cytosines in the viral minus-strand cDNA to uracils, which results in the alternation of guanines to adenine in the nascent viral DNA (i.e., G-to-A mutation) [6,7,9]. Human cells encode 7 APOBEC3 genes, APOBEC3A, B, C, D, F, G and H [6,8]. Extensive studies using in vitro cell cultures have revealed that certain APOBEC3 proteins, particularly APOBEC3D, APOBEC3F, and APOBEC3G, exhibit robust anti-HIV-1 activity principally depending on their enzymatic activity [6,8]. To counteract the anti-viral actions of APOBEC3 proteins, HIV-1 arms its own weapon, Vif. Vif recruits cellular E3 ubiquitin ligase complex, which is composed of cullin 5 (CUL5), elongin B/C (ELOB/C), and core binding factor beta (CBF-β), and degrades APOBEC3 proteins via the ubiquitin/proteasome-dependent pathway (Figure 1) [7]. Moreover, Izumi et al. revealed that Vif elicits cell cycle arrest at G2 phase (G2 arrest) independently of its anti-APOBEC3 activity (Figure 1) [10]. To investigate the dynamic interplay between endogenous APOBEC3 proteins and Vif in vivo, three previous studies have addressed this issue by using humanized mouse models (Table 1) [39,40,41]. First, Sato et al. inoculated CCR5-tropic wild type (WT) HIV-1 (strain JRCSF) and its vif-deficient derivative into hHSC-transplanted humanized mice (designated to NOG-hCD34 mice) [40]. Though WT HIV-1 efficiently expanded in humanized mice, vif-deficient HIV-1 did not show viremia, strongly suggesting that the replication of vif-deficient HIV-1 in humanized mice is canceled by endogenous APOBEC3 proteins expressed in human CD4+ T cells of humanized mice. In addition, the accumulation of G-to-A mutations in provirus genome was observed, and notably, lethal mutations (i.e., mutations to stop codons) were preferred. Furthermore, the mRNA expression levels of APOBEC3 genes in the human CD4+ T cells of humanized mice were comparable to those in human peripheral blood (PB) [40]. Therefore, this report suggests that endogenous APOBEC3 proteins expressed in human CD4+ T cells can abrogate HIV-1 infection in vivo as a result of accumulating G-to-A mutations in proviral DNA, and that Vif counteracts this robust anti-viral activity of endogenous APOBEC3 proteins even in vivo.

Table 1.
HIV-1 mutants used in the studies of humanized mouse models.
| Gene | Strain | Coreceptor Usage | Mutation Type | Reference a |
|---|---|---|---|---|
| vif | JRCSF | CCR5 | Deletion | [40] |
| JRCSF | CCR5 | Deletion | [39] | |
| JRCSF | CCR5 | Frame shift | [39] | |
| LAI | CXCR4 | Deletion | [39] | |
| NLCSFV3 | CCR5 | DRMR/AAAA substitution (4A) | [41] | |
| NLCSFV3 | CCR5 | YRHHY/AAAAA substitution (5A) | [41] | |
| NLCSFV3 | CCR5 | Both of above (4A5A) | [41] | |
| vpr | JRCSF | CCR5 | Deletion | [42] |
| NL4-3 | CXCR4 | Deletion | [42] | |
| vpu | AD8 | CCR5 | Deletion | [44] |
| NLADA b | CCR5 | Deletion | [43] | |
| NLADA b | CCR5 | S52D, S56D substitution | [43] | |
| nef | LAI | CXCR4 | Deletion | [46] |
| LAI | CXCR4 | Frame shift | [45] | |
| LAI | CXCR4 | fsΔ-1 c | [45] | |
| LAI | CXCR4 | fsΔ-13 c | [45] | |
| LAI | CXCR4 | P72A, P75A substitution | [45] |
a References, which corresponds to those in Figure 1, are shown; b The virus used in this study contains GFP reporter via internal ribosome entry site; c Reverted nef ORFs, which are obtained in the mice infected with HIV-1 carrying a frame shift mutation in nef.
Second, Krisko et al. inoculated WT and certain kinds of vif mutant HIV-1 into BLT humanized mice [39]. Similar to the previous report [40], vif-deficient CCR5-tropic HIV-1 (strain JRCSF) was unable to propagate in BLT mice [39]. On the other hand, 6 out of the 16 BLT mice intravenously inoculated with the virus carrying a frame shift mutation in vif (HIV-1 vifFS, strain JRCSF) exhibited viremia. Since vif open reading frame (ORF) is restored in the six mice displayed viremia, these results further suggest that Vif is prerequisite for viral spread in vivo to counteract APOBEC3-mediated anti-viral effect.
When CCR5-tropic HIV-1 vifFS was directly injected into the spleen, liver, lung, or human thymic organoid of BLT mice, only the mice injected the virus solution into human thymic organoid exhibited systemic viremia with the reversion of vif ORF [39]. Moreover, the authors revealed that the mRNA expression levels of APOBEC3F and APOBEC3G in the human thymocytes of humans and BLT mice was significantly lower than those in the human CD4+ T cells in peripheral tissues [39]. Therefore, these findings suggest that thymocytes can allow the partial replication of CCR5-tropic HIV-1 vifFS and its vif restoration, which leads to the systemic spread of the restored viruses. These further suggest that CCR5-tropic HIV-1 is unable to exhibit systemic infection without Vif regardless of infection route.
In contrast to the observations in CCR5-tropic HIV-1-infected BLT mice, it was surprising that the BLT mice intravenously inoculated with CXCR4-tropic vif-deficient HIV-1 (strain LAI) showed a prolonged (until 14 weeks postinfection [wpi]) viremia [39]. More importantly, this virus spread occurred without vif restoration, suggesting that Vif is dispensable for the replication of CXCR4-tropic vif-deficient HIV-1 in BLT mice. In this regard, CCR5 is limitedly expressed in the thymus, whereas 30%–40% of thymocytes express CXCR4 [71,72,73]. Since human thymocytes express low levels of APOBEC3F and APOBEC3G, as mentioned above, human thymus can be susceptible to CXCR4-tropic vif-deficient HIV-1 propagation. However, it should be noted that the majority of HIV-1 isolated from patients is CCR5-tropic, while CXCR4-tropic HIV-1 can infrequently emerge during the onset of AIDS [74,75]. Since the replication of CCR5-tropic vif-deficient HIV-1 infection in BLT mouse needs the restoration of vif and is achieved only via artificial infection route (i.e., direct injection into the human thymic organoid, which is a unique organ in BLT mouse), it would be infeasible for CCR5-tropic HIV-1 to propagate in vivo via relatively natural infection routes (e.g., intrarectal, intravaginal, or intravenous infections). Further, the observations in CXCR4-tropic vif-deficient HIV-1-infected BLT mice may occur only the late stage of HIV-1 infection in patients.
Third, Sato et al. have recently utilized three kinds of site-directed Vif mutants: DRMR/AAAA (4A), YRHHY/AAAAA (5A), double mutant (4A5A), respectively [41]. Vif interacts with APOBEC3D and APOBEC3F via 14DRMR17 motif, while interacts with APOBEC3G via 4°YRHHY44 motif [76,77]. Hence, 4A HIV-1 is susceptible only to APOBEC3D and APOBEC3F, while 5A HIV-1 is susceptible only to APOBEC3G. By using these CCR5-tropic viruses (strain NLCSFV3) and NOG-hCD34 humanized mouse model, the authors demonstrated that endogenous APOBEC3D, APOBEC3F, and APOBEC3G exert strong anti-HIV-1 activity in vivo [41]. In addition, the growth kinetics of 4A HIV-1 negatively correlated with the expression level of APOBEC3F but not of APOBEC3D, suggesting that endogenous APOBEC3F more critically modulates 4A HIV-1 replication in vivo than APOBEC3D. It was of particularly noteworthy that the viral RNA in the plasma of 4A HIV-1-infected mice was significantly diversified compared to those of WT, 5A, and 4A5A HIV-1-infected mice [41]. Furthermore, a mutated virus (E25K mutation in the V3 region of envelope glycoprotein), which is capable of using both CCR5 and CXCR4 as entry coreceptor, has specifically emerged in 4A HIV-1-infected mice [41]. Altogether, these findings suggest that endogenous APOBEC3D, APOBEC3F, and APOBEC3G fundamentally are intrinsic restriction factors against HIV-1 in vivo, but, at the same time, that APOBEC3D and APOBEC3F are capable of promoting viral diversification and evolution in vivo. This is the first report in vivo demonstrating that endogenous APOBEC3D and APOBEC3F potently promote viral diversification and evolution, which can be beneficial for viruses (e.g., emergence of quasispecies resistant to anti-HIV-1 drugs anti-viral immunity).
3. Viral Protein R (Vpr)
Vpr is a small (96 amino acids) protein, but potentially possesses multiple biological functions. Major roles of Vpr in vitro are G2 arrest and apoptosis (Figure 1) [11,12]. Although the mechanisms of action of Vpr leading to G2 arrest and apoptosis remain controversial [11], a paper has recently suggested that Vpr induces the activation of the cellular structure-specific endonuclease regulator SLX4 complex, which results in G2 arrest (Figure 1) [18]. Also, Vpr has the potential to enhance HIV-1 long terminal repeat-driven transcription [16,17]. In the field of immunology, Vpr expressed in the infected cells upregulates UL16 binding protein 2 (ULBP2; also known as NKG2D ligand), a counter receptor for natural killer cell-specific receptor, which leads to natural killer cell-mediated killing [78,79]. Moreover, Vpr recruits a cellular E3 ubiquitin ligase complex, which is composed of cullin 4 (CUL4), damage-specific DNA binding protein 1 (DDB1), and Vpr binding protein (VPRBP), and degrades some cellular proteins such as Uracil-DNA glycosylase (UNG; also known as UNG2) [13,14] and single-strand-selective monofunctional uracil-DNA glycosylase 1 (SMUG1) (Figure 1) [15]. When compared to the observations in Vif (described above) and Vpu (described below), however, the virological significance of Vpr-mediated ubiquitin ligase complex still remains unsolved.
To address the role of Vpr in HIV-1 infection in vivo and its contribution to disease development, Sato et al. inoculated CCR5-tropic vpr-deficient HIV-1 (strain JRCSF) into NOG-hCD34 humanized mice (Table 1) [42]. CCR5-tropic vpr-deficient HIV-1-infected mice showed a significantly lower level of viremia during the acute phase of infection (4 and 7 days postinfection [dpi]) compared with WT HIV-1 [42]. In addition, the level of infected Tregs in vpr-deficient HIV-1-infected mice was significantly lower than that in WT HIV-1-infected mice [42]. Moreover, WT but not vpr-deficient CCR5-tropic HIV-1-infected mice displayed the acute depletion of Tregs in PB and spleen [42]. Furthermore, Vpr-dependent G2 cell cycle arrest and apoptosis are predominantly observed in infected Tregs [42]. Importantly, these were observed in the mice infected with CCR5-tropic HIV-1 (strain JRCSF), whereas there were no significant differences in the case of CXCR4-tropic HIV-1 (strain NL4-3) [42]. These findings suggest that the Vpr-dependent Treg depletion is dependent on viral coreceptor usage. In this regard, Tregs are highly susceptible to CCR5-tropic HIV-1 infection because the CCR5 expression levels on Tregs are higher than those on naive and memory CD4+ T cells [42,80]. Also, Tregs are more susceptible to Vpr-mediated G2 arrest and apoptosis because they are actively proliferating. On the other hand, CXCR4 is broadly expressed on all CD4+ T cell subsets [42,80,81]. Therefore, the Vpr-dependent G2 arrest and apoptosis can be preferentially triggered by CCR5-tropic but not CXCR4-tropic HIV-1 in vivo.
It is known that Treg plays a crucial role in the maintenance of immune homeostasis [82]. In CCR5-tropic HIV-1-infected mice, Vpr-dependent depletion of Treg resulted in immune activation [42], which is a hallmark in the patients infected with HIV-1 [83]. Altogether, these findings suggest that Vpr enhances CCR5-tropic but not CXCR4-tropic HIV-1 replication mediating G2 arrest and apoptosis in vivo by exploiting Treg during the acute phase of infection. This Vpr-dependent Treg depletion may lead to immune activation and provide a pool of activated CD4+ T cells, which supports subsequent HIV-1 expansion in vivo.
4. Viral Protein U (Vpu)
Vpu is a transmembrane protein and has been classically recognized as a “viroporin”, which works as ion channel (Figure 1) [19,20]. In addition, Vpu degrades some host proteins such as CD4 molecule, the receptor for HIV-1 entry, through the ubiquitin/proteasome pathway [84]. Vpu also downregulates signaling lymphocyte activation molecule family member 6 (SLAMF6; also called NTB-A), a transmembrane protein potently inducing natural killer cell-mediated killing [24] and CD1d molecule [23] from the cell surface of infected cells (Figure 1).
It was known that certain human CD4+ T cell lines (e.g., Jurkat cells), primary CD4+ T cells, monocyte-derived macrophages, and HeLa cells are incapable of producing the vpu-deficient HIV-1 virions [21,22]. Neil et al. and Van Damme et al. identified that the cellular factor, bone marrow stromal cell antigen 2 (BST2; also known as CD317, HM1.24 and tetherin) [25,26]. BST2 is an interferon-stimulated protein and is endogenously expressed on human CD4+ T cells and macrophages [85,86]. On the other hand, Vpu downregulates BST2 from the cell surface and counteracts BST2-mediated anti-viral activity (Figure 1) [26,86]. The Vpu-mediated BST2 downregulation is dependent on β-transducin repeat-containing protein 1 (BTRC; also called β-TrCP1), an E3 ubiquitin ligase, similar to the manner by which CD4 is downregulated [84,87,88,89,90]. Moreover, it has been recently revealed that Vpu inhibits the activation of NFκB signaling [27,28]. These observations were brought from in vitro studies using cell culture systems, however, the role of Vpu in HIV-1 replication in vivo, particularly its antagonism of BST2 in vivo, remains unresolved.
Sato et al. [44] and Dave et al. [43] investigated the role of Vpu in HIV-1 expansion in vivo using humanized mouse models (Table 1). In the former paper, NOG-hCD34 humanized mice were inoculated with WT or vpu-deficient HIV-1 (strain AD8) at a relatively high dose (300,000 TCID50) [44]. The authors revealed that the viral load of vpu-deficient HIV-1 was 8.5-fold lower than in that of WT HIV-1 at 7 dpi, suggesting that vpu-deficient HIV-1 more slowly propagates in humanized mice than WT HIV-1 during the initial phase of infection [44]. At 7 dpi, although the percentage of Gag-positive cells (i.e., virus-producing cells) in the spleen of vpu-deficient HIV-1-infected mice was similar to that of WT HIV-1-infected mice, it was of particularly noteworthy that the amount of cell-free virions in the spleen of vpu-deficient HIV-1-infected mice was quite lower (61.8-fold) than in that of WT HIV-1-infected mice [44]. Moreover, the authors revealed that the expression levels of BST2 and CD4, but not SLAMF6, on the surface of Gag-positive cells in the spleen of WT HIV-1-infected mice were significantly lower than in those of vpu-deficient HIV-1 at 7 dpi [44]. These findings suggest that Vpu downregulates BST2 and CD4 from the surface of virus-producing cells to promote the release of nascent virions, which augments the initial burst of HIV-1 replication in vivo.
In the latter paper [43], hHSC-transplanted humanized mice were infected with WT HIV-1 or vpu-deficient HIV-1 (strain NLADA) at a relatively low dose (5,000 TCID50). Until 14 wpi, the viral load in the plasma of vpu-deficient HIV-1-infected mice was ~5-150-fold lower than that of WT HIV-1-infected mice until 14 wpi [43]. Similar to the former paper [44], BST2 downregulation was detected on the surface of Gag-positive cells in WT HIV-1 infected mice. On the other hand, in vpu-deficient HIV-1-infected mice, the expression level of surface BST2 on Gag-positive cells was higher than that of Gag-negative cells [43]. Such BST2 upregulation on the uninfected cells was reported in the former paper during the chronic phase of infection (21 dpi) [44] and the individuals infected HIV-1 [91]. Because BST2 is an interferon-stimulated gene, the BST2 upregulation can be triggered by type I interferon, which is induced by HIV-1 infection. Taken together, these findings suggest that Vpu downregulates surface BST2 in vivo for the promotion of viral production and propagation regardless of input viral dose.
To better understand the association of BTRC with the role of Vpu, Dave et al. [43] used a mutant virus, HIV-1 VpuS52D/S56D. This Vpu is incapable of recruiting BTRC and thereby is unable to degrade BST2. The authors inoculated WT HIV-1, vpu-deficient HIV-1, or HIV-1 VpuS52D/S56D into humanized mice at a relatively high dose (~500,000 TCID50) [43]. At 21 dpi, although vpu-deficient HIV-1-infected mice exhibited severely lower viremia (~15-fold) compared to WT HIV-1-infected mice, the decrease in the viral load of HIV-1 VpuS52D/S56D comparing to WT HIV-1 was relatively mild (~3-fold) [43]. Moreover, HIV-1 VpuS52D/S56D partially downregulated surface BST2 (20%–30%) on Gag-positive cells of infected mice, whereas vpu-deficient HIV-1 was unable to downregulate BST2 on the surface of Gag-positive cells [43]. Since WT HIV-1 strongly downregulated surface BST2 (50%–60%) compared to vpu-deficient HIV-1 and HIV-1 VpuS52D/S56D, these findings suggest that the efficacy of BST2 downregulation associates with the level of viral spread in vivo. However, it should be considered that the mutations of these serine residues in Vpu also abrogates downmodulation of CD4 [92] and inhibition of NFκB signaling [28]. Therefore, the observations in the humanized mice infected with HIV-1 VpuS52D/S56D [43] may not be solely ascribed to the lack of tetherin counteraction.
Notably, Sato et al. [44] further revealed that the efficacy of vpu-deficient HIV-1 infection in humanized mice was significantly lower than that of WT HIV-1. This observation suggests that Vpu potently enhances the efficacy of infection in humans, which leads to the promotion of human-to-human HIV-1 transmission. It is of interest that Vpu proteins of pandemic HIV-1 (group M) possess anti-BST2 ability, while those of non-pandemic HIV-1 (groups N, O, and P) do not or less [2,4,93,94,95]. Therefore, the worldwide epidemic of HIV-1 group M might be attributed to the Vpu-mediated BST2 antagonism to some extent.
5. Negative Factor (Nef)
In vitro investigations have revealed that Nef is a pluripotent protein. For instance, Nef downregulates CD4 [30,35], MHC class I [30,32], CCR5/CXCR4 coreceptors [29,30,31], and CD28 [33] from the surface of infected cells (Figure 1). On the other hand, Nef upregulates CD74, the invariant chain of MHC class II [34]. In addition, Nef potently enhances the infectivity of released virions [35,36], though the molecular mechanism of this action remains unclear. Moreover, Nef induces the activation of cellular protein kinases such as p21 protein (Cdc42/Rac)-activated kinase 2 (PAK2) and a tyrosine kinase, hematopoietic cell kinase (HCK) (Figure 1) [37,38].
In vitro studies using activated human primary CD4+ T cells and ex vivo studies using human tonsil tissue cultures have suggested that CD4 downregulation is more critical for Nef to promote viral replication than MHC class I downregulation [96,97,98]. Importantly, the patients infected with nef-defective HIV-1 failed to develop AIDS [99,100,101,102,103,104]. Given that a long-term non-progressor, who has been infected with attenuate nef-defective viruses, exhibited acute CD4 T cell decrease in PB through the superinfection of nef-proficient virus [105], these notions strongly suggest that Nef closely associates with viral pathogenesis and disease progression in vivo.
To directly investigate the roles of Nef in HIV-1 replication and pathogenesis in vivo, a BLT humanized mouse model and nef-deficient HIV-1 (strain LAI) were used (Table 1) [46]. Although WT HIV-1-infected mice exhibited a profound loss of human CD4+ T cells and thymocytes, nef-deficient HIV-1-infected mice showed neither CD4+ T cell decrease nor thymopathy [46]. Also, the growth of nef-deficient HIV-1 was clearly lower than that of WT HIV-1 [46], suggesting that Nef is necessary for elevated viral replication in vivo, which results in the depletion of thymocytes.
Another paper examined whether nef-defective virus recovers Nef function by using HIV-1 (strain LAI) with a frame shift mutation in nef (HIV-1 nefFS) and a BLT humanized mouse model (Table 1) [45]. Watkins et al. detected two restored nef ORFs, which are designated to fsΔ-1 and fsΔ-13 respectively, in the PB of BLT humanized mice infected with HIV-1 nefFS at 8 wpi [45]. In vitro assays revealed that MHC class I but not CD4 molecule can be downregulated by fsΔ-1 and fsΔ-13, and that the infectivity and replication kinetics of the viruses possessing these two mutated Nef proteins were comparable to that of parental HIV-1 [45]. However, in BLT mice, the growth of HIV-1 fsΔ-1 and HIV-1 fsΔ-13 was ~3-fold lower than WT HIV-1, and the level of systemic CD4+ T cell decrease and thymopathy by HIV-1 fsΔ-1 and HIV-1 fsΔ-13 infections (~50% reduction) were milder than WT HIV-1 (~90% reduction) [45]. These results suggest the importance of CD4 downregulation by Nef for efficient viral growth and pathogenicity.
To explore the roles of Nef other than CD4 downregulation in viral replication kinetics in vivo and pathogenesis, Watkins et al. used the other mutant, HIV-1 NefP72A/P75A [45]. This Nef has mutations in the highly conserved SH3 binding domain (72PQVPLR77), and thereby, is unable to interact with several cellular proteins such as PAK2 and HCK [37,106,107]. Yet, NefP72A/P75A is capable of downregulating CD4 molecules [107,108]. In BLT humanized mice, HIV-1 NefP72A/P75A efficiently expanded comparable to WT HIV-1, and the level of systemic CD4+ T cell decease by HIV-1 NefP72A/P75A infection was similar to WT HIV-1 [45]. Altogether, these findings suggest that the in vivo phenotype of Nef is highly dependent on its ability to downregulate CD4 molecules but minimally on the interaction with cellular proteins via SH3 domain. However, it should be noted that these two previous studies focusing on the roles on Nef in vivo [45,46] were performed by using CXCR4-tropic HIV-1 (strain LAI) (Table 1). It is known that CCR5-tropic viruses are predominant in patients, while CXCR4-tropic viruses occasionally emerge at the end stage of HIV-1 infection [74,75]. Therefore, it would be important to assess the in vivo phenotype of Nef not only by CXCR4-tropic HIV-1 but also by using CCR5-tropic HIV-1 and humanized mouse models.
6. Future Perspective
Here we summarized the current knowledge of the roles of HIV-1 accessory proteins in viral replication and pathogenesis in humanized mouse models. As summarized in Figure 1, there are some overlaps and discrepancies in the observations between in vitro and in vivo. This indicates that certain findings in in vitro studies may not reflect the bona fide roles of HIV-1 accessory proteins in viral infection, replication, and pathogenesis in vivo, and further suggests that the findings brought from in vitro experiments should be verified by in vivo experiments using humanized mouse models. Recently, humanized mouse models have been utilized for the evaluation of anti-HIV-1 prevention/therapeutic strategies in vivo [109,110]. Moreover, these animal models are unique and robust experimental models to elucidate the “authentic” functions of HIV-1 proteins in the dynamics of viral infection in vivo, which can lead to the detailed knowledge of HIV-1 infection and the development of novel anti-viral strategies. Furthermore, it should be noted that laboratory-adapted molecular clones of HIV-1 (e.g., strains LAI, AD8, NL4-3, and their derivatives) have been used in most of humanized mouse studies so far (Table 1). However, recent studies have shown that especially accessory proteins are often not fully functional in these laboratory-adapted strains since they are often dispensable for replication in vitro [111,112]. This also raises a possibility that the roles of accessory proteins, which have been brought from the studies using laboratory-adapted molecular clones, may be underestimated. Therefore, we highlight the importance of analyzing clones of primary isolates (e.g., transmitted/founder and chronic viruses) to investigate the bona fide roles of Vpr, Vif, Vpu and Nef in vivo. Future basic scientific investigations focusing on the host-virus interaction including the roles of HIV-1 accessory proteins, using humanized mouse models will shed light on the not-yet-identified but crucial aspects of the dynamics of HIV-1 infection.
Acknowledgments
We would like to thank Dong Sung An (University of California, Los Angeles, CA, USA) for proofreading the manuscript and Kotubu Misawa for the dedicated supports. This work was funded in-part by CREST, Japan Science and Technology Agency (to K.S.); Imai Memorial Trust for AIDS Research (to K.S.); Ichiro Kanehara Foundation (to K.S.); Kanae Foundation for the Promotion of Medical Science (to K.S.); Suzuken Memorial Foundation (to K.S.); Uehara Memorial Foundation (to K.S.); and a Grant-in-Aid for Scientific Research on Innovative Areas 24115008 from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to Y.K.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barre-Sinoussi, F.; Chermann, J.C.; Rey, F.; Nugeyre, M.T.; Chamaret, S.; Gruest, J.; Dauguet, C.; Axler-Blin, C.; Vezinet-Brun, F.; Rouzioux, C.; Rozenbaum, W.; Montagnier, L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983, 220, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.C.; Sarin, P.S.; Gelmann, E.P.; Robert-Guroff, M.; Richardson, E.; Kalyanaraman, V.S.; Mann, D.; Sidhu, G.D.; Stahl, R.E.; Zolla-Pazner, S.; Leibowitch, J.; Popovic, M. Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS). Science 1983, 220, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Freed, E.O.; Martin, M.A. HIVs and Their Replication. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, 2007; Volume 2, pp. 2107–2185. [Google Scholar]
- Kirchhoff, F. Immune evasion and counteraction of restriction factors by HIV-1 and other primate lentiviruses. Cell Host Microbe 2010, 8, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Malim, M.H.; Bieniasz, P.D. HIV Restriction Factors and Mechanisms of Evasion. Cold Spring Harb. Perspect. Med. 2012, 2, a006940. [Google Scholar] [CrossRef] [PubMed]
- Albin, J.S.; Harris, R.S. Interactions of host APOBEC3 restriction factors with HIV-1 in vivo: implications for therapeutics. Expert Rev. Mol. Med. 2010, 12, e4. [Google Scholar] [CrossRef] [PubMed]
- Desimmie, B.A.; Delviks-Frankenberrry, K.A.; Burdick, R.C.; Qi, D.; Izumi, T.; Pathak, V.K. Multiple APOBEC3 restriction factors for HIV-1 and one Vif to rule them all. J. Mol. Biol. 2014, 426, 1220–1245. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.; Ode, H.; Iwatani, Y. Structural features of antiviral APOBEC3 proteins are linked to their functional activities. Front Microbiol. 2011, 2, 258. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, A.M.; Gaddis, N.C.; Choi, J.D.; Malim, M.H. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 2002, 418, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Izumi, T.; Io, K.; Matsui, M.; Shirakawa, K.; Shinohara, M.; Nagai, Y.; Kawahara, M.; Kobayashi, M.; Kondoh, H.; Misawa, N.; Koyanagi, Y.; Uchiyama, T.; Takaori-Kondo, A. HIV-1 viral infectivity factor interacts with TP53 to induce G2 cell cycle arrest and positively regulate viral replication. Proc. Natl. Acad. Sci. U S A 2010, 107, 20798–20803. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.L.; Le Rouzic, E.; Planelles, V. HIV-1 Vpr: mechanisms of G2 arrest and apoptosis. Exp. Mol. Pathol. 2008, 85, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Goh, W.C.; Rogel, M.E.; Kinsey, C.M.; Michael, S.F.; Fultz, P.N.; Nowak, M.A.; Hahn, B.H.; Emerman, M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat. Med. 1998, 4, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Hrecka, K.; Gierszewska, M.; Srivastava, S.; Kozaczkiewicz, L.; Swanson, S.K.; Florens, L.; Washburn, M.P.; Skowronski, J. Lentiviral Vpr usurps Cul4-DDB1[VprBP] E3 ubiquitin ligase to modulate cell cycle. Proc. Natl. Acad. Sci. U S A 2007, 104, 11778–11783. [Google Scholar] [CrossRef] [PubMed]
- Schrofelbauer, B.; Hakata, Y.; Landau, N.R. HIV-1 Vpr function is mediated by interaction with the damage-specific DNA-binding protein DDB1. Proc. Natl. Acad. Sci. U S A 2007, 104, 4130–4135. [Google Scholar] [CrossRef] [PubMed]
- Schrofelbauer, B.; Yu, Q.; Zeitlin, S.G.; Landau, N.R. Human immunodeficiency virus type 1 Vpr induces the degradation of the UNG and SMUG uracil-DNA glycosylases. J. Virol. 2005, 79, 10978–10987. [Google Scholar] [CrossRef] [PubMed]
- Forget, J.; Yao, X.J.; Mercier, J.; Cohen, E.A. Human immunodeficiency virus type 1 vpr protein transactivation function: mechanism and identification of domains involved. J. Mol. Biol. 1998, 284, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, B.E.; Khalili, K.; Mercer, W.E.; Denisova, L.; Amini, S. Cooperative actions of HIV-1 Vpr and p53 modulate viral gene transcription. J. Biol. Chem. 1998, 273, 20052–20057. [Google Scholar] [CrossRef] [PubMed]
- Laguette, N.; Bregnard, C.; Hue, P.; Basbous, J.; Yatim, A.; Larroque, M.; Kirchhoff, F.; Constantinou, A.; Sobhian, B.; Benkirane, M. Premature activation of the SLX4 complex by Vpr promotes G2/M arrest and escape from innate immune sensing. Cell 2014, 156, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Ewart, G.D.; Sutherland, T.; Gage, P.W.; Cox, G.B. The Vpu protein of human immunodeficiency virus type 1 forms cation-selective ion channels. J. Virol. 1996, 70, 7108–7115. [Google Scholar] [PubMed]
- Gonzalez, M.E.; Carrasco, L. Viroporins. FEBS Lett 2003, 552, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Gottlinger, H.G.; Dorfman, T.; Cohen, E.A.; Haseltine, W.A. Vpu protein of human immunodeficiency virus type 1 enhances the release of capsids produced by gag gene constructs of widely divergent retroviruses. Proc. Natl. Acad. Sci. U S A 1993, 90, 7381–7385. [Google Scholar] [CrossRef] [PubMed]
- Schubert, U.; Clouse, K.A.; Strebel, K. Augmentation of virus secretion by the human immunodeficiency virus type 1 Vpu protein is cell type independent and occurs in cultured human primary macrophages and lymphocytes. J. Virol. 1995, 69, 7699–7711. [Google Scholar] [PubMed]
- Moll, M.; Andersson, S.K.; Smed-Sorensen, A.; Sandberg, J.K. Inhibition of lipid antigen presentation in dendritic cells by HIV-1 Vpu interference with CD1d recycling from endosomal compartments. Blood 2010, 116, 1876–1884. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.H.; Sowrirajan, B.; Davis, Z.B.; Ward, J.P.; Campbell, E.M.; Planelles, V.; Barker, E. Degranulation of natural killer cells following interaction with HIV-1-infected cells is hindered by downmodulation of NTB-A by Vpu. Cell Host Microbe 2010, 8, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Neil, S.J.; Zang, T.; Bieniasz, P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 2008, 451, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, N.; Goff, D.; Katsura, C.; Jorgenson, R.L.; Mitchell, R.; Johnson, M.C.; Stephens, E.B.; Guatelli, J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 2008, 3, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Galao, R.P.; Le Tortorec, A.; Pickering, S.; Kueck, T.; Neil, S.J. Innate sensing of HIV-1 assembly by Tetherin induces NFκB-dependent proinflammatory responses. Cell Host Microbe 2012, 12, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Sauter, D.; Hotter, D.; Van Driessche, B.; Sturzel, C.M.; Kluge, S.F.; Wildum, S.; Yu, H.; Baumann, B.; Wirth, T.; Plantier, J.C.; Leoz, M.; Hahn, B.H.; Van Lint, C.; Kirchhoff, F. Differential regulation of NF-κB-mediated proviral and antiviral host gene expression by primate lentiviral Nef and Vpu proteins. Cell Rep. 2015, 10, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Hrecka, K.; Swigut, T.; Schindler, M.; Kirchhoff, F.; Skowronski, J. Nef proteins from diverse groups of primate lentiviruses downmodulate CXCR4 to inhibit migration to the chemokine stromal derived factor 1. J. Virol. 2005, 79, 10650–10659. [Google Scholar] [CrossRef] [PubMed]
- Landi, A.; Iannucci, V.; Nuffel, A.V.; Meuwissen, P.; Verhasselt, B. One protein to rule them all: modulation of cell surface receptors and molecules by HIV Nef. Curr. HIV Res. 2011, 9, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Michel, N.; Allespach, I.; Venzke, S.; Fackler, O.T.; Keppler, O.T. The Nef protein of human immunodeficiency virus establishes superinfection immunity by a dual strategy to downregulate cell-surface CCR5 and CD4. Curr. Biol. 2005, 15, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, O.; Marechal, V.; Le Gall, S.; Lemonnier, F.; Heard, J.M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 1996, 2, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Swigut, T.; Shohdy, N.; Skowronski, J. Mechanism for down-regulation of CD28 by Nef. EMBO J 2001, 20, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Keppler, O.T.; Tibroni, N.; Venzke, S.; Rauch, S.; Fackler, O.T. Modulation of specific surface receptors and activation sensitization in primary resting CD4+ T lymphocytes by the Nef protein of HIV-1. J. Leukoc. Biol. 2006, 79, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, M.A.; Warmerdam, M.T.; Atchison, R.E.; Miller, M.D.; Greene, W.C. Dissociation of the CD4 downregulation and viral infectivity enhancement functions of human immunodeficiency virus type 1 Nef. J. Virol. 1995, 69, 4112–4121. [Google Scholar] [PubMed]
- Pizzato, M.; Helander, A.; Popova, E.; Calistri, A.; Zamborlini, A.; Palu, G.; Gottlinger, H.G. Dynamin 2 is required for the enhancement of HIV-1 infectivity by Nef. Proc. Natl. Acad. Sci. U S A 2007, 104, 6812–6817. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, K.C.; Mukerji, J.; Gabuzda, D. Nef-mediated enhancement of cellular activation and human immunodeficiency virus type 1 replication in primary T cells is dependent on association with p21-activated kinase 2. Retrovirology 2011, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Trible, R.P.; Emert-Sedlak, L.; Smithgall, T.E. HIV-1 Nef selectively activates Src family kinases Hck, Lyn, and c-Src through direct SH3 domain interaction. J. Biol. Chem. 2006, 281, 27029–27038. [Google Scholar] [CrossRef] [PubMed]
- Krisko, J.F.; Martinez-Torres, F.; Foster, J.L.; Garcia, J.V. HIV restriction by APOBEC3 in humanized mice. PLoS Pathog. 2013, 9, e1003242. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Izumi, T.; Misawa, N.; Kobayashi, T.; Yamashita, Y.; Ohmichi, M.; Ito, M.; Takaori-Kondo, A.; Koyanagi, Y. Remarkable lethal G-to-A mutations in vif-proficient HIV-1 provirus by individual APOBEC3 proteins in humanized mice. J. Virol. 2010, 84, 9546–9556. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takeuchi, J. S.; Misawa, N.; Izumi, T.; Kobayashi, T.; Kimura, Y.; Iwami, S.; Takaori-Kondo, A.; Hu, W.S.; Aihara, K.; Ito, M.; An, D.S.; Pathak, V.K.; Koyanagi, Y. APOBEC3D and APOBEC3F potently promote HIV-1 diversification and evolution in humanized mouse model. PLoS Pathog. 2014, 10, e1004453. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Misawa, N.; Iwami, S.; Satou, Y.; Matsuoka, M.; Ishizaka, Y.; Ito, M.; Aihara, K.; An, D.S.; Koyanagi, Y. HIV-1 Vpr accelerates viral replication during acute infection by exploitation of proliferating CD4+ T cells in vivo. PLoS Pathog. 2013, 9, e1003812. [Google Scholar] [CrossRef] [PubMed]
- Dave, V.P.; Hajjar, F.; Dieng, M.M.; Haddad, E.; Cohen, E.A. Efficient BST2 antagonism by Vpu is critical for early HIV-1 dissemination in humanized mice. Retrovirology 2013, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Misawa, N.; Fukuhara, M.; Iwami, S.; An, D.S.; Ito, M.; Koyanagi, Y. Vpu augments the initial burst phase of HIV-1 propagation and downregulates BST2 and CD4 in humanized mice. J. Virol. 2012, 86, 5000–5013. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.L.; Zou, W.; Denton, P.W.; Krisko, J.F.; Foster, J.L.; Garcia, J.V. In vivo analysis of highly conserved Nef activities in HIV-1 replication and pathogenesis. Retrovirology 2013, 10, e125. [Google Scholar] [CrossRef]
- Zou, W.; Denton, P.W.; Watkins, R.L.; Krisko, J.F.; Nochi, T.; Foster, J.L.; Garcia, J.V. Nef functions in BLT mice to enhance HIV-1 replication and deplete CD4+CD8+ thymocytes. Retrovirology 2012, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A.; Travers, P.; Walport, M.; Shlomchik, M.J. T cell-mediated immunity. In Immunobiology: The Immune System in Health and Disease, 6th ed.; Garland Science: New York, USA, 2005; pp. 25–51. [Google Scholar]
- Glushakova, S.; Baibakov, B.; Margolis, L.B.; Zimmerberg, J. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat. Med. 1995, 1, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Introini, A.; Vanpouille, C.; Lisco, A.; Grivel, J.C.; Margolis, L. Interleukin-7 facilitates HIV-1 transmission to cervico-vaginal tissue ex vivo. PLoS Pathog. 2013, 9, e1003148. [Google Scholar] [CrossRef] [PubMed]
- Merbah, M.; Arakelyan, A.; Edmonds, T.; Ochsenbauer, C.; Kappes, J.C.; Shattock, R.J.; Grivel, J.C.; Margolis, L.B. HIV-1 expressing the envelopes of transmitted/founder or control/reference viruses have similar infection patterns of CD4 T-cells in human cervical tissue ex vivo. PLoS One 2012, 7, e50839. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.J.; Wang, Y.; Huo, Z.; Giemza, R.; Babaahmady, K.; Rahman, D.; Shattock, R.J.; Singh, M.; Lehner, T. Effect of vaginal immunization with HIVgp140 and HSP70 on HIV-1 replication and innate and T cell adaptive immunity in women. J. Virol. 2014, 88, 11648–11657. [Google Scholar] [CrossRef] [PubMed]
- Adjali, O.; Montel-Hagen, A.; Swainson, L.; Marty, S.; Vicente, R.; Mongellaz, C.; Jacquet, C.; Zimmermann, V.; Taylor, N. In vivo and ex vivo gene transfer in thymocytes and thymocyte precursors. Methods Mol. Biol. 2009, 506, 171–190. [Google Scholar] [PubMed]
- Mosier, D. E.; Gulizia, R.J.; Baird, S.M.; Wilson, D.B.; Spector, D.H.; Spector, S.A. Human immunodeficiency virus infection of human-PBL-SCID mice. Science 1991, 251, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Gauduin, M.C.; Parren, P.W.; Weir, R.; Barbas, C.F.; Burton, D.R.; Koup, R.A. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat. Med. 1997, 3, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, J.; Shpitz, B.; Gallinger, S.; Hozumi, N. Human primary immune response in SCID mice engrafted with human peripheral blood lymphocytes. J. Immunol. 1994, 152, 3806–3813. [Google Scholar] [PubMed]
- Shultz, L.D.; Ishikawa, F.; Greiner, D.L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 2007, 7, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Traggiai, E.; Chicha, L.; Mazzucchelli, L.; Bronz, L.; Piffaretti, J.C.; Lanzavecchia, A.; Manz, M.G. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 2004, 304, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Baenziger, S.; Tussiwand, R.; Schlaepfer, E.; Mazzucchelli, L.; Heikenwalder, M.; Kurrer, M.O.; Behnke, S.; Frey, J.; Oxenius, A.; Joller, H.; Aguzzi, A.; Manz, M.G.; Speck, R.F. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2-/- γc-/- mice. Proc. Natl. Acad. Sci. U S A 2006, 103, 15951–15956. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Hiramatsu, H.; Kobayashi, K.; Suzue, K.; Kawahata, M.; Hioki, K.; Ueyama, Y.; Koyanagi, Y.; Sugamura, K.; Tsuji, K.; Heike, T.; Nakahata, T. NOD/SCID/γcnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood 2002, 100, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Brehm, M.A.; Garcia-Martinez, J.V.; Greiner, D.L. Humanized mice for immune system investigation: progress, promise and challenges. Nat. Rev. Immunol. 2012, 12, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Hatziioannou, T.; Evans, D.T. Animal models for HIV/AIDS research. Nat. Rev. Microbiol. 2012, 10, 852–867. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Koyanagi, Y. The mouse is out of the bag: insights and perspectives on HIV-1-infected humanized mouse models. Exp. Biol. Med. 2011, 236, 977–985. [Google Scholar] [CrossRef]
- Ito, R.; Takahashi, T.; Katano, I.; Ito, M. Current advances in humanized mouse models. Cell Mol. Immunol. 2012, 9, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Melkus, M.W.; Estes, J.D.; Padgett-Thomas, A.; Gatlin, J.; Denton, P.W.; Othieno, F.A.; Wege, A.K.; Haase, A.T.; Garcia, J.V. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 2006, 12, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Tonomura, N.; Habiro, K.; Shimizu, A.; Sykes, M.; Yang, Y.G. Antigen-specific human T-cell responses and T cell-dependent production of human antibodies in a humanized mouse model. Blood 2008, 111, 4293–4296. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.; Greiner, D.L.; Shultz, L.D. Humanized SCID mouse models for biomedical research. In Humanized mice; Nomura, T., Watanabe, T., Habu, S., Eds.; Springer: Heidelberg, Germany, 2008; pp. 25–51. [Google Scholar]
- Willinger, T.; Rongvaux, A.; Strowig, T.; Manz, M.G.; Flavell, R.A. Improving human hemato-lymphoid-system mice by cytokine knock-in gene replacement. Trends Immunol. 2011, 32, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Rongvaux, A.; Willinger, T.; Martinek, J.; Strowig, T.; Gearty, S.V.; Teichmann, L.L.; Saito, Y.; Marches, F.; Halene, S.; Palucka, A.K.; Manz, M.G.; Flavell, R.A. Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 2014, 32, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Saito, Y.; Hijikata, A.; Tanaka, S.; Watanabe, T.; Hasegawa, T.; Mochizuki, S.; Kunisawa, J.; Kiyono, H.; Koseki, H.; Ohara, O.; Saito, T.; Taniguchi, S.; Shultz, L.D.; Ishikawa, F. Membrane-bound human SCF/KL promotes in vivo human hematopoietic engraftment and myeloid differentiation. Blood 2012, 119, 2768–2777. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Saito, Y.; Najima, Y.; Tanaka, S.; Ochi, T.; Tomizawa, M.; Doi, T.; Sone, A.; Suzuki, N.; Fujiwara, H.; Yasukawa, M.; Ishikawa, F. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2rγnull humanized mice. Proc. Natl. Acad. Sci. U S A 2010, 107, 13022–13027. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, R.D.; Beckerman, K.P.; Schall, T.J.; McCune, J.M. CXCR4 and CCR5 expression delineates targets for HIV-1 disruption of T cell differentiation. J. Immunol. 1998, 161, 3702–3710. [Google Scholar] [PubMed]
- Kitchen, S.G.; Zack, J.A. Distribution of the human immunodeficiency virus coreceptors CXCR4 and CCR5 in fetal lymphoid organs: implications for pathogenesis in utero. AIDS Res. Hum. Retroviruses 1999, 15, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Zamarchi, R.; Allavena, P.; Borsetti, A.; Stievano, L.; Tosello, V.; Marcato, N.; Esposito, G.; Roni, V.; Paganin, C.; Bianchi, G.; Titti, F.; Verani, P.; Gerosa, G.; Amadori, A. Expression and functional activity of CXCR-4 and CCR-5 chemokine receptors in human thymocytes. Clin. Exp. Immunol. 2002, 127, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Connor, R.I.; Sheridan, K.E.; Ceradini, D.; Choe, S.; Landau, N.R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 1997, 185, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Scarlatti, G.; Tresoldi, E.; Bjorndal, A.; Fredriksson, R.; Colognesi, C.; Deng, H.K.; Malnati, M.S.; Plebani, A.; Siccardi, A.G.; Littman, D.R.; Fenyo, E.M.; Lusso, P. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat. Med. 1997, 3, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.A.; Pathak, V.K. Identification of two distinct human immunodeficiency virus type 1 Vif determinants critical for interactions with human APOBEC3G and APOBEC3F. J. Virol. 2007, 81, 8201–8210. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Pathak, V.K. Identification of specific determinants of human APOBEC3F, APOBEC3C, and APOBEC3DE and African green monkey APOBEC3F that interact with HIV-1 Vif. J. Virol. 2010, 84, 12599–12608. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.; Davis, Z.; DeHart, J.; Zimmerman, E.; Bosque, A.; Brunetta, E.; Mavilio, D.; Planelles, V.; Barker, E. HIV-1 Vpr triggers natural killer cell-mediated lysis of infected cells through activation of the ATR-mediated DNA damage response. PLoS Pathog. 2009, 5, e1000613. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.; Sindhu, S.; Pham, T.N.; Belzile, J.P.; Cohen, E.A. HIV-1 Vpr up-regulates expression of ligands for the activating NKG2D receptor and promotes NK cell-mediated killing. Blood 2010, 115, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Miyara, M.; Yoshioka, Y.; Kitoh, A.; Shima, T.; Wing, K.; Niwa, A.; Parizot, C.; Taflin, C.; Heike, T.; Valeyre, D.; Mathian, A.; Nakahata, T.; Yamaguchi, T.; Nomura, T.; Ono, M.; Amoura, Z.; Gorochov, G.; Sakaguchi, S. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009, 30, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Sato, K.; Misawa, N.; Kitayama, H.; Fujino, H.; Hiramatsu, H.; Heike, T.; Nakahata, T.; Tanaka, Y.; Ito, M.; Koyanagi, Y. Selective infection of CD4+ effector memory T lymphocytes leads to preferential depletion of memory T lymphocytes in R5 HIV-1-infected humanized NOD/SCID/IL-2Rγnull mice. Virology 2009, 394, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Miyara, M.; Costantino, C.M.; Hafler, D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, J. M.; Silvestri, G.; Douek, D.C. Nonprogressive and progressive primate immunodeficiency lentivirus infections. Immunity 2010, 32, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Levesque, K.; Finzi, A.; Binette, J.; Cohen, E.A. Role of CD4 receptor down-regulation during HIV-1 infection. Curr. HIV Res. 2004, 2, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Blasius, A.L.; Giurisato, E.; Cella, M.; Schreiber, R.D.; Shaw, A.S.; Colonna, M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 2006, 177, 3260–3265. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.T.; Serra-Moreno, R.; Singh, R.K.; Guatelli, J.C. BST-2/tetherin: a new component of the innate immune response to enveloped viruses. Trends Microbiol. 2010, 18, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.L.; Gustin, J.K.; Viswanathan, K.; Mansouri, M.; Moses, A.V.; Fruh, K. The great escape: viral strategies to counter BST-2/tetherin. PLoS Pathog. 2010, 6, e1000913. [Google Scholar] [CrossRef] [PubMed]
- Goffinet, C.; Allespach, I.; Homann, S.; Tervo, H.M.; Habermann, A.; Rupp, D.; Oberbremer, L.; Kern, C.; Tibroni, N.; Welsch, S.; Krijnse-Locker, J.; Banting, G.; Krausslich, H.G.; Fackler, O.T.; Keppler, O.T. HIV-1 antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host Microbe 2009, 5, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.S.; Katsura, C.; Skasko, M.A.; Fitzpatrick, K.; Lau, D.; Ruiz, A.; Stephens, E.B.; Margottin-Goguet, F.; Benarous, R.; Guatelli, J.C. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via β-TrCP and endo-lysosomal trafficking. PLoS Pathog. 2009, 5, e1000450. [Google Scholar] [CrossRef] [PubMed]
- Willey, R.L.; Maldarelli, F.; Martin, M.A.; Strebel, K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 1992, 66, 7193–7200. [Google Scholar] [PubMed]
- Homann, S.; Smith, D.; Little, S.; Richman, D.; Guatelli, J. Upregulation of BST-2/tetherin by HIV infection in vivo. J. Virol. 2011, 85, 10659–10668. [Google Scholar] [CrossRef] [PubMed]
- Schubert, U.; Strebel, K. Differential activities of the human immunodeficiency virus type 1-encoded Vpu protein are regulated by phosphorylation and occur in different cellular compartments. J. Virol. 1994, 68, 2260–2271. [Google Scholar] [PubMed]
- Sauter, D.; Schindler, M.; Specht, A.; Landford, W.N.; Munch, J.; Kim, K. A.; Votteler, J.; Schubert, U.; Bibollet-Ruche, F.; Keele, B.F.; Takehisa, J.; Ogando, Y.; Ochsenbauer, C.; Kappes, J.C.; Ayouba, A.; Peeters, M.; Learn, G.H.; Shaw, G.; Sharp, P.M.; Bieniasz, P.; Hahn, B.H.; Hatziioannou, T.; Kirchhoff, F. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe 2009, 6, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Sauter, D.; Hue, S.; Petit, S.J.; Plantier, J.C.; Towers, G.J.; Kirchhoff, F.; Gupta, R.K. HIV-1 Group P is unable to antagonize human tetherin by Vpu, Env or Nef. Retrovirology 2011, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Lopez, L.A.; Exline, C.M.; Haworth, K.G.; Cannon, P.M. Lack of adaptation to human tetherin in HIV-1 group O and P. Retrovirology 2011, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Fackler, O.T.; Moris, A.; Tibroni, N.; Giese, S.I.; Glass, B.; Schwartz, O.; Krausslich, H.G. Functional characterization of HIV-1 Nef mutants in the context of viral infection. Virology 2006, 351, 322–339. [Google Scholar] [CrossRef] [PubMed]
- Glushakova, S.; Munch, J.; Carl, S.; Greenough, T.C.; Sullivan, J.L.; Margolis, L.; Kirchhoff, F. CD4 down-modulation by human immunodeficiency virus type 1 Nef correlates with the efficiency of viral replication and with CD4+ T-cell depletion in human lymphoid tissue ex vivo. J. Virol. 2001, 75, 10113–10117. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, C.A.; Tobiume, M.; Zhou, J.; Unutmaz, D.; Aiken, C. Nef-mediated downregulation of CD4 enhances human immunodeficiency virus type 1 replication in primary T lymphocytes. J. Virol. 2002, 76, 4625–4633. [Google Scholar] [CrossRef] [PubMed]
- Churchill, M. J.; Rhodes, D.I.; Learmont, J.C.; Sullivan, J.S.; Wesselingh, S.L.; Cooke, I.R.; Deacon, N.J.; Gorry, P.R. Longitudinal analysis of human immunodeficiency virus type 1 nef/long terminal repeat sequences in a cohort of long-term survivors infected from a single source. J. Virol. 2006, 80, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Gorry, P.R.; McPhee, D.A.; Verity, E.; Dyer, W.B.; Wesselingh, S.L.; Learmont, J.; Sullivan, J.S.; Roche, M.; Zaunders, J.J.; Gabuzda, D.; Crowe, S.M.; Mills, J.; Lewin, S.R.; Brew, B.J.; Cunningham, A.L.; Churchill, M.J. Pathogenicity and immunogenicity of attenuated, nef-deleted HIV-1 strains in vivo. Retrovirology 2007, 4, e66. [Google Scholar] [CrossRef]
- Greenough, T.C.; Sullivan, J.L.; Desrosiers, R.C. Declining CD4 T-cell counts in a person infected with nef-deleted HIV-1. N. Engl. J. Med. 1999, 340, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Shima, T.; Nishizawa, M.; Sudo, K.; Iwamuro, S.; Okabe, T.; Takebe, Y.; Imai, M. Identification of attenuated variants of HIV-1 circulating recombinant form 01_AE that are associated with slow disease progression due to gross genetic alterations in the nef/long terminal repeat sequences. J. Infect. Dis. 2005, 192, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.I.; Ashton, L.; Solomon, A.; Carr, A.; Cooper, D.; Kaldor, J.; Deacon, N. Characterization of three nef-defective human immunodeficiency virus type 1 strains associated with long-term nonprogression. Australian Long-Term Nonprogressor Study Group. J. Virol. 2000, 74, 10581–10588. [Google Scholar]
- Salvi, R.; Garbuglia, A.R.; Di Caro, A.; Pulciani, S.; Montella, F.; Benedetto, A. Grossly defective nef gene sequences in a human immunodeficiency virus type 1-seropositive long-term nonprogressor. J. Virol. 1998, 72, 3646–3657. [Google Scholar] [PubMed]
- Braibant, M.; Xie, J.; Samri, A.; Agut, H.; Autran, B.; Barin, F. Disease progression due to dual infection in an HLA-B57-positive asymptomatic long-term nonprogressor infected with a nef-defective HIV-1 strain. Virology 2010, 405, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.S.; Baugh, L.L.; Denial, S.J.; Watkins, R.L.; Liu, M.; Garcia, J.V.; Foster, J.L. Overlapping effector interfaces define the multiple functions of the HIV-1 Nef polyproline helix. Retrovirology 2012, 9, e47. [Google Scholar] [CrossRef]
- Manninen, A.; Hiipakka, M.; Vihinen, M.; Lu, W.; Mayer, B.J.; Saksela, K. SH3-Domain binding function of HIV-1 Nef is required for association with a PAK-related kinase. Virology 1998, 250, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.L.; Denial, S.J.; Temple, B.R.; Garcia, J.V. Mechanisms of HIV-1 Nef function and intracellular signaling. J. Neuroimmune Pharmacol. 2011, 6, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Balazs, A. B.; Ouyang, Y.; Hong, C.M.; Chen, J.; Nguyen, S.M.; Rao, D.S.; An, D.S.; Baltimore, D. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat. Med. 2014, 20, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Holt, N.; Wang, J.; Kim, K.; Friedman, G.; Wang, X.; Taupin, V.; Crooks, G.M.; Kohn, D.B.; Gregory, P.D.; Holmes, M.C.; Cannon, P.M. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat. Biotechnol. 2010, 28, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Pickering, S.; Hue, S.; Kim, E.Y.; Reddy, S.; Wolinsky, S.M.; Neil, S.J. Preservation of tetherin and CD4 counter-activities in circulating Vpu alleles despite extensive sequence variation within HIV-1 infected individuals. PLoS Pathog. 2014, 10, e1003895. [Google Scholar] [CrossRef] [PubMed]
- Iwabu, Y.; Kinomoto, M.; Tatsumi, M.; Fujita, H.; Shimura, M.; Tanaka, Y.; Ishizaka, Y.; Nolan, D.; Mallal, S.; Sata, T.; Tokunaga, K. Differential anti-APOBEC3G activity of HIV-1 Vif proteins derived from different subtypes. J. Biol. Chem. 2010, 285, 35350–35358. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).