Chimeric Rabies Virus-Like Particles Containing Membrane-Anchored GM-CSF Enhances the Immune Response against Rabies Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Viruses
2.2. DNA Construction and Recombinant Baculovirus (rBVs) Generation
| Primer | Sequence (5'-3') | Restriction Enzyme Site |
|---|---|---|
| MSP-GMF | TTTGGATCCATGAAGTTCCTGGTGAACGTGGCTC | BamHI |
| MSP-GMR | GGGGAATTCCTTTTGCACAGGCTTCTTGCACTCG | EcoRI |
| EG-TMCTF | CCCCGAATTCTATGTATTACTGAGTGCAGG | EcoRI |
| EG-TMCTR | TTTTAAGCTTTCACAGTCTGGTCTCACCCC | HindIII |
| SPGMF | GGGCTCGAGATGAAGTTCCTGGTGAACGTGGCTC | XhoI |
| SPGMR | TTTGCTAGCTTACAGGCGGGTCTCGCCACCGGAC | NheI |
2.3. Immunofluorescence Assay (IFA)
2.4. Production and Characterization of EVLP-G
2.5. Immunization and Virus Challenge
2.6. Antibody Assay
2.7. IFN-γ and IL-4 Enzyme-Linked Immunospot Assays (ELISpot)
2.8. Flow Cytometry Assays for Intracellular Cytokine Staining (ICS)
2.9. Flow Cytometry Assays for B Cells and DCs
2.10. Laboratory Facility and Ethics Statement
3. Results
3.1. Construction and Generation of rBVs Expressing GM-CSF

3.2. Production and Characterization of EVLP-G
3.3. Antibody Responses Induced by EVLP-G
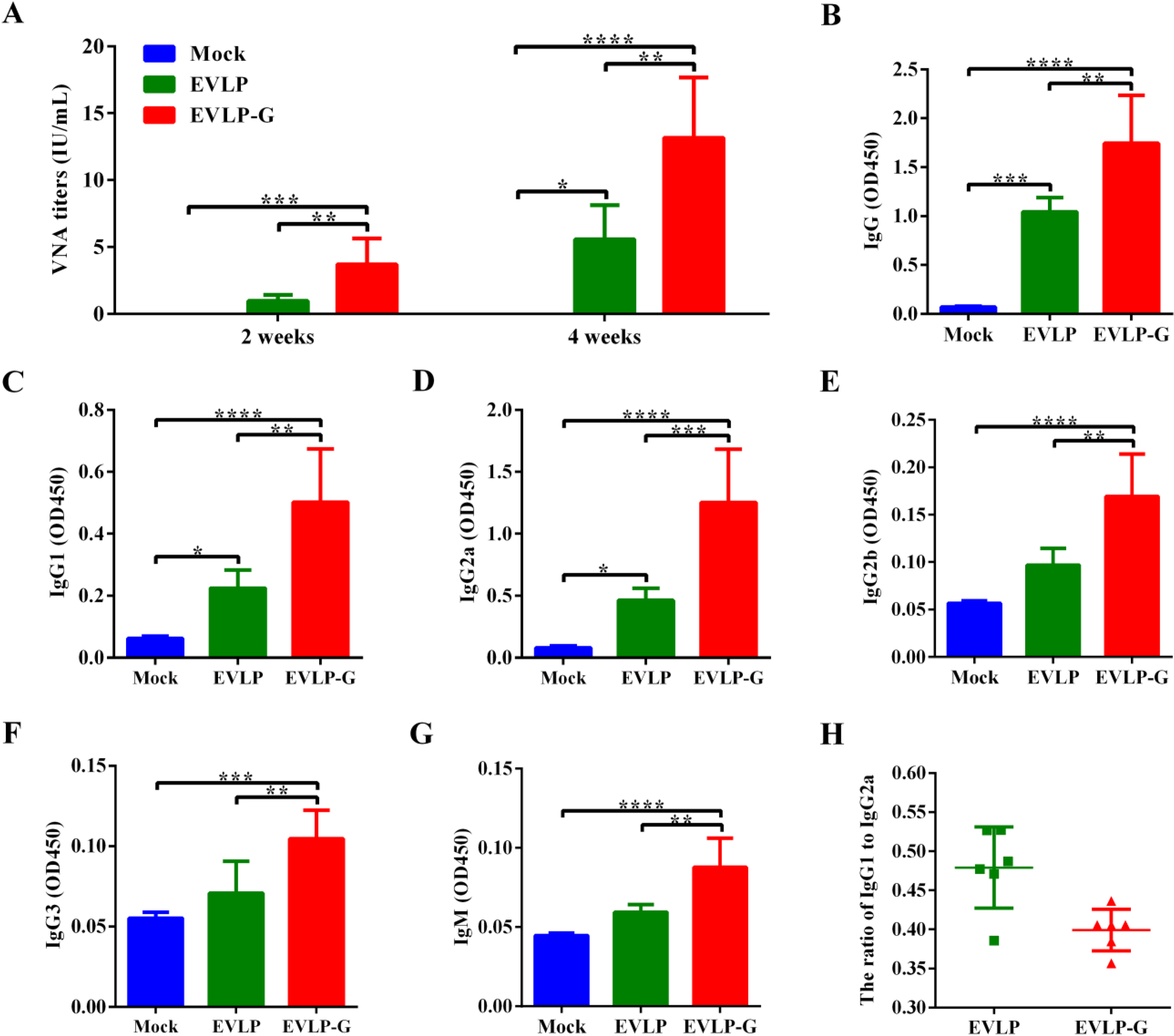
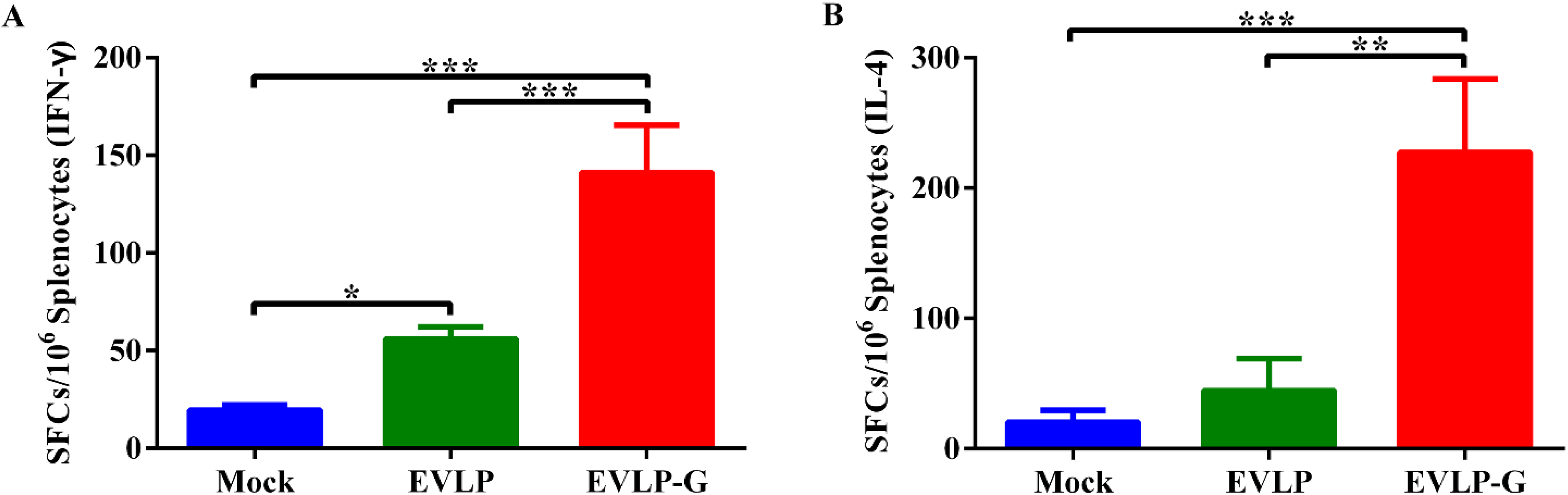
3.4. Antigen-Specific Cellular Immune Responses Induced by EVLP-G
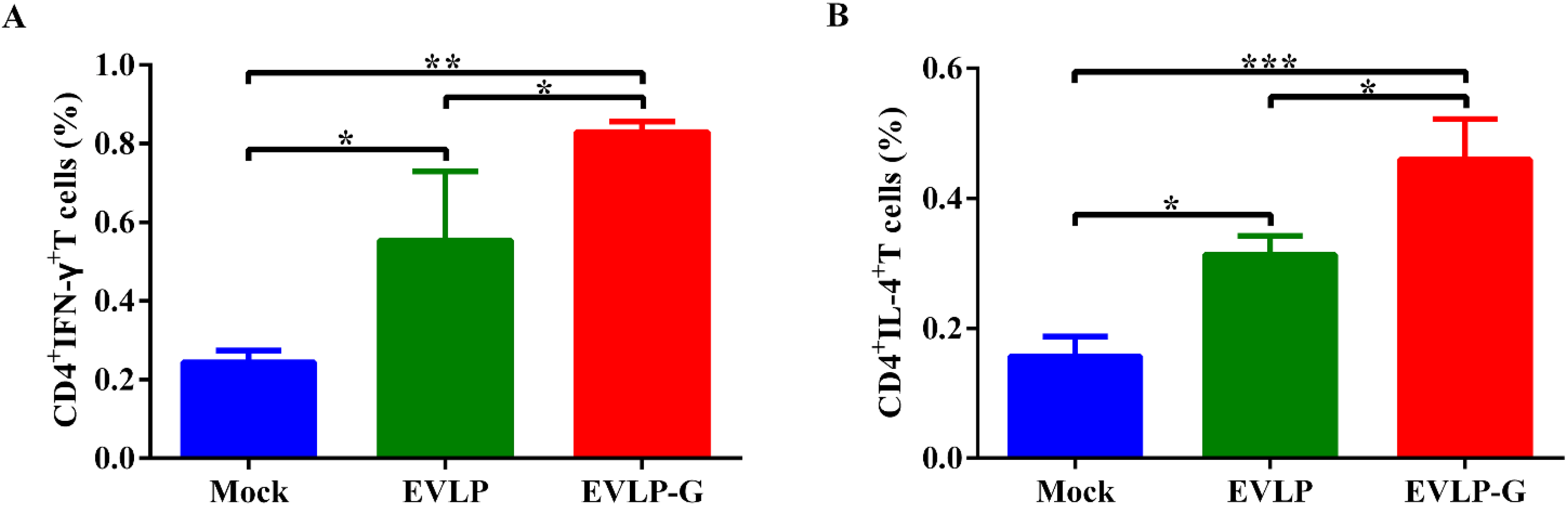
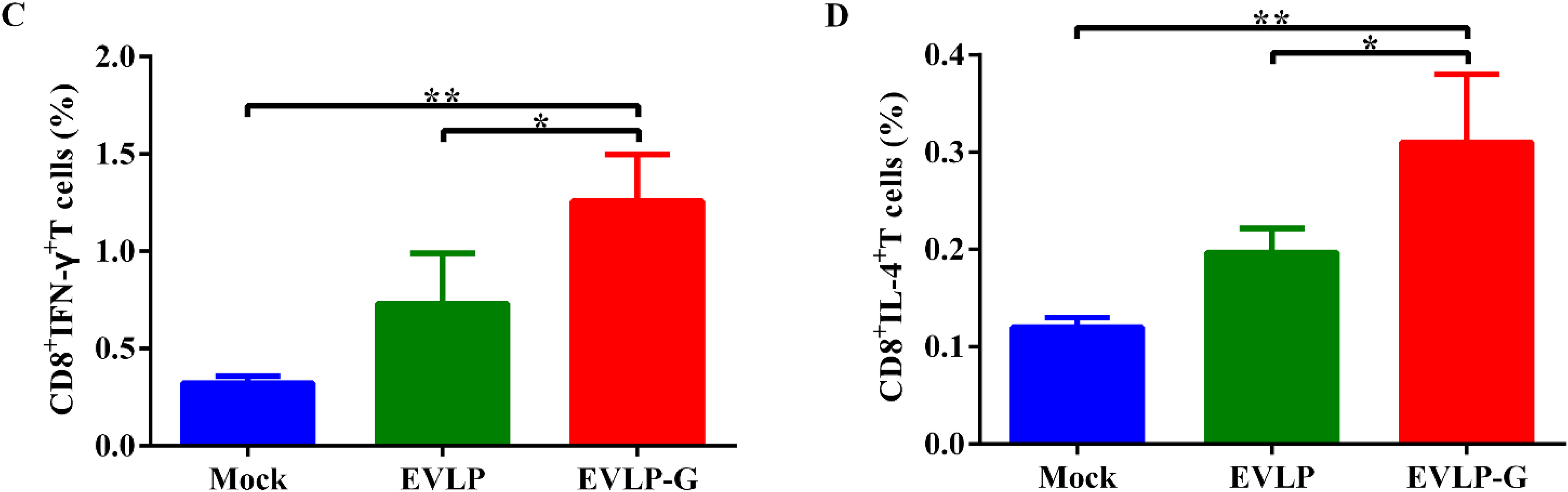
3.5. EVLP-G-Induced Recruitment and/or Activation of B Cells and DCs in Lymph Nodes
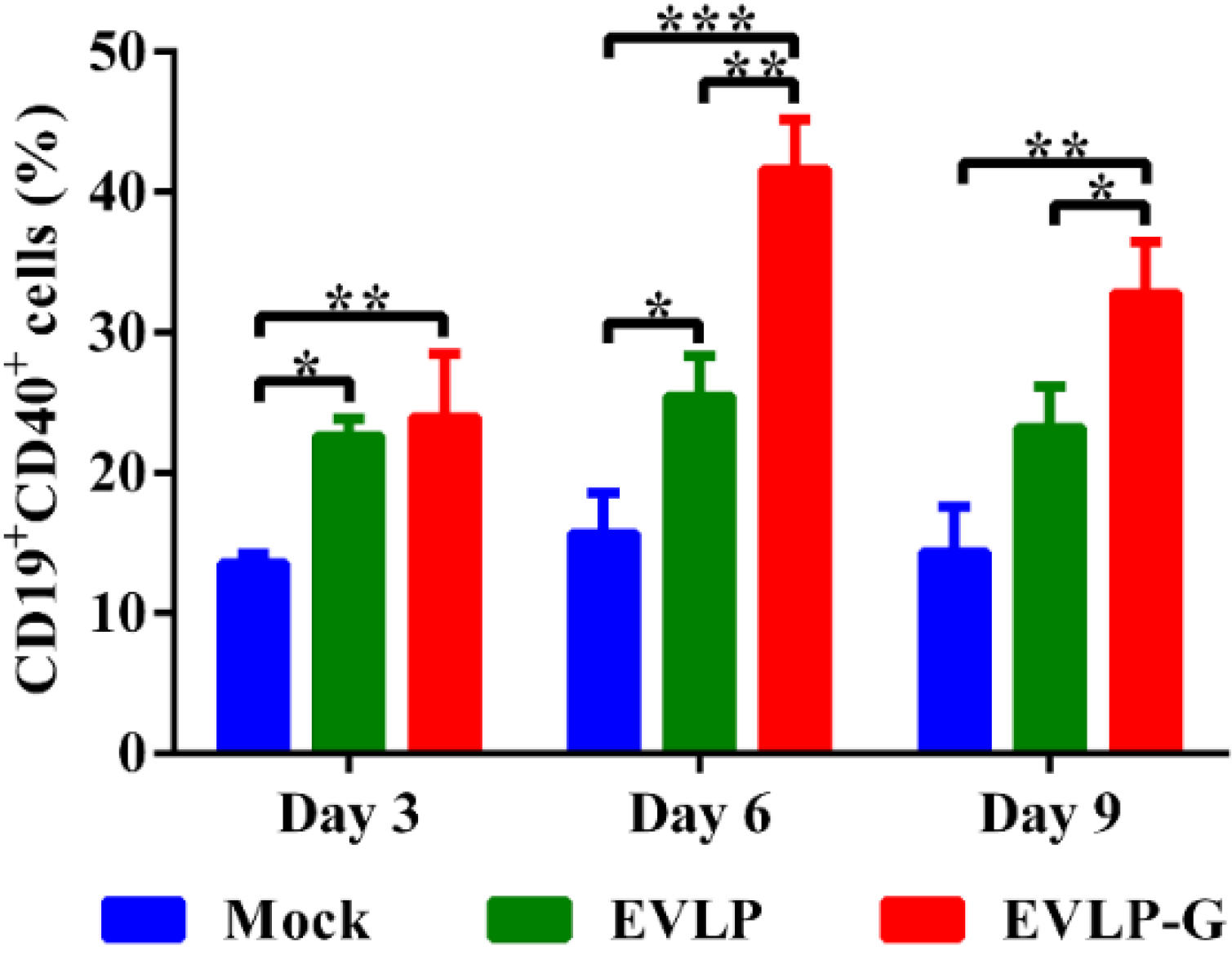
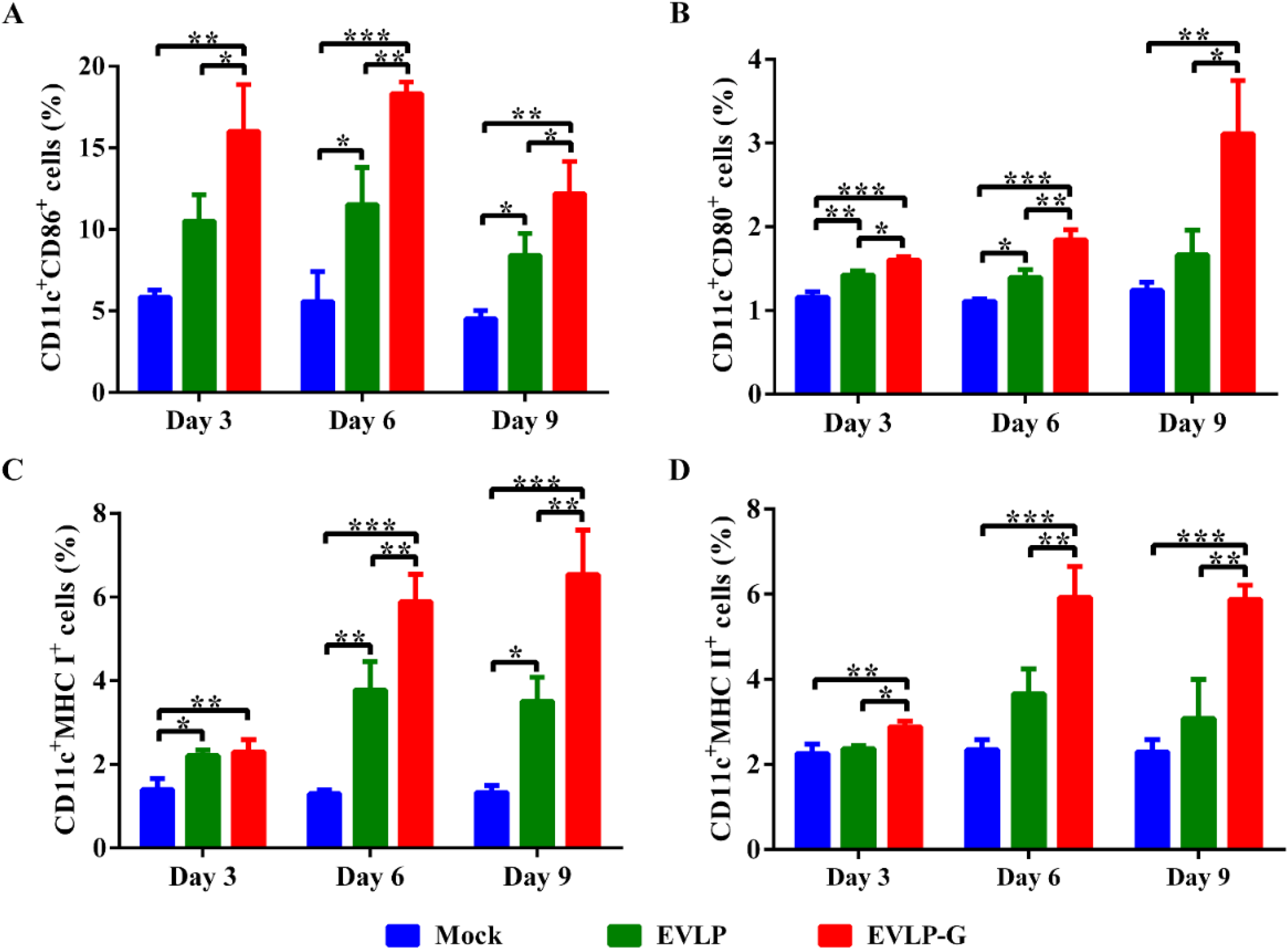
3.6. Challenge Test
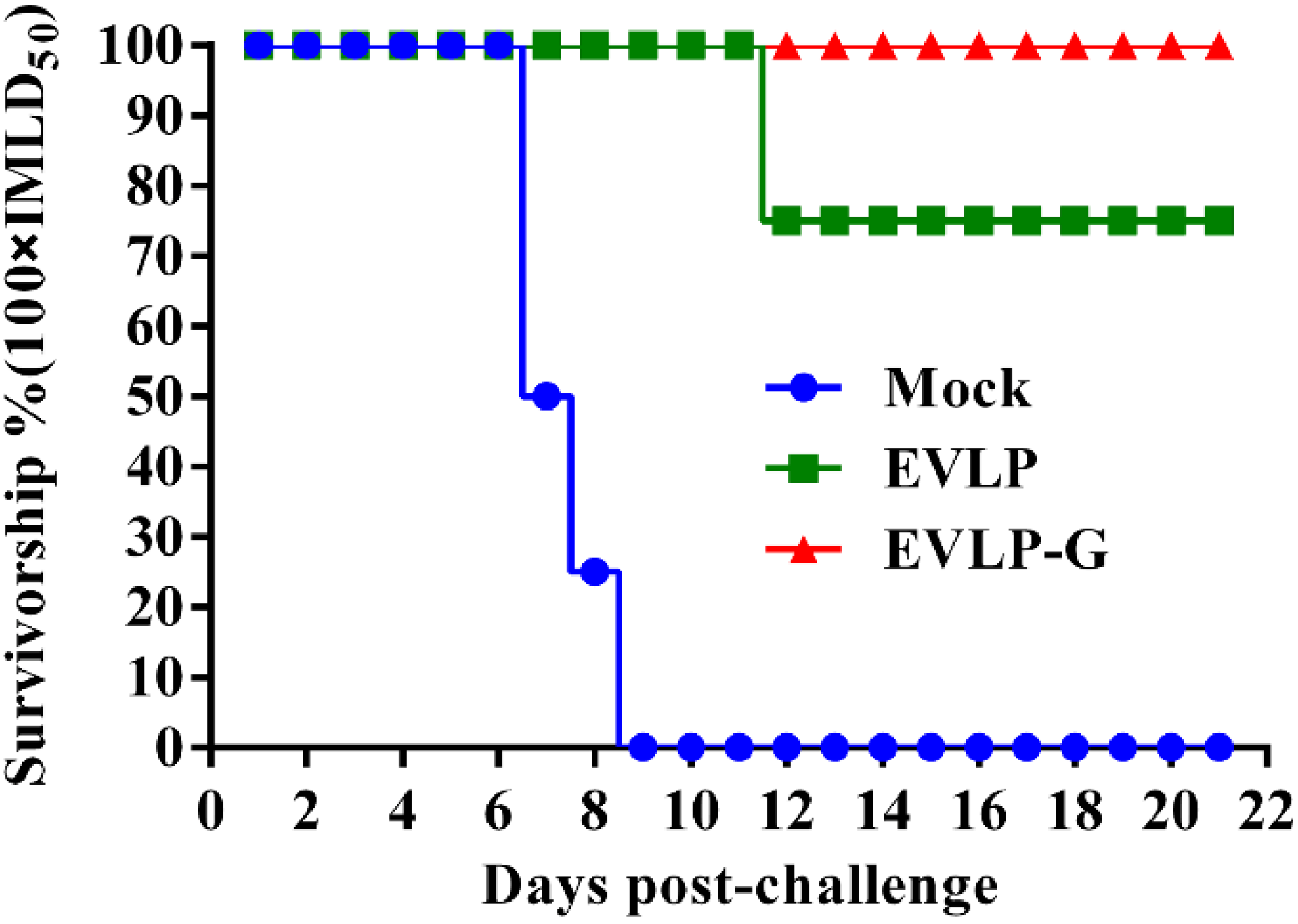
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Willoughby, R.E., Jr.; Tieves, K.S.; Hoffman, G.M.; Ghanayem, N.S.; Amlie-Lefond, C.M.; Schwabe, M.J.; Chusid, M.J.; Rupprecht, C.E. Survival after treatment of rabies with induction of coma. N. Engl. J. Med. 2005, 352, 2508–2514. [Google Scholar] [CrossRef] [PubMed]
- WHO. Who Rabies Fact Sheet; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Fu, Z.F. Rabies and rabies research: Past, present and future. Vaccine 1997, 15, S20–S24. [Google Scholar] [CrossRef] [PubMed]
- Bourhy, H.; Dacheux, L.; Strady, C.; Mailles, A. Rabies in europe in 2005. Euro Surveill. 2005, 10, 213–216. [Google Scholar] [PubMed]
- Dorfmeier, C.L.; Lytle, A.G.; Dunkel, A.L.; Gatt, A.; McGettigan, J.P. Protective vaccine-induced CD4(+) t cell-independent B cell responses against rabies infection. J. Virol. 2012, 86, 11533–11540. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, N.; Streatfield, S.J.; Yusibov, V. Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine 2012, 31, 58–83. [Google Scholar] [CrossRef] [PubMed]
- Moron, G.; Rueda, P.; Casal, I.; Leclerc, C. CD8alpha− CD11b+ dendritic cells present exogenous virus-like particles to CD8+ T cells and subsequently express CD8alpha and CD205 molecules. J. Exp. Med. 2002, 195, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Kurt-Jones, E.A.; Popova, L.; Kwinn, L.; Haynes, L.M.; Jones, L.P.; Tripp, R.A.; Walsh, E.E.; Freeman, M.W.; Golenbock, D.T.; Anderson, L.J.; et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 2000, 1, 398–401. [Google Scholar] [CrossRef]
- Pushko, P.; Pearce, M.B.; Ahmad, A.; Tretyakova, I.; Smith, G.; Belser, J.A.; Tumpey, T.M. Influenza virus-like particle can accommodate multiple subtypes of hemagglutinin and protect from multiple influenza types and subtypes. Vaccine 2011, 29, 5911–5918. [Google Scholar] [CrossRef] [PubMed]
- Tyler, M.; Tumban, E.; Peabody, D.S.; Chackerian, B. The use of hybrid virus-like particles to enhance the immunogenicity of a broadly protective HPV vaccine. Biotechnol. Bioeng. 2014, 111, 2398–2406. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Song, Y.; Li, X.; Huang, X.; Liu, J.; Ding, H.; Zhu, P.; Zhou, P. HIV-1 virus-like particles produced by stably transfected drosophila s2 cells: A desirable vaccine component. J. Virol. 2012, 86, 7662–7676. [Google Scholar] [CrossRef] [PubMed]
- Raghunandan, R. Virus-like particles: Innate immune stimulators. Expert Rev. Vaccines 2011, 10, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Becker, Y. Immunological and regulatory functions of uninfected and virus infected immature and mature subtypes of dendritic cells—A review. Virus Genes 2003, 26, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Toriumi, H.; Wang, H.; Kuang, Y.; Guo, X.; Morimoto, K.; Fu, Z.F. Expression of MIP-1alpha (CCL3) by a recombinant rabies virus enhances its immunogenicity by inducing innate immunity and recruiting dendritic cells and B cells. J. Virol. 2010, 84, 9642–9648. [Google Scholar] [CrossRef] [PubMed]
- Brilot, F.; Strowig, T.; Munz, C. Nk cells interactions with dendritic cells shape innate and adaptive immunity. Front. Biosci. J. Virtual Libr. 2008, 13, 6443–6454. [Google Scholar] [CrossRef]
- Bodey, G.P. The potential role of granulocyte-macrophage colony stimulating factor in therapy of fungal infections: A commentary. Eur. J. Clin. Microbiol. Infect. Dis. 1994, 13, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Jager, E.; Ringhoffer, M.; Dienes, H.P.; Arand, M.; Karbach, J.; Jager, D.; Ilsemann, C.; Hagedorn, M.; Oesch, F.; Knuth, A. Granulocyte-macrophage-colony-stimulating factor enhances immune responses to melanoma-associated peptides in vivo. Int. J. Cancer 1996, 67, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Disis, M.L.; Bernhard, H.; Shiota, F.M.; Hand, S.L.; Gralow, J.R.; Huseby, E.S.; Gillis, S.; Cheever, M.A. Granulocyte-macrophage colony-stimulating factor: An effective adjuvant for protein and peptide-based vaccines. Blood 1996, 88, 202–210. [Google Scholar] [PubMed]
- Ramsburg, E.; Publicover, J.; Buonocore, L.; Poholek, A.; Robek, M.; Palin, A.; Rose, J.K. A vesicular stomatitis virus recombinant expressing granulocyte-macrophage colony-stimulating factor induces enhanced t-cell responses and is highly attenuated for replication in animals. J. Virol. 2005, 79, 15043–15053. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M. The dendritic cell system and its role in immunogenicity. Ann. Rev. Immunol. 1991, 9, 271–296. [Google Scholar] [CrossRef]
- Daro, E.; Pulendran, B.; Brasel, K.; Teepe, M.; Pettit, D.; Lynch, D.H.; Vremec, D.; Robb, L.; Shortman, K.; McKenna, H.J.; et al. Polyethylene glycol-modified GM-CSF expands CD11b(high)CD11c(high) but notCD11b(low)CD11c(high) murine dendritic cells in vivo: A comparative analysis with Flt3 ligand. J. Immunol. 2000, 165, 49–58. [Google Scholar] [CrossRef]
- Lai, L.; Vodros, D.; Kozlowski, P.A.; Montefiori, D.C.; Wilson, R.L.; Akerstrom, V.L.; Chennareddi, L.; Yu, T.; Kannanganat, S.; Ofielu, L.; et al. GM-CSF DNA: An adjuvant for higher avidity IgG, rectal Iga, and increased protection against the acute phase of a SHIV-89.6p challenge by a DNA/MVA immunodeficiency virus vaccine. Virology 2007, 369, 153–167. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, G.; Ren, G.; Gnanadurai, C.W.; Li, Z.; Chai, Q.; Yang, Y.; Leyson, C.M.; Wu, W.; Cui, M.; et al. Recombinant rabies viruses expressing gm-csf or flagellin are effective vaccines for both intramuscular and oral immunizations. PLOS ONE 2013, 8, e63384. [Google Scholar] [CrossRef]
- Janke, M.; Peeters, B.; de Leeuw, O.; Moorman, R.; Arnold, A.; Fournier, P.; Schirrmacher, V. Recombinant newcastle disease virus (NDV) with inserted gene coding for gm-csf as a new vector for cancer immunogene therapy. Gene Ther. 2007, 14, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Qi, Y.; Zheng, X.; Feng, H.; Wang, H.; Gao, Y.; Guo, X.; Yang, S.; Xia, X. Rabies virus-like particles assembled in sf9 cells induce strong humoral and cellular immune responses against a lethal rabies virus challenge in mice and dogs. PLOS ONE. submitted.
- Li, L. The Analysis of the Growth Characteristics and the Animal Infection Characteristics of Different Host-Derived Street Rabies Virus. Master Thesis, Jilin University, Changchun, China, 2014. [Google Scholar]
- Cliquet, F.; Aubert, M.; Sagne, L. Development of a fluorescent antibody virus neutralisation test (favn test) for the quantitation of rabies-neutralising antibody. J. Immunol. Methods 1998, 212, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, K.; Xin, K.Q.; Katoh, H.; Sumino, K.; Hagiwara, E.; Kawamoto, S.; Okuda, K.; Miyagi, Y.; Aoki, I.; Nishioka, K.; et al. The timing of GM-CSF expression plasmid administration influences the Th1/Th2 response induced by an HIV-1-specific DNA vaccine. J. Immunol. 2000, 164, 3102–3111. [Google Scholar] [CrossRef]
- Quan, F.S.; Kim, Y.; Lee, S.; Yi, H.; Kang, S.M.; Bozja, J.; Moore, M.L.; Compans, R.W. Viruslike particle vaccine induces protection against respiratory syncytial virus infection in mice. J. Infect. Dis. 2011, 204, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wang, H.; Wu, H.; Yang, F.; Tripp, R.A.; Hogan, R.J.; Fu, Z.F. Rabies virus expressing dendritic cell-activating molecules enhances the innate and adaptive immune response to vaccination. J. Virol. 2011, 85, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Win, S.J.; Ward, V.K.; Dunbar, P.R.; Young, S.L.; Baird, M.A. Cross-presentation of epitopes on virus-like particles via the mhc i receptor recycling pathway. Immunol. Cell biol. 2011, 89, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Tessier, D.C.; Thomas, D.Y.; Khouri, H.E.; Laliberte, F.; Vernet, T. Enhanced secretion from insect cells of a foreign protein fused to the honeybee melittin signal peptide. Gene 1991, 98, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Z.; Liu, W.; Kang, S.M.; Alam, M.; Huang, C.; Ye, L.; Sun, Y.; Li, Y.; Kothe, D.L.; Pushko, P.; et al. Incorporation of high levels of chimeric human immunodeficiency virus envelope glycoproteins into virus-like particles. J. Virol. 2007, 81, 10869–10878. [Google Scholar] [CrossRef]
- Encke, J.; Bernardin, J.; Geib, J.; Barbakadze, G.; Bujdoso, R.; Stremmel, W. Genetic vaccination with Flt3-l and GM-CSF as adjuvants: Enhancement of cellular and humoral immune responses that results in protective immunity in a murine model of hepatitis c virus infection. World J. Gastroenterol. 2006, 12, 7118–7125. [Google Scholar] [PubMed]
- Spearman, P.; Kalams, S.; Elizaga, M.; Metch, B.; Chiu, Y.L.; Allen, M.; Weinhold, K.J.; Ferrari, G.; Parker, S.D.; McElrath, M.J.; et al. Safety and immunogenicity of a CTL multiepitope peptide vaccine for HIV with or without GM-CSF in a phase I trial. Vaccine 2009, 27, 243–249. [Google Scholar] [CrossRef]
- Banerjee, K.; Klasse, P.J.; Sanders, R.W.; Pereyra, F.; Michael, E.; Lu, M.; Walker, B.D.; Moore, J.P. IgG subclass profiles in infected HIV type 1 controllers and chronic progressors and in uninfected recipients of env vaccines. AIDS Res. Hum. Retrovir. 2010, 26, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.S.; Madiga, M.C.; Moore, P.L.; Mlisana, K.; Abdool Karim, S.S.; Binley, J.M.; Shaw, G.M.; Mascola, J.R.; Morris, L. Broad neutralization of human immunodeficiency virus type 1 mediated by plasma antibodies against the gp41 membrane proximal external region. J. Virol. 2009, 83, 11265–11274. [Google Scholar] [CrossRef] [PubMed]
- Visciano, M.L.; Tagliamonte, M.; Tornesello, M.L.; Buonaguro, F.M.; Buonaguro, L. Effects of adjuvants on IgG subclasses elicited by virus-like particles. J. Trans. Med. 2012, 10, e4. [Google Scholar] [CrossRef]
- Klimovich, V.B. IgM and its receptors: Structural and functional aspects. Biochem. Biokhimiia 2011, 76, 534–549. [Google Scholar] [CrossRef]
- Dorfmeier, C.L.; Shen, S.; Tzvetkov, E.P.; McGettigan, J.P. Reinvestigating the role of IgM in rabies virus postexposure vaccination. J. Virol. 2013, 87, 9217–9222. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, P. Friendly and dangerous signals: Is the tissue in control? Nat. Immunol. 2007, 8, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Ahlers, J.D.; Belyakov, I.M. Molecular pathways regulating CD4(+) T cell differentiation, anergy and memory with implications for vaccines. Trends Mol. Med. 2010, 16, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.P.; Mills, K.H. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013, 34, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.C.; Yashiro-Ohtani, Y.; Del Bianco, C.; Knoblock, D.M.; Blacklow, S.C.; Pear, W.S. Notch directly regulates GATA3 expression during T helper 2 cell differentiation. Immunity 2007, 27, 100–110. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; Qi, Y.; Wang, H.; Zheng, X.; Gao, Y.; Li, N.; Yang, S.; Xia, X. Chimeric Rabies Virus-Like Particles Containing Membrane-Anchored GM-CSF Enhances the Immune Response against Rabies Virus. Viruses 2015, 7, 1134-1152. https://doi.org/10.3390/v7031134
Kang H, Qi Y, Wang H, Zheng X, Gao Y, Li N, Yang S, Xia X. Chimeric Rabies Virus-Like Particles Containing Membrane-Anchored GM-CSF Enhances the Immune Response against Rabies Virus. Viruses. 2015; 7(3):1134-1152. https://doi.org/10.3390/v7031134
Chicago/Turabian StyleKang, Hongtao, Yinglin Qi, Hualei Wang, Xuexing Zheng, Yuwei Gao, Nan Li, Songtao Yang, and Xianzhu Xia. 2015. "Chimeric Rabies Virus-Like Particles Containing Membrane-Anchored GM-CSF Enhances the Immune Response against Rabies Virus" Viruses 7, no. 3: 1134-1152. https://doi.org/10.3390/v7031134
APA StyleKang, H., Qi, Y., Wang, H., Zheng, X., Gao, Y., Li, N., Yang, S., & Xia, X. (2015). Chimeric Rabies Virus-Like Particles Containing Membrane-Anchored GM-CSF Enhances the Immune Response against Rabies Virus. Viruses, 7(3), 1134-1152. https://doi.org/10.3390/v7031134






