Chikungunya Virus Replication in Salivary Glands of the Mosquito Aedes albopictus
Abstract
:1. Introduction

2. Results
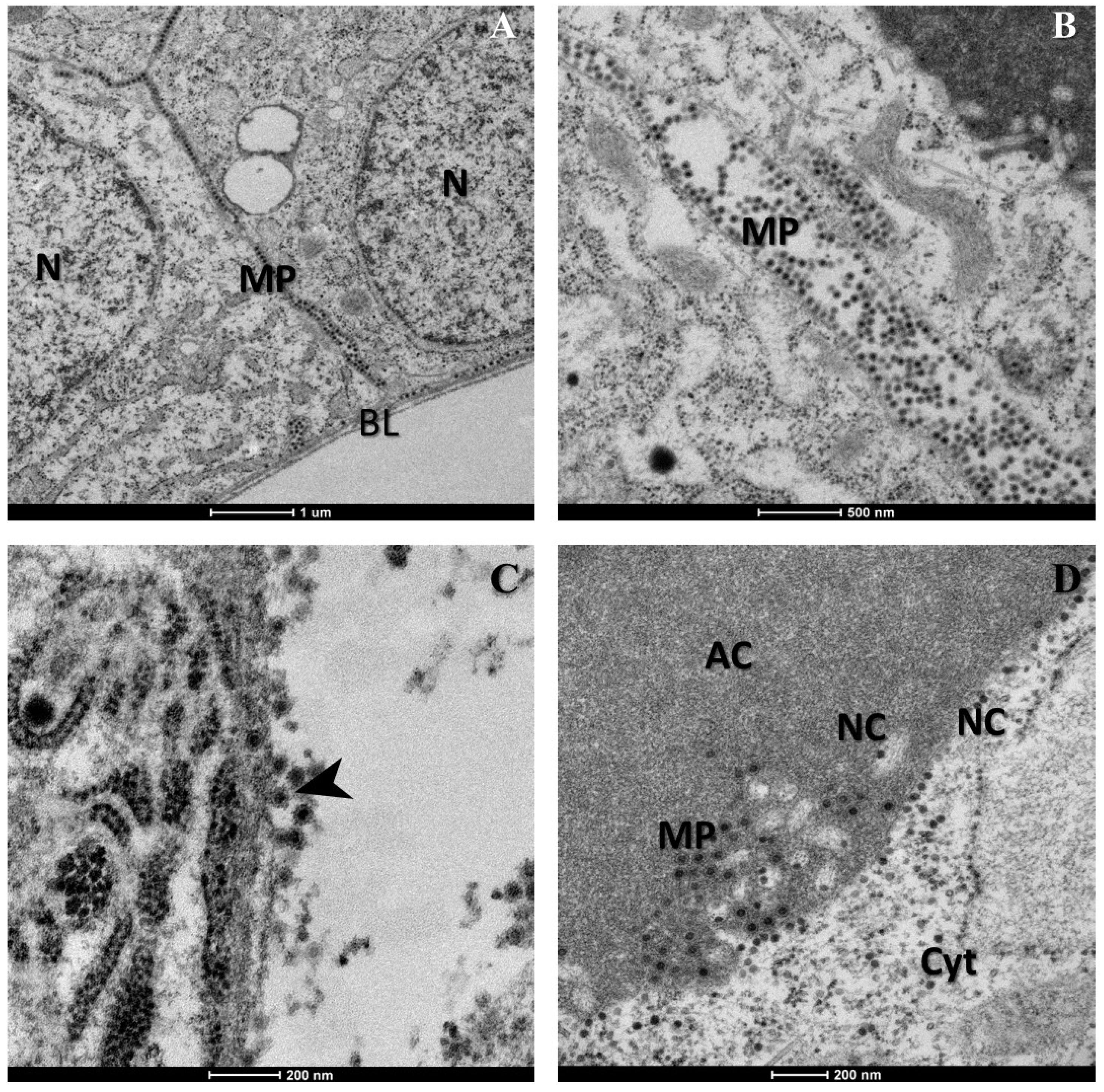
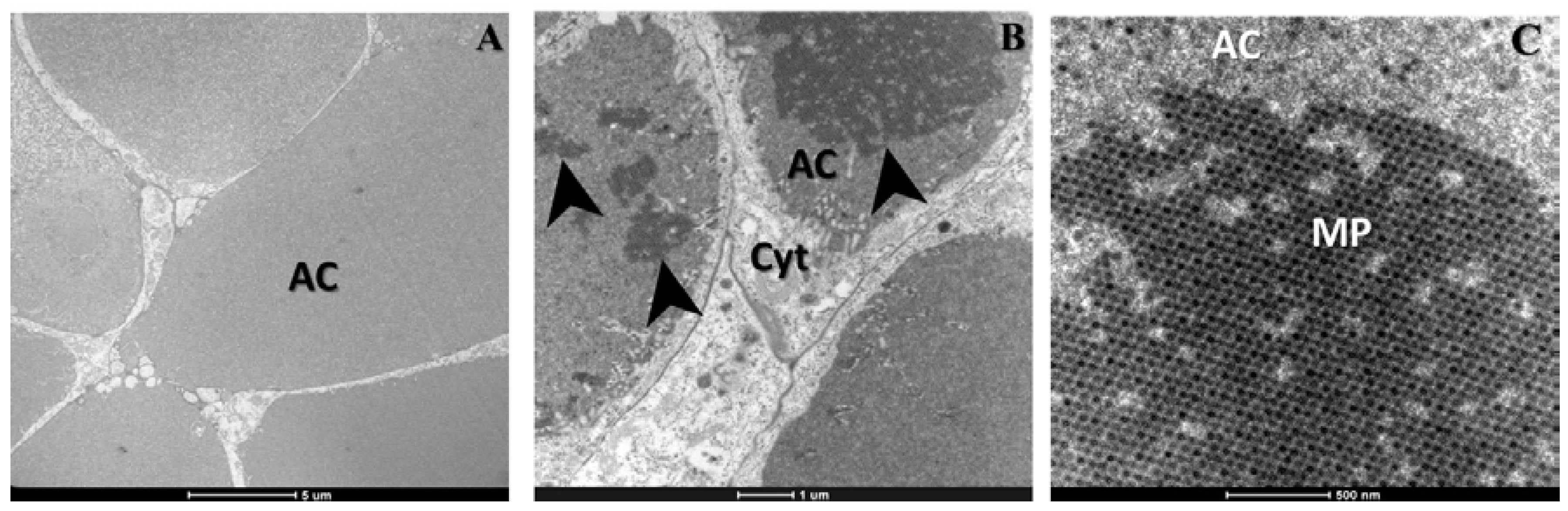

3. Discussion
4. Materials and Methods
4.1. Mosquito Rearing and Oral Infection
4.2. Salivary Glands Dissection and Treatment for Transmission Electron Microscopy
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coffey, L.; Failloux, A.-B.; Weaver, S. Chikungunya Virus-Vector Interactions. Viruses 2014, 6, 4628–4663. [Google Scholar] [CrossRef] [PubMed]
- Mangiafico, J.A. Chikungunya virus infection and transmission in five species of mosquito. Am. J. Trop. Med. Hyg. 1971, 20, 642–645. [Google Scholar] [PubMed]
- Hardy, J.L.; Houk, E.J.; Kramer, L.D.; Reeves, W.C. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu. Rev. Entomol. 1983, 28, 229–262. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Clements, A.N. The Physiology of Mosquitoes; The Macmillan Company, Ed.; Pergamon Press: New York, NY, USA, 1963. [Google Scholar]
- Jobling, B.; Lewis, D.J. Anatomical Drawings of Biting Flies; British Museum (Natural History) in Association with the Wellcome Trust: London, UK, 1987. [Google Scholar]
- Franz, A.; Kantor, A.; Passarelli, A.; Clem, R. Tissue barriers to arbovirus infection in mosquitoes. Viruses 2015, 7, 3741–3767. [Google Scholar] [CrossRef] [PubMed]
- Soekiman, S.; Matsumura, T.; Yamanishi, H. Multiplication of Chikungunya virus in salivary glands of Aedes albopictus (Oahu strain) mosquitoes: An electron microscopic study. Jpn. J. Med. Sci. Biol. 1986, 39, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Forrester, N.; Coffey, L.; Weaver, S. Arboviral bottlenecks and challenges to maintaining diversity and fitness during mosquito transmission. Viruses 2014, 6, 3991–4004. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [PubMed]
- Chevillon, C.; Briant, L.; Renaud, F.; Devaux, C. The Chikungunya threat: An ecological and evolutionary perspective. Trends Microbiol. 2008, 16, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, O.; Albert, M.L. Biology and pathogenesis of chikungunya virus. Nat. Rev. Microbiol. 2010, 8, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Bowers, D.F.; Coleman, C.G.; Brown, D.T.; Bowers, D.F.; Coleman, C.G.; Brown, D.T. Sindbis virus-associated pathology in Aedes albopictus (Diptera: Culicidae ). J. Med. Entomol. 2003, 40, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.D.; Ebel, G.D. Dynamics of flavivirus infection in mosquitoes. Adv. Virus Res. 2003, 60, 187–232. [Google Scholar] [PubMed]
- Vega-Rúa, A.; Zouache, K.; Girod, R.; Failloux, A.-B.; Lourenço-de-Oliveira, R. High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya virus. J. Virol. 2014, 88, 6294–6306. [Google Scholar] [CrossRef] [PubMed]
- Schuffenecker, I.; Iteman, I.; Michault, A.; Murri, S.; Frangeul, L.; Vaney, M.C.; Lavenir, R.; Pardigon, N.; Reynes, J.M.; Pettinelli, F.; et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006, 3, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Vazeille, M.; Moutailler, S.; Coudrier, D.; Rousseaux, C.; Khun, H.; Huerre, M.; Thiria, J.; Dehecq, J.S.; Fontenille, D.; Schuffenecker, I.; et al. Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS ONE 2007, 2, e1168. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.A.; Vanlandingham, D.L.; McGee, C.E.; Higgs, S. A single mutation in Chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007, 3, 1895–1906. [Google Scholar] [CrossRef] [PubMed]
- Dubrulle, M.; Mousson, L.; Moutailier, S.; Vazeille, M.; Failloux, A.B. Chikungunya virus and Aedes mosquitoes: Saliva is infectious as soon as two days after oral infection. PLoS ONE 2009, 4, e5895. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rua, A.; Zouache, K.; Caro, V.; Diancourt, L.; Delaunay, P.; Grandadam, M.; Failloux, A.B. High efficiency of temperate Aedes albopictus to transmit Chikungunya and Dengue viruses in the southeast of France. PLoS ONE 2013, 8, e59716. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-Rúa, A.; Schmitt, C.; Bonne, I.; Krijnse Locker, J.; Failloux, A.-B. Chikungunya Virus Replication in Salivary Glands of the Mosquito Aedes albopictus. Viruses 2015, 7, 5902-5907. https://doi.org/10.3390/v7112917
Vega-Rúa A, Schmitt C, Bonne I, Krijnse Locker J, Failloux A-B. Chikungunya Virus Replication in Salivary Glands of the Mosquito Aedes albopictus. Viruses. 2015; 7(11):5902-5907. https://doi.org/10.3390/v7112917
Chicago/Turabian StyleVega-Rúa, Anubis, Christine Schmitt, Isabelle Bonne, Jacomine Krijnse Locker, and Anna-Bella Failloux. 2015. "Chikungunya Virus Replication in Salivary Glands of the Mosquito Aedes albopictus" Viruses 7, no. 11: 5902-5907. https://doi.org/10.3390/v7112917
APA StyleVega-Rúa, A., Schmitt, C., Bonne, I., Krijnse Locker, J., & Failloux, A.-B. (2015). Chikungunya Virus Replication in Salivary Glands of the Mosquito Aedes albopictus. Viruses, 7(11), 5902-5907. https://doi.org/10.3390/v7112917





