Low Proviral Load is Associated with Indeterminate Western Blot Patterns in Human T-Cell Lymphotropic Virus Type 1 Infected Individuals: Could Punctual Mutations be Related?
Abstract
:1. Introduction
2. Results
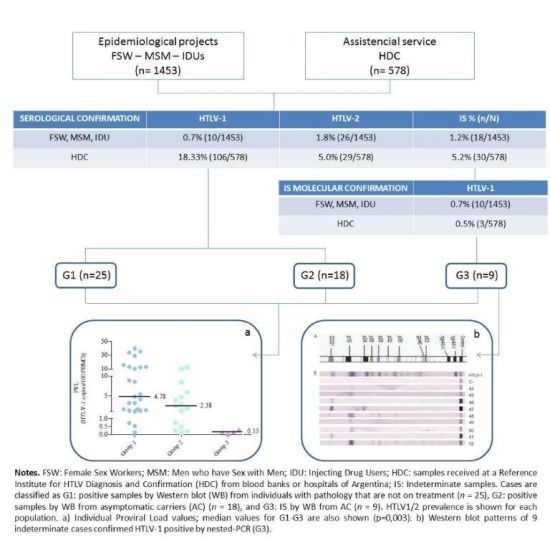
2.1. Prevalence Studies
| Reactive by PA or ELISA | Indeterminate samples (IS) n (%) | IS Confirmed HTLV-1+ by n-PCR n (%) | IS Confirmed HTLV-2+ by n-PCR n (%) | Total HTLV-1 Prevalence % (n/N) | Total HTLV-2 Prevalence % (n/N) | |
|---|---|---|---|---|---|---|
| MSM (N=667) | 26 | 11 (1.65) | 3 (27.28) | 0 (0) | 0.45% (3/667) c | 0% (0/667)c |
| IDU (N=173) | 36 | 4 (2.31 ) | 4 (100 ) | 2 b (100 ) | 4.62% (8/173) c | 15.6% (27/173) c |
| FSW (N=613) | 25 | 3 (2.12) | 3 (23.10 ) | 0 (0 ) | 1.46% (9/613) c | 0.2% (1/613) c |
| HDC (N=578) | 207 | 30 (5.19 ) | 3 (15.79) a | 2 (10.53)a | 18.8% (109/578) | 5.36% (31/578) |
| Total | 294 | 48 (16.33) | 13 (35.13) | 4 (10.81) | 6.35% (129/2031) | 2.90% (59/2031) |
| WB Indeterminate Banding Pattern | N | HTLV-1/2 Negative | HTLV-1/2 Positive | Not Performed |
|---|---|---|---|---|
| GD21 | 6 | 3 | 1 | 2 |
| GD21 + others | 7 | 3 | 3 | 1 |
| rgp46-1 and/or 2 | 4 | 1 | 1 | 2 |
| p19 | 2 | 2 | 0 | 0 |
| p19 + p24 | 4 | 2 | 0 | 2 |
| p19 + others | 1 | 0 | 0 | 1 |
| HGIP | 6 | 3 | 0 | 3 |
| Total | 30 | 14 | 5 | 11 |
2.2. Performance of the qPCR
2.3. PVL Values
2.4. PVL Distribution
| Sample N° (Sequence Code) | Group | Age | Gender | PVL |
|---|---|---|---|---|
| 1 | 1- Leukemia | 53 | M | 33.9768 |
| 2 | 1- Leukemia | 66 | F | 40.1995 |
| 3 (ATL1) | 1- Lymphoma | 48 | F | 1.2974 |
| 4 (ATL2) | 1- Leukemia | 67 | F | 12.4920 |
| 5 | 1- HAM/TSP | 43 | F | 0.7081 |
| 6 | 1- HAM/TSP | 14 | F | 8.9051 |
| 7 | 1- HAM/TSP | 27 | F | 3.1244 |
| 8 | 1- HAM/TSP | 38 | F | 1.6633 |
| 9 | 1- HAM/TSP | 52 | F | 8.5679 |
| 10 | 1- HAM/TSP | 65 | M | 5.3882 |
| 11 | 1- HAM/TSP | 37 | F | 1.2808 |
| 12 | 1- HAM/TSP | 51 | F | 4.7829 |
| 13 (Neu28) | 1- HAM/TSP | 42 | F | 13.0850 |
| 14 (Neu14) | 1- HAM/TSP | 39 | F | 1.0119 |
| 15 | 1- HAM/TSP | 52 | M | 35.0971 |
| 16 | 1- HAM/TSP | 59 | F | 12.8610 |
| 17 | 1- HAM/TSP | 50 | M | 1.3508 |
| 18 | 1- HAM/TSP | 56 | F | 29.5394 |
| 19 | 1- HAM/TSP | NA | M | 0.1183 |
| 20 | 1- HAM/TSP | 26 | F | 3.1234 |
| 21 | 1- HAM/TSP | 71 | F | 10.4542 |
| 22 | 1- HAM/TSP | 35 | M | 0.5227 |
| 23 | 1- HAM/TSP | 67 | F | 1.6661 |
| 24 | 1- HAM/TSP | 52 | M | 15.5252 |
| 25 | 1- HAM/TSP | 49 | M | 4.0326 |
| 26 | 2 | 50 | M | 12.4340 |
| 27 | 2 | 64 | M | 1.8929 |
| 28 | 2 | 50 | M | 0.0832 |
| 29 | 2 | 46 | F | 1.2681 |
| 30 (ASYAR3) | 2 | 35 | M | 4.6143 |
| 31 (ASYAR2) | 2 | 26 | F | 0.7502 |
| 32 | 2 | 47 | F | 0.2476 |
| 33 | 2 | 25 | M | 8.4668 |
| 34 | 2 | 33 | M | 2.3861 |
| 35 | 2 | 39 | F | 2.8778 |
| 36 (ASYAR1) | 2 | 47 | F | 0.4813 |
| 37 | 2 | 52 | F | <3 copies/ reaction |
| 38 | 2 | NA | F | <3 copies/ reaction |
| 39 | 2 | 38 | F | 0.1832 |
| 40 (BDAR20) | 2 | 38 | M | 3.9461 |
| 41 | 2 | 57 | M | 5.9663 |
| 42 | 2 | NA | M | <3 copies/ reaction |
| 43 | 2 | 46 | M | 10.5358 |
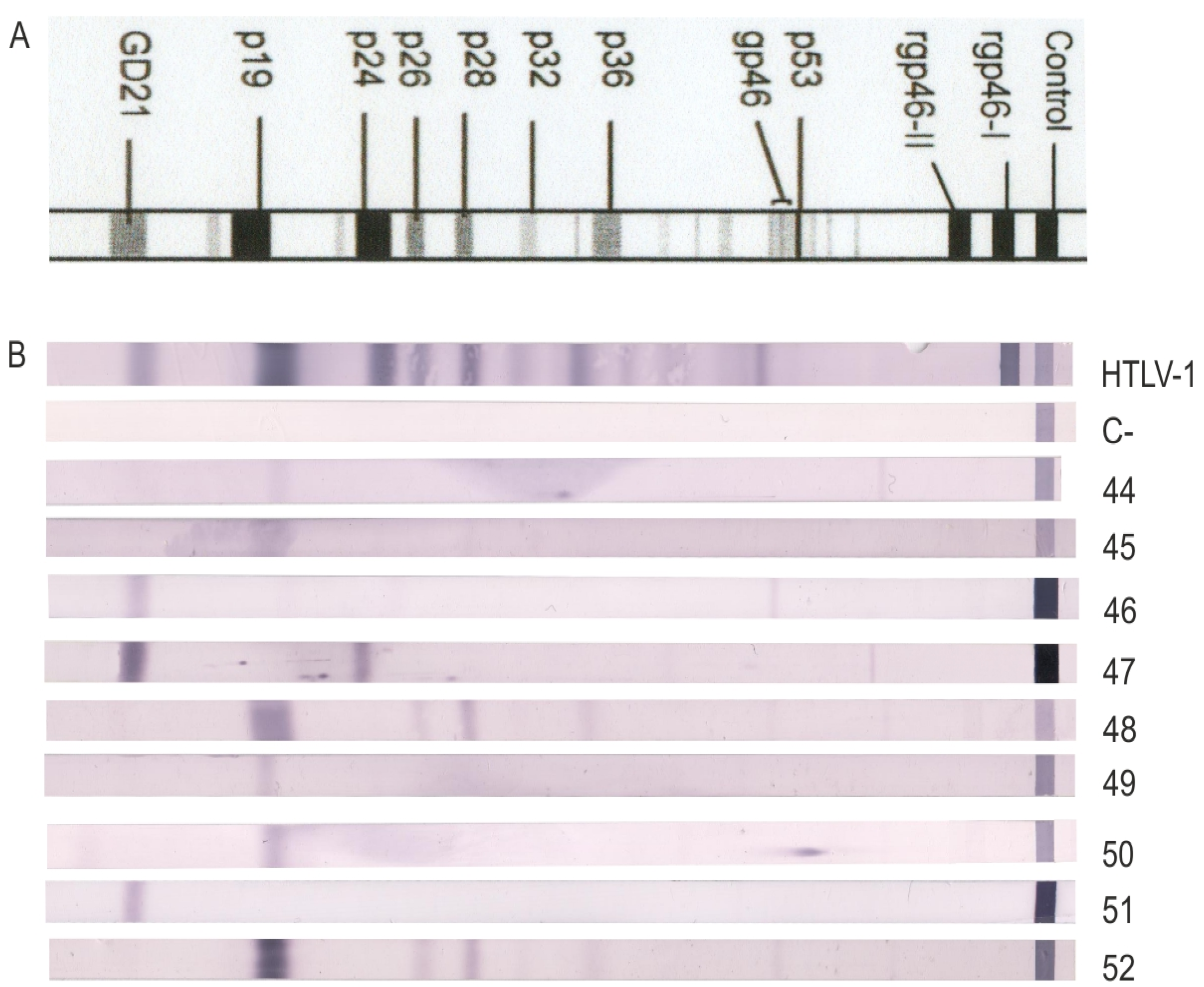
| 44 | 3 (p19) | 59 | M | 0.0013 |
| 45 | 3 (p19) | 24 | M | 0.3365 |
| 46 (BDAR21) | 3 (GD21) | 28 | M | 0.1493 |
| 47 (BDAR18) | 3 (p24, GD21) | 52 | M | 0.1452 |
| 48 (FSW8) | 3 (HGIP) | 32 | F | <3 copies/ reaction |
| 49 (FSW9) | 3 (p19) | 52 | F | <3 copies/ reaction |
| 50 (FSW7) | 3 (p19) | 25 | F | <3 copies/ reaction |
| 51 (BDAR19) | 3 (GD21) | 31 | M | <3 copies/ reaction |
| 52 | 3 (HGIP) | 21 | M | pol not detected |

| PVL Range | G1 (n=25) | G2 (n=18) | G3 (n=8) |
|---|---|---|---|
| <1 | 12% | 44.4% | 100% |
| 1–10 | 52% | 44.4% | - |
| >10 | 36% | 11.2% | - |
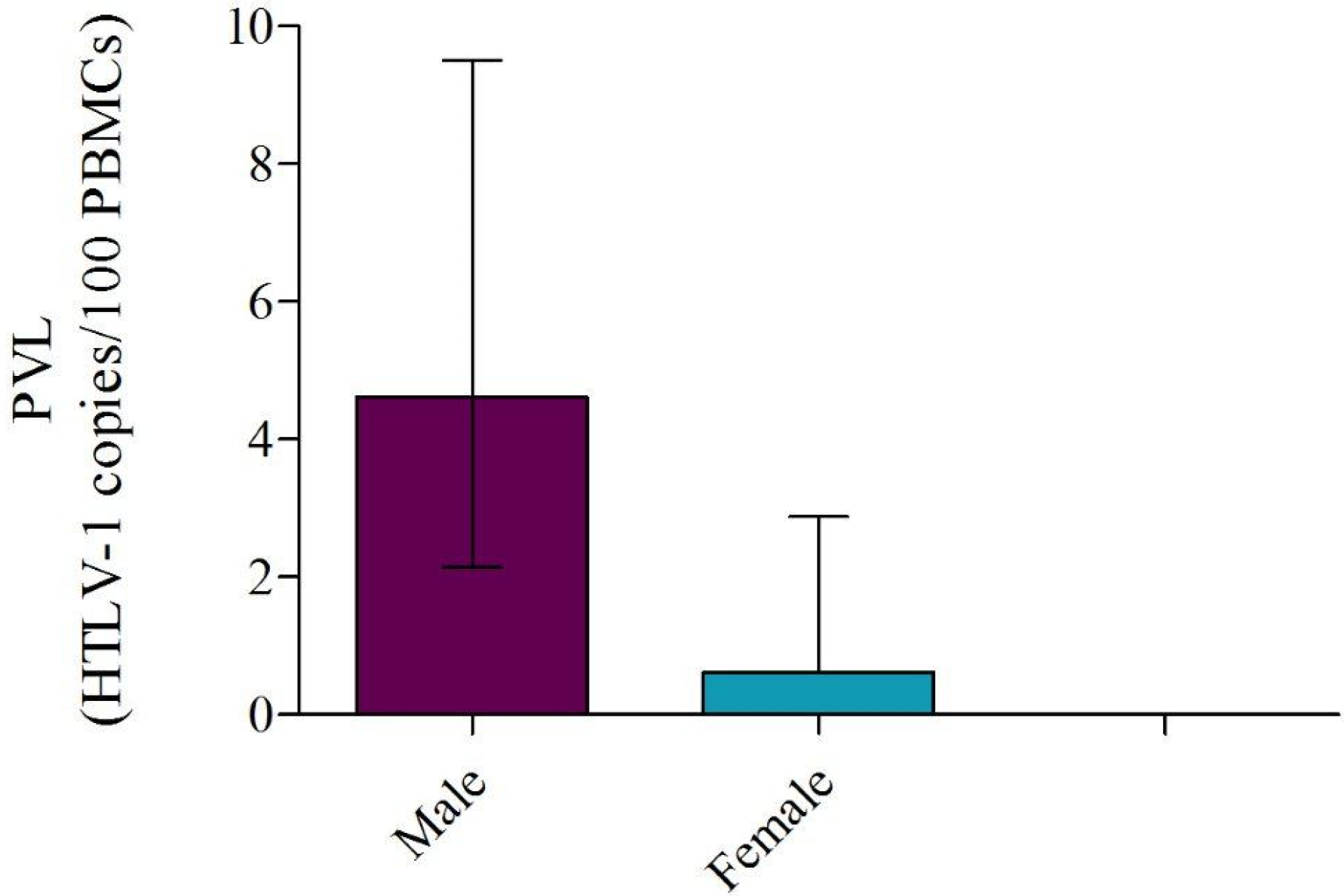
2.5. PVL Association with Gender, Age, and Optical Density
2.6. HTLV-1 Phylogeny
2.7. Sequence Analysis
| Punctual Mutation | Sequence N° | Region | Punctual Mutation | Sequence N° | Region |
|---|---|---|---|---|---|
| 8295G>A | 46- 48 | LTR; U-3 | 8718C>T | 51 | LTR; R |
| 8367C>A | Geographical | LTR; U-3 | 8779T>C | 47 | LTR; R |
| 8381G>A | 48 | LTR; U-3 | 8822G>A | 46 | LTR; R |
| 8391G>A | 46 | LTR; U-3 | 8828A>G | 47 | LTR; R |
| 8392G>A | 46 | LTR; U-3 | 8912T>C | 46- 47 | LTR; U-5 |
| 8420C>T | 49 | LTR; U-3; TRE-1; dr | 8955G>A | 47 | LTR; U-5 |
| 8428_8429insA | Geographical | LTR; U-3; TRE-1 | 7383C>T | 46 | tax |
| 8446G>A | Geographical | LTR; U-3; TRE-1 | 7398C>T | 46- 47 | tax |
| 8471G>T | 50 | LTR; U-3; TRE-1; cr | 7401C>T | Geographical | tax |
| 8509A>G | Geographical | LTR; U-3; TRE-2 | 7431G>A | 47 | tax |
| 8509_8511delA | Geographical | LTR; U-3; TRE-2 | 7448A>C | 46 | tax |
| 8522T>C | 50 | LTR; U-3; TRE-2 | 7780A>G | 47 | tax |
| 8545G>A | 50 | LTR; U-3; TRE-2 | 7914T>C | Geographical | tax |
| 8546T>C | Geographical | LTR; U-3; TRE-2 | 7920C>T | Geographical | tax |
| 8606C>G | Geographical | LTR; U-3 | 7933C>T | 47 | tax |
| 8606C>A | 49- 51 | LTR; U-3 | 7982C>T | Geographical | tax |
| 8632G>A | 46 | LTR; U-3 | 8001A>G | 46 | tax |
| 8655G>T | 46 | LTR; U-3 | 8231G>A | Geographical | tax |
| 8665C>T | 46- 48 | LTR; R |
3. Materials and Methods
3.1. Samples
3.2. Diagnostic Algorithm
3.3. DNA Quantitation
3.4. Molecular Analysis
3.5. Accession Numbers
3.6.Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gessain, A.; Cassar, O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Roucoux, D.F.; Murphy, E.L. The epidemiology and disease outcomes of human T-lymphotropic virus type II. AIDS Rev. 2004, 6, 144–154. [Google Scholar] [PubMed]
- Gessain, A.; Mahieux, R. Epidemiology, origin and genetic diversity of HTLV-1 retrovirus and STLV-1 simian affiliated retrovirus. Bull. Soc. Pathol. Exot. 2000, 93, 163–171. [Google Scholar] [PubMed]
- Vidal, A.U.; Gessain, A.; Yoshida, M.; Tekaia, F.; Garin, B.; Guillemain, B.; Schulz, T.; Farid, R.; Thé, G. Phylogenetic classification of human T cell leukaemia/lymphoma virus type I genotypes in five major molecular and geographical subtypes. J. Gen. Virol. 1994, 75, 3655–3666. [Google Scholar] [CrossRef] [PubMed]
- Van Dooren, S.; Gotuzzo, E.; Salemi, M.; Watts, D.; Audenaert, E.; Duwe, S.; Ellerbrok, H.; Grassmann, R.; Hagelberg, E.; Desmyter, J.; et al. Evidence for a post-Columbian introduction of human T-cell lymphotropic virus in Latin America. J. Gen. Virol. 1998, 79, 2695–2708. [Google Scholar] [CrossRef] [PubMed]
- Biglione, M.M.; Astarloa, L.; Salomón, H.E. Referent HTLV-I/II Argentina Group. High prevalence of HTLV-I and HTLV-II among blood donors in Argentina: A South American health concern. AIDS Res. Hum. Retrovir. 2005, 21, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gastaldello, R.; Hall, W.W.; Gallego, S. Seroepidemiology of HTLV-I/II in Argentina: An overview. J. Acquir. Immune Defic. Syndr. 2004, 35, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Licensure of Screening Tests for Antibody to Human T-Lymphotropic Virus Type 1. MMWR 1988, 37, 736–747. [PubMed]
- Khabbaz, R.F.; Heneine, W.; Grindon, A.; Hartley, T.M.; Shulman, G.; Kaplan, J. Indeterminate HTLV serologic results in U.S. blood donors: Are they due to HTLV-I or HTLV-II? J. Acquir. Immune Defic. Syndr. 1992, 5, 400–404. [Google Scholar] [PubMed]
- Berini, C.A.; Eirin, M.E.; Pando, M.A.; Biglione, M.M. Human T-cell lymphotropic virus types I and II (HTLV-I and -II) infection among seroindeterminate cases in Argentina. J. Med. Virol. 2007, 79, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Filippone, C.; Bassot, S.; Betsem, E.; Tortevoye, P.; Guillotte, M.; Mercereau-Puijalon, O.; Plancoulaine, S.; Calattini, S.; Gessain, A. A new and frequent human T-cell leukemia virus indeterminate Western blot pattern: Epidemiological determinants and PCR results in central African inhabitants. J. Clin. Microbiol. 2012, 50, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Prince, H.E.; Gross, M. Impact of initial screening for human T-cell lymphotropic virus (HTLV) antibodies on efficiency of HTLV Western blotting. Clin. Diagn. Lab. Immunol. 2001, 8. [Google Scholar] [CrossRef]
- Mahieux, R.; Horal, P.; Mauclère, P.; Mercereau-Puijalon, O.; Guillotte, M.; Meertens, L.; Murphy, E.; Gessain, A. Human T-cell lymphotropic virus type 1 gag indeterminate western blot patterns in Central Africa: Relationship to Plasmodium falciparum infection. J. Clin. Microbiol. 2000, 38, 4049–4057. [Google Scholar] [PubMed]
- Porter, K.R.; Liang, L.; Long, J.W.; Bangs, M.J.; Anthony, R.; Andersen, E.M.; Hayes, C.G. Evidence for anti-Plasmodium falciparumantibodies that cross-react with human T-lymphotropic virus type I proteins in a population in Irian Jaya, Indonesia. Clin. Diagn. Lab Immunol. 1994, 1, 11–15. [Google Scholar] [PubMed]
- Hayes, C.G.; Burans, J.P.; Oberst, R.B. Antibodies to human T lymphotropic virus type I in a population from the Philippines: Evidence for cross-reactivity with Plasmodium falciparum. J. Infect. Dis. 1991, 163, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Abrams, A.; Akahata, Y.; Jacobson, S. The prevalence and significance of HTLV-I/II seroindeterminate Western blot patterns. Viruses 2011, 3, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Netto, E.C.; Brites, C. Characteristics of Chronic Pain and Its Impact on Quality of Life of Patients with HTLV-1-associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP). Clin. J. Pain. 2011, 27, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Smith, M.R. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990, 4, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Heneine, W.; Khabbaz, R.F.; Lal, R.B.; Kaplan, J.E. Sensitive and specific polymerase chain reaction assays for diagnosis of human T-cell lymphotropic virus type I (HTLV-I) and HTLV-II infections in HTLV-I/II-seropositive individuals. J. Clin. Microbiol. 1992, 30, 1605–1607. [Google Scholar] [PubMed]
- Tuke, P.W.; Luton, P.; Garson, J.A. Differential diagnosis of HTLV-I and HTLV-II infections by restriction enzyme analysis of 'nested' PCR products. J. Virol. Methods 1992, 40, 163–173. [Google Scholar] [CrossRef]
- Furtado, M.; Andrade, R.G.; Romanelli, L.C.; Ribeiro, M.A.; Ribas, J.G.; Torres, E.B.; Barbosa-Stancioli, E.F.; Proietti, A.B.; Martins, M.L. Monitoring the HTLV-1 proviral load in the peripheral blood of asymptomatic carriers and patients with HTLV-associated myelopathy/tropical spastic paraparesis from a Brazilian cohort: ROC curve analysis to establish the threshold for risk disease. J. Med. Virol. 2012, 84, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Usuku, K.; Matsumoto, W.; Kodama, D.; Takenouchi, N.; Moritoyo, T.; Hashiguchi, S.; Ichinose, M.; Bangham, C.R.; Izumo, S.; et al. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: High proviral load strongly predisposes to HAM/TSP. J. Neurovirol. 1998, 4, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Olindo, S.; Lézin, A.; Cabre, P.; Merle, H.; Saint-Vil, M.; Edimonana-Kaptue, M.; Signate, A.; Césaire, R.; Smadja, D. HTLV-1 proviral load in peripheral blood mononuclear cells quantified in 100 HAM/TSP patients: A marker of disease progression. J. Neurol Sci. 2005, 237, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.A.; Magri, M.C.; Caterino-de-Araujo, A. The best algorithm to confirm the diagnosis of HTLV-1 and HTLV-2 in at-risk individuals from São Paulo, Brazil. J. Virol. Methods 2011, 173, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.; Oliveira, A.L.; Coughlan, S.; de Venecia, C.; Schor, D.; Leite, A.C.; Araújo, A.Q.; Hall, W.W. Multiplex real-time PCR for the detection and quantitation of HTLV-1 and HTLV-2 proviral load: Addressing the issue of indeterminate HTLV results. J. Clin. Virol. 2011, 52, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Berini, C.A.; Pando, M.A.; Bautista, C.T.; Eirin, M.E.; Martinez-Peralta, L.; Weissenbacher, M.; Avila, M.M.; Biglione, M.M. HTLV-1/2 among high-risk groups in Argentina: Molecular diagnosis and prevalence of different sexual transmitted infections. J. Med.Virol. 2007, 79, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Mangano, A.M.; Remesar, M.; del Pozo, A.; Sen, L. Human T lymphotropic virus types I and II proviral sequences in Argentinian blood donors with indeterminate Western blot patterns. J. Med. Virol. 2004, 74, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Eirin, M.E. Epidemiología molecular del virus linfotrópico T-humano tipo 1 (HTLV-1) en Argentina: Análisis étnico-geográfico y variabilidad viral. Doctoral Thesis, Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires, CABA, Argentina, 2011. [Google Scholar]
- Gascuel, O. BIONJ: An improved version of the NJ algorithm based on a simple model of sequence data. Mol. Biol. Evol. 1997, 14, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Cesaire, R.; Bera, O.; Maier, H.; Martial, J.; Ouka, M.; Kerob-Bauchet, B.; Ould Amar, A.K.; Vernant, J.C. Seroindeterminate patterns and seroconversions to human T-lymphotropic virus type I positivity in blood donors from Martinique, French West Indies. Transfusion 1999, 39, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Rouet, F.; Meertens, L.; Courouble, G.; Herrmann-Storck, C.; Pabingui, R.; Chancerel, B.; Abid, A.; Strobel, M.; Mauclere, P.; Gessain, A. Serological, epidemiological, and molecular differences between human T-cell lymphotropic virus Type 1 (HTLV-1)-seropositive healthy carriers and persons with HTLV-I Gag indeterminate Western blot patterns from the Caribbean. J. Clin. Microbiol. 2001, 39, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- The HTLV European Research Network. Seroepidemiology of the human T-cell leukaemia/lymphoma viruses in Europe. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1996, 13, 68–77. [PubMed]
- Olah, I.; Fukumori, L.M.; Smid, J.; de Oliveira, A.C.; Duarte, A.J.; Casseb, J. Neither molecular diversity of the envelope, immunosuppression status, nor proviral load causes indeterminate HTLV western blot profiles in samples from human T-cell lymphotropic virus type 2 (HTLV-2)-infected individuals. J. Med. Virol. 2010, 82, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Hisada, M.; Maloney, E.; Yoshihisa, Y.; Hanchard, B.; Wilks, R.; Rios, M.; Jacobson, S. Human T Lymphotropic Virus Types I and II Western Blot Seroindeterminate Status and Its Association with Exposure to Prototype HTLV-I. J. Infect. Dis. 2006, 193, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Mangano, A.; Altamirano, N.; Remesar, M.; Bouzas, M.B.; Aulicino, P.; Zapiola, I.; DelPozo, A.; Sen, L. HTLV-I proviral load in Argentinean subjects with indeterminate western blot patterns. Retrovirology 2011, 8 (Suppl 1). [Google Scholar] [CrossRef]
- Demontis, M.A.; Hilburn, S.; Taylor, G.P. Human T cell lymphotropic virus type 1 viral load variability and long-term trends in asymptomatic carriers and in patients with human T cell lymphotropic virus type 1-related diseases. AIDS Res. Hum. Retrovir. 2013, 29, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Vakili, R.; Sabet, F.; Aahmadi, S.; Boostani, R.; Rafatpanah, H.; Shamsian, A.; Rahim Rezaee, S.A. Human T-lymphotropic Virus Type I (HTLV-I) Proviral Load and Clinical Features in Iranian HAM/TSP Patients: Comparison of HTLV-I Proviral Load in HAM/TSP Patients. Iran J. Basic Med. Sci. 2013, 16, 268–272. [Google Scholar] [PubMed]
- Hodson, A.; Laydon, D.; Bain, B.J.; Fields, P.A.; Taylor, G.P. Pre-morbid human T-lymphotropicvirus type I proviral load, rather than percentage of abnormal lymphocytes, is associated with an increased risk of aggressive adult T-cell leukemia/lymphoma. Haematologica 2013, 98, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Montanheiro, P.; Olah, I.; Fukumori, L.M.I.; Smid, J.; Penalva de Oliveira, A.C.; Kanzaki, L.I.B.; Fonseca, L.A.; Duarte, A.J.; Casseb, J. Low DNA HTLV-2 proviral load among women in Sao Paulo City. Virus Res. 2008, 135, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Hisada, M.; Miley, W.J.; Biggar, R.J. Provirus load is lower in human T lymphotropic virus (HTLV)-II carriers than in HTLV-I carriers: A key difference in viral pathogenesis? J. Infect. Dis. 2005, 191, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Manns, A.; Miley, W.J.; Wilks, R.J.; Morgan, O.; Hanchard, B.; Wharfe, G.; Cranston, B.; Maloney, E.; Welles, S.; Blattner, W.A.; et al. Quantitative Proviral DNA and Antibody Levels in the Natural History of HTLV-I Infection. J. Infect. Dis. 1999, 180, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, M.; Kozako, T.; Sawada, T.; Matsushita, K.; Ozaki, A.; Hamada, H.; Kawada, H.; Yoshimitsu, M.; Tokunaga, M.; Haraguchi, K.; et al. Anti-HTLV-1 tax antibody and tax-specific cytotoxic T lymphocyte are associated with a reduction in HTLV-1 proviral load in asymptomatic carriers. J. Med.Virol. 2007, 79, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Eirin, M.E.; Dilernia, D.A.; Berini, C.A.; Jones, L.R.; Pando, M.A.; Biglione, M.M. Divergent strains of human T-lymphotropic virus type 1 (HTLV-1) within the Cosmopolitan subtype in Argentina. AIDS Res. Hum.Retrovir. 2008, 24, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Bosselut, R.; Lim, F.; Romond, P.C.; Frampton, J.; Brady, J.; Ghysdael, J. Myb protein binds to multiple sites in the human T cell lymphotropic virus type 1 long terminal repeat and transactivates LTR-mediated expression. Virology 1992, 186, 764–769. [Google Scholar] [CrossRef]
- Marriott, S.J.; Boros, I.; Duvall, J.F.; Brady, J.N. Indirect binding of human T-cell leukemia virus type I tax1 to a responsive element in the viral long terminal repeat. Mol. Cell. Biol. 1989, 9, 4152–4160. [Google Scholar] [CrossRef] [PubMed]
- Numata, N.; Ohtani, K.; Niki, M.; Nakamura, M.; Sugamura, K. Synergism between two distinct elements of the HTLV-I enhancer during activation by the trans-activator of HTLV-I. New Biol. 1991, 3, 896–906. [Google Scholar] [PubMed]
- Datta, S.; Kothari, N. H.; Fan, H. In vivo genomic footprinting of the human T-cell leukemia virus type 1 (HTLV-1) long terminal repeat enhancer sequences in HTLV-1-infected human T-cell lines with different levels of Tax I activity. J. Virol. 2000, 74, 8277–8285. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.M.; Segurado, A.C. Molecular evidence of human T-cell lymphotropic virus types 1 and 2 (HTLV-1 and HTLV-2) infections in HTLV seroindeterminate individuals from São Paulo, Brazil. J. Clin. Virol. 2009, 44, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Thorstensson, R.; Albert, J.; Andersson, S. Strategies for diagnosis of HTLV-I and –II. Transfusion 2002, 42, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.P.; Switzer, W.M.; Murphy, E.L.; Thomson, R.; Heneine, W. Absence of evidence of infection with divergent primate T-lymphotropic viruses in United States blood donors who have seroindeterminate HTLV test results. Transfusion 2000, 40, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, G; Massoud, G.; Leibovitch, E.C.; Caruso, B.; Johnson, K.; Ohayon, J.; Fenton, K.; Cortese, I.; Jacobson, S. Digital droplet PCR (ddPCR) for the precise quantification of human T-lymphotropic virus 1 proviral loads in peripheral blood and cerebrospinal fluid of HAM/TSP patients and identification of viral mutations. J. Neurovirol. 2014, 20, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Hayden, R.T.; Gu, Z.; Abdul-Ali, D.; Shi, L.; Pounds, S.; Caliendo, A.M. Comparison of droplet digital PCR to real-time PCR for quantitative detection of cytomegalovirus. J. Clin. Microbiol. 2013, 51, 540–546. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cánepa, C.; Salido, J.; Ruggieri, M.; Fraile, S.; Pataccini, G.; Berini, C.; Biglione, M. Low Proviral Load is Associated with Indeterminate Western Blot Patterns in Human T-Cell Lymphotropic Virus Type 1 Infected Individuals: Could Punctual Mutations be Related? Viruses 2015, 7, 5643-5658. https://doi.org/10.3390/v7112897
Cánepa C, Salido J, Ruggieri M, Fraile S, Pataccini G, Berini C, Biglione M. Low Proviral Load is Associated with Indeterminate Western Blot Patterns in Human T-Cell Lymphotropic Virus Type 1 Infected Individuals: Could Punctual Mutations be Related? Viruses. 2015; 7(11):5643-5658. https://doi.org/10.3390/v7112897
Chicago/Turabian StyleCánepa, Camila, Jimena Salido, Matías Ruggieri, Sindy Fraile, Gabriela Pataccini, Carolina Berini, and Mirna Biglione. 2015. "Low Proviral Load is Associated with Indeterminate Western Blot Patterns in Human T-Cell Lymphotropic Virus Type 1 Infected Individuals: Could Punctual Mutations be Related?" Viruses 7, no. 11: 5643-5658. https://doi.org/10.3390/v7112897
APA StyleCánepa, C., Salido, J., Ruggieri, M., Fraile, S., Pataccini, G., Berini, C., & Biglione, M. (2015). Low Proviral Load is Associated with Indeterminate Western Blot Patterns in Human T-Cell Lymphotropic Virus Type 1 Infected Individuals: Could Punctual Mutations be Related? Viruses, 7(11), 5643-5658. https://doi.org/10.3390/v7112897