Rabbit Hemorrhagic Disease Virus Detected in Pico, Azores, Portugal, Revealed a Unique Endemic Strain with More Than 17 Years of Independent Evolution
Abstract
:1. Introduction
2. Materials and Methods
3. Results
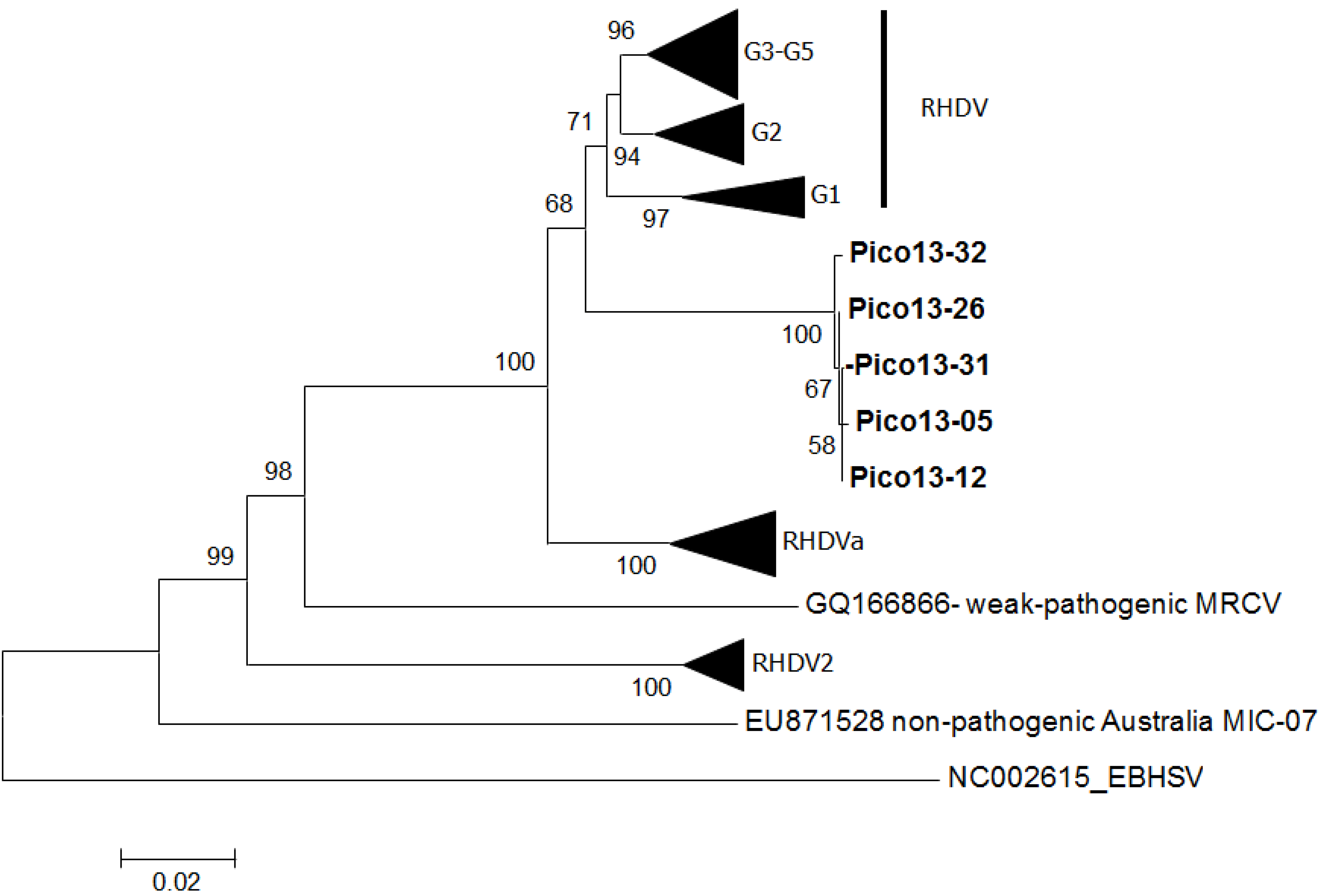
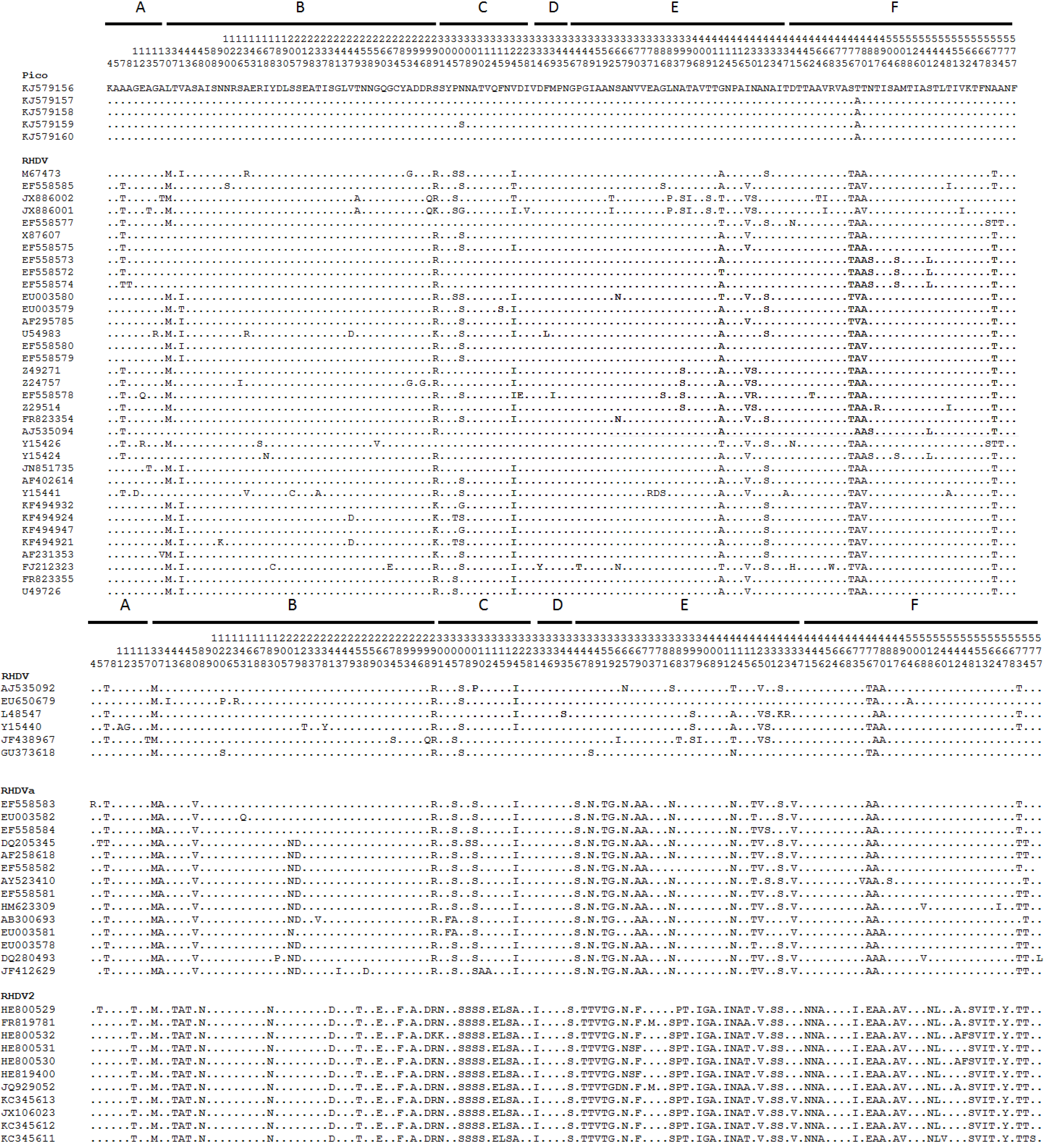
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Abrantes, J.; van der Loo, W.; le Pendu, J.; Esteves, P.J. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): A review. Vet. Res. 2012, 43, 12. [Google Scholar] [CrossRef]
- Correa, A.J. História do descobrimento das Ilhas dos açores e sua denominação de Ilhas Flamengas. In Revista Michaelense; S. Miguel: Açores, Portugal, 1918. [Google Scholar]
- Van der Loo, W.; Mougel, F.; Sánchez, M.S.; Bouton, C.; Castien, E.; Fonseca, A.; Ferrand, N.; Soriguer, R.; Monnerot, M. Cytonuclear disequilibria in wild populations of rabbit (Oryctolagus cuniculus L.) suggest unequal allele turnover rates at the b locus (IGKC1). Immunogenetics 1999, 49, 629–643. [Google Scholar] [CrossRef]
- Esteves, P.J.; Lanning, D.; Zhai, S.-K.; Ferrand, N.; Knight, K.L.; van der Loo, W. Allelic variation at the VHa locus in natural populations of rabbit (Oryctolagus cuniculus, L.). J. Immunol. 2004, 172, 1044–1053. [Google Scholar] [CrossRef]
- Ferrand, N. Inferring the evolutionary history of the European rabbit (Oryctolagus cuniculus) from molecular markers. In Lagomorph Biology: Evolution, Ecology, and Conservation, 1st ed.; Alves, P.C., Ferrand, N., Hackländer, K., Eds.; Springer: Berlin, Germany, 2008; pp. 47–63. [Google Scholar]
- Capucci, L.; Fusi, P.; Lavazza, A.; Pacciarini, M.L.; Rossi, C. Detection and preliminary characterization of a new rabbit calicivirus related to rabbit hemorrhagic disease virus but nonpathogenic. J. Virol. 1996, 70, 8614–8623. [Google Scholar]
- Strive, T.; Wright, J.D.; Robinson, A.J. Identification and partial characterisation of a new Lagovirus in Australian wild rabbits. Virology 2009, 384, 97–105. [Google Scholar] [CrossRef]
- Bergin, I.L.; Wise, A.G.; Bolin, S.R.; Mullaney, T.P.; Kiupel, M.; Maes, R.K. Novel calicivirus identified in rabbits, Michigan, USA. Emerg. Infect. Dis. 2009, 15, 1955–1962. [Google Scholar] [CrossRef]
- Abrantes, J.; Esteves, P.J. Not-so-novel michigan rabbit calicivirus. Emerg. Infect. Dis. 2010, 8, 1331–1332. [Google Scholar] [CrossRef]
- Le Gall-Reculé, G.; Zwingelstein, F.; Fages, M.P.; Bertagnoli, S.; Gelfi, J.; Aubineau, J.; Roobrouck, A.; Botti, G.; Lavazza, A.; Marchandeau, S. Characterisation of a non-pathogenic and non-protective infectious rabbit lagovirus related to RHDV. Virology 2011, 410, 395–402. [Google Scholar] [CrossRef]
- Le Gall-Reculé, G.; Zwingelstein, F.; Laurent, S.; de Boisséson, C.; Portejoie, Y.; Rasschaert, D. Phylogenetic analysis of rabbit haemorrhagic disease virus in France between 1993 and 2000, and the characterisation of RHDV antigenic variants. Arch. Virol. 2003, 148, 65–81. [Google Scholar] [CrossRef]
- Capucci, L.; Fallacara, F.; Grazioli, S.; Lavazza, A.; Pacciarini, M.L.; Brocchi, E. A further step in the evolution of rabbit hemorrhagic disease virus: The appearance of the first consistent antigenic variant. Virus Res. 1998, 58, 115–126. [Google Scholar] [CrossRef]
- Le Gall-Reculé, G.; Zwingelstein, F.; Boucher, S.; le Normand, B.; Plassiart, G.; Portejoie, Y.; Decors, A.; Bertagnoli, S.; Guérin, J.L.; Marchandeau, S. Detection of a new variant of rabbit haemorrhagic disease virus in France. Vet. Rec. 2011, 168, 137–138. [Google Scholar]
- Dalton, K.P.; Nicieza, I.; Balseiro, A.; Muguerza, M.A.; Rosell, J.M.; Casais, R.; Álvarez, Á.L.; Parra, F. Variant rabbit hemorrhagic disease virus in young rabbits, Spain. Emerg. Infect. Dis. 2012, 18, 2009–2012. [Google Scholar] [CrossRef]
- Abrantes, J.; Lopes, A.M.; Dalton, K.P.; Melo, P.; Correia, J.J.; Ramada, M.; Alves, P.C.; Parra, F.; Esteves, P.J. New variant of rabbit hemorrhagic disease virus, Portugal, 2012–2013. Emerg. Infect. Dis. 2013, 19, 1900–1902. [Google Scholar]
- Le Gall-Reculé, G.; Lavazza, A.; Marchandeau, S.; Bertagnoli, S.; Zwingelstein, F.; Cavadini, P.; Martinelli, N.; Lombardi, G.; Guérin, J.L.; Lemaitre, E.; et al. Emergence of a new lagovirus related to Rabbit Haemorrhagic Disease Virus. Vet. Res. 2013, 44, 81. [Google Scholar] [CrossRef]
- Muller, A.; Freitas, J.; Silva, E.; le Gall-Reculé, G.; Zwingelstein, F.; Abrantes, J.; Esteves, P.J.; Alves, P.C.; van der Loo, W.; Kolodziejek, J.; et al. Evolution of rabbit haemorrhagic disease virus (RHDV) in the European rabbit (Oryctolagus cuniculus) from the Iberian Peninsula. Vet. Microbiol. 2009, 135, 368–373. [Google Scholar] [CrossRef]
- Alda, F.; Gaitero, T.; Suárez, M.; Merchán, T.; Rocha, G.; Doadrio, I. Evolutionary history and molecular epidemiology of rabbit haemorrhagic disease virus in the Iberian Peninsula and Western Europe. BMC Evol. Biol. 2010, 10, 347. [Google Scholar] [CrossRef]
- Abrantes, J.; Lopes, A.M.; Esteves, P.J. Complete genomic sequences of rabbit hemorrhagic disease virus G1 strains isolated in the European rabbit original range. J. Virol. 2012, 86, 13886. [Google Scholar]
- Dalton, K.P.; Nicieza, I.; Abrantes, J.; Esteves, P.J.; Parra, F. Spread of new variant RHDV in domestic rabbits on the Iberian Peninsula. Vet. Microbiol. 2014, 169, 67–73. [Google Scholar] [CrossRef]
- Calvete, C.; Sarto, P.; Calvo, A.J.; Maonroy, F.; Calvo, J.H. Could the new rabbit haemorrhagic disease virus variant (RHDVb) be fully replacing classical RHD strains in the Iberian Peninsula? World Rabbit Sci. 2014, 22, 91. [Google Scholar] [CrossRef]
- Carvalho, G. Contribuição para o estudo duma população de coelhos selvagens Oryctolagus cuniculuss na ilha de Sta Maria e o impacto do RVHD na população local; Relatórios e comunicaçãoes do Departamento de Biologia, Universidade dos Açores: Ponta Delgada, Portugal, 1992; Volume 19; pp. 61–67. [Google Scholar]
- Carvalho, G.; Ferrand, N.; Fonseca, A.; Branco, M.; Azevedo, M.; Mendes, R.; Batista, P.; Mântua, P. Estudo de uma população de coelhos selvagens, Oryctolagus cuniculus, (L.), na ilha de S. Jorge-Açores; Relatórios e comunicaçãoes do Departamento de Biologia, Universidade dos Açores: Ponta Delgada, Portugal, 1994; Volume 21; pp. 8–20. [Google Scholar]
- Carvalho, G.; Fonseca, A.; Cruz, A.; Célio, P.; Mantua, P.; Simões, C.; Silva, S.; Arruda, G. Estudo preliminar de alguns parâmetros de uma população de coelho selvagem (Oryctolagus cuniculus) da ilha do Faial-Açores; Relatórios e comunicaçãoes do Departamento de Biologia, Universidade dos Açores: Ponta Delgada, Portugal, 1994; Volume 22; pp. 49–60. [Google Scholar]
- Abrantes, J.; Esteves, P.J.; van der Loo, W. Evidence for recombination in the major capsid gene VP60 of the rabbit haemorrhagic disease virus (RHDV). Arch. Virol. 2008, 153, 329–335. [Google Scholar] [CrossRef]
- Forrester, N.L.; Moss, S.R.; Turner, S.L.; Schirrmeier, H.; Gould, E.A. Recombination in Rabbit haemorrhagic disease virus; possible impact on evolution and epidemiology. Virology 2008, 376, 390–399. [Google Scholar] [CrossRef]
- Martin, D.P.; Lemey, P.; Lott, M.; Moulton, V.; Posada, D.; Lefeuvre, P. RDP3: A flexible and fast computer program for analyzing recombination. Bioinformatics 2010, 26, 2462–2463. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Neill, J.D. Nucleotide sequence of the capsid protein gene of two serotypes of San Miguel sea lion virus: Identification of conserved and non-conserved amino acid sequences among calicivirus capsid proteins. Virus Res. 1992, 24, 211–222. [Google Scholar] [CrossRef]
- Esteves, P.J.; Abrantes, J.; Carneiro, M.; Muller, A.; Thompson, G.; van der Loo, W. Detection of positive selection in the major capsid protein VP60 of the rabbit haemorrhagic disease virus (RHDV). Virus Res. 2008, 137, 253–256. [Google Scholar] [CrossRef]
- Kovaliski, J.; Sinclair, R.; Mutze, G.; Peacock, D.; Strive, T.; Abrantes, J.; Esteves, P.J.; Holmes, E.C. Molecular epidemiology of Rabbit Haemorrhagic Disease Virus in Australia: When one became many. Mol. Ecol. 2013. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Esteves, P.J.; Lopes, A.M.; Magalhães, M.J.; Pinheiro, A.; Gonçalves, D.; Abrantes, J. Rabbit Hemorrhagic Disease Virus Detected in Pico, Azores, Portugal, Revealed a Unique Endemic Strain with More Than 17 Years of Independent Evolution. Viruses 2014, 6, 2698-2707. https://doi.org/10.3390/v6072698
Esteves PJ, Lopes AM, Magalhães MJ, Pinheiro A, Gonçalves D, Abrantes J. Rabbit Hemorrhagic Disease Virus Detected in Pico, Azores, Portugal, Revealed a Unique Endemic Strain with More Than 17 Years of Independent Evolution. Viruses. 2014; 6(7):2698-2707. https://doi.org/10.3390/v6072698
Chicago/Turabian StyleEsteves, Pedro J., Ana M. Lopes, Maria J. Magalhães, Ana Pinheiro, David Gonçalves, and Joana Abrantes. 2014. "Rabbit Hemorrhagic Disease Virus Detected in Pico, Azores, Portugal, Revealed a Unique Endemic Strain with More Than 17 Years of Independent Evolution" Viruses 6, no. 7: 2698-2707. https://doi.org/10.3390/v6072698
APA StyleEsteves, P. J., Lopes, A. M., Magalhães, M. J., Pinheiro, A., Gonçalves, D., & Abrantes, J. (2014). Rabbit Hemorrhagic Disease Virus Detected in Pico, Azores, Portugal, Revealed a Unique Endemic Strain with More Than 17 Years of Independent Evolution. Viruses, 6(7), 2698-2707. https://doi.org/10.3390/v6072698







