Development and Application of Quantitative Detection Method for Viral Hemorrhagic Septicemia Virus (VHSV) Genogroup IVa
Abstract
:1. Introduction
2. Results and Discussion
2.1. Plasmid Construction for Standard and Primer Efficiency
2.2. Comparison of qRT-PCR and TCID50 Method (In Vitro)
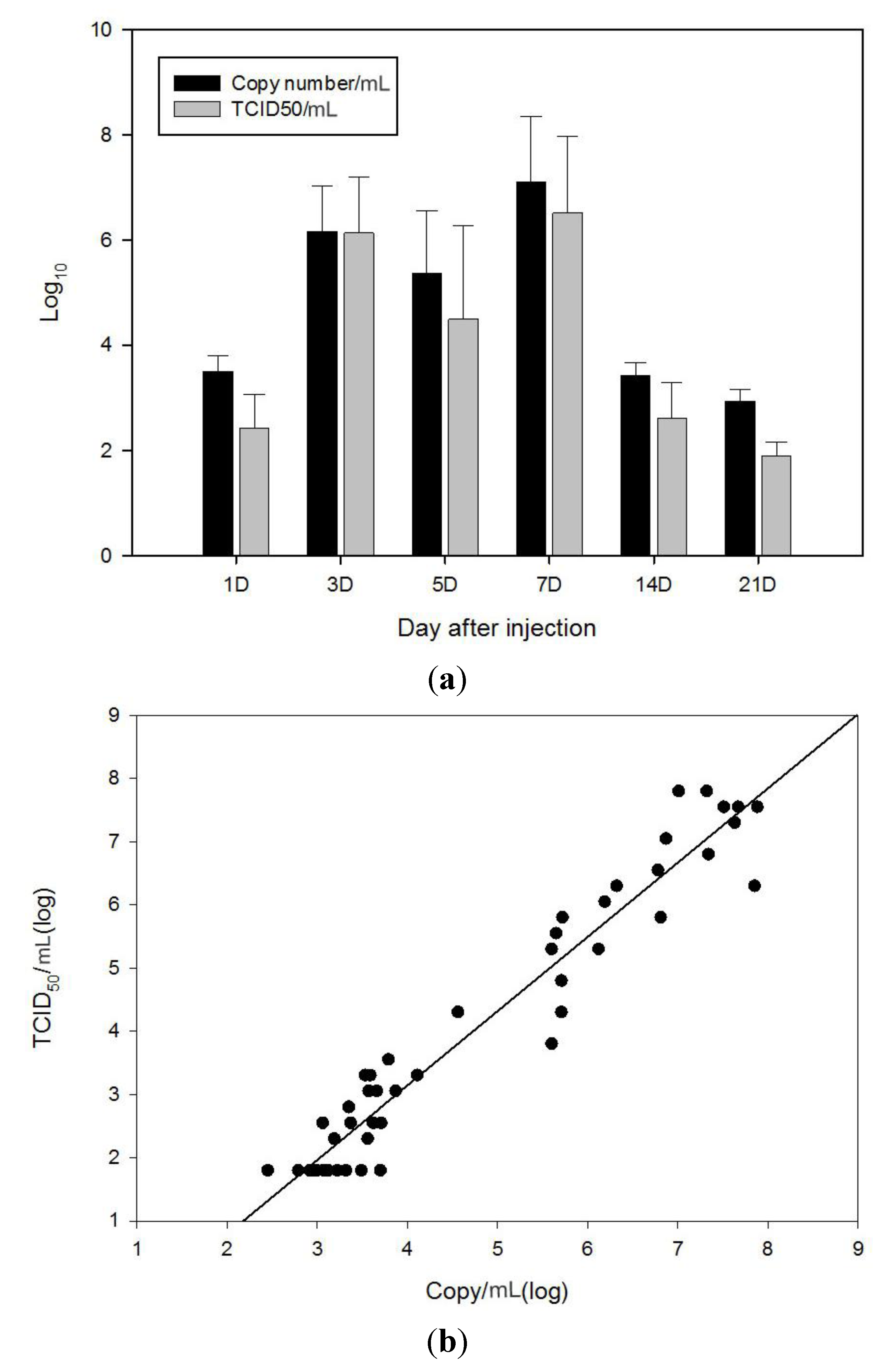
2.3. Comparison of qRT-PCR and TCID50 Method with VHSV Challenged Olive Flounder (In Vivo)
3. Experimental Section
3.1. Virus and Cells
3.2. Viral RNA Extraction and cDNA Synthesis
3.3. Gene Cloning
3.4. Quantitative RT-PCR (qRT-PCR)
3.5. Comparison qRT-PCR and TCID50 Method (In Vitro)
3.6. Comparison of qRT-PCR and TCID50 Method with VHSV Challenged Olive Flounder (In Vivo)
4. Conclusions
Supplementary Files
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Kim, S.M.; Lee, J.I.; Hong, M.J.; Park, H.S.; Park, S.I. Genetic relationship of the VHSV (viral hemorrhagic septicemia virus) isolated from cultured olive flounder, Paralichthys olivaceus in Korea. J. Fish Pathol. 2003, 16, 1–12. [Google Scholar]
- Kim, W.S.; Kim, S.R.; Kim, D.W.; Kim, J.O.; Park, M.A.; Kitamura, S.I.; Kim, H.Y.; Kim, D.H.; Han, H.J.; Jung, S.J.; et al. An outbreak of VHSV (viral haemorrhagic septicaemia virus) infection in farmed olive flounder Paralichthys olivaceus in Korea. Aquaculture 2009, 296, 165–168. [Google Scholar] [CrossRef]
- Kim, W.S.; Jung, S.J.; Kim, J.O.; Kim, D.W.; Kim, J.H.; Oh, M.J. Genetic positioning of Korean viral hemorrhagic septicemia virus (VHSV) from cultured and wild marine fishes. J. Fish Pathol. 2011, 24, 1–9. [Google Scholar] [CrossRef]
- Isshiki, I.; Nishizawa, T.; Kobayashi, T.; Nagano, T.; Miyzaki, T. An outbreak of VHSV (viral haemorrhagic septicemia virus) infection in farmed Japanese flounder Paralichthys olivaceus in Japan. Dis. Aquat. Org. 2001, 47, 87–99. [Google Scholar] [CrossRef]
- Skall, H.F.; Olesen, N.J.; Mellergaard, S. Viral haemorrhagic septicaemia virus in marine fish and its implications for fish farming—A review. J. Fish Dis. 2005, 28, 509–529. [Google Scholar] [CrossRef]
- Smail, A.; Snow, M. Viral haemorrhagic septicaemia. In Fish Disease and Disorders, 2nd ed.; Woo, P.T.K., et Bruno, D.W., Eds.; CAB International: New York, NY, USA, 2011; Volume 3, pp. 110–142. [Google Scholar]
- Wolf, K. Viral Hemorrhagic Septicemia Virus, Fish Viruses and Fish Viral Diseases; Cornell University Press: Ithaca, NY, USA, 1988; pp. 217–249. [Google Scholar]
- Trdo, N.; Benmansour, A.; Calisher, C.; Dietzgen, R.G.; Fang, R.X.; Jackson, A.O.; Kurath, G.; Nadin-Davis, S.; Tesh, R.B.; Walker, P.J. Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses; Elsevier/Academic Press: London, UK, 2005; pp. 623–644. [Google Scholar]
- Brunson, R.; True, K.; Yancey, J. VHS virus isolated at Makah National Fish Hatchery. Am. Fish Soc. Newsl. 1989, 17, 3–4. [Google Scholar]
- Hopper, K. The isolation of VHSV from Chinook salmon at Glenwood Springs, Orcas Island, Washington. Am. Fish Soc. Newsl. 1989, 17, 1–2. [Google Scholar]
- Meyers, T.R.; Winton, J.R. Viral hemorrhagic septicemia in North America. Annu. Rev. Fish Dis. 1995, 5, 3–24. [Google Scholar] [CrossRef]
- Meyers, T.R.; Short, S.; Lipson, K. Isolation of the North American strain of viral hemorrhagic septicemia virus (VHSV) associated with epizootic mortality in two new host species of Alaskan marine fish. Dis. Aquat. Org. 1999, 38, 81–86. [Google Scholar] [CrossRef]
- Mortensen, H.F.; Heuer, O.E.; Lorenzen, N.; Otte, L.; Olesen, N.J. Isolation of viral haemorrhagic septicaemia virus (VHSV) from wild marine fish species in Baltic Sea, Kattegat, Skagerrak and the North Sea. Virus Res. 1999, 63, 95–106. [Google Scholar]
- Nishizawa, T.; Iida, H.; Takano, R.; Isshiki, T.; Nakajima, K.; Muroga, K. Genetic relatedness among Japanese, American and European isolates of viral hemorrhagic septicemia virus (VHSV) based on partial G and P genes. Dis. Aquat. Org. 2002, 48, 143–148. [Google Scholar] [CrossRef]
- Schlotfeldt, H.J.; Ahne, W.; Jorgensen, P.E.V.; Glende, W. Occurrence of viral haemorrhagic septicaemia in turbot (Scophthalmus maximus)—A natural outbreak. Bull. Eur. Assoc. Fish Pathol. 1991, 11, 105–107. [Google Scholar]
- Takano, R.; Nishizawa, T.; Arimoto, M.; Muroga, K. Isolation of viral hemorrhagic septicemia virus (VHSV) from wild Japanese flounder, Paralichthys olivaceus. Bull. Eur. Assoc. Fish Pathol. 2000, 20, 186–193. [Google Scholar]
- OIE. Manual of Diagnostic Tests for Aquatic Animals. Viral Hemorrhagic Septicemia Virus (VHSV); Office International des Eizooties: Paris, France, 2009. [Google Scholar]
- Kim, J.O.; Kim, W.S.; Nishizawa, T.; Oh, M.J. Complete genome sequence of viral hemorrhagic septicemia virus isolated from an olive flounder in South Korea. Genome Announc. 2013, 1. [Google Scholar] [CrossRef]
- Mackay, I.M. Real-time PCR in the microbiology laboratory. Clin. Microbiol. Infec. 2004, 10, 190–212. [Google Scholar] [CrossRef]
- Bland, F.; Snow, M.; Garver, K.A.; Matejusova, I. Genotype-specific Taqman(®) assays for the detection and rapid characterisation of European strains of viral haemorrhagic septicaemia virus. J. Virol. Methods 2012, 187, 209–214. [Google Scholar]
- Jonstrup, S.P.; Kahns, S.; Skall, H.F.; Boutrup, T.S.; Olesen, N.J. Development and validation of a novel Taqman-based real-time RT-PCR assay suitable for demonstrating freedom from viral haemorrhagic septicaemia virus. J. Fish Dis. 2013, 36, 9–23. [Google Scholar] [CrossRef]
- Hope, K.; Casey, R.; Groocock, G. Comparison of quantitative RT-PCR with cell culture to detect viral hemorrhagic septicemia virus (VHSV) IVb infections in the Great Lakes. J. Aquat. Anim. Health. 2010, 22, 50–61. [Google Scholar] [CrossRef]
- Cornwell, E.R.; Eckerlin, G.E.; Getchell, R.G.; Groocock, G.H.; Thompson, T.M.; Batts, W.N.; Casey, R.N.; Kurath, G.; Winton, J.R.; Bowser, P.R.; et al. Detection of viral hemorrhagic septicemia virus by quantitative reverse transcription polymerase chain reaction from two fish species at two sites in Lake Superior. J. Aquat. Anim. Health. 2011, 23, 207–217. [Google Scholar] [CrossRef]
- Pierce, L.R.; Willey, J.C.; Crawford, E.L.; Palsule, V.V.; Leaman, D.W.; Faisal, M.; Kim, R.K.; Shepherd, B.S.; Stanoszek, L.M.; Stepien, C.A. A new StaRT-PCR approach to detect and quantify fish Viral Hemorrhagic Septicemia virus (VHSv): Enhanced quality control with internal standards. J. Virol. Methods 2013, 189, 129–142. [Google Scholar] [CrossRef]
- Primer3Plus. Available online: http://primer3plus.com/cgi-bin/dev/primer3plus.cgi/ (accessed on 10 July 2012).
- Reed, L.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidem. 1938, 27, 493–497. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, J.-O.; Kim, W.-S.; Kim, S.-W.; Han, H.-J.; Kim, J.W.; Park, M.A.; Oh, M.-J. Development and Application of Quantitative Detection Method for Viral Hemorrhagic Septicemia Virus (VHSV) Genogroup IVa. Viruses 2014, 6, 2204-2213. https://doi.org/10.3390/v6052204
Kim J-O, Kim W-S, Kim S-W, Han H-J, Kim JW, Park MA, Oh M-J. Development and Application of Quantitative Detection Method for Viral Hemorrhagic Septicemia Virus (VHSV) Genogroup IVa. Viruses. 2014; 6(5):2204-2213. https://doi.org/10.3390/v6052204
Chicago/Turabian StyleKim, Jong-Oh, Wi-Sik Kim, Si-Woo Kim, Hyun-Ja Han, Jin Woo Kim, Myoung Ae Park, and Myung-Joo Oh. 2014. "Development and Application of Quantitative Detection Method for Viral Hemorrhagic Septicemia Virus (VHSV) Genogroup IVa" Viruses 6, no. 5: 2204-2213. https://doi.org/10.3390/v6052204
APA StyleKim, J.-O., Kim, W.-S., Kim, S.-W., Han, H.-J., Kim, J. W., Park, M. A., & Oh, M.-J. (2014). Development and Application of Quantitative Detection Method for Viral Hemorrhagic Septicemia Virus (VHSV) Genogroup IVa. Viruses, 6(5), 2204-2213. https://doi.org/10.3390/v6052204



