Hantavirus Gn and Gc Envelope Glycoproteins: Key Structural Units for Virus Cell Entry and Virus Assembly
Abstract
:1. Introduction
2. Structure of the Hantavirus Glycoproteins
3. Roles of the Glycoproteins during Virus Cell Entry
3.1. Interaction of the Glycoproteins with Cellular Receptors
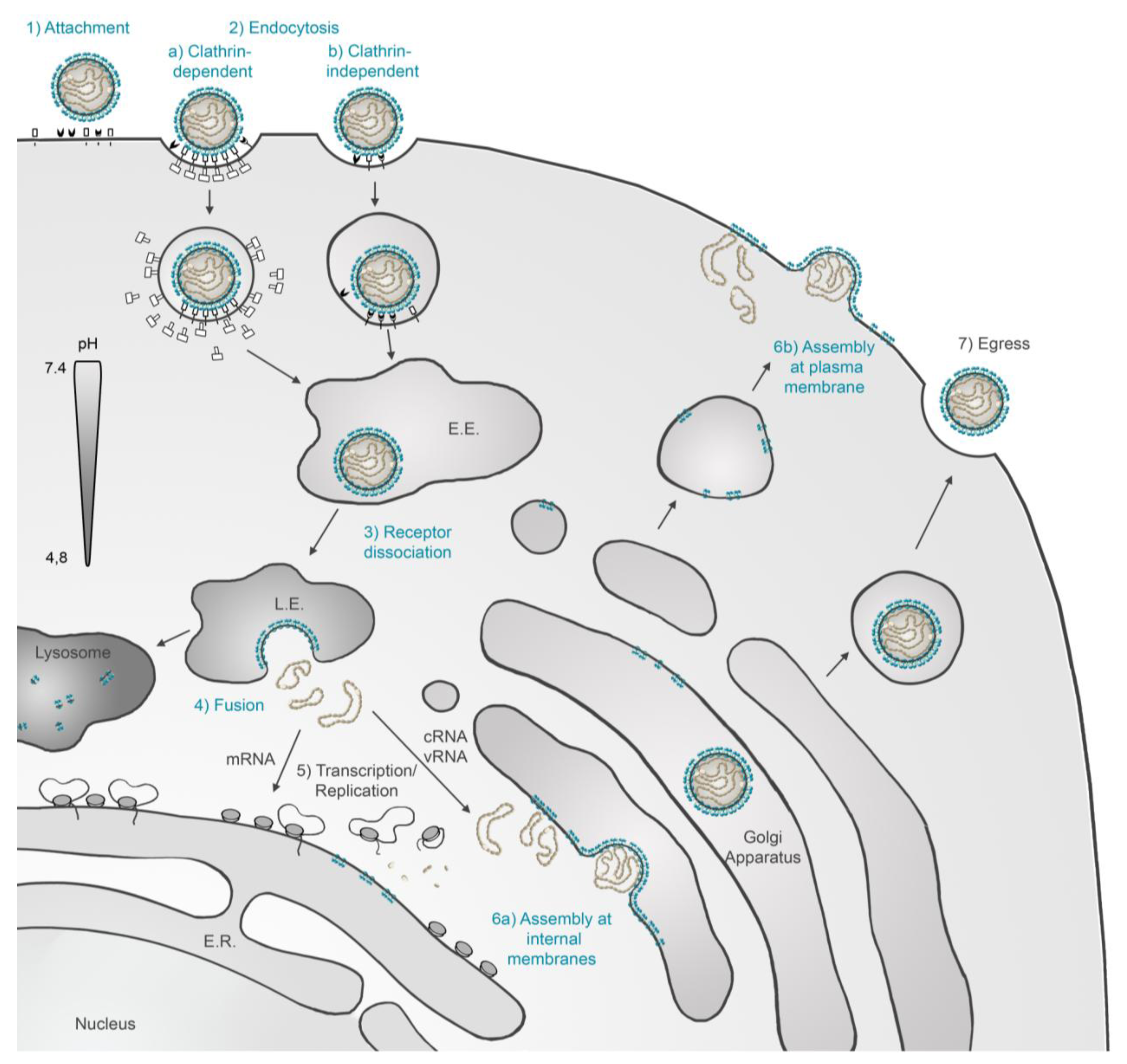
3.2. Endocytic Uptake and Pathways of Hantaviruses
3.3. Glycoprotein-Mediated Membrane Fusion
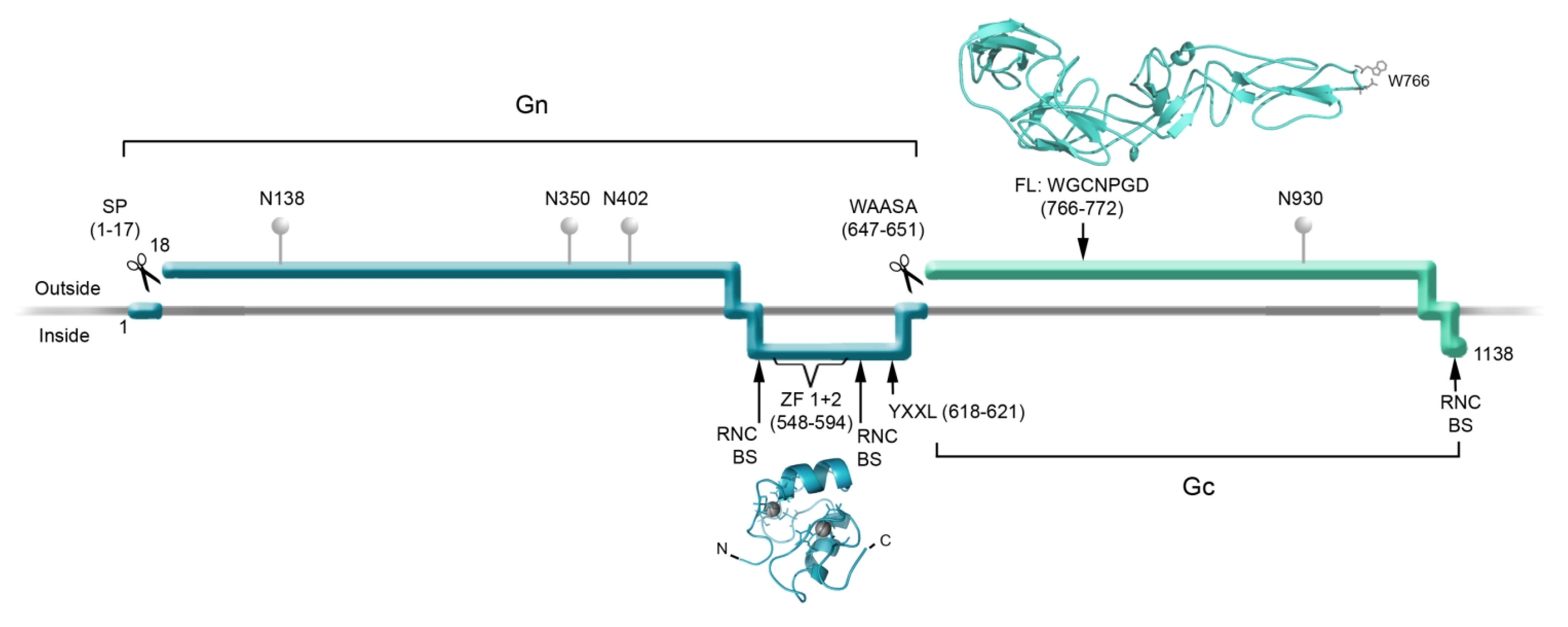
4. Biogenesis of the Viral Glycoproteins
5. Roles of the Glycoproteins during Virus Cell Exit
5.1. Interactions of the Glycoproteins with Internal Viral Components
5.2. Roles of the Glycoproteins during Virus Budding
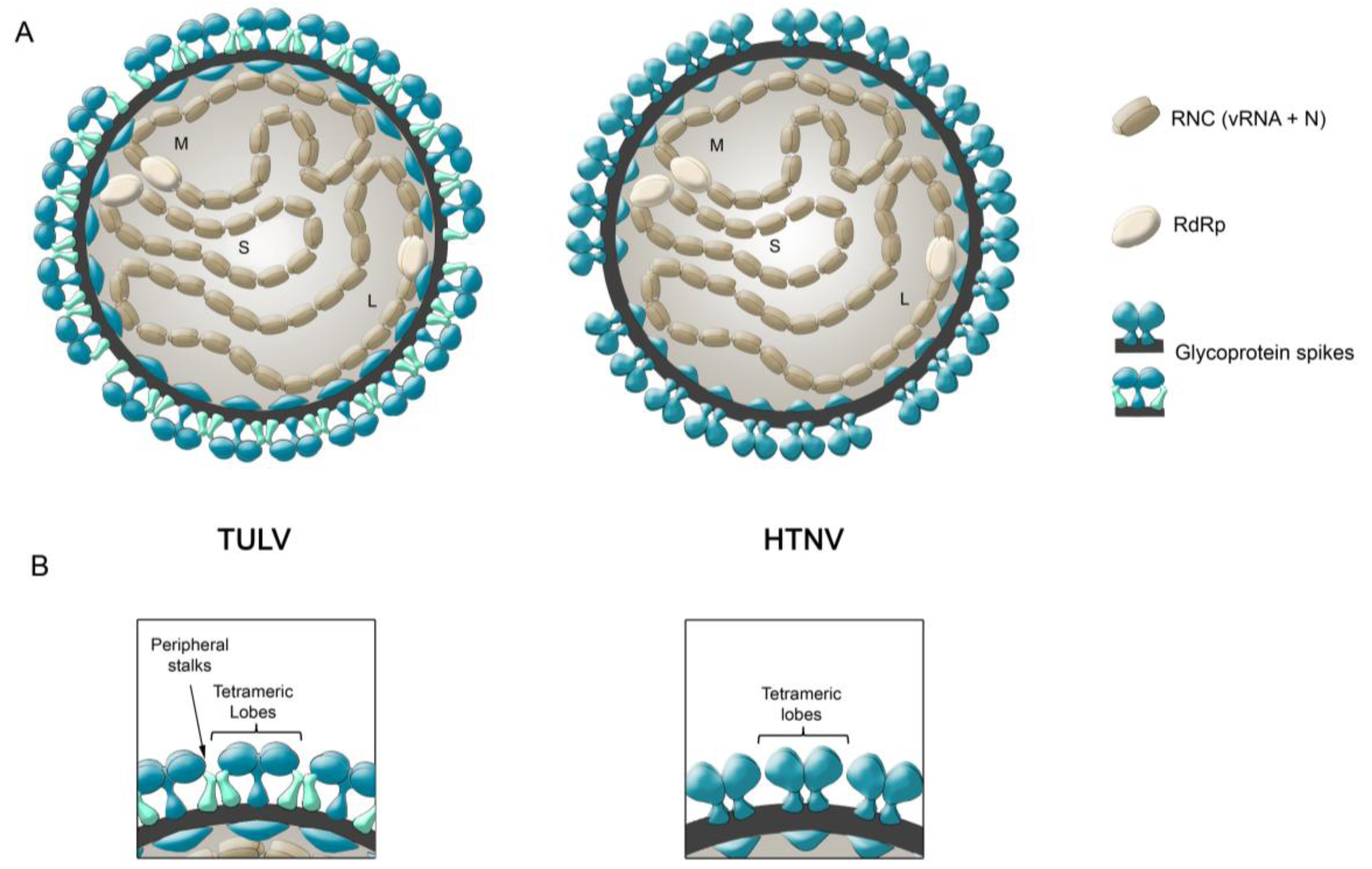
6. Glycoprotein Arrangements on Hantavirus Particles
- (i)
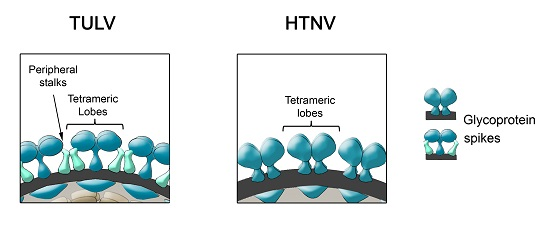
- During Gn and Gc biogenesis, the glycoproteins seem to require hetero-oligomerization in order to exit the ER (see above). Before entering the secretory pathway, fusion proteins must shield their fusion peptides; this is usually achieved through intermolecular interactions [58]. In the case of class II fusion proteins this interaction occurs with a companion protein [118]. Hence, to hide the fusion loop peptide of Gc, it is likely that this region interacts with Gn; alternatively it may interact with another Gc molecule in a juxtaposed position. In this regard, it is interesting to note that the residue substitution D121N, located in the fusion loop candidate of ANDV Gc, abrogates its incorporation onto lentivirus particles [31]. It can be speculated that this substitution may impede the intermolecular interactions of the fusion loop with Gn or Gc, which may be involved in the Gn/Gc quaternary assembly. In the context of the spike arrangements, it is therefore more likely that Gc forms part of the more buried, peripheral stalks. However, the volume of the electronic density occupied by the peripheral stalks seems to be too small to accommodate a class II fusion protein. Hence, if hantavirus Gc is arranged in a “class II fold”, then it must occupy also part of the electron density corresponding to the tetrameric lobes
- (ii)
- It has been known for a long time that Gn forms SDS-resistant oligomers, even under reducing conditions [71,83,84,85]. Using reducing agents and cross-linking techniques, Hepojoki and colleagues [71] demonstrated that one of the Gn oligomeric arrangements corresponds to a tetramer. If, the most exposed tetrameric lobes on the virus surface, thus correspond to Gn tetramers, then Gn may in turn correspond to the protein that establishes the first interactions with cell surface molecules during virus attachment to the c
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Elliott, R.M. Molecular biology of the Bunyaviridae. J. Gen. Virol. 1990, 71, 501–522. [Google Scholar]
- Jonsson, C.B.; Figueiredo, L.T.; Vapalahti, O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 2010, 23, 412–441. [Google Scholar]
- Krautkramer, E.; Zeier, M.; Plyusnin, A. Hantavirus infection: An emerging infectious disease causing acute renal failure. Kidney Int. 2013, 83, 23–27. [Google Scholar] [CrossRef]
- Macneil, A.; Nichol, S.T.; Spiropoulou, C.F. Hantavirus pulmonary syndrome. Virus Res. 2011, 162, 138–147. [Google Scholar] [CrossRef]
- Welsch, S.; Muller, B.; Krausslich, H.G. More than one door—Budding of enveloped viruses through cellular membranes. FEBS Lett. 2007, 581, 2089–2097. [Google Scholar] [CrossRef]
- Lyles, D.S. Assembly and budding of negative-strand RNA viruses. Adv. Virus Res. 2013, 85, 57–90. [Google Scholar]
- Yamauchi, Y.; Helenius, A. Virus entry at a glance. J. Cell Sci. 2013, 126, 1289–1295. [Google Scholar] [CrossRef]
- Marsh, M.; Helenius, A. Virus entry: Open sesame. Cell 2006, 124, 729–740. [Google Scholar] [CrossRef]
- Smith, A.E.; Helenius, A. How viruses enter animal cells. Science 2004, 304, 237–242. [Google Scholar] [CrossRef]
- Lee, H.W.; Cho, H.J. Electron microscope appearance of Hantaan virus, the causative agent of Korean haemorrhagic fever. Lancet 1981, 1, 1070–1072. [Google Scholar] [CrossRef]
- McCormick, J.B.; Sasso, D.R.; Palmer, E.L.; Kiley, M.P. Morphological identification of the agent of Korean haemorrhagic fever (Hantaan virus) as a member of the Bunyaviridae. Lancet 1982, 1, 765–768. [Google Scholar]
- Elliott, L.H.; Ksiazek, T.G.; Rollin, P.E.; Spiropoulou, C.F.; Morzunov, S.; Monroe, M.; Goldsmith, C.S.; Humphrey, C.D.; Zaki, S.R.; Krebs, J.W.; et al. Isolation of the causative agent of hantavirus pulmonary syndrome. Am. J. Trop. Med. Hyg. 1994, 51, 102–108. [Google Scholar]
- Schmaljohn, C.S.; Hasty, S.E.; Harrison, S.A.; Dalrymple, J.M. Characterization of Hantaan virions, the prototype virus of hemorrhagic fever with renal syndrome. J. Infect. Dis. 1983, 148, 1005–1012. [Google Scholar] [CrossRef]
- Martin, M.L.; Lindsey-Regnery, H.; Sasso, D.R.; McCormick, J.B.; Palmer, E. Distinction between Bunyaviridae genera by surface structure and comparison with Hantaan virus using negative stain electron microscopy. Arch. Virol. 1985, 86, 17–28. [Google Scholar] [CrossRef]
- Goldsmith, C.S.; Elliott, L.H.; Peters, C.J.; Zaki, S.R. Ultrastructural characteristics of Sin Nombre virus, causative agent of hantavirus pulmonary syndrome. Arch. Virol. 1995, 140, 2107–2122. [Google Scholar] [CrossRef]
- Battisti, A.J.; Chu, Y.K.; Chipman, P.R.; Kaufmann, B.; Jonsson, C.B.; Rossmann, M.G. Structural studies of Hantaan virus. J. Virol. 2011, 85, 835–841. [Google Scholar] [CrossRef]
- Huiskonen, J.T.; Hepojoki, J.; Laurinmaki, P.; Vaheri, A.; Lankinen, H.; Butcher, S.J.; Grunewald, K. Electron cryotomography of Tula hantavirus suggests a unique assembly paradigm for enveloped viruses. J. Virol. 2010, 84, 4889–4897. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Freiberg, A.N.; Sherman, M.B.; Morais, M.C.; Holbrook, M.R.; Watowich, S.J. Three-dimensional organization of Rift Valley fever virus revealed by cryoelectron tomography. J. Virol. 2008, 82, 10341–10348. [Google Scholar]
- Overby, A.K.; Pettersson, R.F.; Grunewald, K.; Huiskonen, J.T. Insights into bunyavirus architecture from electron cryotomography of Uukuniemi virus. Proc. Natl. Acad. Sci. USA 2008, 105, 2375–2379. [Google Scholar]
- Sherman, M.B.; Freiberg, A.N.; Holbrook, M.R.; Watowich, S.J. Single-particle cryo-electron microscopy of Rift Valley fever virus. Virology 2009, 387, 11–15. [Google Scholar] [CrossRef]
- Huiskonen, J.T.; Overby, A.K.; Weber, F.; Grunewald, K. Electron cryo-microscopy and single-particle averaging of Rift Valley fever virus: Evidence for GN-GC glycoprotein heterodimers. J. Virol. 2009, 83, 3762–3769. [Google Scholar] [CrossRef]
- Bowden, T.A.; Bitto, D.; McLees, A.; Yeromonahos, C.; Elliott, R.M.; Huiskonen, J.T. Orthobunyavirus ultrastructure and the curious tripodal glycoprotein spike. PLoS Pathog. 2013, 9, e1003374. [Google Scholar] [CrossRef]
- Estrada, D.F.; Boudreaux, D.M.; Zhong, D.; St Jeor, S.C.; de Guzman, R.N. The hantavirus glycoprotein G1 tail contains dual CCHC-type classical zinc fingers. J. Biol. Chem. 2009, 284, 8654–8660. [Google Scholar]
- Estrada, D.F.; Conner, M.; Jeor, S.C.; Guzman, R.N. The structure of the hantavirus zinc finger domain is conserved and represents the only natively folded region of the Gn cytoplasmic tail. Front. Microbiol. 2011, 2, e251. [Google Scholar]
- Estrada, D.F.; de Guzman, R.N. Structural characterization of the Crimean-Congo hemorrhagic fever virus Gn tail provides insight into virus assembly. J. Biol. Chem. 2011, 286, 21678–21686. [Google Scholar] [CrossRef]
- Dessau, M.; Modis, Y. Crystal structure of glycoprotein C from Rift Valley fever virus. Proc. Natl. Acad. Sci. USA 2013, 110, 1696–1701. [Google Scholar] [CrossRef]
- Garry, C.E.; Garry, R.F. Proteomics computational analyses suggest that the carboxyl terminal glycoproteins of Bunyaviruses are class II viral fusion protein (beta-penetrenes). Theor. Biol. Med. Model. 2004, 1. [Google Scholar] [CrossRef]
- Tischler, N.D.; Gonzalez, A.; Perez-Acle, T.; Rosemblatt, M.; Valenzuela, P.D. Hantavirus Gc glycoprotein: Evidence for a class II fusion protein. J. Gen. Virol. 2005, 86, 2937–2947. [Google Scholar] [CrossRef]
- Plassmeyer, M.L.; Soldan, S.S.; Stachelek, K.M.; Martin-Garcia, J.; Gonzalez-Scarano, F. California serogroup Gc (G1) glycoprotein is the principal determinant of pH-dependent cell fusion and entry. Virology 2005, 338, 121–132. [Google Scholar] [CrossRef]
- Cifuentes-Muñoz, N.; Barriga, G.P.; Valenzuela, P.D.T.; Tischler, N.D. Aromatic and polar residues spanning the candidate fusion peptide of Andes virus are essential for membrane fusion and Infection. J. Gen. Virol. 2011, 92, 552–563. [Google Scholar] [CrossRef]
- Vaney, M.C.; Rey, F.A. Class II enveloped viruses. Cell. Microbiol. 2011, 13, 1451–1459. [Google Scholar] [CrossRef]
- Rey, F.A.; Heinz, F.X.; Mandl, C.; Kunz, C.; Harrison, S.C. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 1995, 375, 291–298. [Google Scholar] [CrossRef]
- Kielian, M. Class II virus membrane fusion proteins. Virology 2006, 344, 38–47. [Google Scholar] [CrossRef]
- DuBois, R.M.; Vaney, M.C.; Tortorici, M.A.; Kurdi, R.A.; Barba-Spaeth, G.; Krey, T.; Rey, F.A. Functional and evolutionary insight from the crystal structure of rubella virus protein E1. Nature 2013, 493, 552–556. [Google Scholar] [CrossRef]
- Kielian, M.; Rey, F.A. Virus membrane-fusion proteins: More than one way to make a hairpin. Nat. Rev. Microbiol. 2006, 4, 67–76. [Google Scholar] [CrossRef]
- Grove, J.; Marsh, M. The cell biology of receptor-mediated virus entry. J. Cell Biol. 2011, 195, 1071–1082. [Google Scholar] [CrossRef]
- Gavrilovskaya, I.N.; Shepley, M.; Shaw, R.; Ginsberg, M.H.; Mackow, E.R. beta3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl. Acad. Sci. USA 1998, 95, 7074–7079. [Google Scholar]
- Gavrilovskaya, I.N.; Brown, E.J.; Ginsberg, M.H.; Mackow, E.R. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by beta3 integrins. J. Virol. 1999, 73, 3951–3959. [Google Scholar]
- Choi, Y.; Kwon, Y.C.; Kim, S.I.; Park, J.M.; Lee, K.H.; Ahn, B.Y. A hantavirus causing hemorrhagic fever with renal syndrome requires gC1qR/p32 for efficient cell binding and infection. Virology 2008, 381, 178–183. [Google Scholar] [CrossRef]
- Krautkramer, E.; Zeier, M. Hantavirus causing hemorrhagic fever with renal syndrome enters from the apical surface and requires decay-accelerating factor (DAF/CD55). J. Virol. 2008, 82, 4257–4264. [Google Scholar] [CrossRef]
- Kim, T.Y.; Choi, Y.; Cheong, H.S.; Choe, J. Identification of a cell surface 30 kDa protein as a candidate receptor for Hantaan virus. J. Gen. Virol. 2002, 83, 767–773. [Google Scholar]
- Mou, D.L.; Wang, Y.P.; Huang, C.X.; Li, G.Y.; Pan, L.; Yang, W.S.; Bai, X.F. Cellular entry of Hantaan virus A9 strain: Specific interactions with beta3 integrins and a novel 70 kDa protein. Biochem. Biophys. Res. Commun. 2006, 339, 611–617. [Google Scholar] [CrossRef]
- Matthys, V.S.; Gorbunova, E.E.; Gavrilovskaya, I.N.; Mackow, E.R. Andes virus recognition of human and Syrian hamster beta3 integrins is determined by an L33P substitution in the PSI domain. J. Virol. 2010, 84, 352–360. [Google Scholar] [CrossRef]
- Raymond, T.; Gorbunova, E.; Gavrilovskaya, I.N.; Mackow, E.R. Pathogenic hantaviruses bind plexin-semaphorin-integrin domains present at the apex of inactive, bent alphavbeta3 integrin conformers. Proc. Natl. Acad. Sci. USA 2005, 102, 1163–1168. [Google Scholar] [CrossRef]
- Simon, M.; Johansson, C.; Mirazimi, A. Crimean-Congo hemorrhagic fever virus entry and replication is clathrin-, pH- and cholesterol-dependent. J. Gen. Virol. 2009, 90, 210–215. [Google Scholar] [CrossRef]
- Cifuentes-Muñoz, N.; Darlix, J.L.; Tischler, N.D. Development of a lentiviral vector system to study the Andes virus glycoproteins. Virus Res. 2010, 153, 29–35. [Google Scholar] [CrossRef]
- Waarts, B.L.; Bittman, R.; Wilschut, J. Sphingolipid and cholesterol dependence of alphavirus membrane fusion. Lack of correlation with lipid raft formation in target liposomes. J. Biol. Chem. 2002, 277, 38141–38147. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Smit, J.M.; Bittman, R.; Wilschut, J. Low-pH-dependent fusion of Sindbis virus with receptor-free cholesterol- and sphingolipid-containing liposomes. J. Virol. 1999, 73, 8476–8484. [Google Scholar]
- Jin, M.; Park, J.; Lee, S.; Park, B.; Shin, J.; Song, K.J.; Ahn, T.I.; Hwang, S.Y.; Ahn, B.Y.; Ahn, K. Hantaan virus enters cells by clathrin-dependent receptor-mediated endocytosis. Virology 2002, 294, 60–69. [Google Scholar] [CrossRef]
- Buranda, T.; BasuRay, S.; Swanson, S.; Agola, J.; Bondu, V.; Wandinger-Ness, A. Rapid parallel flow cytometry assays of active GTPases using effector beads. Anal. Biochem. 2013, 442, 149–157. [Google Scholar] [CrossRef]
- Garrison, A.R.; Radoshitzky, S.R.; Kota, K.P.; Pegoraro, G.; Ruthel, G.; Kuhn, J.H.; Altamura, L.A.; Kwilas, S.A.; Bavari, S.; Haucke, V.; et al. Crimean-Congo hemorrhagic fever virus utilizes a clathrin- and early endosome-dependent entry pathway. Virology 2013, 444, 45–54. [Google Scholar] [CrossRef]
- Hollidge, B.S.; Nedelsky, N.B.; Salzano, M.V.; Fraser, J.W.; Gonzalez-Scarano, F.; Soldan, S.S. Orthobunyavirus entry into neurons and other mammalian cells occurs via clathrin-mediated endocytosis and requires trafficking into early endosomes. J. Virol. 2012, 86, 7988–8001. [Google Scholar] [CrossRef]
- Lozach, P.Y.; Mancini, R.; Bitto, D.; Meier, R.; Oestereich, L.; Overby, A.K.; Pettersson, R.F.; Helenius, A. Entry of bunyaviruses into mammalian cells. Cell Host Microbe 2010, 7, 488–499. [Google Scholar] [CrossRef]
- Simon, M.; Johansson, C.; Lundkvist, A.; Mirazimi, A. Microtubule-dependent and microtubule-independent steps in Crimean-Congo hemorrhagic fever virus replication cycle. Virology 2009, 385, 313–322. [Google Scholar] [CrossRef]
- Harrison, S.C. Viral membrane fusion. Nat. Struct. Mol. Biol. 2008, 15, 690–698. [Google Scholar] [CrossRef]
- White, J.M.; Delos, S.E.; Brecher, M.; Schornberg, K. Structures and mechanisms of viral membrane fusion proteins: Multiple variations on a common theme. Crit. Rev. Biochem. Mol. Biol. 2008, 43, 189–219. [Google Scholar] [CrossRef]
- Allison, S.L.; Schalich, J.; Stiasny, K.; Mandl, C.W.; Kunz, C.; Heinz, F.X. Oligomeric rearrangement of tick-borne encephalitis virus envelope proteins induced by an acidic pH. J. Virol. 1995, 69, 695–700. [Google Scholar]
- Ogino, M.; Yoshimatsu, K.; Ebihara, H.; Araki, K.; Lee, B.H.; Okumura, M.; Arikawa, J. Cell fusion activities of Hantaan virus envelope glycoproteins. J. Virol. 2004, 78, 10776–10782. [Google Scholar] [CrossRef]
- PyMOL Molecular Graphics System, version 0.99rc6; Schrödinger, LLC: Portland, OR, USA, 2006.
- Arikawa, J.; Takashima, I.; Hashimoto, N. Cell fusion by haemorrhagic fever with renal syndrome (HFRS) viruses and its application for titration of virus infectivity and neutralizing antibody. Arch. Virol. 1985, 86, 303–313. [Google Scholar] [CrossRef]
- Strandin, T.; Hepojoki, J.; Wang, H.; Vaheri, A.; Lankinen, H. Inactivation of hantaviruses by N-ethylmaleimide preserves virion integrity. J. Gen. Virol. 2011, 92, 1189–1198. [Google Scholar]
- Qin, Z.L.; Zheng, Y.; Kielian, M. Role of conserved histidine residues in the low-pH dependence of the Semliki Forest virus fusion protein. J. Virol. 2009, 83, 4670–4677. [Google Scholar] [CrossRef]
- Delos, S.E.; La, B.; Gilmartin, A.; White, J.M. Studies of the “chain reversal regions” of the avian sarcoma/leukosis virus (ASLV) and ebolavirus fusion proteins: Analogous residues are important, and a His residue unique to EnvA affects the pH dependence of ASLV entry. J. Virol. 2010, 84, 5687–5694. [Google Scholar] [CrossRef]
- Carneiro, F.A.; Stauffer, F.; Lima, C.S.; Juliano, M.A.; Juliano, L.; da Poian, A.T. Membrane fusion induced by vesicular stomatitis virus depends on histidine protonation. J. Biol. Chem. 2003, 278, 13789–13794. [Google Scholar]
- Fritz, R.; Stiasny, K.; Heinz, F.X. Identification of specific histidines as pH sensors in flavivirus membrane fusion. J. Cell Biol. 2008, 183, 353–361. [Google Scholar] [CrossRef]
- De Boer, S.M.; Kortekaas, J.; Spel, L.; Rottier, P.J.; Moormann, R.J.; Bosch, B.J. Acid-activated structural reorganization of the Rift Valley fever virus Gc fusion protein. J. Virol. 2012, 86, 13642–13652. [Google Scholar] [CrossRef]
- Bressanelli, S.; Stiasny, K.; Allison, S.L.; Stura, E.A.; Duquerroy, S.; Lescar, J.; Heinz, F.X.; Rey, F.A. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 2004, 23, 728–738. [Google Scholar] [CrossRef]
- Gibbons, D.L.; Vaney, M.C.; Roussel, A.; Vigouroux, A.; Reilly, B.; Lepault, J.; Kielian, M.; Rey, F.A. Conformational change and protein-protein interactions of the fusion protein of Semliki Forest virus. Nature 2004, 427, 320–325. [Google Scholar] [CrossRef]
- Hepojoki, J.; Strandin, T.; Vaheri, A.; Lankinen, H. Interactions and oligomerization of hantavirus glycoproteins. J. Virol. 2010, 84, 227–242. [Google Scholar] [CrossRef]
- Epand, R.M. Fusion peptides and the mechanism of viral fusion. Biochim. Biophys. Acta 2003, 1614, 116–121. [Google Scholar] [CrossRef]
- Nieva, J.L.; Agirre, A. Are fusion peptides a good model to study viral cell fusion? Biophys. Acta 2003, 1614, 104–115. [Google Scholar] [CrossRef]
- Klein, D.E.; Choi, J.L.; Harrison, S.C. Structure of a dengue virus envelope protein late-stage fusion intermediate. J. Virol. 2013, 87, 2287–2293. [Google Scholar] [CrossRef]
- Roman-Sosa, G.; Kielian, M. The interaction of alphavirus E1 protein with exogenous domain III defines stages in virus-membrane fusion. J. Virol. 2011, 85, 12271–12279. [Google Scholar] [CrossRef]
- Stiasny, K.; Brandler, S.; Kossl, C.; Heinz, F.X. Probing the flavivirus membrane fusion mechanism by using monoclonal antibodies. J. Virol. 2007, 81, 11526–11531. [Google Scholar]
- Schmaljohn, C.S.; Schmaljohn, A.L.; Dalrymple, J.M. Hantaan virus M RNA: Coding strategy, nucleotide sequence, and gene order. Virology 1987, 157, 31–39. [Google Scholar] [CrossRef]
- Rapoport, T.A.; Rolls, M.M.; Jungnickel, B. Approaching the mechanism of protein transport across the ER membrane. Curr. Opin. Cell Biol. 1996, 8, 499–504. [Google Scholar] [CrossRef]
- Schmaljohn, C.S.; Nichol, S.T. Bunyaviridae. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 1741–1789. [Google Scholar]
- Lober, C.; Anheier, B.; Lindow, S.; Klenk, H.D.; Feldmann, H. The Hantaan virus glycoprotein precursor is cleaved at the conserved pentapeptide WAASA. Virology 2001, 289, 224–229. [Google Scholar] [CrossRef]
- Ruusala, A.; Persson, R.; Schmaljohn, C.S.; Pettersson, R.F. Coexpression of the membrane glycoproteins G1 and G2 of Hantaan virus is required for targeting to the Golgi complex. Virology 1992, 186, 53–64. [Google Scholar] [CrossRef]
- Deyde, V.M.; Rizvanov, A.A.; Chase, J.; Otteson, E.W.; St Jeor, S.C. Interactions and trafficking of Andes and Sin Nombre Hantavirus glycoproteins G1 and G2. Virology 2005, 331, 307–315. [Google Scholar] [CrossRef]
- Antic, D.; Wright, K.E.; Kang, C.Y. Maturation of Hantaan virus glycoproteins G1 and G2. Virology 1992, 189, 324–328. [Google Scholar]
- Persson, R.; Pettersson, R.F. Formation and intracellular transport of a heterodimeric viral spike protein complex. J. Cell Biol. 1991, 112, 257–266. [Google Scholar] [CrossRef]
- Shi, X.; Elliott, R.M. Analysis of N-linked glycosylation of hantaan virus glycoproteins and the role of oligosaccharide side chains in protein folding and intracellular trafficking. J. Virol. 2004, 78, 5414–5422. [Google Scholar]
- Johansson, P.; Olsson, M.; Lindgren, L.; Ahlm, C.; Elgh, F.; Holmstrom, A.; Bucht, G. Complete gene sequence of a human Puumala hantavirus isolate, Puumala Umea/hu: Sequence comparison and characterisation of encoded gene products. Virus Res. 2004, 105, 147–155. [Google Scholar]
- Tischler, N.D.; Fernandez, J.; Muller, I.; Martinez, R.; Galeno, H.; Villagra, E.; Mora, J.; Ramirez, E.; Rosemblatt, M.; Valenzuela, P.D. Complete sequence of the genome of the human isolate of Andes virus CHI-7913: Comparative sequence and protein structure analysis. Biol. Res. 2003, 36, 201–210. [Google Scholar]
- Isegawa, Y.; Tanishita, O.; Ueda, S.; Yamanishi, K. Association of serine in position 1124 of Hantaan virus glycoprotein with virulence in mice. J. Gen. Virol. 1994, 75, 3273–3278. [Google Scholar] [CrossRef]
- Pettersson, R.; Melin, L. Synthesis, assembly and intracellular transport of bunyaviridae membrane proteins. In The Bunyaviridae; Elliott, R.M., Ed.; Plenum Press: New York, NY, USA, 1996; pp. 159–188. [Google Scholar]
- Matsuoka, Y.; Ihara, T.; Bishop, D.H.; Compans, R.W. Intracellular accumulation of Punta Toro virus glycoproteins expressed from cloned cDNA. Virology 1988, 167, 251–260. [Google Scholar] [CrossRef]
- Spiropoulou, C. Molecular biology of Hantavirus infection. In Bunyaviridae: Molecular and Cellular Biology; Plyusnin, A., Elliott, R.M., Eds.; Caister Academic Press: Norfolk, UK, 2011; pp. 41–60. [Google Scholar]
- Spiropoulou, C.F.; Goldsmith, C.S.; Shoemaker, T.R.; Peters, C.J.; Compans, R.W. Sin Nombre virus glycoprotein trafficking. Virology 2003, 308, 48–63. [Google Scholar] [CrossRef]
- Ravkov, E.V.; Nichol, S.T.; Compans, R.W. Polarized entry and release in epithelial cells of Black Creek Canal virus, a New World hantavirus. J. Virol. 1997, 71, 1147–1154. [Google Scholar]
- Xu, F.; Yang, Z.; Wang, L.; Lee, Y.L.; Yang, C.C.; Xiao, S.Y.; Xiao, H.; Wen, L. Morphological characterization of hantavirus HV114 by electron microscopy. Intervirology 2007, 50, 166–172. [Google Scholar]
- Veesler, D.; Johnson, J.E. Virus maturation. Annu. Rev. Biophys. 2012, 41, 473–496. [Google Scholar] [CrossRef]
- Hepojoki, J.; Strandin, T.; Wang, H.; Vapalahti, O.; Vaheri, A.; Lankinen, H. Cytoplasmic tails of hantavirus glycoproteins interact with the nucleocapsid protein. J. Gen. Virol. 2010, 91, 2341–2350. [Google Scholar] [CrossRef]
- Wang, H.; Alminaite, A.; Vaheri, A.; Plyusnin, A. Interaction between hantaviral nucleocapsid protein and the cytoplasmic tail of surface glycoprotein Gn. Virus Res. 2010, 151, 205–212. [Google Scholar] [CrossRef]
- Overby, A.K.; Pettersson, R.F.; Neve, E.P. The glycoprotein cytoplasmic tail of Uukuniemi virus (Bunyaviridae) interacts with ribonucleoproteins and is critical for genome packaging. J. Virol. 2007, 81, 3198–3205. [Google Scholar] [CrossRef]
- Ribeiro, D.; Borst, J.W.; Goldbach, R.; Kormelink, R. Tomato spotted wilt virus nucleocapsid protein interacts with both viral glycoproteins Gn and Gc in planta. Virology 2009, 383, 121–130. [Google Scholar] [CrossRef]
- Snippe, M.; Willem Borst, J.; Goldbach, R.; Kormelink, R. Tomato spotted wilt virus Gc and N proteins interact in vivo. Virology 2007, 357, 115–123. [Google Scholar] [CrossRef]
- Strandin, T.; Hepojoki, J.; Vaheri, A. Cytoplasmic tails of bunyavirus Gn glycoproteins-Could they act as matrix protein surrogates? Virology 2013, 437, 73–80. [Google Scholar] [CrossRef]
- Miller, J.; McLachlan, A.D.; Klug, A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985, 4, 1609–1614. [Google Scholar]
- Klug, A.; Rhodes, D. Zinc fingers: A novel protein fold for nucleic acid recognition. Cold Spring Harb. Symp. Quant. Biol. 1987, 52, 473–482. [Google Scholar] [CrossRef]
- Gamsjaeger, R.; Liew, C.K.; Loughlin, F.E.; Crossley, M.; Mackay, J.P. Sticky fingers: Zinc-fingers as protein-recognition motifs. Trends Biochem. Sci. 2007, 32, 63–70. [Google Scholar] [CrossRef]
- De Guzman, R.N.; Wu, Z.R.; Stalling, C.C.; Pappalardo, L.; Borer, P.N.; Summers, M.F. Structure of the HIV-1 nucleocapsid protein bound to the SL3 psi-RNA recognition element. Science 1998, 279, 384–388. [Google Scholar] [CrossRef]
- Parisi, G.; Echave, J.; Ghiringhelli, D.; Romanowski, V. Computational characterisation of potential RNA-binding sites in arenavirus nucleocapsid proteins. Virus Genes 1996, 13, 247–254. [Google Scholar] [CrossRef]
- Tortorici, M.A.; Ghiringhelli, P.D.; Lozano, M.E.; Albarino, C.G.; Romanowski, V. Zinc-binding properties of Junin virus nucleocapsid protein. J. Gen. Virol. 2001, 82, 121–128. [Google Scholar]
- Strandin, T.; Hepojoki, J.; Wang, H.; Vaheri, A.; Lankinen, H. The cytoplasmic tail of hantavirus Gn glycoprotein interacts with RNA. Virology 2011, 418, 12–20. [Google Scholar] [CrossRef]
- Piper, M.E.; Sorenson, D.R.; Gerrard, S.R. Efficient cellular release of Rift Valley fever virus requires genomic RNA. PLoS One 2011, 6, e18070. [Google Scholar]
- Acuna, R.; Cifuentes-Munoz, N.; Marquez, C.L.; Bulling, M.; Klingstrom, J.; Mancini, R.; Lozach, P.Y.; Tischler, N.D. Hantavirus Gn and Gc glycoproteins self-assemble into virus-like particles. J. Virol. 2014, 88, 2344–2348. [Google Scholar] [CrossRef]
- Betenbaugh, M.; Yu, M.; Kuehl, K.; White, J.; Pennock, D.; Spik, K.; Schmaljohn, C. Nucleocapsid- and virus-like particles assemble in cells infected with recombinant baculoviruses or vaccinia viruses expressing the M and the S segments of Hantaan virus. Virus Res. 1995, 38, 111–124. [Google Scholar] [CrossRef]
- Li, C.; Liu, F.; Liang, M.; Zhang, Q.; Wang, X.; Wang, T.; Li, J.; Li, D. Hantavirus-like particles generated in CHO cells induce specific immune responses in C57BL/6 mice. Vaccine 2010, 28, 4294–4300. [Google Scholar] [CrossRef]
- Overby, A.K.; Popov, V.; Neve, E.P.; Pettersson, R.F. Generation and analysis of infectious virus-like particles of Uukuniemi virus (Bunyaviridae): A useful system for studying bunyaviral packaging and budding. J. Virol. 2006, 80, 10428–10435. [Google Scholar] [CrossRef]
- Mandell, R.B.; Koukuntla, R.; Mogler, L.J.; Carzoli, A.K.; Freiberg, A.N.; Holbrook, M.R.; Martin, B.K.; Staplin, W.R.; Vahanian, N.N.; Link, C.J.; et al. A replication-incompetent Rift Valley fever vaccine: Chimeric virus-like particles protect mice and rats against lethal challenge. Virology 2010, 397, 187–198. [Google Scholar] [CrossRef]
- Geimonen, E.; Fernandez, I.; Gavrilovskaya, I.N.; Mackow, E.R. Tyrosine residues direct the ubiquitination and degradation of the NY-1 hantavirus G1 cytoplasmic tail. J. Virol. 2003, 77, 10760–10868. [Google Scholar] [CrossRef]
- Sen, N.; Sen, A.; Mackow, E.R. Degrons at the C terminus of the pathogenic but not the nonpathogenic hantavirus G1 tail direct proteasomal degradation. J. Virol. 2007, 81, 4323–4330. [Google Scholar] [CrossRef]
- Wang, H.; Strandin, T.; Hepojoki, J.; Lankinen, H.; Vaheri, A. Degradation and aggresome formation of the Gn tail of the apathogenic Tula hantavirus. J. Gen. Virol. 2009, 90, 2995–3001. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef]
- Zhang, W.; Mukhopadhyay, S.; Pletnev, S.V.; Baker, T.S.; Kuhn, R.J.; Rossmann, M.G. Placement of the structural proteins in Sindbis virus. J. Virol. 2002, 76, 11645–11658. [Google Scholar]
- Albertini, A.A.; Merigoux, C.; Libersou, S.; Madiona, K.; Bressanelli, S.; Roche, S.; Lepault, J.; Melki, R.; Vachette, P.; Gaudin, Y. Characterization of monomeric intermediates during VSV glycoprotein structural transition. PLoS Pathog. 2012, 8, e1002556. [Google Scholar] [CrossRef]
- Korkut, A.; Hendrickson, W.A. Structural plasticity and conformational transitions of HIV envelope glycoprotein gp120. PLoS One 2012, 7, e52170. [Google Scholar] [CrossRef]
- Hepojoki, J.; Strandin, T.; Lankinen, H.; Vaheri, A. Hantavirus structure—Molecular interactions behind the scene. J. Gen. Virol. 2012, 93, 1631–1644. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cifuentes-Muñoz, N.; Salazar-Quiroz, N.; Tischler, N.D. Hantavirus Gn and Gc Envelope Glycoproteins: Key Structural Units for Virus Cell Entry and Virus Assembly. Viruses 2014, 6, 1801-1822. https://doi.org/10.3390/v6041801
Cifuentes-Muñoz N, Salazar-Quiroz N, Tischler ND. Hantavirus Gn and Gc Envelope Glycoproteins: Key Structural Units for Virus Cell Entry and Virus Assembly. Viruses. 2014; 6(4):1801-1822. https://doi.org/10.3390/v6041801
Chicago/Turabian StyleCifuentes-Muñoz, Nicolás, Natalia Salazar-Quiroz, and Nicole D. Tischler. 2014. "Hantavirus Gn and Gc Envelope Glycoproteins: Key Structural Units for Virus Cell Entry and Virus Assembly" Viruses 6, no. 4: 1801-1822. https://doi.org/10.3390/v6041801
APA StyleCifuentes-Muñoz, N., Salazar-Quiroz, N., & Tischler, N. D. (2014). Hantavirus Gn and Gc Envelope Glycoproteins: Key Structural Units for Virus Cell Entry and Virus Assembly. Viruses, 6(4), 1801-1822. https://doi.org/10.3390/v6041801