Development of Lectin-Linked Immunomagnetic Separation for the Detection of Hepatitis A Virus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of pH and Metal Ions on the Binding of HAV to Oyster Digestive Cells
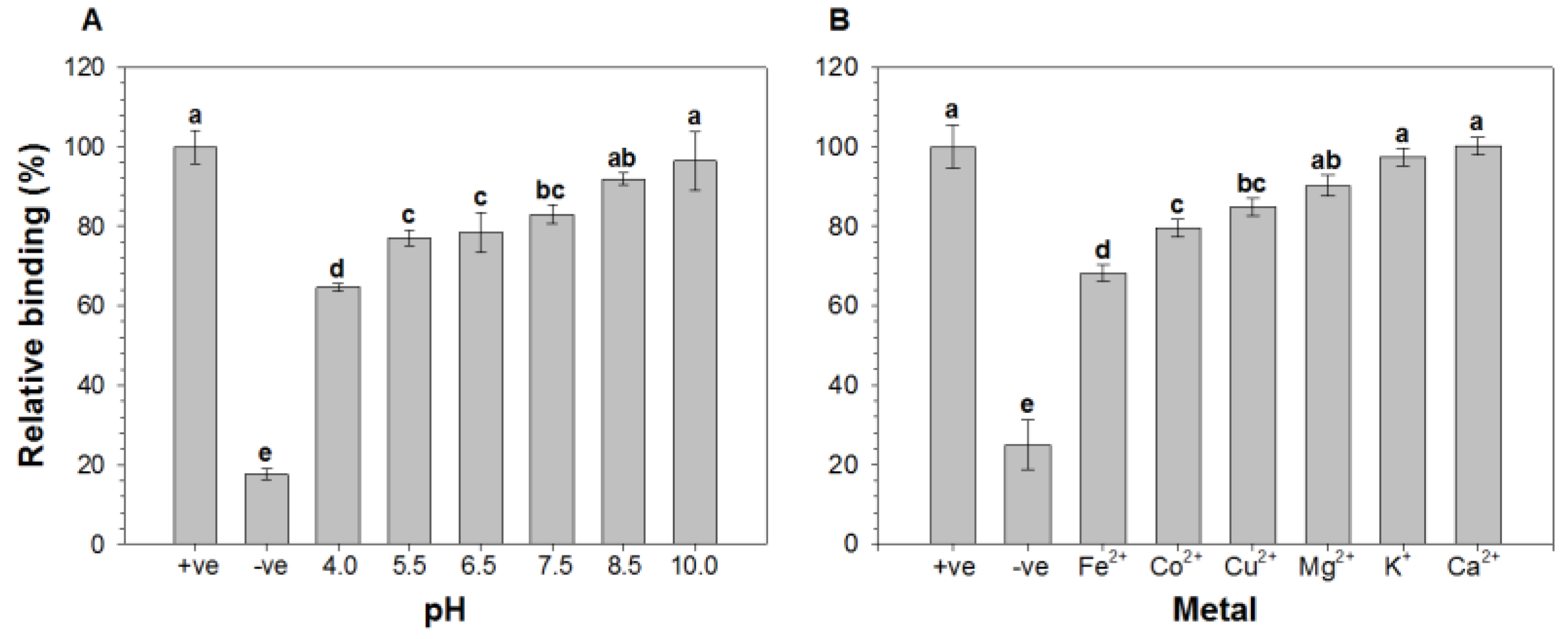
2.2. Comparison of the Relative Binding of HAV to Lectins
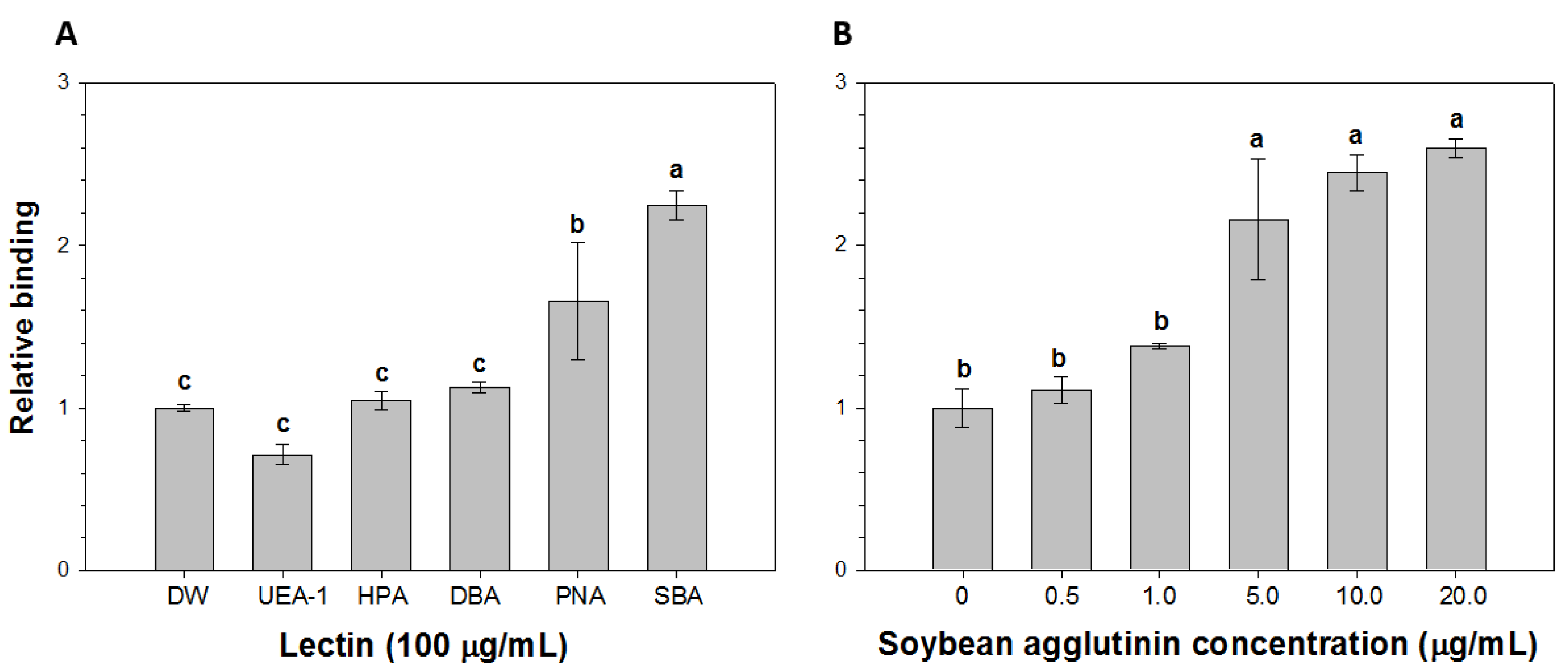
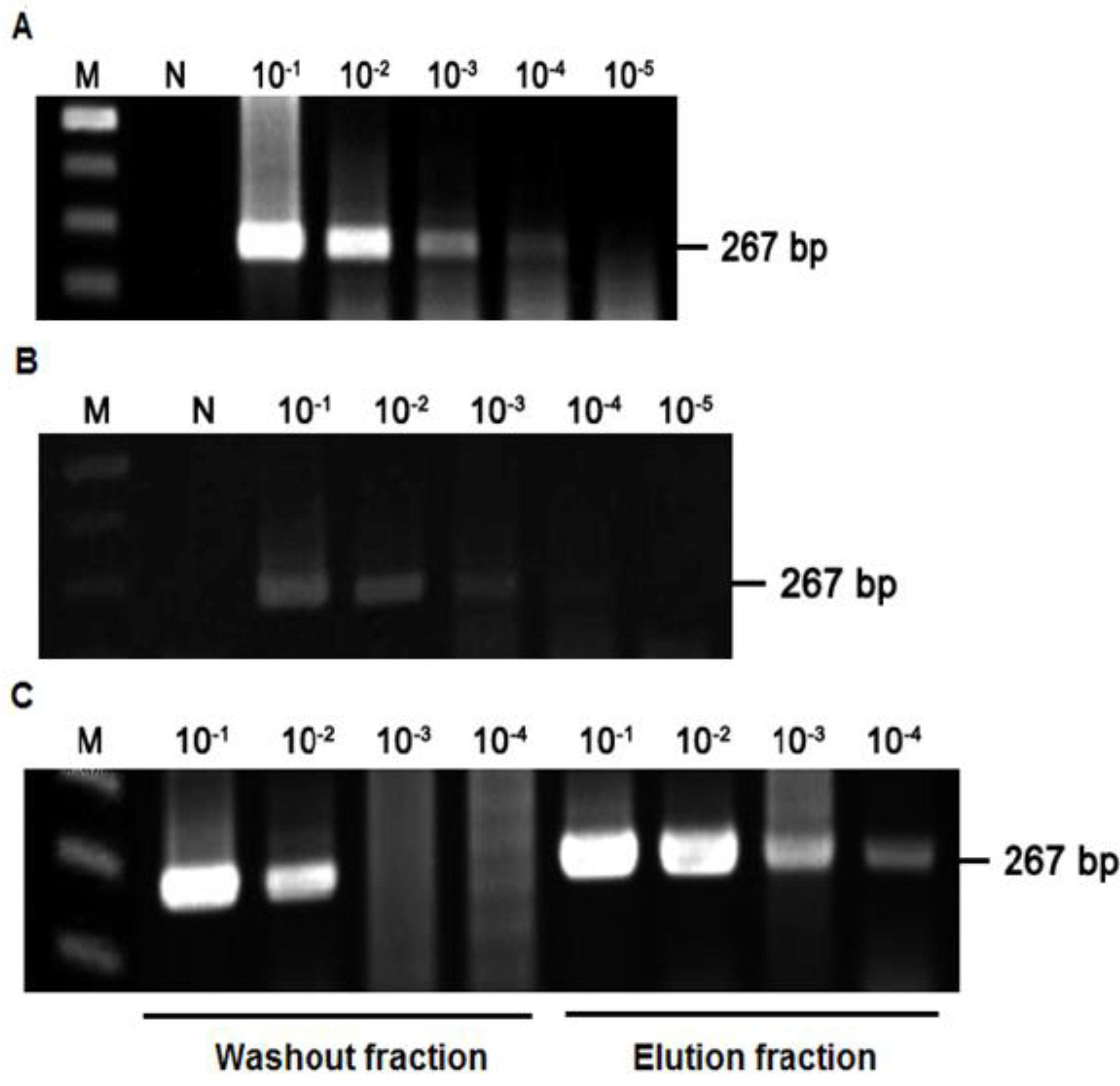
2.3. Detection of HAV Using SMS Combined with RT-PCR
3. Experimental Section
3.1. Oyster Digestive Cells and Virus
3.2. Relative Binding of HAV to Oyster Digestive Cells Determined by ELISA
3.3. Relative Binding of HAV to Lectin Determined by ELISA
3.4. Soybean Agglutinin Linked-Magnetic Bead Separation (SMS)
3.5. RT-PCR Assay
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Cliver, D.O. Epidemiology of viral foodborne disease. J. Food Prot. 1994, 57, 263–266. [Google Scholar]
- Costa-Mattioli, M.; Cristina, J.; Romero, H.; Perez-Bercof, R.; Casane, D.; Colina, R.; Garcia, L.; Vega, I.; Glikman, G.; Romanowsky, V.; et al. Molecular evolution of hepatitis A virus: A new classification based on the complete VP1 protein. J. Virol. 2002, 76, 9516–9525. [Google Scholar] [CrossRef]
- Lu, L.; Ching, K.Z.; de Paula, V.S.; Nakano, T.; Siegl, G.; Weitz, M.; Robertson, B.H. Characterization of the complete genomic sequence of genotype II hepatitis A virus (CF53/Berne isolate). J. Gen. Virol. 2004, 85, 2943–2952. [Google Scholar] [CrossRef]
- Tian, P.; Engelbrektson, A.L.; Jiang, X.; Zhong, W.; Mandrell, R.E. Norovirus recognizes histo-blood group antigens on gastrointestinal cells of clams, mussels, and oysters: A possible mechanism of bioaccumulation. J. Food Prot. 2007, 70, 2140–2147. [Google Scholar]
- Conaty, S.; Bird, P.; Bell, G.; Kraa, E.; Grohmann, G.; McAnulty, J.M. Hepatitis A in New South Wales, Australia, from consumption of oysters: The first reported outbreak. Epidemiol. Infect. 2000, 124, 121–130. [Google Scholar] [CrossRef]
- Le Guyader, F.S.; Atmar, R.L. Binding and inactivation of viruses on and in food, with a focus on the role of the matrix. In Food-Borne Viruses: Progress and Challenge; Koopmans, M., Bosch, A., Cleaver, D., Eds.; ASM Press: Washington, DC, USA, 2008; Chapter 8; pp. 189–208. [Google Scholar]
- Scholz, E.; Heinricy, U.; Flehmig, B. Acid stability of hepatitis A virus. J. Gen. Virol. 1989, 70, 2481–2485. [Google Scholar] [CrossRef]
- Hamilton, B.S.; Whittaker, G.R.; Daniel, S. Influenza virus-mediated membrane fusion: Determinants of hemagglutinin fusogenic activity and experimental approaches for assessing virus fusion. Viruses 2012, 4, 1144–1168. [Google Scholar] [CrossRef]
- Belouzard, S.; Millet, J.K.; Licitra, B.N.; Whittaker, G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses 2012, 4, 1011–1033. [Google Scholar] [CrossRef]
- Zajac, A.J.; Amphlett, E.M.; Rowlands, D.J.; Sangar, D.V. Parameters influencing the attachment of hepatitis A virus to a variety of continuous cell lines. J. Gen. Virol. 1991, 72, 1667–1675. [Google Scholar] [CrossRef]
- Bishop, N.E.; Anderson, D.A. Early interactions of hepatitis A virus with cultured cells: Viral elution and the effect of pH and calcium ions. Arch. Virol. 1997, 142, 2161–2178. [Google Scholar] [CrossRef]
- Love, D.C.; Casteel, M.J.; Meschke, J.S.; Sobsey, M.D. Methods for recovery of hepatitis A virus (HAV) and other viruses from processed foods and detection of HAV by nested RT-PCR and TaqMan RT-PCR. Int. J. Food Microbiol. 2008, 126, 221–226. [Google Scholar] [CrossRef]
- Kim, D.; Kim, S.R.; Kwon, K.S.; Lee, J.W.; Oh, M.J. Detection of hepatitis A virus from oyster by nested PCR using efficient extraction and concentration method. J. Microbiol. 2008, 46, 436–440. [Google Scholar] [CrossRef]
- Hewitt, J.; Greening, G.E. Survival and persistence of norovirus, hepatitis A virus, and feline calicivirus in marinated mussels. J. Food Prot. 2004, 67, 1743–1750. [Google Scholar]
- Atmar, R.L.; Neill, F.H.; Romalde, J.L.; Le Guyader, F.; Woodley, C.M.; Metcalf, T.G.; Estes, M.K. Detection of Norwalk virus and hepatitis A virus in shellfish tissues with the PCR. Appl. Environ. Microbiol. 1995, 61, 3014–3018. [Google Scholar]
- Guévremont, E.; Brassard, J.; Houde, A.; Simard, C.; Trottier, Y.L. Development of an extraction and concentration procedure and comparison of RT-PCR primer systems for the detection of hepatitis A virus and norovirus GII in green onions. J. Virol. Methods 2006, 134, 130–135. [Google Scholar] [CrossRef]
- Sair, A.I.; D’Souza, D.H.; Moe, C.L.; Jaykus, L.A. Improved detection of human enteric viruses in foods by RT-PCR. J. Virol. Methods 2002, 100, 57–69. [Google Scholar] [CrossRef]
- Costafreda, M.I.; Bosch, A.; Pintó, R.M. Development, evaluation, and standardization of a real-time TaqMan reverse transcription-PCR assay for quantification of hepatitis A virus in clinical and shellfish samples. Appl. Environ. Microbiol. 2006, 72, 3846–3855. [Google Scholar] [CrossRef]
- Shan, X.C.; Wolffs, P.; Griffiths, M.W. Rapid and quantitative detection of hepatitis A virus from green onion and strawberry rinses by use of real-time reverse transcription-PCR. Appl. Environ. Microbiol. 2005, 71, 5624–5626. [Google Scholar] [CrossRef]
- Shieh, Y.S.C.; Calci, K.R.; Baric, R.S. A method to detect low levels of enteric viruses in contaminated oysters. Appl. Environ. Microbiol. 1999, 65, 4709–4714. [Google Scholar]
- Richards, G.P. Limitations of molecular biological techniques for assessing the virological safety of foods. J. Food Prot. 1999, 62, 691–697. [Google Scholar]
- Jaykus, L.A.; de Leon, R.; Sobsey, M.D. A virion concentration method for detection of human enteric viruses in oysters by PCR and oligoprobe hybridization. Appl. Environ. Microbiol. 1996, 62, 2074–2080. [Google Scholar]
- Mullendore, J.L.; Sobsey, M.D.; Shieh, Y.S.C. Improved method for the recovery of hepatitis A virus from oysters. J. Virol. Methods 2001, 94, 25–35. [Google Scholar] [CrossRef]
- Park, Y.; Cho, Y.H.; Jee, Y.; Ko, G. Immunomagnetic separation combined with real-time reverse transcriptase PCR assays for detection of norovirus in contaminated food. Appl. Environ. Microbiol. 2008, 74, 4226–4230. [Google Scholar] [CrossRef]
- Hwang, Y.C.; Leong, O.M.; Chen, W.; Yates, M.V. Comparison of a reporter assay and immunomagnetic separation real-time reverse transcription-PCR for the detection of enteroviruses in seeded environmental water samples. Appl. Environ. Microbiol. 2007, 73, 2338–2340. [Google Scholar] [CrossRef]
- Monceyron, C.; Grinde, B. Detection of hepatitis A virus in clinical and environmental samples by immunomagnetic separation and PCR. J. Virol. Methods 1994, 46, 157–166. [Google Scholar] [CrossRef]
- Casas, N.; Suñén, E. Detection of enteroviruses, hepatitis A virus and rotaviruses in sewage by means of an immunomagnetic capture reverse transcription-PCR assay. Microbiol. Res. 2002, 157, 169–175. [Google Scholar] [CrossRef]
- Jothikumar, N.; Cliver, D.O.; Mariam, T.W. Immunomagnetic capture PCR for rapid concentration and detection of hepatitis A virus from environmental samples. Appl. Environ. Microbiol. 1998, 64, 504–508. [Google Scholar]
- Yang, W.; Gu, A.Z.; Zeng, S.Y.; Li, D.; He, M.; Shi, H.C. Development of a combined immunomagnetic separation and quantitative reverse transcription-PCR assay for sensitive detection of infectious rotavirus in water samples. J. Microbiol. Methods 2011, 84, 447–453. [Google Scholar]
- López‐Sabater, E.I.; Deng, M.Y.; Cliver, D. Magnetic immunoseparation PCR assay (MIPA) for detection of hepatitis A virus (HAV) in American oyster (Crassostrea virginica). Lett. Appl. Microbiol. 1997, 24, 101–104. [Google Scholar]
- Goldstein, I.J.; Hughes, R.C.; Monsigny, M.; Osawa, T.; Sharon, N. What should be called a lectin? Nature 1980, 285. [Google Scholar] [CrossRef]
- Slifkin, M.; Doyle, R.J. Lectins and their application to clinical microbiology. Clin. Microbiol. Rev. 1990, 3, 197–218. [Google Scholar]
- Olofsson, S.; Jeansson, S.; Lycke, E. Unusual lectin-binding properties of a herpes simplex virus type 1-specific glycoprotein. J. Virol. 1981, 38, 564–570. [Google Scholar]
- Uematsu, J.; Koyama, A.; Takano, S.; Ura, Y.; Tanemura, M.; Kihira, S.; Yamamoto, H.; Kawano, M.; Tsurudome, M.; O’Brien, M.; et al. Legume lectins inhibit human parainfluenza virus type 2 infection by interfering with the entry. Viruses 2012, 4, 1104–1115. [Google Scholar] [CrossRef]
- Müller, W.; Renneisen, K.; Kreuter, M.H.; Schröder, H.; Winkler, I. The D-mannose-specific lectin from Gerardia savaglia blocks binding of human immunodeficiency virus type I to H9 cells and human lymphocytes in vitro. J. Acquir. Immune Defic. Syndr. 1988, 1, 453–458. [Google Scholar]
- Hunter, E.; Hill, E.; Hardwick, M.; Bhown, A.; Schwartz, D.E.; Tizard, R. Complete sequence of the Rous sarcoma virus env gene: Identification of structural and functional regions of its product. J. Virol. 1983, 46, 920–936. [Google Scholar]
- Malkinson, M.; Orgad, U.; Becker, Y. Use of lectins to detect and differentiate subtypes of Marek’s disease virus and turkey herpesvirus glycoproteins in tissue culture. J. Virol. Methods 1986, 13, 129–133. [Google Scholar] [CrossRef]
- Drake, D.; Taylor, K.G.; Bleiweis, A.S.; Doyle, R.J. Specificity of the glucan-binding lectin of Streptococcus cricetus. Infect. Immun. 1988, 56, 1864–1872. [Google Scholar]
- Cole, H.B.; Ezzell, J.W., Jr.; Keller, K.F.; Doyle, R.J. Differentiation of Bacillus anthracis and other Bacillus species by lectins. J. Clin. Microbiol. 1984, 19, 48–53. [Google Scholar]
- Porter, J.; Robinson, J.; Pickup, R.; Edwards, C. An evaluation of lectin-mediated magnetic bead cell sorting for the targeted separation of enteric bacteria. J. Appl. Microbiol. 1998, 84, 722–732. [Google Scholar]
- Corbel, M.J.; Gill, K.P.W. Lectin agglutination of thermophilic Campylobacter species. Vet. Microbiol. 1987, 15, 163–173. [Google Scholar] [CrossRef]
- Bishop, N.E. Conformational changes in the hepatitis A virus capsid in response to acidic conditions. J. Med. Microbiol. 1999, 48, 443–450. [Google Scholar] [CrossRef]
- Di Girolamo, R.; Liston, J.; Matches, J. Ionic bonding, the mechanism of viral uptake by shellfish mucus. Appl. Environ. Microbiol. 1977, 33, 19–25. [Google Scholar]
- Victoria, M.; Guimarães, F.; Fumian, T.; Ferreira, F.; Vieira, C.; Leite, J.P.; Miagostovich, M. Evaluation of an adsorption-elution method for detection of astrovirus and norovirus in environmental waters. J. Virol. Methods 2009, 156, 73–76. [Google Scholar] [CrossRef]
- Sobsey, M.D.; Wallis, C.; Henderson, M.; Melnick, J.L. Concentration of enteroviruses from large volumes of water. Appl. Microbiol. 1973, 26, 529–534. [Google Scholar]
- Sánchez, G.; Aragonès, L.; Costafreda, M.I.; Ribes, E.; Bosch, A.; Pintó, R.M. Capsid region involved in hepatitis A virus binding to glycophorin A of the erythrocyte membrane. J. Virol. 2004, 78, 9807–9813. [Google Scholar] [CrossRef]
- Stapleton, J.T.; Frederick, J.; Meyer, B. Hepatitis A virus attachment to cultured cell lines. J. Infect. Dis. 1991, 164, 1098–1103. [Google Scholar] [CrossRef]
- Keyaerts, E.; Vijgen, L.; Pannecouque, C.; Van Damme, E.; Peumans, W.; Egberink, H.; Balzarini, J.; Van Ranst, M. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antivir. Res. 2007, 75, 179–187. [Google Scholar] [CrossRef]
- Le Guyader, F.S.; Loisy, F.; Atmar, R.L.; Hutson, A.M.; Estes, M.K.; Ruvoën-Clouet, N.; Pommepuy, M.; Le Pendu, J. Norwalk virus–specific binding to oyster digestive tissues. Emerg. Infect. Dis. 2006, 12, 931–936. [Google Scholar] [CrossRef]
- Jothikumar, N.; Lowther, J.A.; Henshilwood, K.; Lees, D.N.; Hill, V.R.; Vinjé, J. Rapid and sensitive detection of noroviruses by using TaqMan-based one-step reverse transcription-PCR assays and application to naturally contaminated shellfish samples. Appl. Environ. Microbiol. 2005, 71, 1870–1875. [Google Scholar] [CrossRef]
- Statistical Package for Social Sciences, Version 12.0; IBM Corp.: Somers, NY, USA, 2008.
- Microsoft Office Excel, Microsoft Corp.: Redmond, WA, USA, 2010.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ko, S.-M.; Kwon, J.; Vaidya, B.; Choi, J.S.; Lee, H.-M.; Oh, M.-J.; Bae, H.-J.; Cho, S.-Y.; Oh, K.-S.; Kim, D. Development of Lectin-Linked Immunomagnetic Separation for the Detection of Hepatitis A Virus. Viruses 2014, 6, 1037-1048. https://doi.org/10.3390/v6031037
Ko S-M, Kwon J, Vaidya B, Choi JS, Lee H-M, Oh M-J, Bae H-J, Cho S-Y, Oh K-S, Kim D. Development of Lectin-Linked Immunomagnetic Separation for the Detection of Hepatitis A Virus. Viruses. 2014; 6(3):1037-1048. https://doi.org/10.3390/v6031037
Chicago/Turabian StyleKo, Sang-Mu, Joseph Kwon, Bipin Vaidya, Jong Soon Choi, Hee-Min Lee, Myung-Joo Oh, Hyeun-Jong Bae, Se-Young Cho, Kyung-Seo Oh, and Duwoon Kim. 2014. "Development of Lectin-Linked Immunomagnetic Separation for the Detection of Hepatitis A Virus" Viruses 6, no. 3: 1037-1048. https://doi.org/10.3390/v6031037
APA StyleKo, S.-M., Kwon, J., Vaidya, B., Choi, J. S., Lee, H.-M., Oh, M.-J., Bae, H.-J., Cho, S.-Y., Oh, K.-S., & Kim, D. (2014). Development of Lectin-Linked Immunomagnetic Separation for the Detection of Hepatitis A Virus. Viruses, 6(3), 1037-1048. https://doi.org/10.3390/v6031037



