“Ménage à Trois”: The Evolutionary Interplay between JSRV, enJSRVs and Domestic Sheep
Abstract
:1. Introduction
2. Jaagsiekte Sheep Retrovirus (JSRV)
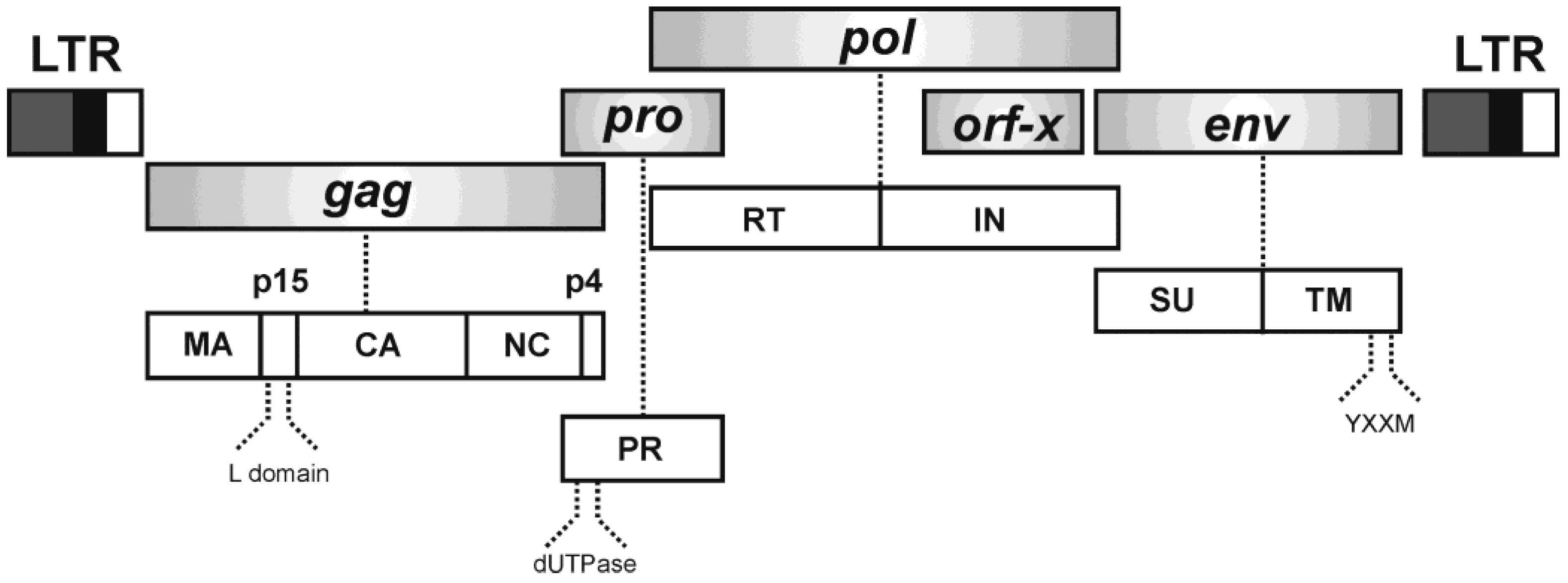
3. Ovine Pulmonary Adenocarcinoma
4. Endogenous Sheep Betaretroviruses: enJSRVs
5. enJSRVs and Sheep Reproductive Biology

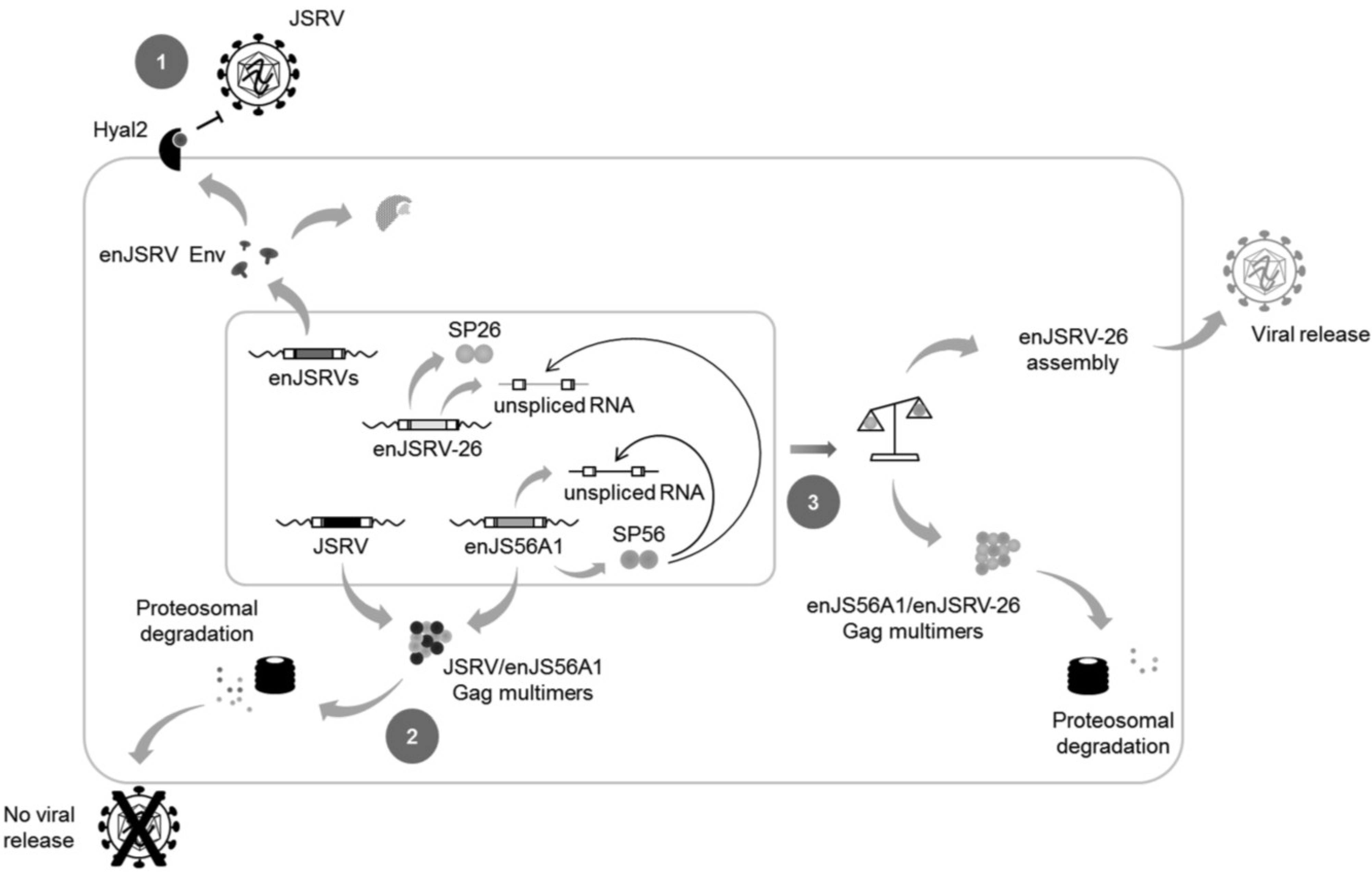
6. enJSRVs and Host Defense
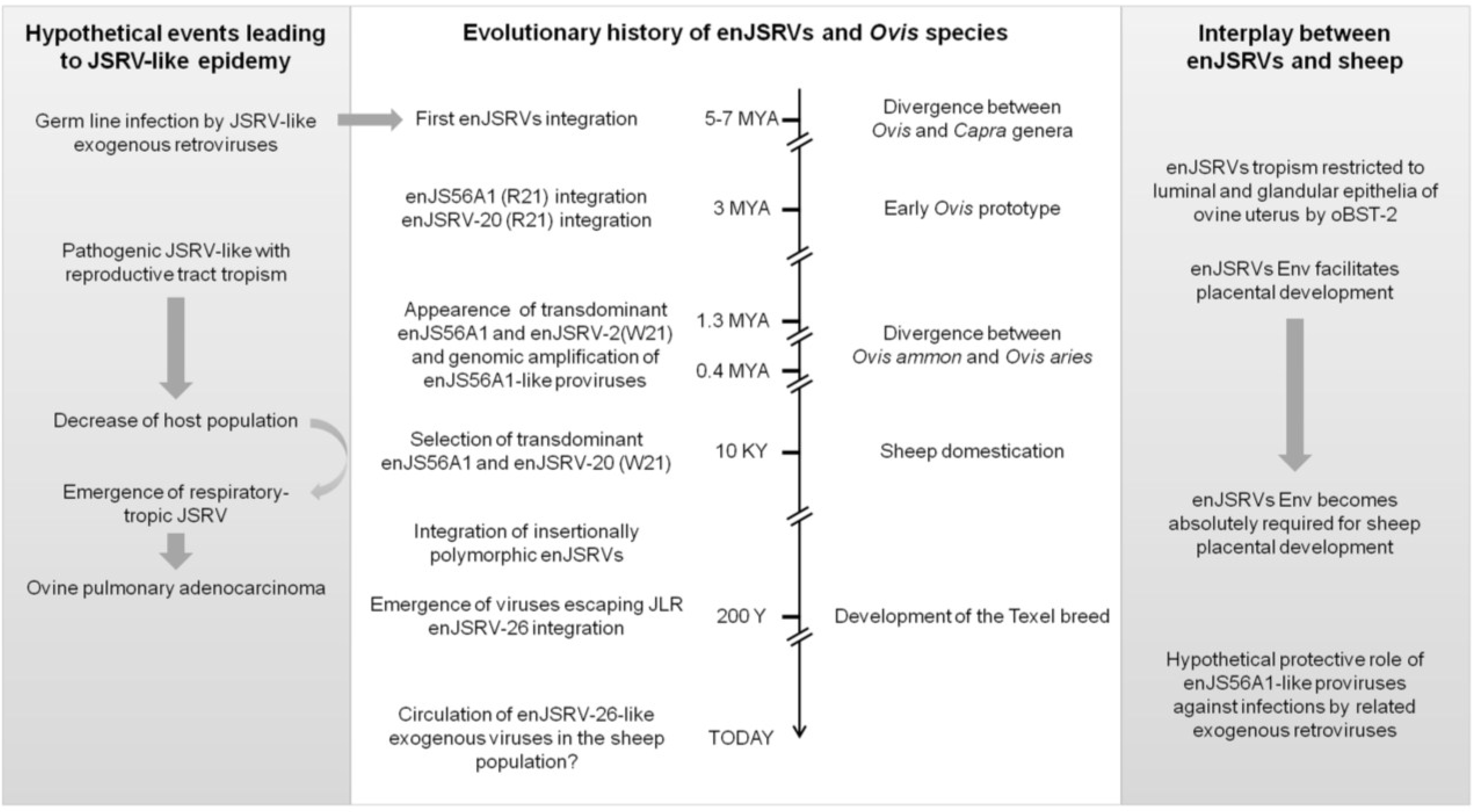
7. The Evolutionary History of enJSRVs and Their Hosts
8. It Takes All the Running You Can Do, to Keep in the Same Place
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stoye, J.P. Studies of endogenous retroviruses reveal a continuing evolutionary saga. Nat. Rev. Microbiol. 2012, 10, 395–406. [Google Scholar] [PubMed]
- Jern, P.; Coffin, J.M. Effects of retroviruses on host genome function. Annu. Rev. Genet. 2008, 42, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Gifford, R.; Tristem, M. The evolution, distribution and diversity of endogenous retroviruses. Virus Genes 2003, 26, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.Y.; Yu, S.L.; Seo, D.W.; Jung, K.C.; Cho, I.C.; Lim, H.T.; Jin, D.I.; Lee, J.H. Characterization of insertional variation of porcine endogenous retroviruses in six different pig breeds. Asian-Australas. J. Anim. Sci. 2012, 25, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Kimsa, M.C.; Strzalka-Mrozik, B.; Kimsa, M.W.; Gola, J.; Nicholson, P.; Lopata, K.; Mazurek, U. Porcine endogenous retroviruses in xenotransplantation--molecular aspects. Viruses 2014, 6, 2062–2083. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, F.; Caporale, M.; Varela, M.; Biek, R.; Chessa, B.; Alberti, A.; Golder, M.; Mura, M.; Zhang, Y.P.; Yu, L.; et al. A paradigm for virus-host coevolution: Sequential counter-adaptations between endogenous and exogenous retroviruses. PLoS Pathog. 2007, 3, e170. [Google Scholar] [CrossRef] [PubMed]
- Elleder, D.; Kim, O.; Padhi, A.; Bankert, J.G.; Simeonov, I.; Schuster, S.C.; Wittekindt, N.E.; Motameny, S.; Poss, M. Polymorphic integrations of an endogenous gammaretrovirus in the mule deer genome. J. Virol. 2012, 86, 2787–2796. [Google Scholar] [CrossRef] [PubMed]
- Tarlinton, R.E.; Meers, J.; Young, P.R. Retroviral invasion of the koala genome. Nature 2006, 442, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Palmarini, M.; Sharp, J.M.; de las Heras, M.; Fan, H. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J. Virol. 1999, 73, 6964–6972. [Google Scholar] [PubMed]
- De las Heras, M.; Gonzalez, L.; Sharp, J.M. Pathology of ovine pulmonary adenocarcinoma. Curr. Top. Microbiol. Immunol. 2003, 275, 25–54. [Google Scholar]
- Caporale, M.; Martineau, H.; de las Heras, M.; Murgia, C.; Huang, R.; Centorame, P.; di Francesco, G.; di Gialleonardo, L.; Spencer, T.E.; Griffiths, D.J.; et al. Host species barriers to Jaagsiekte sheep retrovirus replication and carcinogenesis. J. Virol. 2013, 87, 10752–10762. [Google Scholar] [CrossRef] [PubMed]
- Palmarini, M.; Fan, H. Retrovirus-induced ovine pulmonary adenocarcinoma, an animal model for lung cancer. J. Natl. Cancer Inst. 2001, 93, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Miller, A.D. Oncogenic transformation by the jaagsiekte sheep retrovirus envelope protein. Oncogene 2007, 26, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Caporale, M.; Cousens, C.; Centorame, P.; Pinoni, C.; De las Heras, M.; Palmarini, M. Expression of the jaagsiekte sheep retrovirus envelope glycoprotein is sufficient to induce lung tumors in sheep. J. Virol. 2006, 80, 8030–8037. [Google Scholar] [CrossRef] [PubMed]
- Wootton, S.K.; Halbert, C.L.; Miller, A.D. Sheep retrovirus structural protein induces lung tumours. Nature 2005, 434, 904–907. [Google Scholar] [CrossRef] [PubMed]
- York, D.F.; Vigne, R.; Verwoerd, D.W.; Querat, G. Nucleotide sequence of the jaagsiekte retrovirus, an exogenous and endogenous type D and B retrovirus of sheep and goats. J. Virol. 1992, 66, 4930–4939. [Google Scholar] [PubMed]
- York, D.F.; Vigne, R.; Verwoerd, D.W.; Querat, G. Isolation, identification, and partial cDNA cloning of genomic RNA of jaagsiekte retrovirus, the etiological agent of sheep pulmonary adenomatosis. J. Virol. 1991, 65, 5061–5067. [Google Scholar] [PubMed]
- Palmarini, M.; Fan, H. Molecular biology of jaagsiekte sheep retrovirus. Curr. Top. Microbiol. Immunol. 2003, 275, 81–115. [Google Scholar] [PubMed]
- DeMartini, J.C.; Bishop, J.V.; Allen, T.E.; Jassim, F.A.; Sharp, J.M.; de las Heras, M.; Voelker, D.R.; Carlson, J.O. Jaagsiekte sheep retrovirus proviral clone JSRV(JS7), derived from the JS7 lung tumor cell line, induces ovine pulmonary carcinoma and is integrated into the surfactant protein A gene. J. Virol. 2001, 75, 4239–4246. [Google Scholar] [CrossRef] [PubMed]
- Palmarini, M.; Murgia, C.; Fan, H. Spliced and prematurely polyadenylated Jaagsiekte sheep retrovirus-specific RNAs from infected or transfected cells. Virology 2002, 294, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Murcia, P.R.; Arnaud, F.; Palmarini, M. The transdominant endogenous retrovirus enJS56A1 associates with and blocks intracellular trafficking of Jaagsiekte sheep retrovirus Gag. J. Virol. 2007, 81, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Mura, M.; Murcia, P.; Caporale, M.; Spencer, T.E.; Nagashima, K.; Rein, A.; Palmarini, M. Late viral interference induced by transdominant Gag of an endogenous retrovirus. Proc. Natl. Acad. Sci. USA 2004, 101, 11117–11122. [Google Scholar] [CrossRef] [PubMed]
- Gottwein, E.; Bodem, J.; Muller, B.; Schmechel, A.; Zentgraf, H.; Krausslich, H.G. The Mason-Pfizer monkey virus PPPY and PSAP motifs both contribute to virus release. J. Virol. 2003, 77, 9474–9485. [Google Scholar] [CrossRef] [PubMed]
- Freed, E.O. Viral late domains. J. Virol. 2002, 76, 4679–4687. [Google Scholar] [CrossRef] [PubMed]
- Spencer, T.E.; Mura, M.; Gray, C.A.; Griebel, P.J.; Palmarini, M. Receptor usage and fetal expression of ovine endogenous betaretroviruses: Implications for coevolution of endogenous and exogenous retroviruses. J. Virol. 2003, 77, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Palmarini, M.; Maeda, N.; Murgia, C.; De-Fraja, C.; Hofacre, A.; Fan, H. A phosphatidylinositol 3-kinase docking site in the cytoplasmic tail of the Jaagsiekte sheep retrovirus transmembrane protein is essential for envelope-induced transformation of NIH 3T3 cells. J. Virol. 2001, 75, 11002–11009. [Google Scholar] [CrossRef] [PubMed]
- Cianciolo, G.J.; Copeland, T.D.; Oroszlan, S.; Snyderman, R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science 1985, 230, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Henzy, J.E.; Coffin, J.M. Betaretroviral envelope subunits are noncovalently associated and restricted to the mammalian class. J. Virol. 2013, 87, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Bu, Z.; Vzorov, A.; Taylor, D.; Compans, R.W.; Yang, C. Surface stability and immunogenicity of the human immunodeficiency virus envelope glycoprotein: Role of the cytoplasmic domain. J. Virol. 2004, 78, 13409–13419. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Miller, A.D. Transformation of madin-darby canine kidney epithelial cells by sheep retrovirus envelope proteins. J. Virol. 2005, 79, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Lerman, M.I.; Miller, A.D. Putative phosphatidylinositol 3-kinase (PI3K) binding motifs in ovine betaretrovirus Env proteins are not essential for rodent fibroblast transformation and PI3K/Akt activation. J. Virol. 2003, 77, 7924–7935. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.E.; Sherrill, K.J.; Crispell, S.M.; Perrott, M.R.; Carlson, J.O.; DeMartini, J.C. The jaagsiekte sheep retrovirus envelope gene induces transformation of the avian fibroblast cell line DF-1 but does not require a conserved SH2 binding domain. J. Gen. Virol. 2002, 83, 2733–2742. [Google Scholar] [PubMed]
- Zavala, G.; Pretto, C.; Chow, Y.H.; Jones, L.; Alberti, A.; Grego, E.; De las Heras, M.; Palmarini, M. Relevance of Akt phosphorylation in cell transformation induced by Jaagsiekte sheep retrovirus. Virology 2003, 312, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.; Chow, Y.H.; Sturkie, C.; Murcia, P.; Palmarini, M. Association of RON tyrosine kinase with the Jaagsiekte sheep retrovirus envelope glycoprotein. Virology 2006, 350, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Suau, F.; Cottin, V.; Archer, F.; Croze, S.; Chastang, J.; Cordier, G.; Thivolet-Bejui, F.; Mornex, J.F.; Leroux, C. Telomerase activation in a model of lung adenocarcinoma. Eur. Respir. J. 2006, 27, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- De Las Heras, M.; Ortin, A.; Benito, A.; Summers, C.; Ferrer, L.M.; Sharp, J.M. In-situ demonstration of mitogen-activated protein kinase Erk 1/2 signalling pathway in contagious respiratory tumours of sheep and goats. J. Comp. Pathol. 2006, 135, 1–10. [Google Scholar]
- Maeda, N.; Fu, W.; Ortin, A.; de las Heras, M.; Fan, H. Roles of the Ras-MEK-mitogen-activated protein kinase and phosphatidylinositol 3-kinase-Akt-mTOR pathways in Jaagsiekte sheep retrovirus-induced transformation of rodent fibroblast and epithelial cell lines. J. Virol. 2005, 79, 4440–4450. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.M.; DeMartini, J.C. Natural history of JSRV in sheep. Curr. Top. Microbiol. Immunol. 2003, 275, 55–79. [Google Scholar] [PubMed]
- Demartini, J.C.; Rosadio, R.H.; Lairmore, M.D. The etiology and pathogenesis of ovine pulmonary carcinoma (sheep pulmonary adenomatosis). Vet. Microbiol. 1988, 17, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Ortin, A.; Minguijon, E.; Dewar, P.; Garcia, M.; Ferrer, L.M.; Palmarini, M.; Gonzalez, L.; Sharp, J.M.; de las Heras, M. Lack of a specific immune response against a recombinant capsid protein of Jaagsiekte sheep retrovirus in sheep and goats naturally affected by enzootic nasal tumour or sheep pulmonary adenomatosis. Vet. Immunol. Immunopathol. 1998, 61, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Grego, E.; de Meneghi, D.; Alvarez, V.; Benito, A.A.; Minguijon, E.; Ortin, A.; Mattoni, M.; Moreno, B.; Perez de Villarreal, M.; Alberti, A.; et al. Colostrum and milk can transmit jaagsiekte retrovirus to lambs. Vet. Microbiol. 2008, 130, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Caporale, M.; Centorame, P.; Giovannini, A.; Sacchini, F.; di Ventura, M.; de las Heras, M.; Palmarini, M. Infection of lung epithelial cells and induction of pulmonary adenocarcinoma is not the most common outcome of naturally occurring JSRV infection during the commercial lifespan of sheep. Virology 2005, 338, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Murgia, C.; Caporale, M.; Ceesay, O.; Di Francesco, G.; Ferri, N.; Varasano, V.; de las Heras, M.; Palmarini, M. Lung adenocarcinoma originates from retrovirus infection of proliferating type 2 pneumocytes during pulmonary post-natal development or tissue repair. PLoS Pathog. 2011, 7, e1002014. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.M.; Herring, A.J. Sheep pulmonary adenomatosis: Demonstration of a protein which cross-reacts with the major core proteins of Mason-Pfizer monkey virus and mouse mammary tumour virus. J. Gen. Virol. 1983, 64 Pt 10, 2323–2327. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.P.; DeMartini, J.C.; Ameghino, E.; Caletti, E. Coexistence of pulmonary adenomatosis and progressive pneumonia in sheep in the central sierra of Peru. Am. J. Vet. Res. 1983, 44, 1334–1338. [Google Scholar] [PubMed]
- Dawson, M.; Done, S.H.; Venables, C.; Jenkins, C.E. Maedi-visna and sheep pulmonary adenomatosis: A study of concurrent infection. Br. Vet. J. 1990, 146, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Craigie, R. The road to chromatin-nuclear entry of retroviruses. Nat. Rev. Microbiol. 2007, 5, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Murgia, C.; Liu, S.L.; Mura, M.; Cousens, C.; Sharp, M.; Miller, A.D.; Palmarini, M. Envelope-induced cell transformation by ovine betaretroviruses. J. Virol. 2002, 76, 5387–5394. [Google Scholar] [CrossRef] [PubMed]
- Cousens, C.; Maeda, N.; Murgia, C.; Dagleish, M.P.; Palmarini, M.; Fan, H. In vivo tumorigenesis by Jaagsiekte sheep retrovirus (JSRV) requires Y590 in Env TM, but not full-length orfX open reading frame. Virology 2007, 367, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Palmarini, M.; Murgia, C.; Fan, H. Direct transformation of rodent fibroblasts by jaagsiekte sheep retrovirus DNA. Proc. Natl. Acad. Sci. USA 2001, 98, 4449–4454. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.K.; Duh, F.M.; Vigdorovich, V.; Danilkovitch-Miagkova, A.; Lerman, M.I.; Miller, A.D. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc. Natl. Acad. Sci. USA 2001, 98, 4443–4448. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Fan, H. Signal transduction pathways utilized by enzootic nasal tumor virus (ENTV-1) envelope protein in transformation of rat epithelial cells resemble those used by jaagsiekte sheep retrovirus. Virus Genes 2008, 36, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Palmarini, M.; Holland, M.J.; Cousens, C.; Dalziel, R.G.; Sharp, J.M. Jaagsiekte retrovirus establishes a disseminated infection of the lymphoid tissues of sheep affected by pulmonary adenomatosis. J. Gen. Virol. 1996, 77 Pt 12, 2991–2998. [Google Scholar] [CrossRef] [PubMed]
- Rajya, B.S.; Singh, C.M. The Pathology of Pneumonia and Associated Respiratory Disease of Sheep and Goats. I. Occurrence of jagziekte and maedi in sheep and goats in India. Am. J. Vet. Res. 1964, 25, 61–67. [Google Scholar] [PubMed]
- Nobel, T.A. Pulmonary adenomatosies (jaagsiekte) in sheep with special reference to its occurrence in Israel. Refu. Vet. (Israel) 1958, 15, 98–101. [Google Scholar]
- Banerjee, M.; Gupta, P.P. Note on maedi and jaagsiekte in sheep and goats in Ludhiana area of Punjab. Indian J. Anim. Sci. 1979, 12, 1102–1105. [Google Scholar]
- Stamp, J.T.; Nisbet, D.I. Pneumonia of sheep. J. Comp. Pathol. 1963, 73, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.; Benito, A.; Ortin, A.; Garcia de Jalon, J.A.; Gonzalez, L.; Norval, M.; Sharp, J.M.; de las Heras, M. The distribution of immune cells in the lungs of classical and atypical ovine pulmonary adenocarcinoma. Vet. Immunol. Immunopathol. 2012, 146, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Palmarini, M.; Hallwirth, C.; York, D.; Murgia, C.; de Oliveira, T.; Spencer, T.; Fan, H. Molecular cloning and functional analysis of three type D endogenous retroviruses of sheep reveal a different cell tropism from that of the highly related exogenous jaagsiekte sheep retrovirus. J. Virol. 2000, 74, 8065–8076. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhu, R.Y.; Stedman, K.; Cousens, C.; Carlson, J.; Sharp, J.M.; DeMartini, J.C. Unique long terminal repeat U3 sequences distinguish exogenous jaagsiekte sheep retroviruses associated with ovine pulmonary carcinoma from endogenous loci in the sheep genome. J. Virol. 1996, 70, 3159–3168. [Google Scholar] [PubMed]
- Spencer, T.E.; Stagg, A.G.; Joyce, M.M.; Jenster, G.; Wood, C.G.; Bazer, F.W.; Wiley, A.A.; Bartol, F.F. Discovery and characterization of endometrial epithelial messenger ribonucleic acids using the ovine uterine gland knockout model. Endocrinology 1999, 140, 4070–4080. [Google Scholar] [PubMed]
- Palmarini, M.; Gray, C.A.; Carpenter, K.; Fan, H.; Bazer, F.W.; Spencer, T.E. Expression of endogenous betaretroviruses in the ovine uterus: Effects of neonatal age, estrous cycle, pregnancy, and progesterone. J. Virol. 2001, 75, 11319–11327. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, K.A.; Palmarini, M.; Varela, M.; Burghardt, R.C.; Hayashi, K.; Farmer, J.L.; Spencer, T.E. Endogenous retroviruses regulate periimplantation placental growth and differentiation. Proc. Natl. Acad. Sci. USA 2006, 103, 14390–14395. [Google Scholar] [CrossRef] [PubMed]
- Mangeney, M.; Renard, M.; Schlecht-Louf, G.; Bouallaga, I.; Heidmann, O.; Letzelter, C.; Richaud, A.; Ducos, B.; Heidmann, T. Placental syncytins: Genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins. Proc. Natl. Acad. Sci. USA 2007, 104, 20534–20539. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.; Spencer, T.E.; Palmarini, M.; Arnaud, F. Friendly viruses: The special relationship between endogenous retroviruses and their host. Ann. NY Acad. Sci. 2009, 1178, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Black, S.G.; Arnaud, F.; Palmarini, M.; Spencer, T.E. Endogenous retroviruses in trophoblast differentiation and placental development. Am. J. Reprod. Immunol. 2010, 64, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, F.; Black, S.G.; Murphy, L.; Griffiths, D.J.; Neil, S.J.; Spencer, T.E.; Palmarini, M. Interplay between ovine bone marrow stromal cell antigen 2/tetherin and endogenous retroviruses. J. Virol. 2010, 84, 4415–4425. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.; Varela, M.; Desloire, S.; Ftaich, N.; Murgia, C.; Golder, M.; Neil, S.; Spencer, T.E.; Wootton, S.K.; Lavillette, D.; et al. The sheep tetherin paralog, oBST2B, blocks envelope glycoprotein incorporation into nascent retroviral virions. J. Virol. 2014. [Google Scholar] [CrossRef]
- Jouvenet, N.; Neil, S.J.; Zhadina, M.; Zang, T.; Kratovac, Z.; Lee, Y.; McNatt, M.; Hatziioannou, T.; Bieniasz, P.D. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 2009, 83, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Kaletsky, R.L.; Francica, J.R.; Agrawal-Gamse, C.; Bates, P. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc. Natl. Acad. Sci. USA 2009, 106, 2886–2891. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.; Viswanathan, K.; Douglas, J.L.; Hines, J.; Gustin, J.; Moses, A.V.; Fruh, K. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2009, 83, 9672–9681. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, T.; Noda, T.; Urata, S.; Kawaoka, Y.; Yasuda, J. Inhibition of Lassa and Marburg virus production by tetherin. J. Virol. 2009, 83, 2382–2385. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Chen, Z.J. Intrinsic antiviral immunity. Nat. Immunol. 2012, 13, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, N.; Goff, D.; Katsura, C.; Jorgenson, R.L.; Mitchell, R.; Johnson, M.C.; Stephens, E.B.; Guatelli, J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 2008, 3, 245–252. [Google Scholar]
- Neil, S.J.; Zang, T.; Bieniasz, P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 2008, 451, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Spencer, T.E.; Wu, G. Interferons and progesterone for establishment and maintenance of pregnancy: Interactions among novel cell signaling pathways. Reprod. Biol. 2008, 8, 179–211. [Google Scholar] [CrossRef] [PubMed]
- Spencer, T.E.; Johnson, G.A.; Bazer, F.W.; Burghardt, R.C.; Palmarini, M. Pregnancy recognition and conceptus implantation in domestic ruminants: Roles of progesterone, interferons and endogenous retroviruses. Reprod. Fertil. Dev. 2007, 19, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Hassanin, A.; Douzery, E.J. Molecular and morphological phylogenies of ruminantia and the alternative position of the moschidae. Syst. Biol. 2003, 52, 206–228. [Google Scholar] [CrossRef] [PubMed]
- Black, S.G.; Arnaud, F.; Burghardt, R.C.; Satterfield, M.C.; Fleming, J.A.; Long, C.R.; Hanna, C.; Murphy, L.; Biek, R.; Palmarini, M.; et al. Viral particles of endogenous betaretroviruses are released in the sheep uterus and infect the conceptus trophectoderm in a transspecies embryo transfer model. J. Virol. 2010, 84, 9078–9085. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, F.; Murcia, P.R.; Palmarini, M. Mechanisms of late restriction induced by an endogenous retrovirus. J. Virol. 2007, 81, 11441–11451. [Google Scholar] [CrossRef] [PubMed]
- Armezzani, A.; Arnaud, F.; Caporale, M.; di Meo, G.; Iannuzzi, L.; Murgia, C.; Palmarini, M. The signal peptide of a recently integrated endogenous sheep betaretrovirus envelope plays a major role in eluding gag-mediated late restriction. J. Virol. 2011, 85, 7118–7128. [Google Scholar] [CrossRef] [PubMed]
- Chessa, B.; Pereira, F.; Arnaud, F.; Amorim, A.; Goyache, F.; Mainland, I.; Kao, R.R.; Pemberton, J.M.; Beraldi, D.; Stear, M.J.; et al. Revealing the history of sheep domestication using retrovirus integrations. Science 2009, 324, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Martins, H.; Villesen, P. Improved integration time estimation of endogenous retroviruses with phylogenetic data. PLoS One 2011, 6, e14745. [Google Scholar] [CrossRef] [PubMed]
- Goodier, J.L.; Kazazian, H.H., Jr. Retrotransposons revisited: The restraint and rehabilitation of parasites. Cell 2008, 135, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Redelsperger, F.; Cornelis, G.; Vernochet, C.; Tennant, B.C.; Catzeflis, F.; Mulot, B.; Heidmann, O.; Heidmann, T.; Dupressoir, A. Capture of syncytin-Mar1, a fusogenic endogenous retroviral envelope gene involved in placentation in the Rodentia squirrel-related clade. J. Virol. 2014, 88, 7915–7928. [Google Scholar] [CrossRef] [PubMed]
- Dupressoir, A.; Marceau, G.; Vernochet, C.; Benit, L.; Kanellopoulos, C.; Sapin, V.; Heidmann, T. Syncytin-A and syncytin-B, two fusogenic placenta-specific murine envelope genes of retroviral origin conserved in Muridae. Proc. Natl. Acad. Sci. USA 2005, 102, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.; Heidmann, O.; Degrelle, S.A.; Vernochet, C.; Lavialle, C.; Letzelter, C.; Bernard-Stoecklin, S.; Hassanin, A.; Mulot, B.; Guillomot, M.; et al. Captured retroviral envelope syncytin gene associated with the unique placental structure of higher ruminants. Proc. Natl. Acad. Sci. USA 2013, 110, E828–E837. [Google Scholar] [CrossRef] [PubMed]
- Blaise, S.; de Parseval, N.; Benit, L.; Heidmann, T. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 13013–13018. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, K.A.; Palmarini, M.; Spencer, T.E. Ovine endogenous betaretroviruses (enJSRVs) and placental morphogenesis. Placenta 2006, 27 (Suppl. A), S135–S140. [Google Scholar] [CrossRef]
- Nitta, T.; Hofacre, A.; Hull, S.; Fan, H. Identification and mutational analysis of a Rej response element in Jaagsiekte sheep retrovirus RNA. J. Virol. 2009, 83, 12499–12511. [Google Scholar] [CrossRef] [PubMed]
- Hofacre, A.; Nitta, T.; Fan, H. Jaagsiekte sheep retrovirus encodes a regulatory factor, Rej, required for synthesis of Gag protein. J. Virol. 2009, 83, 12483–12498. [Google Scholar] [CrossRef] [PubMed]
- Caporale, M.; Arnaud, F.; Mura, M.; Golder, M.; Murgia, C.; Palmarini, M. The signal peptide of a simple retrovirus envelope functions as a posttranscriptional regulator of viral gene expression. J. Virol. 2009, 83, 4591–4604. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.; Halani, N.; Mertz, J.A.; Ali, A.F.; Lozano, M.M.; Dudley, J.P. Retroviral Rem protein requires processing by signal peptidase and retrotranslocation for nuclear function. Proc. Natl. Acad. Sci. USA 2010, 107, 12287–12292. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ren, X.M.; Xing, J.J.; Zheng, A.C. The nucleolus and viral infection. Virol. Sin. 2010, 25, 151–157. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armezzani, A.; Varela, M.; Spencer, T.E.; Palmarini, M.; Arnaud, F. “Ménage à Trois”: The Evolutionary Interplay between JSRV, enJSRVs and Domestic Sheep. Viruses 2014, 6, 4926-4945. https://doi.org/10.3390/v6124926
Armezzani A, Varela M, Spencer TE, Palmarini M, Arnaud F. “Ménage à Trois”: The Evolutionary Interplay between JSRV, enJSRVs and Domestic Sheep. Viruses. 2014; 6(12):4926-4945. https://doi.org/10.3390/v6124926
Chicago/Turabian StyleArmezzani, Alessia, Mariana Varela, Thomas E. Spencer, Massimo Palmarini, and Frédérick Arnaud. 2014. "“Ménage à Trois”: The Evolutionary Interplay between JSRV, enJSRVs and Domestic Sheep" Viruses 6, no. 12: 4926-4945. https://doi.org/10.3390/v6124926
APA StyleArmezzani, A., Varela, M., Spencer, T. E., Palmarini, M., & Arnaud, F. (2014). “Ménage à Trois”: The Evolutionary Interplay between JSRV, enJSRVs and Domestic Sheep. Viruses, 6(12), 4926-4945. https://doi.org/10.3390/v6124926



