Virus-Like Particles of Chimeric Recombinant Porcine Circovirus Type 2 as Antigen Vehicle Carrying Foreign Epitopes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses, Cells and Plasmids
2.2. Construction of Recombinant Baculoviruses
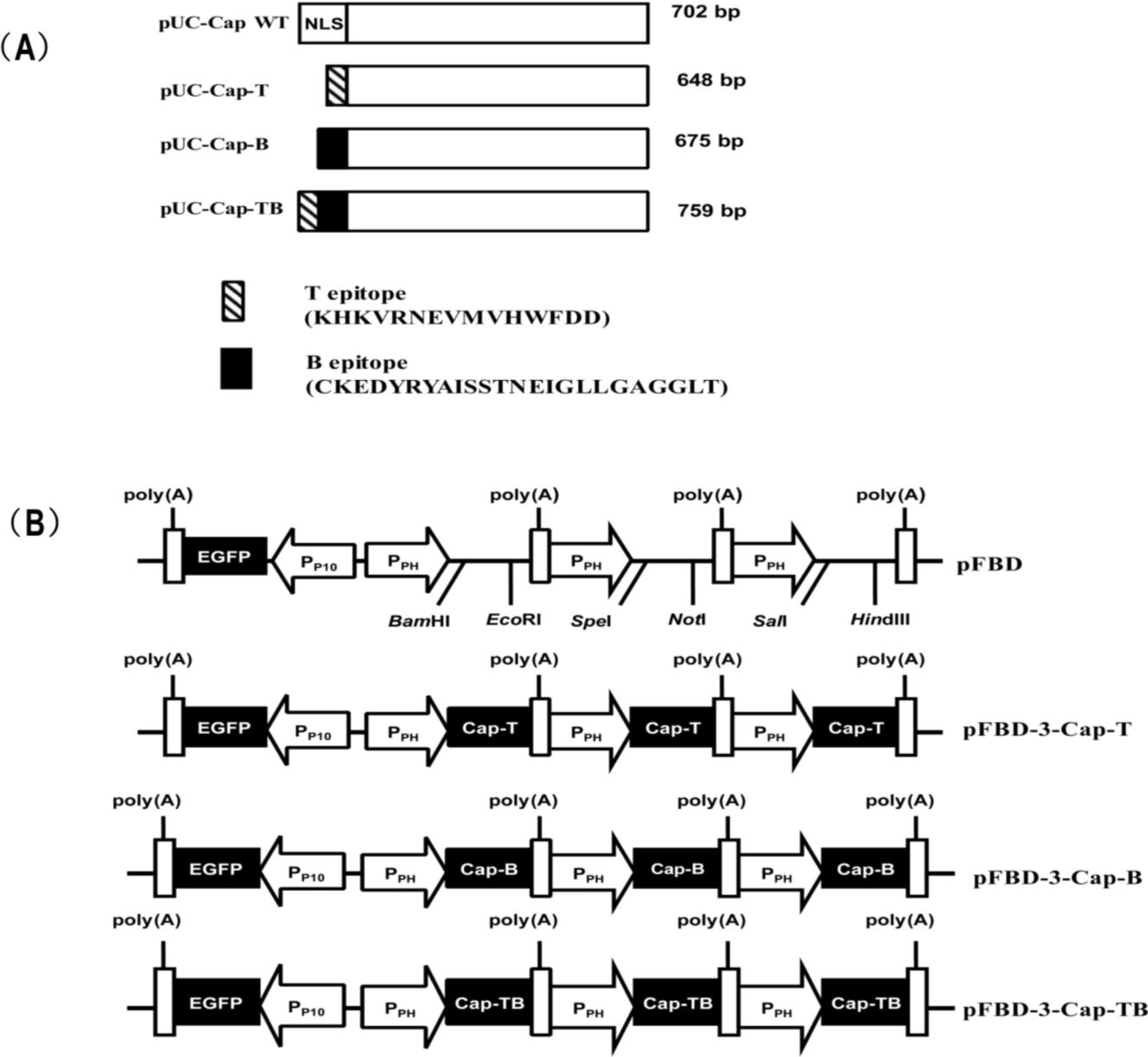
| Genes | Name | Primer sequence (5'–3') | Restriction site |
|---|---|---|---|
| Cap-T a | 1-F | TTGGATCCGCCACCATGAAACACAAAGTGAGGAATGAAGT | BamHI |
| 2-F | TTACTAGTGCCACCATGAAACACAAAGTGAGGAATGAAGT | SpeI | |
| 3-F | TTGTCGACGCCACCATGAAACACAAAGTGAGGAATGAAGT | SalI | |
| Cap-B b | 4-F | TTGGATCCGCCACCATGTGCAAGGAAGATTACAGGT | BamHI |
| 5-F | TTACTAGTGCCACCATGTGCAAGGAAGATTACAGGT | SpeI | |
| 6-F | TTGTCGACGCCACCATGTGCAAGGAAGATTACAGGT | SalI | |
| Cap c | 1-R | TTGAATTCTTACTTTGGGTTCAGTGGAGGGTCCT | EcoRI |
| 2-R | TTGCGGCCGCTTACTTTGGGTTCAGTGGAGGGTCCT | NotI | |
| 3-R | TTAAGCTTTTACTTTGGGTTCAGTGGAGGGTCCT | HindIII |
2.3. Western Blot
2.4. Confocal Microscopy
2.5. Electron Microscopy
2.6. Immunization of Mice
| Groups | Number of mice | Recombinant proteins | Type and composition | Immunization | Number of inoculation | Concentration |
|---|---|---|---|---|---|---|
| A | 6 | Cap-T | W/O/W, ISA 206 VG | IM | 2 | 0.2 µg/µL |
| B | 6 | Cap-B | W/O/W, ISA 206 VG | IM | 2 | 0.2 µg/µL |
| C | 6 | Cap-TB | W/O/W, ISA 206 VG | IM | 2 | 0.2 µg/µL |
| D | 6 | PBS | PBS | IM | 2 | - |
2.7. Indirect ELISA
2.8. Virus Neutralization Assay
2.9. IFN-γ Release Assays
2.10. Cytotoxicity Assay
2.11. Statistical Analysis
3. Results
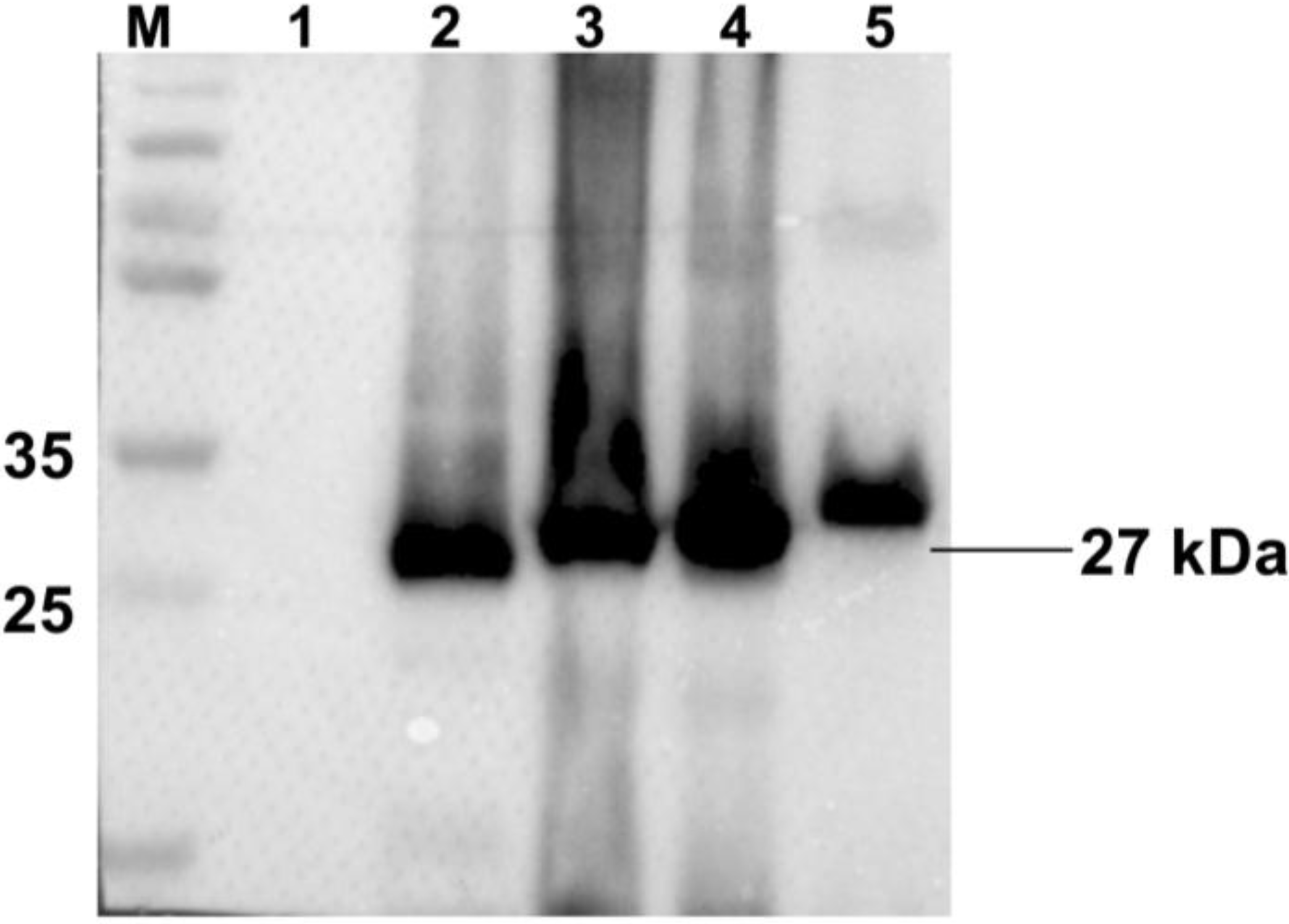
3.1. Expression of Recombinant Proteins
3.2. Electron Microscopy Analysis
3.3. PCV2-Specific Humoral Immune Responses
3.4. CSFV Peptide-Specific Immune Response
3.5. Cellular Immune Response
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Zhang, H.; Cao, H.; Wu, Z.; Cui, Y. A review of molecular characterization of classical swine fever virus (CSFV). J. Vet. Med. 2011, 66, 89–95. [Google Scholar]
- Wu, C.W.; Chien, M.S.; Liu, T.Y.; Lin, G.J.; Lee, W.C.; Huang, C. Characterization of the monoclonal antibody against classical swine fever virus glycoprotein Erns and its application to an indirect sandwich ELISA. Appl. Microbiol. Biotechnol. 2011, 92, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.; Fukusho, A.; Lefevre, P.C.; Lipowski, A.; Pejsak, Z.; Roehe, P.; Westergaard, J. Classical swine fever: The global situation. Vet. Microbial. 2000, 73, 103–119. [Google Scholar] [CrossRef]
- Pereda, A.; Greiser-Wilke, I.; Schmitt, B.; Rincon, M.; Mogollon, J.; Sabogal, Z.; Lora, A.; Sanguinetti, H.; Piccone, M. Phylogenetic analysis of classical swine fever virus (CSFV) field isolates from outbreaks in South and Central America. Virus Res. 2005, 110, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kaden, V.; Lange, E.; Polster, U.; Klopfleisch, R.; Teifke, J. Studies on the virulence of two field isolates of the classical Swine Fever virus genotype 2.3 rostock in wild boars of different age groups. J. Vet. Med. Ser. B 2004, 51, 202–208. [Google Scholar] [CrossRef]
- Edwards, S.; Paton, D. Antigenic differences among pestiviruses. Vet. Clin. North Am. Food Anim. Pract. 1995, 11, 563–577. [Google Scholar] [PubMed]
- Chen, N.; Tong, C.; Li, D.; Wan, J.; Yuan, X.; Li, X.; Peng, J.; Fang, W. Antigenic analysis of classical swine fever virus E2 glycoprotein using pig antibodies identifies residues contributing to antigenic variation of the vaccine C-strain and group 2 strains circulating in China. Virol. J. 2010, 7, e378. [Google Scholar] [CrossRef]
- Tarradas, J.; Monsó, M.; Fraile, L.; de la Torre, B.G.; Muñoz, M.; Rosell, R.; Riquelme, C.; Pérez, L.J.; Nofrarías, M.; Domingo, M. A T-cell epitope on NS3 non-structural protein enhances the B and T cell responses elicited by dendrimeric constructions against CSFV in domestic pigs. Vet. Immunol. Immunopathol. 2012, 150, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X.; Zhou, Y.J.; Yu, H.; Li, L.; Wang, Y.X.; Tong, W.; Hou, J.W.; Xu, Y.Z.; Zhu, J.P.; Xu, A.T. A novel dendrimeric peptide induces high level neutralizing antibodies against classical swine fever virus in rabbits. Vet. Microbial. 2012, 156, 200–204. [Google Scholar] [CrossRef]
- Dong, X.N.; Chen, Y.H. Marker vaccine strategies and candidate CSFV marker vaccines. Vaccine 2007, 25, 205–230. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.N.; Qi, Y.; Ying, J.; Chen, X.; Chen, Y.H. Candidate peptide-vaccine induced potent protection against CSFV and identified a principal sequential neutralizing determinant on E2. Vaccine 2006, 24, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Tarradas, J.; Monsó, M.; Munoz, M.; Rosell, R.; Fraile, L.; Frías, M.T.; Domingo, M.; Andreu, D.; Sobrino, F.; Ganges, L. Partial protection against classical swine fever virus elicited by dendrimeric vaccine-candidate peptides in domestic pigs. Vaccine 2011, 29, 4422–4429. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, R.; Magar, R.; D’Allaire, S. Genetic characterization and phylogenetic analysis of porcine circovirus type 2 (PCV2) strains from cases presenting various clinical conditions. Virus Res. 2002, 90, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Grau-Roma, L.; Crisci, E.; Sibila, M.; Lopez-Soria, S.; Nofrarias, M.; Cortey, M.; Fraile, L.; Olvera, A.; Segalés, J. A proposal on porcine circovirus type 2 (PCV2) genotype definition and their relation with postweaning multisystemic wasting syndrome (PMWS) occurrence. Vet. Microbial. 2008, 128, 23–35. [Google Scholar] [CrossRef]
- Fort, M.; Sibila, M.; Allepuz, A.; Mateu, E.; Roerink, F.; Segalés, J. Porcine circovirus type 2 (PCV2) vaccination of conventional pigs prevents viremia against PCV2 isolates of different genotypes and geographic origins. Vaccine 2008, 26, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Khayat, R.; Brunn, N.; Speir, J.A.; Hardham, J.M.; Ankenbauer, R.G.; Schneemann, A.; Johnson, J.E. The 2.3-angstrom structure of porcine circovirus 2. J. Virol. 2011, 85, 7856–7862. [Google Scholar] [CrossRef]
- Wu, P.C.; Lin, W.L.; Wu, C.M.; Chi, J.N.; Chien, M.S.; Huang, C. Characterization of porcine circovirus type 2 (PCV2) capsid particle assembly and its application to virus-like particle vaccine development. Appl. Microbial. Biotechnol. 2012, 95, 1501–1507. [Google Scholar] [CrossRef]
- Yin, S.; Sun, S.; Yang, S.; Shang, Y.; Cai, X.; Liu, X. Self-assembly of virus-like particles of porcine circovirus type 2 capsid protein expressed from Escherichia coli. Virol. J. 2010, 7, e166. [Google Scholar] [CrossRef]
- Fort, M.; Sibila, M.; Perez-Martin, E.; Nofrarias, M.; Mateu, E.; Segalés, J. One dose of a porcine circovirus 2 (PCV2) sub-unit vaccine administered to 3-week-old conventional piglets elicits cell-mediated immunity and significantly reduces PCV2 viremia in an experimental model. Vaccine 2009, 27, 4031–4037. [Google Scholar] [CrossRef] [PubMed]
- Crisci, E.; Bárcena, J.; Montoya, M. Virus-like particles: The new frontier of vaccines for animal viral infections. Vet. Immunol. Immunopathol. 2012, 148, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Tegerstedt, K.; Franzén, A.V.; Andreasson, K.; Joneberg, J.; Heidari, S.; Ramqvist, T.; Dalianis, T. Murine polyomavirus virus-like particles (VLPs) as vectors for gene and immune therapy and vaccines against viral infections and cancer. Anticancer Res. 2005, 25, 2601–2608. [Google Scholar] [PubMed]
- Zeltins, A. Construction and characterization of virus-like particles: A review. Mol. Biotechnol. 2013, 53, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ge, S.; Li, L.; Wu, X.; Liu, Z.; Wang, Z. Virus-like particles: Potential veterinary vaccine immunogens. Res. Vet. Sci. 2012, 93, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Sedlik, C.; Saron, M.F.; Sarraseca, J.; Casal, I.; Leclerc, C. Recombinant parvovirus-like particles as an antigen carrier: A novel nonreplicative exogenous antigen to elicit protective antiviral cytotoxic T cells. Proc. Natl. Acad. Sci. USA 1997, 94, 7503–7508. [Google Scholar] [CrossRef] [PubMed]
- Pushko, P.; Tumpey, T.M.; Bu, F.; Knell, J.; Robinson, R.; Smith, G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine 2005, 23, 5751–5759. [Google Scholar] [CrossRef] [PubMed]
- Fort, M.; Olvera, A.; Sibila, M.; Segalés, J.; Mateu, E. Detection of neutralizing antibodies in postweaning multisystemic wasting syndrome (PMWS)-affected and non-PMWS-affected pigs. Vet. Microbial. 2007, 125, 244–255. [Google Scholar] [CrossRef]
- Roques, E.; Girard, A.; Gagnon, C.A.; Archambault, D. Antibody responses induced in mice immunized with recombinant adenovectors expressing chimeric proteins of various porcine pathogens. Vaccine 2013, 31, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Palucha, A.; Loniewska, A.; Satheshkumar, S.; Boguszewska-Chachulska, A.M.; Umashankar, M.; Milner, M.; Haenni, A.L.; Savithri, H.S. Virus-like particles: Models for assembly studies and foreign epitope carriers. Prog. Nucleic Acid Res. Mol. Biol. 2005, 80, 135–168. [Google Scholar] [PubMed]
- Casal, J.I. Use of parvovirus-like particles for vaccination and induction of multiple immune responses. Biotechnol. Appl. Biochem. 1999, 29, 141–150. [Google Scholar] [PubMed]
- Strauss, R.; Hüser, A.; Ni, S.; Tuve, S.; Kiviat, N.; Sow, P.S.; Hofmann, C.; Lieber, A. Baculovirus-based vaccination vectors allow for efficient induction of immune responses against Plasmodium falciparum circumsporozoite protein. Mol. Ther. 2007, 15, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Noad, R.; Roy, P. Virus-like particles as immunogens. Trends Microbial. 2003, 11, 438–444. [Google Scholar] [CrossRef]
- Liu, L.J.; Suzuki, T.; Tsunemitsu, H.; Kataoka, M.; Ngata, N.; Takeda, N.; Wakita, T.; Miyamura, T.; Li, T.C. Efficient production of type 2 porcine circovirus-like particles by a recombinant baculovirus. Arch. Virol. 2008, 153, 2291–2295. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Pan, Y.; Fang, L.; Wang, D.; Wang, S.; Jiang, Y.; Chen, H.; Xiao, S. Construction and immunogenicity of recombinant pseudotype baculovirus expressing the capsid protein of porcine circovirus type 2 in mice. J. Virol. Methods 2008, 150, 21–26. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Qian, P.; Liu, L.; Qian, S.; Chen, H.; Li, X. Virus-Like Particles of Chimeric Recombinant Porcine Circovirus Type 2 as Antigen Vehicle Carrying Foreign Epitopes. Viruses 2014, 6, 4839-4855. https://doi.org/10.3390/v6124839
Zhang H, Qian P, Liu L, Qian S, Chen H, Li X. Virus-Like Particles of Chimeric Recombinant Porcine Circovirus Type 2 as Antigen Vehicle Carrying Foreign Epitopes. Viruses. 2014; 6(12):4839-4855. https://doi.org/10.3390/v6124839
Chicago/Turabian StyleZhang, Huawei, Ping Qian, Lifeng Liu, Suhong Qian, Huanchun Chen, and Xiangmin Li. 2014. "Virus-Like Particles of Chimeric Recombinant Porcine Circovirus Type 2 as Antigen Vehicle Carrying Foreign Epitopes" Viruses 6, no. 12: 4839-4855. https://doi.org/10.3390/v6124839
APA StyleZhang, H., Qian, P., Liu, L., Qian, S., Chen, H., & Li, X. (2014). Virus-Like Particles of Chimeric Recombinant Porcine Circovirus Type 2 as Antigen Vehicle Carrying Foreign Epitopes. Viruses, 6(12), 4839-4855. https://doi.org/10.3390/v6124839




