Implication of Human Endogenous Retrovirus Envelope Proteins in Placental Functions
Abstract
:1. Introduction
2. ERVs and Placenta Development
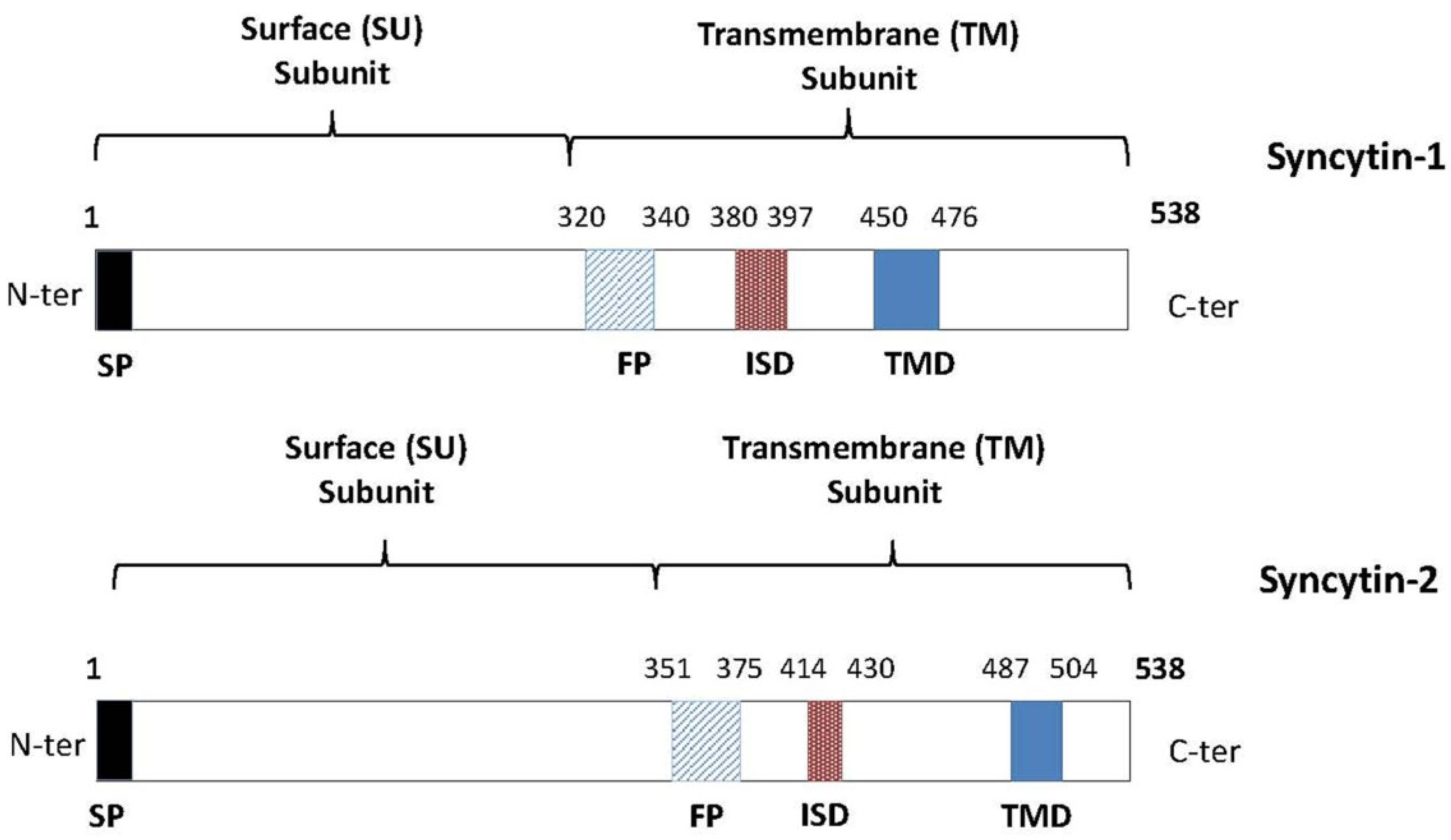
3. Syncytin-1 and Syncytin-2: Potential Mediators of Immune Tolerance
4. Exosomes and the Placenta
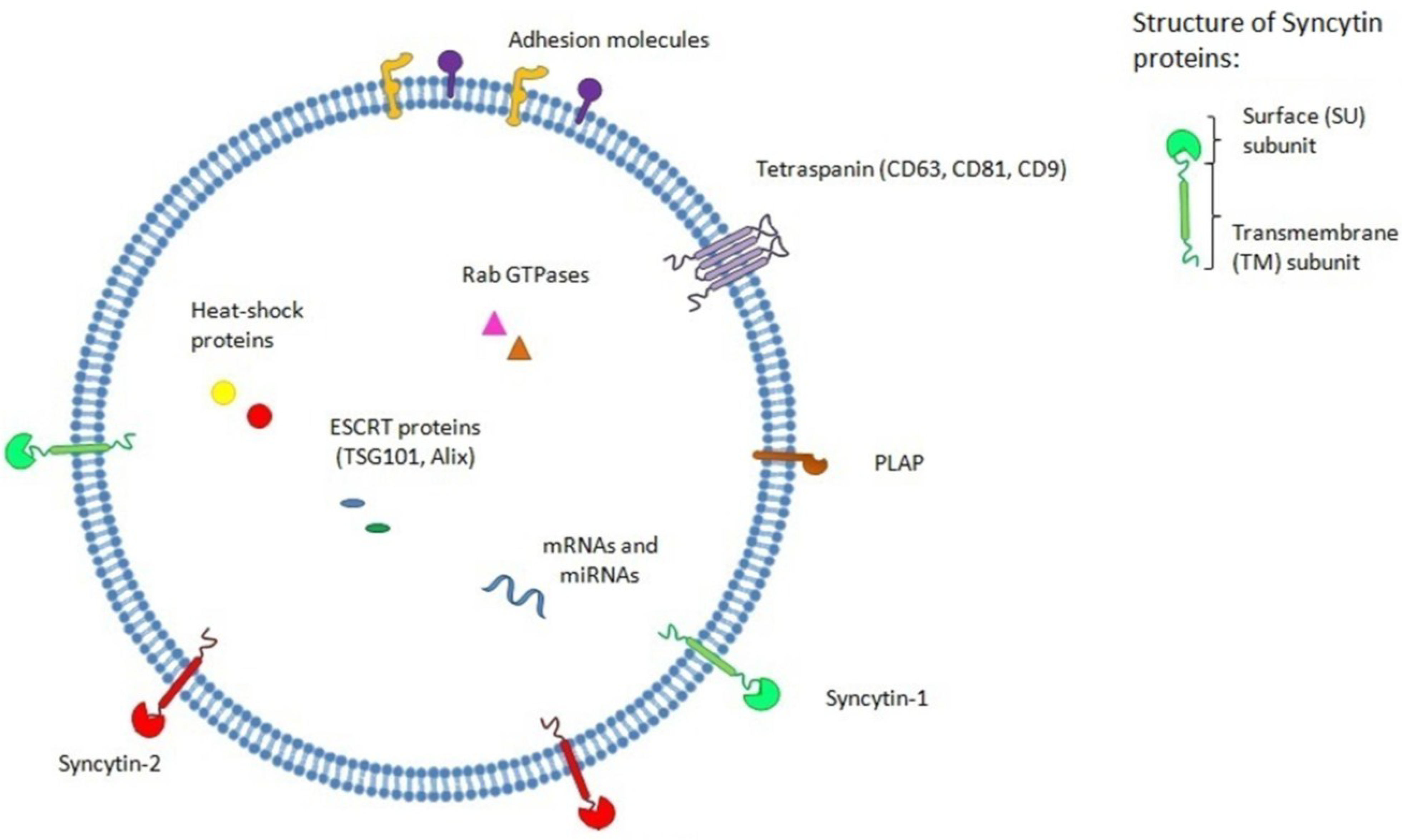
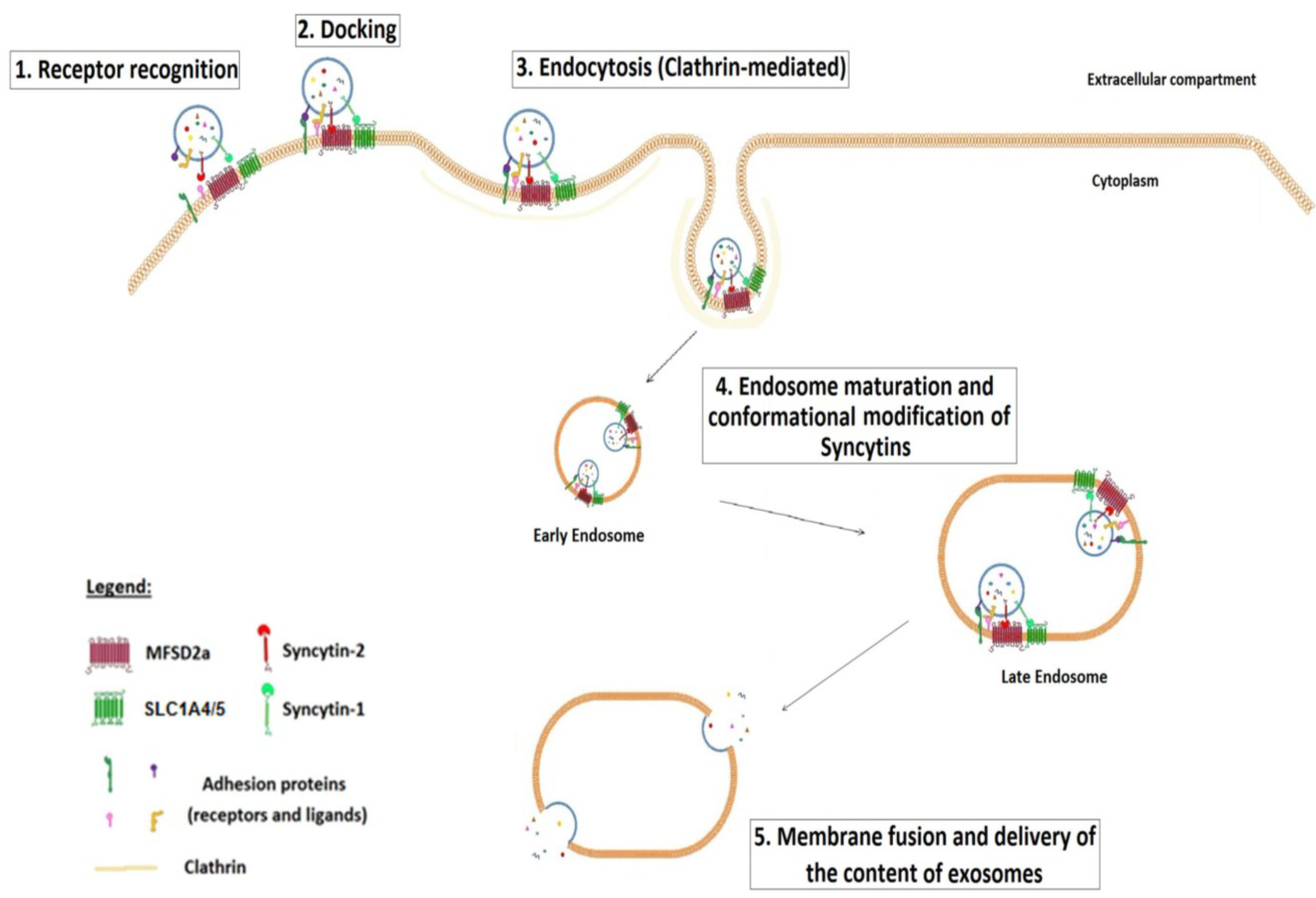
5. Placenta Exosomes, Syncytin and Modulation of the Immune Response
6. Association between Downregulation of Syncytin-1 and -2 and Preeclampsia and Their Use as New Potential Biomarkers
7. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Dewannieux, M.; Heidmann, T. Endogenous retroviruses: Acquisition, amplification and taming of genome invaders. Curr. Opin. Virol. 2013, 3, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Stoye, J.P. Studies of endogenous retroviruses reveal a continuing evolutionary saga. Nat. Rev. Microbiol. 2012, 10, 395–406. [Google Scholar] [PubMed]
- Magiorkinis, G.; Gifford, R.J.; Katzourakis, A.; de Ranter, J.; Belshaw, R. Env-less endogenous retroviruses are genomic superspreaders. Proc. Natl. Acad. Sci. USA 2012, 109, 7385–7390. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.; Andersson, G. Beneficial role of human endogenous retroviruses: Facts and hypotheses. Scand. J. Immunol. 1998, 48, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Blikstad, V.; Benachenhou, F.; Sperber, G.O.; Blomberg, J. Evolution of human endogenous retroviral sequences: A conceptual account. Cell. Mol. Life Sci. 2008, 65, 3348–3365. [Google Scholar] [CrossRef] [PubMed]
- Kurth, R.; Bannert, N. Beneficial and detrimental effects of human endogenous retroviruses. Int. J. Cancer J. Int. Cancer 2010, 126, 306–314. [Google Scholar] [CrossRef]
- Blomberg, J.; Benachenhou, F.; Blikstad, V.; Sperber, G.; Mayer, J. Classification and nomenclature of endogenous retroviral sequences (ervs): Problems and recommendations. Gene 2009, 448, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Belshaw, R.; Dawson, A.L.; Woolven-Allen, J.; Redding, J.; Burt, A.; Tristem, M. Genomewide screening reveals high levels of insertional polymorphism in the human endogenous retrovirus family herv-k(hml2): Implications for present-day activity. J. Virol. 2005, 79, 12507–12514. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, R.P.; Wildschutte, J.H.; Russo, C.; Coffin, J.M. Identification, characterization, and comparative genomic distribution of the herv-k (hml-2) group of human endogenous retroviruses. Retrovirology 2011, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Antony, J.M.; van Marle, G.; Opii, W.; Butterfield, D.A.; Mallet, F.; Yong, V.W.; Wallace, J.L.; Deacon, R.M.; Warren, K.; Power, C. Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat. Neurosci. 2004, 7, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Lower, R.; Lower, J.; Kurth, R. The viruses in all of us: Characteristics and biological significance of human endogenous retrovirus sequences. Proc. Natl. Acad. Sci. USA 1996, 93, 5177–5184. [Google Scholar] [CrossRef]
- Wang-Johanning, F.; Frost, A.R.; Jian, B.; Azerou, R.; Lu, D.W.; Chen, D.T.; Johanning, G.L. Detecting the expression of human endogenous retrovirus e envelope transcripts in human prostate adenocarcinoma. Cancer 2003, 98, 187–197. [Google Scholar] [PubMed]
- Wang-Johanning, F.; Frost, A.R.; Jian, B.; Epp, L.; Lu, D.W.; Johanning, G.L. Quantitation of herv-k env gene expression and splicing in human breast cancer. Oncogene 2003, 22, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Muyan, M.; Boime, I. Secretion of chorionic gonadotropin from human trophoblasts. Placenta 1997, 18, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Handwerger, S.; Freemark, M. The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development. J. Pediatr. Endocrinol. Metab. 2000, 13, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.C.; Guibourdenche, J.; Frendo, J.L.; Pidoux, G.; Evain-Brion, D. Placental growth hormones. Endocrine 2002, 19, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Suano, A.; Hamilton, A.B.; Betz, A.G. Gimme shelter: The immune system during pregnancy. Immunol. Rev. 2011, 241, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, O. Children's immunology, what can we learn from animal studies (1): Decidual cells induce specific immune system of feto-maternal interface. J. Toxicol. Sci. 2009, 34, SP331–SP339. [Google Scholar] [PubMed]
- Warning, J.C.; McCracken, S.A.; Morris, J.M. A balancing act: Mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction 2011, 141, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Blaise, S.; de Parseval, N.; Benit, L.; Heidmann, T. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 13013–13018. [Google Scholar] [CrossRef] [PubMed]
- Blaise, S.; de Parseval, N.; Heidmann, T. Functional characterization of two newly identified human endogenous retrovirus coding envelope genes. Retrovirology 2005, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Lee, X.; Li, X.; Veldman, G.M.; Finnerty, H.; Racie, L.; LaVallie, E.; Tang, X.Y.; Edouard, P.; Howes, S.; et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 2000, 403, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Moreau, J.; Landry, S.; LeBellego, F.; Toufaily, C.; Rassart, E.; Lafond, J.; Barbeau, B. Syncytin-2 plays an important role in the fusion of human trophoblast cells. J. Mol. Biol. 2009, 392, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, U.; Germeyer, A.; Stengel, S.; Kapp, M.; Denner, J. Human endogenous retrovirus k (herv-k) is expressed in villous and extravillous cytotrophoblast cells of the human placenta. J. Reprod. Immunol. 2011, 91, 1–8. [Google Scholar] [PubMed]
- Blond, J.L.; Beseme, F.; Duret, L.; Bouton, O.; Bedin, F.; Perron, H.; Mandrand, B.; Mallet, F. Molecular characterization and placental expression of herv-w, a new human endogenous retrovirus family. J. Virol. 1999, 73, 1175–1185. [Google Scholar] [PubMed]
- Blond, J.L.; Lavillette, D.; Cheynet, V.; Bouton, O.; Oriol, G.; Chapel-Fernandes, S.; Mandrand, B.; Mallet, F.; Cosset, F.L. An envelope glycoprotein of the human endogenous retrovirus herv-w is expressed in the human placenta and fuses cells expressing the type d mammalian retrovirus receptor. J. Virol. 2000, 74, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Frendo, J.L.; Olivier, D.; Cheynet, V.; Blond, J.L.; Bouton, O.; Vidaud, M.; Rabreau, M.; Evain-Brion, D.; Mallet, F. Direct involvement of herv-w env glycoprotein in human trophoblast cell fusion and differentiation. Mol. Cell. Biol. 2003, 23, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Hayward, M.D.; Potgens, A.J.; Drewlo, S.; Kaufmann, P.; Rasko, J.E. Distribution of human endogenous retrovirus type w receptor in normal human villous placenta. Pathology 2007, 39, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Muir, A.; Lever, A.M.; Moffett, A. Human endogenous retrovirus-w envelope (syncytin) is expressed in both villous and extravillous trophoblast populations. J. Gen. Virol. 2006, 87, 2067–2071. [Google Scholar] [CrossRef] [PubMed]
- Malassine, A.; Handschuh, K.; Tsatsaris, V.; Gerbaud, P.; Cheynet, V.; Oriol, G.; Mallet, F.; Evain-Brion, D. Expression of herv-w env glycoprotein (syncytin) in the extravillous trophoblast of first trimester human placenta. Placenta 2005, 26, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Handwerger, S. A placenta-specific enhancer of the human syncytin gene. Biol. Reprod. 2005, 73, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Richardson, B.D.; Hubert, M.A.; Handwerger, S. Isolation and characterization of the human syncytin gene promoter. Biol. Reprod. 2004, 70, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Shen, K.; Lin, M.; Chen, P.; Lin, C.; Chang, G.D.; Chen, H. Gcma regulates the syncytin-mediated trophoblastic fusion. J. Biol. Chem. 2002, 277, 50062–50068. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Chang, G.D.; Chen, H. A novel cyclic amp/epac1/camki signaling cascade promotes gcm1 desumoylation and placental cell fusion. Mol. Cell. Biol. 2011, 31, 3820–3831. [Google Scholar] [CrossRef] [PubMed]
- Prudhomme, S.; Oriol, G.; Mallet, F. A retroviral promoter and a cellular enhancer define a bipartite element which controls env ervwe1 placental expression. J. Virol. 2004, 78, 12157–12168. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.W.; Li, J.; Brost, B.C.; Xia, X.Y.; Chen, H.B.; Wang, C.X.; Jiang, S.W. Decreased expression and altered methylation of syncytin-1 gene in human placentas associated with preeclampsia. Curr. Pharm. Des. 2014, 20, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Matouskova, M.; Blazkova, J.; Pajer, P.; Pavlicek, A.; Hejnar, J. Cpg methylation suppresses transcriptional activity of human syncytin-1 in non-placental tissues. Exp. Cell Res. 2006, 312, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Nellaker, C.; Sabunciyan, S.; Yolken, R.H.; Jones-Brando, L.; Johansson, A.S.; Owe-Larsson, B.; Karlsson, H. Transcriptional derepression of the ervwe1 locus following influenza a virus infection. J. Virol. 2014, 88, 4328–4337. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, J.; Montgiraud, C.; Oriol, G.; Pichon, J.P.; Ruel, K.; Tsatsaris, V.; Gerbaud, P.; Frendo, J.L.; Evain-Brion, D.; Mallet, F. Comparative methylation of ervwe1/syncytin-1 and other human endogenous retrovirus ltrs in placenta tissues. DNA Res.: Int. J. Rapid Publ. Rep. Genes Genomes 2009, 16, 195–211. [Google Scholar]
- Trejbalova, K.; Blazkova, J.; Matouskova, M.; Kucerova, D.; Pecnova, L.; Vernerova, Z.; Heracek, J.; Hirsch, I.; Hejnar, J. Epigenetic regulation of transcription and splicing of syncytins, fusogenic glycoproteins of retroviral origin. Nucleic Acids Res. 2011, 39, 8728–8739. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Boyd, C.A. Changes in expression and function of syncytin and its receptor, amino acid transport system b(0) (asct2), in human placental choriocarcinoma bewo cells during syncytialization. Placenta 2002, 23, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.; Lavillette, D.; Kelly, S.M.; Kabat, D. N-linked glycosylation and sequence changes in a critical negative control region of the asct1 and asct2 neutral amino acid transporters determine their retroviral receptor functions. J. Virol. 2003, 77, 2936–2945. [Google Scholar] [CrossRef] [PubMed]
- Sommerfelt, M.A. Retrovirus receptors. J. Gen. Virol. 1999, 80, 3049–3064. [Google Scholar] [PubMed]
- Huppertz, B.; Bartz, C.; Kokozidou, M. Trophoblast fusion: Fusogenic proteins, syncytins and adams, and other prerequisites for syncytial fusion. Micron 2006, 37, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Malassine, A.; Blaise, S.; Handschuh, K.; Lalucque, H.; Dupressoir, A.; Evain-Brion, D.; Heidmann, T. Expression of the fusogenic herv-frd env glycoprotein (syncytin 2) in human placenta is restricted to villous cytotrophoblastic cells. Placenta 2007, 28, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Y.; Wang, L.J.; Chen, C.P.; Chen, L.F.; Chen, Y.H.; Chen, H. Gcm1 regulation of the expression of syncytin 2 and its cognate receptor mfsd2a in human placenta. Biol. Reprod. 2010, 83, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Esnault, C.; Priet, S.; Ribet, D.; Vernochet, C.; Bruls, T.; Lavialle, C.; Weissenbach, J.; Heidmann, T. A placenta-specific receptor for the fusogenic, endogenous retrovirus-derived, human syncytin-2. Proc. Natl. Acad. Sci. USA 2008, 105, 17532–17537. [Google Scholar] [CrossRef] [PubMed]
- Toufaily, C.; Vargas, A.; Lemire, M.; Lafond, J.; Rassart, E.; Barbeau, B. Mfsd2a, the syncytin-2 receptor, is important for trophoblast fusion. Placenta 2013, 34, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Thiery, M.; Lafond, J.; Barbeau, B. Transcriptional and functional studies of human endogenous retrovirus envelope envp(b) and envv genes in human trophoblasts. Virology 2012, 425, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kjeldbjerg, A.L.; Villesen, P.; Aagaard, L.; Pedersen, F.S. Gene conversion and purifying selection of a placenta-specific erv-v envelope gene during simian evolution. BMC Evol. Biol. 2008, 8, 266. [Google Scholar] [CrossRef] [PubMed]
- Esnault, C.; Cornelis, G.; Heidmann, O.; Heidmann, T. Differential evolutionary fate of an ancestral primate endogenous retrovirus envelope gene, the envv syncytin, captured for a function in placentation. PLoS Genet. 2013, 9, e1003400. [Google Scholar] [CrossRef] [PubMed]
- Lavialle, C.; Cornelis, G.; Dupressoir, A.; Esnault, C.; Heidmann, O.; Vernochet, C.; Heidmann, T. Paleovirology of 'syncytins', retroviral env genes exapted for a role in placentation. Philos. Trans. R. Soc. Lon. Ser. B Biol. Sci. 2013, 368, 20120507. [Google Scholar] [CrossRef]
- Chuong, E.B. Retroviruses facilitate the rapid evolution of the mammalian placenta. BioEssays 2013, 35, 853–861. [Google Scholar] [PubMed]
- Dupressoir, A.; Marceau, G.; Vernochet, C.; Benit, L.; Kanellopoulos, C.; Sapin, V.; Heidmann, T. Syncytin-a and syncytin-b, two fusogenic placenta-specific murine envelope genes of retroviral origin conserved in muridae. Proc. Natl. Acad. Sci. USA 2005, 102, 725–730. [Google Scholar] [PubMed]
- Dupressoir, A.; Vernochet, C.; Bawa, O.; Harper, F.; Pierron, G.; Opolon, P.; Heidmann, T. Syncytin-a knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc. Natl. Acad. Sci. USA 2009, 106, 12127–12132. [Google Scholar] [PubMed]
- Dupressoir, A.; Vernochet, C.; Harper, F.; Guegan, J.; Dessen, P.; Pierron, G.; Heidmann, T. A pair of co-opted retroviral envelope syncytin genes is required for formation of the two-layered murine placental syncytiotrophoblast. Proc. Natl. Acad. Sci. USA 2011, 108, E1164–E1173. [Google Scholar] [CrossRef] [PubMed]
- Heidmann, O.; Vernochet, C.; Dupressoir, A.; Heidmann, T. Identification of an endogenous retroviral envelope gene with fusogenic activity and placenta-specific expression in the rabbit: A new "syncytin" in a third order of mammals. Retrovirology 2009, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.; Heidmann, O.; Bernard-Stoecklin, S.; Reynaud, K.; Veron, G.; Mulot, B.; Dupressoir, A.; Heidmann, T. Ancestral capture of syncytin-car1, a fusogenic endogenous retroviral envelope gene involved in placentation and conserved in carnivora. Proc. Natl. Acad. Sci. USA 2012, 109, E432–E441. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.; Heidmann, O.; Degrelle, S.A.; Vernochet, C.; Lavialle, C.; Letzelter, C.; Bernard-Stoecklin, S.; Hassanin, A.; Mulot, B.; Guillomot, M.; et al. Captured retroviral envelope syncytin gene associated with the unique placental structure of higher ruminants. Proc. Natl. Acad. Sci. USA 2013, 110, E828–E837. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.; Vernochet, C.; Malicorne, S.; Souquere, S.; Tzika, A.C.; Goodman, S.M.; Catzeflis, F.; Robinson, T.J.; Milinkovitch, M.C.; Pierron, G.; et al. Retroviral envelope syncytin capture in an ancestrally diverged mammalian clade for placentation in the primitive afrotherian tenrecs. Proc. Natl. Acad. Sci. USA 2014. [Google Scholar] [CrossRef]
- Redelsperger, F.; Cornelis, G.; Vernochet, C.; Tennant, B.C.; Catzeflis, F.; Mulot, B.; Heidmann, O.; Heidmann, T.; Dupressoir, A. Capture of syncytin-mar1, a fusogenic endogenous retroviral envelope gene involved in placentation in the rodentia squirrel-related clade. J. Virol. 2014, 88, 7915–7928. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, K.A.; Palmarini, M.; Varela, M.; Burghardt, R.C.; Hayashi, K.; Farmer, J.L.; Spencer, T.E. Endogenous retroviruses regulate periimplantation placental growth and differentiation. Proc. Natl. Acad. Sci. USA 2006, 103, 14390–14395. [Google Scholar] [CrossRef] [PubMed]
- Benit, L.; Dessen, P.; Heidmann, T. Identification, phylogeny, and evolution of retroviral elements based on their envelope genes. J. Virol 2001, 75, 11709–11719. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Chen, L.F.; Yang, S.R.; Chen, C.Y.; Ko, C.C.; Chang, G.D.; Chen, H. Functional characterization of the human placental fusogenic membrane protein syncytin 2. Biol. Reprod. 2008, 79, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Cheynet, V.; Ruggieri, A.; Oriol, G.; Blond, J.L.; Boson, B.; Vachot, L.; Verrier, B.; Cosset, F.L.; Mallet, F. Synthesis, assembly, and processing of the env ervwe1/syncytin human endogenous retroviral envelope. J. Virol. 2005, 79, 5585–5593. [Google Scholar] [CrossRef] [PubMed]
- Cheynet, V.; Oriol, G.; Mallet, F. Identification of the hasct2-binding domain of the env ervwe1/syncytin-1 fusogenic glycoprotein. Retrovirology 2006, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Gong, R.; Peng, X.; Kang, S.; Feng, H.; Huang, J.; Zhang, W.; Lin, D.; Tien, P.; Xiao, G. Structural characterization of the fusion core in syncytin, envelope protein of human endogenous retrovirus family w. Biochem. Biophys. Res. Commun. 2005, 331, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, S.; Good, R.A.; Day-Good, N.K. A potent immunosuppressive retroviral peptide: Cytokine patterns and signaling pathways. Immunol. Res. 2008, 41, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Denner, J. The transmembrane proteins contribute to immunodeficiencies induced by hiv-1 and other retroviruses. AIDS 2014, 28, 1081–1090. [Google Scholar] [PubMed]
- Schlecht-Louf, G.; Renard, M.; Mangeney, M.; Letzelter, C.; Richaud, A.; Ducos, B.; Bouallaga, I.; Heidmann, T. Retroviral infection in vivo requires an immune escape virulence factor encrypted in the envelope protein of oncoretroviruses. Proc. Natl. Acad. Sci. USA 2010, 107, 3782–3787. [Google Scholar] [CrossRef] [PubMed]
- Schlecht-Louf, G.; Mangeney, M.; El-Garch, H.; Lacombe, V.; Poulet, H.; Heidmann, T. A targeted mutation within the feline leukemia virus (felv) envelope protein immunosuppressive domain to improve a canarypox virus-vectored felv vaccine. J. Virol. 2014, 88, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Cianciolo, G.J.; Bogerd, H.P.; Kipnis, R.J.; Copeland, T.D.; Oroszlan, S.; Snyderman, R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to envelope proteins of human and animal retroviruses. Trans. Assoc. Am. Physicians 1985, 98, 30–41. [Google Scholar] [PubMed]
- Haraguchi, S.; Good, R.A.; Cianciolo, G.J.; Engelman, R.W.; Day, N.K. Immunosuppressive retroviral peptides: Immunopathological implications for immunosuppressive influences of retroviral infections. J. Leukoc. Biol. 1997, 61, 654–666. [Google Scholar] [PubMed]
- Haraguchi, S.; Good, R.A.; Day, N.K. Immunosuppressive retroviral peptides: Camp and cytokine patterns. Immunol. Today 1995, 16, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Mangeney, M.; Renard, M.; Schlecht-Louf, G.; Bouallaga, I.; Heidmann, O.; Letzelter, C.; Richaud, A.; Ducos, B.; Heidmann, T. Placental syncytins: Genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins. Proc. Natl. Acad. Sci. USA 2007, 104, 20534–20539. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, J.M.; Schjenken, J.E.; Clifton, V.L.; Vargas, A.; Barbeau, B.; Lowry, P.; Maiti, K.; Smith, R. The endogenous retroviral envelope protein syncytin-1 inhibits lps/pha-stimulated cytokine responses in human blood and is sorted into placental exosomes. Placenta 2012, 33, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Holder, B.S.; Tower, C.L.; Forbes, K.; Mulla, M.J.; Aplin, J.D.; Abrahams, V.M. Immune cell activation by trophoblast-derived microvesicles is mediated by syncytin 1. Immunology 2012, 136, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Record, M. Intercellular communication by exosomes in placenta: A possible role in cell fusion? Placenta 2014, 35, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Sargent, I.L. Microparticles and immunomodulation in pregnancy and pre-eclampsia. J. Reprod. Immunol. 2007, 76, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Tannetta, D.S.; Dragovic, R.A.; Gardiner, C.; Southcombe, J.H.; Collett, G.P.; Sargent, I.L. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta 2012, 33, S48–S54. [Google Scholar] [CrossRef] [PubMed]
- Mincheva-Nilsson, L.; Baranov, V. The role of placental exosomes in reproduction. Am. J. Reprod. Immunol. 2010, 63, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Mincheva-Nilsson, L.; Baranov, V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: Immune modulation for pregnancy success. Am. J. Reprod. Immunol. 2014. [Google Scholar] [CrossRef]
- Vargas, A.; Zhou, S.; Ethier-Chiasson, M.; Flipo, D.; Lafond, J.; Gilbert, C.; Barbeau, B. Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. FASEB J. 2014, 28, 3703–3719. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell. Biol 1983, 97, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.; Heuser, J.; Stahl, P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: Demonstration of a pathway for receptor shedding. Eur. J. Cell Biol. 1984, 35, 256–263. [Google Scholar] [PubMed]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [PubMed]
- Denzer, K.; van Eijk, M.; Kleijmeer, M.J.; Jakobson, E.; de Groot, C.; Geuze, H.J. Follicular dendritic cells carry mhc class ii-expressing microvesicles at their surface. J. Immunol. 2000, 165, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Boussac, M.; Veron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic analysis of dendritic cell-derived exosomes: A secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef] [PubMed]
- Faure, J.; Lachenal, G.; Court, M.; Hirrlinger, J.; Chatellard-Causse, C.; Blot, B.; Grange, J.; Schoehn, G.; Goldberg, Y.; Boyer, V.; et al. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 2006, 31, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Guescini, M.; Genedani, S.; Stocchi, V.; Agnati, L.F. Astrocytes and glioblastoma cells release exosomes carrying mtdna. J. Neural Transm. 2010, 117, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Oh, S.; Ahn, S.M.; Lee, B.H.; Moon, M.H. Proteomic analysis of exosomes from human neural stem cells by flow field-flow fractionation and nanoflow liquid chromatography-tandem mass spectrometry. J. Proteome Res. 2008, 7, 3475–3480. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by msc reduces myocardial ischemia/reperfusion injury. Stem Cell. Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Atay, S.; Gercel-Taylor, C.; Suttles, J.; Mor, G.; Taylor, D.D. Trophoblast-derived exosomes mediate monocyte recruitment and differentiation. Am. J. Reprod. Immunol. 2011, 65, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.S.; Ishibashi, O.; Ishikawa, G.; Ishikawa, T.; Katayama, A.; Mishima, T.; Takizawa, T.; Shigihara, T.; Goto, T.; Izumi, A.; et al. Human villous trophoblasts express and secrete placenta-specific micrornas into maternal circulation via exosomes. Biol. Reprod. 2009, 81, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, J. Potential of cancer cell-derived exosomes in clinical application: A review of recent research advances. Clin. Ther. 2014, 36, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Jensen, S.S.; Lim, J.W. Proteomic profiling of exosomes: Current perspectives. Proteomics 2008, 8, 4083–4099. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell. Biol. 2006. [Google Scholar] [CrossRef]
- Hanson, P.I.; Cashikar, A. Multivesicular body morphogenesis. Annu. Rev. Cell. Dev. Biol. 2012, 28, 337–362. [Google Scholar] [CrossRef] [PubMed]
- Atay, S.; Gercel-Taylor, C.; Kesimer, M.; Taylor, D.D. Morphologic and proteomic characterization of exosomes released by cultured extravillous trophoblast cells. Exp. Cell Res. 2011, 317, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Cantin, R.; Diou, J.; Belanger, D.; Tremblay, A.M.; Gilbert, C. Discrimination between exosomes and hiv-1: Purification of both vesicles from cell-free supernatants. J. Immunol. Methods 2008, 338, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Savina, A.; Vidal, M.; Colombo, M.I. The exosome pathway in k562 cells is regulated by rab11. J. Cell. Sci. 2002, 115, 2505–2515. [Google Scholar] [PubMed]
- Simpson, R.J.; Kalra, H.; Mathivanan, S. Exocarta as a resource for exosomal research. J. Extracell Vesicles 2012, 1. [Google Scholar] [CrossRef]
- Donker, R.B.; Mouillet, J.F.; Chu, T.; Hubel, C.A.; Stolz, D.B.; Morelli, A.E.; Sadovsky, Y. The expression profile of c19mc micrornas in primary human trophoblast cells and exosomes. Mol. Hum. Reprod. 2012, 18, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Mouillet, J.F.; Coyne, C.B.; Sadovsky, Y. Review: Placenta-specific micrornas in exosomes - good things come in nano-packages. Placenta 2014, 35, S69–S73. [Google Scholar] [CrossRef] [PubMed]
- Delorme-Axford, E.; Donker, R.B.; Mouillet, J.F.; Chu, T.; Bayer, A.; Ouyang, Y.; Wang, T.; Stolz, D.B.; Sarkar, S.N.; Morelli, A.E.; et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc. Natl. Acad. Sci. USA 2013, 110, 12048–12053. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Ryan, J.; Sobrevia, L.; Kobayashi, M.; Ashman, K.; Mitchell, M.; Rice, G.E. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One 2013, 8, e68451. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Torres, M.J.; Kobayashi, M.; Scholz-Romero, K.; Sobrevia, L.; Dobierzewska, A.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS One 2014, 9, e98667. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Yee, S.; Scholz-Romero, K.; Kobayashi, M.; Vaswani, K.; Kvaskoff, D.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E. Extravillous trophoblast cells-derived exosomes promote vascular smooth muscle cell migration. Front. Pharmacol. 2014, 5, 175. [Google Scholar] [CrossRef] [PubMed]
- Wurdinger, T.; Gatson, N.N.; Balaj, L.; Kaur, B.; Breakefield, X.O.; Pegtel, D.M. Extracellular vesicles and their convergence with viral pathways. Adv. Virol. 2012, 2012, 767694. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.D.; Akyol, S.; Gercel-Taylor, C. Pregnancy-associated exosomes and their modulation of t cell signaling. J. Immunol. 2006, 176, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Sabapatha, A.; Gercel-Taylor, C.; Taylor, D.D. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am. J. Reprod. Immunol. 2006, 56, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Stenqvist, A.C.; Nagaeva, O.; Baranov, V.; Mincheva-Nilsson, L. Exosomes secreted by human placenta carry functional fas ligand and trail molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J. Immunol. 2013, 191, 5515–5523. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, M.; Stenqvist, A.C.; Nagaeva, O.; Kjellberg, L.; Wulff, M.; Baranov, V.; Mincheva-Nilsson, L. Human placenta expresses and secretes nkg2d ligands via exosomes that down-modulate the cognate receptor expression: Evidence for immunosuppressive function. J. Immunol. 2009, 183, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Mincheva-Nilsson, L.; Nagaeva, O.; Chen, T.; Stendahl, U.; Antsiferova, J.; Mogren, I.; Hernestal, J.; Baranov, V. Placenta-derived soluble mhc class i chain-related molecules down-regulate nkg2d receptor on peripheral blood mononuclear cells during human pregnancy: A possible novel immune escape mechanism for fetal survival. J. Immunol. 2006, 176, 3585–3592. [Google Scholar] [CrossRef] [PubMed]
- Atay, S.; Gercel-Taylor, C.; Taylor, D.D. Human trophoblast-derived exosomal fibronectin induces pro-inflammatory il-1beta production by macrophages. Am. J. Reprod. Immunol. 2011, 66, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Holder, B.S.; Tower, C.L.; Abrahams, V.M.; Aplin, J.D. Syncytin 1 in the human placenta. Placenta 2012, 33, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Genbacev, O.; Joslin, R.; Damsky, C.H.; Polliotti, B.M.; Fisher, S.J. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J. Clin. Invest. 1996, 97, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Redline, R.W.; Patterson, P. Pre-eclampsia is associated with an excess of proliferative immature intermediate trophoblast. Hum. Pathol. 1995, 26, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Toufaily, C.; LeBellego, F.; Rassart, E.; Lafond, J.; Barbeau, B. Reduced expression of both syncytin 1 and syncytin 2 correlates with severity of preeclampsia. Reprod. Sci. 2011, 18, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Knerr, I.; Huppertz, B.; Weigel, C.; Dotsch, J.; Wich, C.; Schild, R.L.; Beckmann, M.W.; Rascher, W. Endogenous retroviral syncytin: Compilation of experimental research on syncytin and its possible role in normal and disturbed human placentogenesis. Mol. Hum. Reprod. 2004, 10, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Wang, K.G.; Chen, C.Y.; Yu, C.; Chuang, H.C.; Chen, H. Altered placental syncytin and its receptor asct2 expression in placental development and pre-eclampsia. Int. J. Obstet. Gynaecol. 2006, 113, 152–158. [Google Scholar] [CrossRef]
- Keith, J.C., Jr.; Pijnenborg, R.; van Assche, F.A. Placental syncytin expression in normal and preeclamptic pregnancies. Am. J. Obstet. Gynecol. 2002, 187, 1122–1123, author reply 1123–1124. [Google Scholar] [CrossRef] [PubMed]
- Knerr, I.; Beinder, E.; Rascher, W. Syncytin, a novel human endogenous retroviral gene in human placenta: Evidence for its dysregulation in preeclampsia and hellp syndrome. Am. J. Obstet. Gynecol. 2002, 186, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Kudaka, W.; Oda, T.; Jinno, Y.; Yoshimi, N.; Aoki, Y. Cellular localization of placenta-specific human endogenous retrovirus (herv) transcripts and their possible implication in pregnancy-induced hypertension. Placenta 2008, 29, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Langbein, M.; Strick, R.; Strissel, P.L.; Vogt, N.; Parsch, H.; Beckmann, M.W.; Schild, R.L. Impaired cytotrophoblast cell-cell fusion is associated with reduced syncytin and increased apoptosis in patients with placental dysfunction. Mol. Reprod. Dev. 2008, 75, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.; Keith, J.C., Jr.; Stumm, N.; Moutsatsos, I.; McCoy, J.M.; Crum, C.P.; Genest, D.; Chin, D.; Ehrenfels, C.; Pijnenborg, R.; et al. Downregulation of placental syncytin expression and abnormal protein localization in pre-eclampsia. Placenta 2001, 22, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Ruebner, M.; Strissel, P.L.; Ekici, A.B.; Stiegler, E.; Dammer, U.; Goecke, T.W.; Faschingbauer, F.; Fahlbusch, F.B.; Beckmann, M.W.; Strick, R. Reduced syncytin-1 expression levels in placental syndromes correlates with epigenetic hypermethylation of the ervw-1 promoter region. PLoS One 2013, 8, e56145. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lokossou, A.G.; Toudic, C.; Barbeau, B. Implication of Human Endogenous Retrovirus Envelope Proteins in Placental Functions. Viruses 2014, 6, 4609-4627. https://doi.org/10.3390/v6114609
Lokossou AG, Toudic C, Barbeau B. Implication of Human Endogenous Retrovirus Envelope Proteins in Placental Functions. Viruses. 2014; 6(11):4609-4627. https://doi.org/10.3390/v6114609
Chicago/Turabian StyleLokossou, Adjimon Gatien, Caroline Toudic, and Benoit Barbeau. 2014. "Implication of Human Endogenous Retrovirus Envelope Proteins in Placental Functions" Viruses 6, no. 11: 4609-4627. https://doi.org/10.3390/v6114609
APA StyleLokossou, A. G., Toudic, C., & Barbeau, B. (2014). Implication of Human Endogenous Retrovirus Envelope Proteins in Placental Functions. Viruses, 6(11), 4609-4627. https://doi.org/10.3390/v6114609




