Ecology of West Nile Virus in North America
Abstract
:1. Introduction
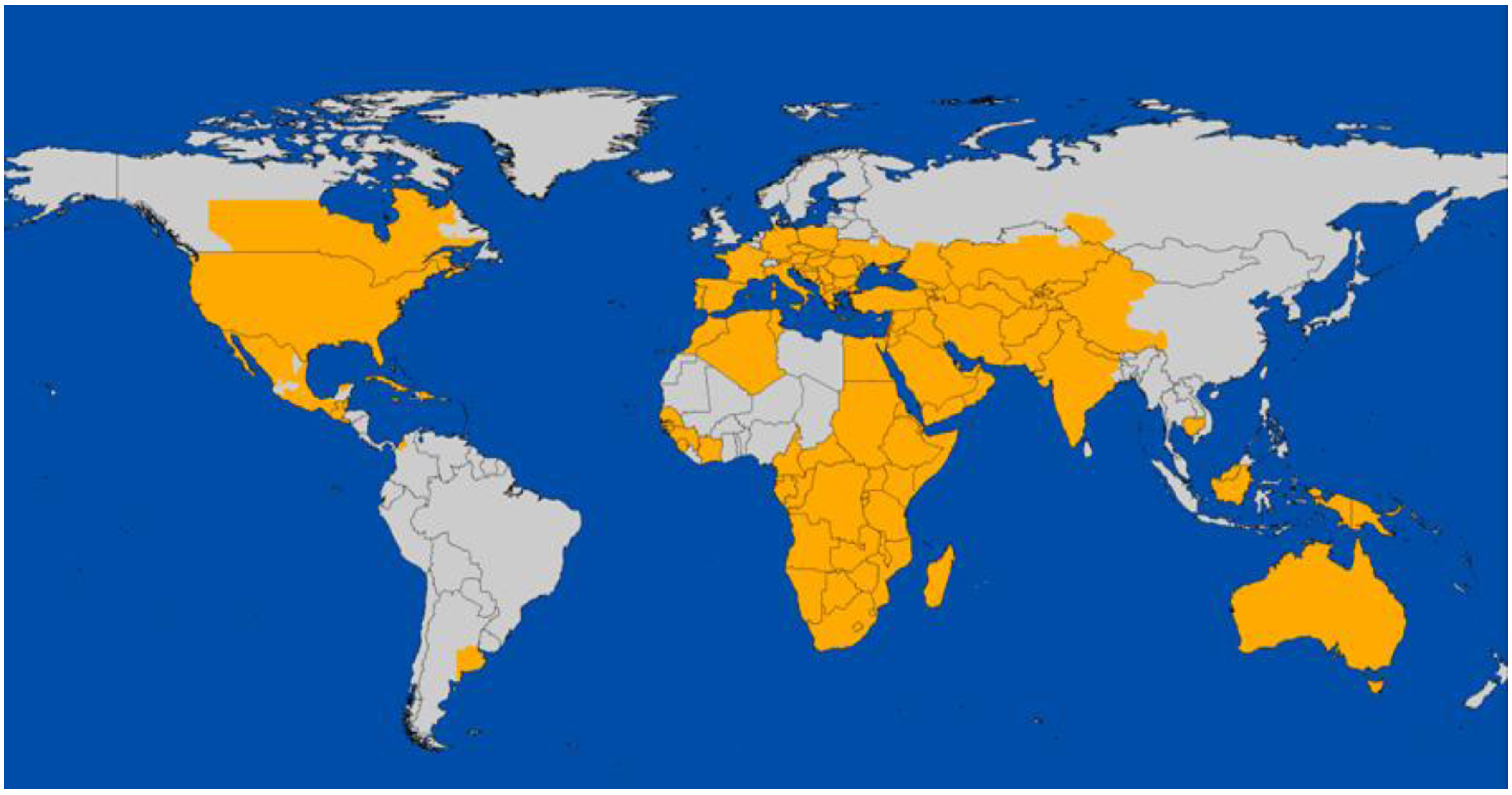
2. Global Distribution
3. Transmission Cycle in the Old World
4. Invasion of North America
4.1. Setting the Stage
4.2. The Invasion
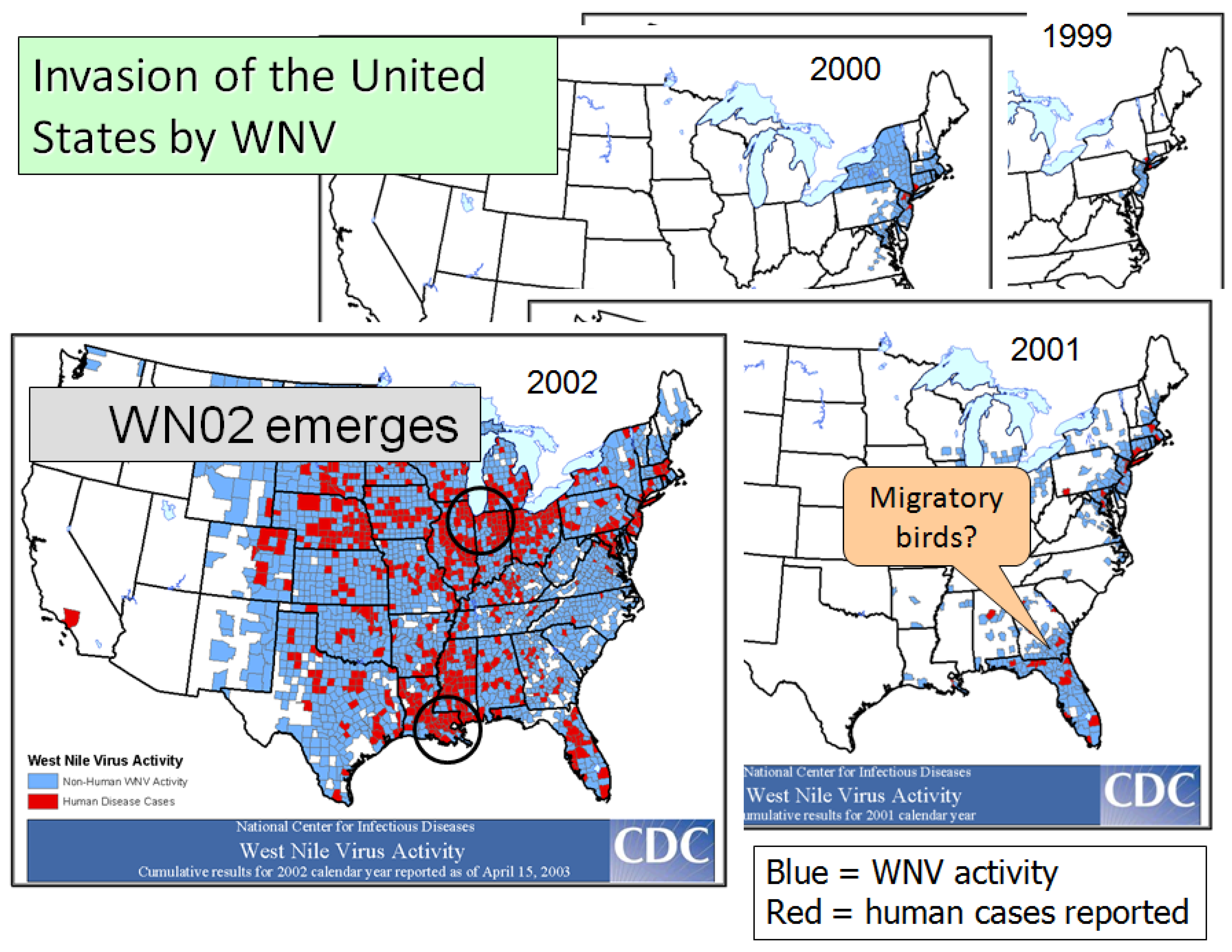
4.3. Movement
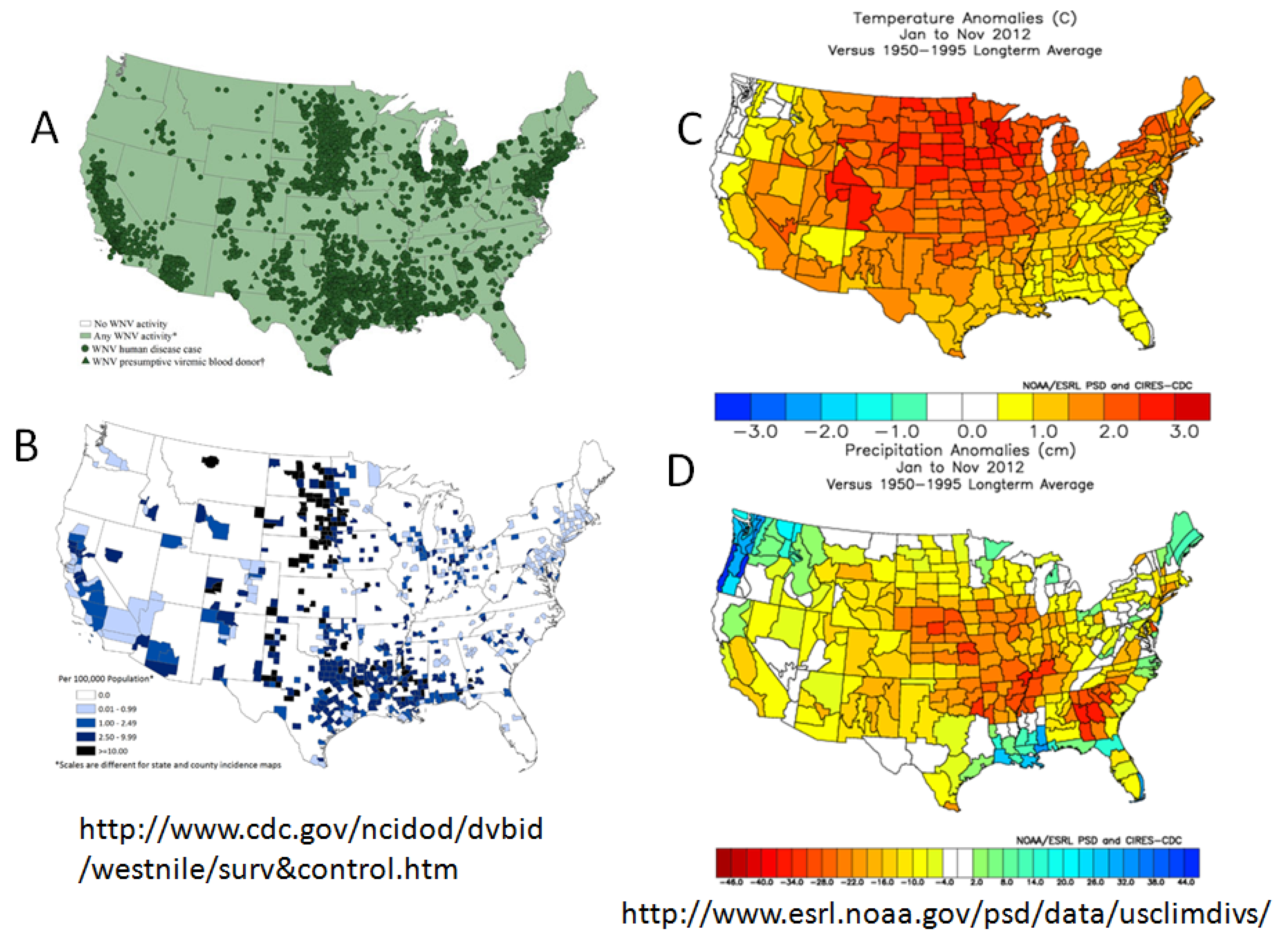
5. Virus Persistence
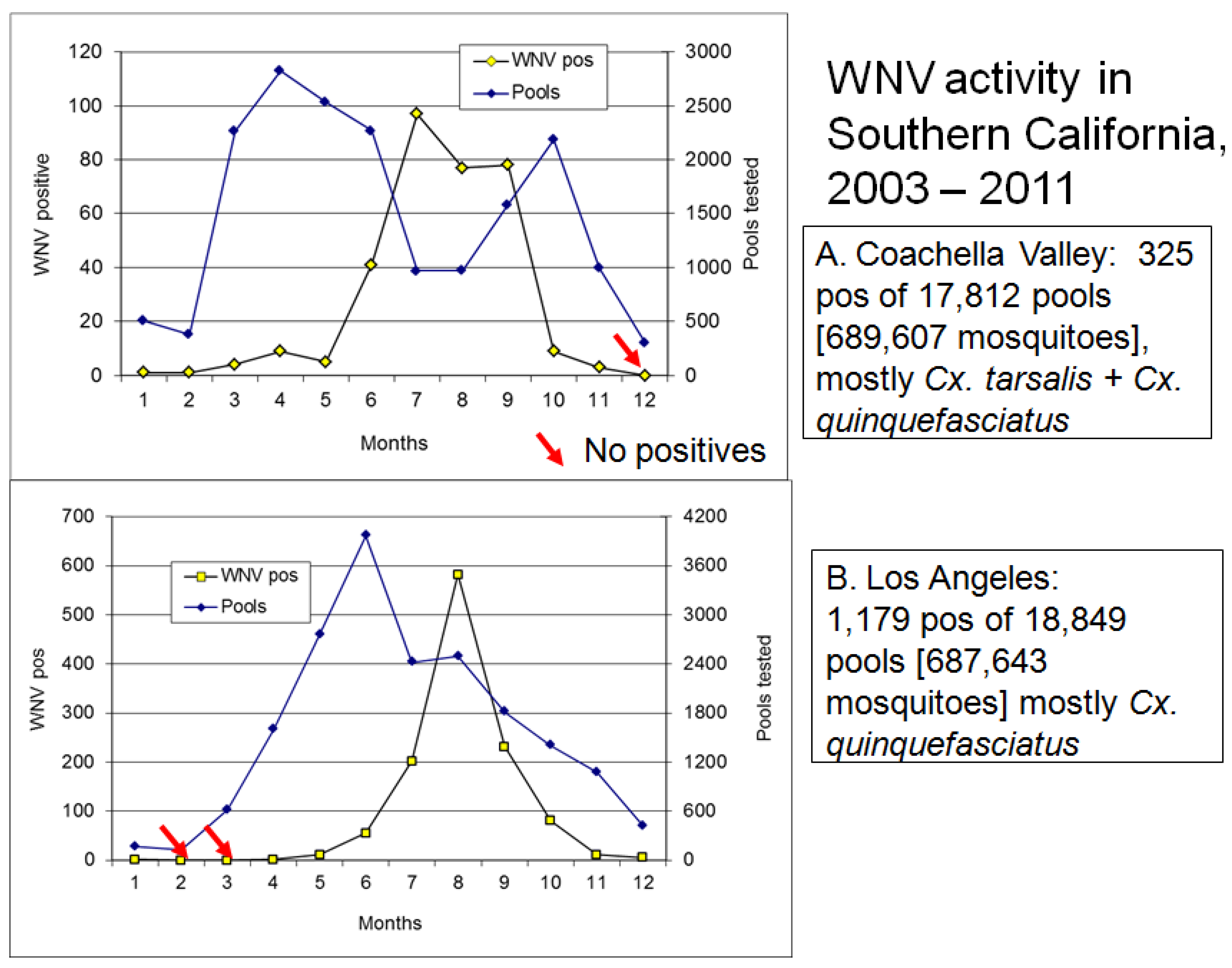
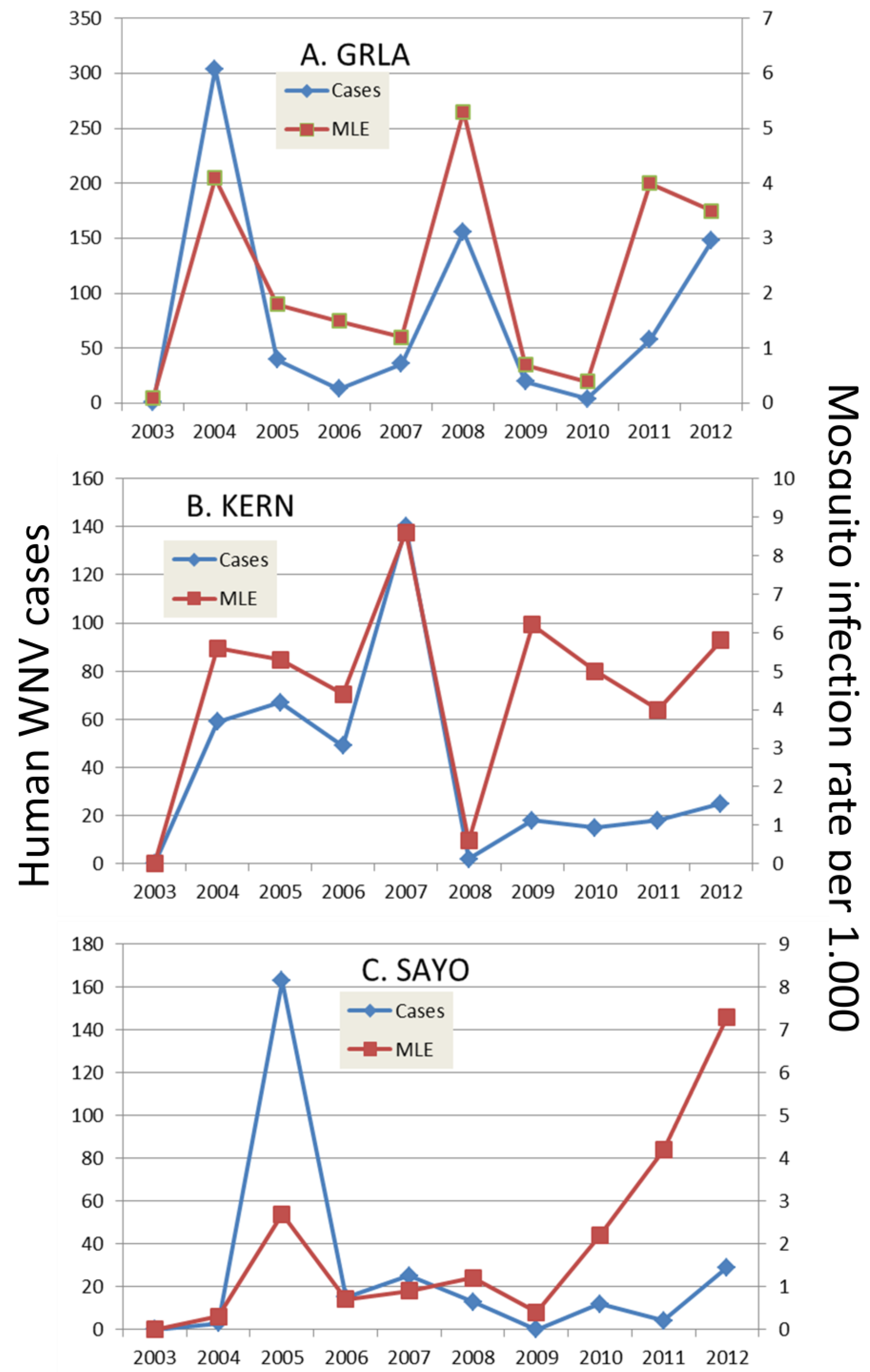
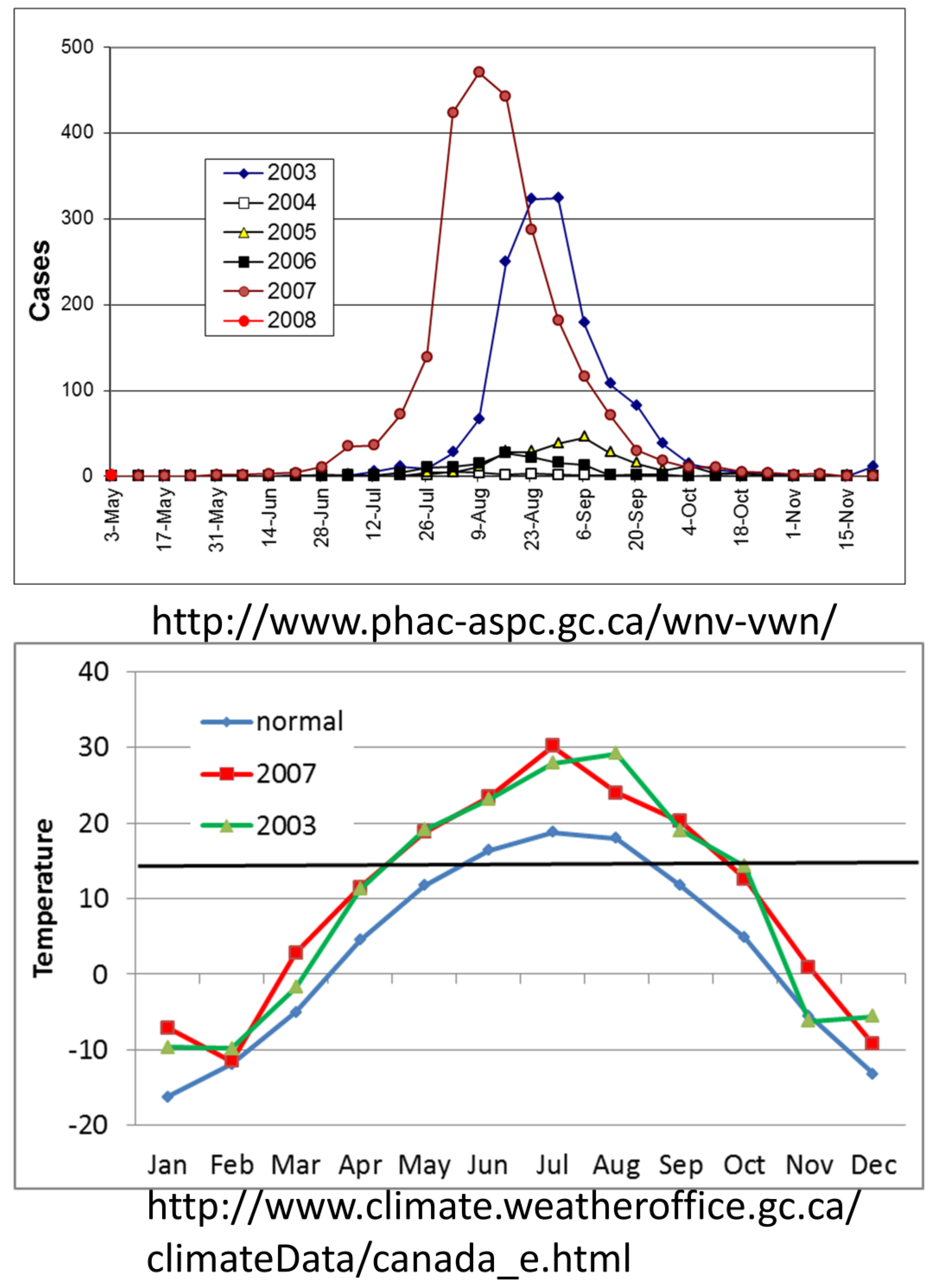
6. Outbreaks
6.1. Onset of Amplification
6.2. Economics and Heath Priorities Impact Transmission Dynamics
7. A look to the Future
Acknowledgments
References
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A neutrophic virus isolated from the blood of a native of Uganda. Am. J. Trop Med. Hyg 1940, 20, 471–492. [Google Scholar]
- Hubalek, Z.; Halouzka, J. West Nile fever—A reemerging mosquito-borne viral disease in Europe. Emerg. Infect. Dis. 1999, 5, 643–650. [Google Scholar] [CrossRef]
- Campbell, G.L.; Marfin, A.A.; Lanciotti, R.S.; Gubler, D.J. West Nile virus. Lancet Infect. Dis. 2002, 2, 519–529. [Google Scholar]
- May, F.J.; Davis, C.T.; Tesh, R.B.; Barrett, A.D. Phylogeography of West Nile virus: From the cradle of evolution in Africa to Eurasia, Australia, and the Americas. J. Virol. 2011, 85, 2964–2974. [Google Scholar] [CrossRef]
- Hayes, C.G. West Nile virus: Uganda, 1937, to New York City, 1999. Ann. N. Y. Acad. Sci. 2001, 951, 25–37. [Google Scholar] [CrossRef]
- Davis, C.T.; Ebel, G.D.; Lanciotti, R.S.; Brault, A.C.; Guzman, H.; Siirin, M.; Lambert, A.; Parsons, R.E.; Beasley, D.W.; Novak, R.J.; et al. Phylogenetic analysis of North American West Nile virus isolates, 2001–2004: Evidence for the emergence of a dominant genotype. Virology 2005, 342, 252–265. [Google Scholar] [CrossRef]
- Granwehr, B.P.; Lillibridge, K.M.; Higgs, S.; Mason, P.W.; Aronson, J.F.; Campbell, G.A.; Barrett, A.D. West Nile virus: Where are we now? Lancet Infect. Dis. 2004, 4, 547–556. [Google Scholar] [CrossRef]
- Beasley, D.W.; Davis, C.T.; Guzman, H.; Vanlandingham, D.L.; Travassos da Rosa, A.P.; Parsons, R.E.; Higgs, S.; Tesh, R.B.; Barrett, A.D. Limited evolution of West Nile virus has occurred during its southwesterly spread in the United States. Virology 2003, 309, 190–195. [Google Scholar] [CrossRef]
- Kramer, L.D.; Styer, L.M.; Ebel, G.D. A Global Perspective on the Epidemiology of West Nile Virus. Annu. Rev. Entomol. 2008, 53, 61–81. [Google Scholar] [CrossRef]
- Mackenzie, J.S. The emergence of two flaviviruses: A discussion of the emrgence of Japanese encephalitis virus in Australia and West Nile virus in North America. Arbo Res. Aust. 2001, 8, 231–236. [Google Scholar]
- Samuel, M.A.; Diamond, M.S. Pathogenesis of West Nile Virus infection: A balance between virulence, innate and adaptive immunity, and viral evasion. J. Virol. 2006, 80, 9349–9360. [Google Scholar] [CrossRef]
- Murray, K.O.; Walker, C.; Gould, E. The virology, epidemiology, and clinical impact of West Nile virus: A decade of advancements in research since its introduction into the Western Hemisphere. Epidemiol. Infect. 2011, 139, 807–817. [Google Scholar]
- Kramer, L.D.; Li, J.; Shi, P.Y. West Nile virus. Lancet Neurol. 2007, 6, 171–181. [Google Scholar] [CrossRef]
- Hayes, E.B.; Sejvar, J.J.; Zaki, S.R.; Lanciotti, R.S.; Bode, A.V.; Campbell, G.L. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg. Infect. Dis. 2005, 11, 1174–1179. [Google Scholar]
- Leis, A.A.; Stokic, D.S. Neuromuscular manifestations of human west nile virus infection. Curr. Treat. Options Neurol. 2005, 7, 15–22. [Google Scholar]
- Petersen, L.R.; Roehrig, J.T. West Nile virus: A reemerging global pathogen. Emerg. Infect. Dis. 2001, 7, 611–614. [Google Scholar]
- Petersen, L.R.; Marfin, A.A. West Nile virus: A primer for the clinician. Ann. Intern. Med. 2002, 137, 173–179. [Google Scholar]
- Petersen, L.R.; Marfin, A.A.; Gubler, D.J. West nile virus. JAMA 2003, 290, 524–528. [Google Scholar]
- Cantile, C.; Del, P.F.; Di, G.G.; Arispici, M. Pathologic and immunohistochemical findings in naturally occuring West Nile virus infection in horses. Vet. Pathol. 2001, 38, 414–421. [Google Scholar] [CrossRef]
- Steele, K.E.; Linn, M.J.; Schoepp, R.J.; Komar, N.; Geisbert, T.W.; Manduca, R.M.; Calle, P.P.; Raphael, B.L.; Clippinger, T.L.; Larsen, T.; et al. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Vet. Pathol. 2000, 37, 208–224. [Google Scholar] [CrossRef]
- Hayes, E.B.; Komar, N.; Nasci, R.S.; Montgomery, S.P.; O'Leary, D.R.; Campbell, G.L. Epidemiology and transmission dynamics of West Nile virus disease. Emerg. Infect. Dis. 2005, 11, 1167–1173. [Google Scholar] [CrossRef]
- Komar, N. West Nile virus: Epidemiology and ecology in North America. Adv. Virus Res. 2003, 61, 185–234. [Google Scholar] [CrossRef]
- Peterson, A.T.; Robbins, A.; Restifo, R.; Howell, J.; Nasci, R. Predictable ecology and geography of West Nile virus transmission in the central United States. J. Vector Ecol. 2008, 33, 342–352. [Google Scholar] [CrossRef]
- Roehrig, J.T.; Layton, M.; Smith, P.; Campbell, G.L.; Nasci, R.; Lanciotti, R.S. The emergence of West Nile virus in North America: Ecology, epidemiology, and surveillance. Curr. Top. Microbiol. Immunol. 2002, 267, 223–240. [Google Scholar] [CrossRef]
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Kilpatrick, A.M. "Bird biting" mosquitoes and human disease: A review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 2011, 11, 1577–1585. [Google Scholar] [CrossRef]
- Kilpatrick, A.M. Globalization, land use, and the invasion of West Nile virus. Science 2011, 334, 323–327. [Google Scholar] [CrossRef]
- Pybus, O.G.; Suchard, M.A.; Lemey, P.; Bernardin, F.J.; Rambaut, A.; Crawford, F.W.; Gray, R.R.; Arinaminpathy, N.; Stramer, S.L.; Busch, M.P.; et al. Unifying the spatial epidemiology and molecular evolution of emerging epidemics. Proc. Natl. Acad. Sci. USA 2012, 109, 15066–15071. [Google Scholar] [CrossRef]
- Rappole, J.H.; Derrickson, S.R.; Hubalek, Z. Migratory birds and spread of West Nile Virus in the Western Hemisphere. Emerg. Infect. Dis. 2000, 6, 319–328. [Google Scholar] [CrossRef]
- Rappole, J.H.; Hubalek, Z. Migratory birds and West Nile virus. J. Appl. Microbiol. 2003, 94, 47S–58S. [Google Scholar] [CrossRef]
- McLean, R.G.; Ubico, S.R.; Docherty, D.E.; Hansen, W.R.; Sileo, L.; McNamara, T.S. West Nile virus transmission and ecology in birds. Ann. N. Y. Acad. Sci. 2001, 951, 54–57. [Google Scholar]
- Reed, K.D.; Meece, J.K.; Henkel, J.S.; Shukla, S.K. Birds, migration and emerging zoonoses: West nile virus, lyme disease, influenza A and enteropathogens. Clin. Med. Res. 2003, 1, 5–12. [Google Scholar] [CrossRef]
- Marra, P.P.; Griffing, S.M.; Caffrey, C.L.; Kilpatrick, A.M.; McLean, R.G.; Brand, C.; Saito, E.K.; Kramer, L.D.; Novak, R. West Nile virus and wildlife. BioScience 2004, 54, 393–402. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; LaDeau, S.L.; Marra, P.P. Ecology of West Nile virus transmission and its impact on birds in the western hemisphere. Auk 2007, 124, 1121–1136. [Google Scholar] [CrossRef]
- Wheeler, S.S.; Barker, C.M.; Armijos, M.V.; Carroll, B.D.; Husted, S.R.; Reisen, W.K. Differential impact of West Nile virus on California birds. Condor 2009, 111, 1–20. [Google Scholar] [CrossRef]
- LaDeau, S.L.; Kilpatrick, A.M.; Marra, P.P. West Nile virus emergence and large-scale declines of North American bird populations. Nature 2007, 447, 710–713. [Google Scholar] [CrossRef]
- Spielman, A. Structure and seasonality of nearctic Culex pipiens populations. Ann. N. Y. Acad. Sci. 2001, 951, 220–234. [Google Scholar] [CrossRef]
- Turell, M.J.; Sardelis, M.R.; Dohm, D.J.; O'Guinn, M.L. Potential North American vectors of West Nile virus. Ann. N. Y. Acad. Sci. 2001, 951, 317–324. [Google Scholar]
- Turell, M.J.; Sardelis, M.R.; O'Guinn, M.L.; Dohm, D.J. Potential vectors of West Nile virus in North America. Curr. Top. Microbiol. Immunol. 2002, 267, 241–252. [Google Scholar]
- Kent, R.J. Molecular methods for arthropod bloodmeal identification and applications to ecological and vector-borne disease studies. Mol. Ecol. Resour. 2009, 9, 4–18. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Roehrig, J.T.; Deubel, V.; Smith, J.; Parker, M.; Steele, K.; Crise, B.; Volpe, K.E.; Crabtree, M.B.; Scherret, J.H.; et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 1999, 286, 2333–2337. [Google Scholar] [CrossRef]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef]
- Hall, R.A.; Scherret, J.H.; Mackenzie, J.S. Kunjin virus: An Australian variant of West Nile? Ann. N. Y. Acad. Sci. 2001, 951, 153–160. [Google Scholar]
- Hayes, C.G.; Baqar, S.; Ahmed, A.; Chowdhry, M.A.; Reisen, W.K. West Nile virus in Pakistan. 1. Sero-epidemiological studies in Punjab Province. Trans. R. Soc. Trop Med. Hyg. 1982, 76, 431–436. [Google Scholar] [CrossRef]
- Monath, T.P. Epidemiology. In St. Louis Encephalitis; Monath, T.P., Ed.; American Public Health Association: Washington, DC, 1980; pp. 239–312. [Google Scholar]
- Jupp, P.G.; McIntosh, B.M.; Blackburn, N.K. Experimental assessment of the vector competence of Culex (Culex) neavei Theobald with West Nile and Sindbis viruses in South Africa. Trans. R. Soc. Trop Med. Hyg. 1986, 80, 226–230. [Google Scholar] [CrossRef]
- Jupp, P.G.; McIntosh, B.M. Quantitative experiments on the vector capability of Culex (Culex) pipiens fatigans Wiedemann with West Nile and Sindbis viruses. J. Med. Entomol. 1970, 7, 353–356. [Google Scholar]
- Taylor, R.M.; Work, T.H.; Hurlbut, H.S.; Rizk, F. A study of the ecology of West Nile virus in Egypt. Am. J. Trop Med. Hyg. 1956, 5, 579–620. [Google Scholar]
- Balenghien, T.; Vazeille, M.; Grandadam, M.; Schaffner, F.; Zeller, H.; Reiter, P.; Sabatier, P.; Fouque, F.; Bicout, D.J. Vector competence of some french Culex and Aedes mosquitoes for west nile virus. Vector Borne Zoonotic Dis. 2008, 8, 589–595. [Google Scholar] [CrossRef]
- Kay, B.H.; Fanning, I.D.; Carley, J.G. The vector competence of Australian Culex annulirostris with Murray Valley encephalitis and Kunjin viruses. Aust. J. Exp. Biol. Med. Sci. 1984, 62 (Pt 5), 641–650. [Google Scholar]
- Kay, B.H.; Fanning, I.D.; Carley, J.G. Vector competence of Culex pipiens quinquefasciatus for Murray Valley encephalitis, Kunjin, and Ross River viruses from Australia. Am. J. Trop Med. Hyg. 1982, 31, 844–848. [Google Scholar]
- Paramasivan, R.; Mishra, A.C.; Mourya, D.T. West Nile virus: The Indian scenario. Indian J. Med. Res. 2003, 118, 101–108. [Google Scholar]
- Ilkal, M.A.; Mavale, M.S.; Prasanna, Y.; Jacob, P.G.; Geevarghese, G.; Banerjee, K. Experimental studies on the vector potential of certain Culex species to West Nile virus. Indian J. Med. Res. 1997, 106, 225–228. [Google Scholar]
- Akhter, R.; Hayes, C.G.; Baqar, S.; Reisen, W.K. West Nile virus in Pakistan. III. Comparative vector capability of Culex tritaeniorhynchus and eight other species of mosquitoes. Trans. R. Soc. Trop Med. Hyg. 1982, 76, 449–453. [Google Scholar] [CrossRef]
- Reisen, W.K.; Hayes, C.G.; Azra, K.; Niaz, S.; Mahmood, F.; Parveen, T.; Boreham, P.F.L. West Nile virus in Pakistan. II. Entomological studies at Changa Manga National Forest, Punjab Province. Trans. R. Soc. Trop Med. Hyg. 1982, 76, 437–448. [Google Scholar] [CrossRef]
- Malkinson, M.; Banet, C. The role of birds in the ecology of West Nile virus in Europe and Africa. Curr. Top. Microbiol. Immunol. 2002, 267, 309–322. [Google Scholar] [CrossRef]
- Rodrigues, F.M.; Guttikar, S.N.; Pinto, B.D. Prevalence of antibodies to Japanese encephalitis and West Nile viruses among wild birds in the Krishna-Godavari Delta, Andhra Pradesh, India. Trans. R. Soc. Trop Med. Hyg. 1981, 75, 258–262. [Google Scholar] [CrossRef]
- Brault, A.C.; Huang, C.Y.; Langevin, S.A.; Kinney, R.M.; Bowen, R.A.; Ramey, W.N.; Panella, N.A.; Holmes, E.C.; Powers, A.M.; Miller, B.R. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nat. Genet. 2007, 39, 1162–1166. [Google Scholar] [CrossRef]
- Malkinson, M.; Banet, C.; Weisman, Y.; Pokamunski, S.; King, R.; Drouet, M.T.; Deubel, V. Introduction of West Nile virus in the Middle East by Migrating White Storks. Emerg. Infect. Dis. 2002, 8, 392–397. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Flather, C.H.; Radeloff, V.C.; Pidgeon, A.M.; Hammer, R.B.; Liu, J.G. Human impacts on regional avian diversity and abundance. Conserv. Biol. 2008, 22, 405–416. [Google Scholar] [CrossRef]
- Pidgeon, A.M.; Radeloff, V.C.; Flather, C.H.; Lepczyk, C.A.; Clayton, M.K.; Hawbaker, T.J.; Hammer, R.B. Associations of forest bird species richness with housing and landscape patterns across the USA. Ecol. Appl. 2007, 17, 1989–2010. [Google Scholar] [CrossRef]
- Loss, S.R.; Hamer, G.L.; Walker, E.D.; Ruiz, M.O.; Goldberg, T.L.; Kitron, U.D.; Brawn, J.D. Avian host community structure and prevalence of West Nile virus in Chicago, Illinois. Oecologia 2009, 159, 415–424. [Google Scholar] [CrossRef]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef]
- Molaei, G.; Andreadis, T.G.; Armstrong, P.M.; Anderson, J.F.; Vossbrinck, C.R. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg. Infect. Dis. 2006, 12, 468–474. [Google Scholar]
- Molaei, G.; Andreadis, T.G.; Armstrong, P.M.; Bueno, R., Jr.; Dennett, J.A.; Real, S.V.; Sargent, C.; Bala, A.; Randle, Y.; Guzman, H.; et al. Host Feeding pattern of Culex quinquefasciatus (Diptera: Culicidae) and its role in transmission of west nile virus in harris county, Texas. Am. J. Trop Med. Hyg. 2007, 77, 73–81. [Google Scholar]
- Molaei, G.; Cummings, R.F.; Su, T.; Armstrong, P.M.; Williams, G.A.; Cheng, M.L.; Webb, J.P.; Andreadis, T.G. Vector-host interactions governing epidemiology of West Nile virus in southern california. Am. J. Trop Med. Hyg. 2010, 83, 1269–1282. [Google Scholar] [CrossRef]
- Thiemann, T.C.; Wheeler, S.S.; Barker, C.M.; Reisen, W.K. Mosquito host selection varies seasonally with host availability and mosquito density. PLoS Negl. Trop Dis. 2011, 5, e1452. [Google Scholar] [CrossRef]
- Thiemann, T.C.; Lemenager, D.A.; Kluh, S.; Carroll, B.D.; Lothrop, H.D.; Reisen, W.K. Spatial variation in host feeding patterns of Culex tarsalis and the Culex pipiens complex (Diptera: Culicidae) in California. J. Med. Entomol. 2012, 49, 903–916. [Google Scholar] [CrossRef]
- Kwan, J.L.; Kluh, S.; Reisen, W.K. Antecedent avian immunity limits tangential transmission of West Nile virus to humans. PLoS One 2012, 7, e34127. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Godsey, M.S.; King, R.J.; Guptill, S.C. Avian diversity and West Nile virus: Testing associations between biodiversity and infectious disease risk. Proc. Biol. Sci. 2006, 273, 109–117. [Google Scholar] [CrossRef]
- Harbach, R.E.; Dahl, C.; White, G.B. Culex (Culex) pipiens Linnaeus(Diptera: Culicidae): concepts, type designation, and description. Proc. Entomol. Soc. Wash. 1985, 87, 1–24. [Google Scholar]
- Fonseca, D.M.; Smith, J.L.; Wilkerson, R.C.; Fleischer, R.C. Pathways of expansion and multiple introductions illustrated by large genetic differentiation among worldwide populations of the southern house mosquito. Am. J. Trop Med. Hyg. 2006, 74, 284–289. [Google Scholar]
- Barr, A.R. The distribution of Culex p. pipiens and C.p. quinquefasciatus in North America. Am. J. Trop Med. Hyg. 1957, 6, 153–165. [Google Scholar]
- Kothera, L.; Zimmerman, E.M.; Richards, C.M.; Savage, H.M. Microsatellite characterization of subspecies and their hybrids in Culex pipiens complex (Diptera: Culicidae) mosquitoes along a north-south transect in the central United States. J. Med. Entomol. 2009, 46, 236–248. [Google Scholar] [CrossRef]
- Edillo, F.; Kiszewski, A.; Manjourides, J.; Pagano, M.; Hutchinson, M.; Kyle, A.; Arias, J.; Gaines, D.; Lampman, R.; Novak, R.; et al. Effects of latitude and longitude on the population structure of Culex pipiens s.l., vectors of West Nile virus in North America. Am. J. Trop Med. Hyg. 2009, 81, 842–848. [Google Scholar] [CrossRef]
- Kothera, L.; Godsey, M.; Mutebi, J.P.; Savage, H.M. A comparison of aboveground and belowground populations of Culex pipiens (Diptera: Culicidae) mosquitoes in Chicago, Illinois, and New York City, New York, using microsatellites. J. Med. Entomol. 2010, 47, 805–813. [Google Scholar] [CrossRef]
- Edman, J.D. Host-feeding patterns of Florida mosquitoes. 3. Culex (Culex) and Culex (Neoculex). J. Med. Entomol. 1974, 11, 95–104. [Google Scholar]
- Edman, J.D.; Taylor, D.J. Culex nigripalpus: Seasonal shift in the bird: mammal feeding rates in a mosquito vector of human encephalitis. Science 1968, 161, 67–68. [Google Scholar]
- Lumsden, L.L. St. Louis encephalitis in 1933. Observations on epidemiological features. Public Health Rep. 1958, 73, 340–353. [Google Scholar] [CrossRef]
- Campbell, G.L.; Ceianu, C.S.; Savage, H.M. Epidemic West Nile encephalitis in Romania: Waiting for history to repeat itself. Ann. N. Y. Acad. Sci. 2001, 951, 94–101. [Google Scholar] [CrossRef]
- Lvov, D.K.; Butenko, A.M.; Gromashevsky, V.L.; Larichev, V.P.; Gaidamovich, S.Y.; Vyshemirsky, O.I.; Zhukov, A.N.; Lazorenko, V.V.; Salko, V.N.; Kovtunov, A.I.; et al. Isolation of two strains of West Nile virus during an outbreak in southern Russia, 1999. Emerg. Infect. Dis. 2000, 6, 373–376. [Google Scholar]
- Lvov, D.K.; Butenko, A.M.; Gromashevsky, V.L.; Kovtunov, A.I.; Prilipov, A.G.; Kinney, R.; Aristova, V.A.; Dzharkenov, A.F.; Samokhvalov, E.I.; Savage, H.M.; et al. West Nile virus and other zoonotic viruses in Russia: Examples of emerging-reemerging situations. Arch. Virol. Suppl. 2004, 18, 85–96. [Google Scholar]
- Zeller, H.G.; Schuffenecker, I. West Nile virus: An overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 147–156. [Google Scholar]
- Eidson, M.; Miller, J.; Kramer, L.; Cherry, B.; Hagiwara, Y.; Ostlund, E.N.; Crom, R.L.; Pedersen, D.D.; Johnson, D.J.; Williams, W.O.; et al. Dead crow densities and human cases of West Nile virus, New York State, 2000. Emerg. Infect. Dis. 2001, 7, 662–669. [Google Scholar]
- Wheeler, S.S.; Vineyard, M.P.; Barker, C.M.; Reisen, W.K. Importance of recrudescent avian infection in West Nile virus overwintering: incomplete antibody neutralization of virus allows infrequent vector infection. J. Med. Entomol. 2012, 49, 895–902. [Google Scholar] [CrossRef]
- Nasci, R.S.; White, D.J.; Stirling, H.; Daniels, T.J.; Falco, R.C.; Campbell, S.; Crans, W.J.; Savage, H.M.; Lanciotti, R.S.; Moore, C.G.; et al. West Nile virus isolates from mosquitoes in New York and New Jersey, 1999. Emerg. Infect. Dis. 2001, 7, 626–630. [Google Scholar]
- Komar, N.; Panella, N.A.; Burns, J.E.; Dusza, S.W.; Mascarenhas, T.M.; Talbot, T.O. Serologic evidence for West Nile virus infection in birds in the New York City vicinity during an outbreak in 1999. Emerg. Infect. Dis. 2001, 7, 621–625. [Google Scholar]
- Julian, K.G.; Eidson, M.; Kipp, A.M.; Weiss, E.; Petersen, L.R.; Miller, J.R.; Hinten, S.R.; Marfin, A.A. Early season crow mortality as a sentinel for West Nile virus disease in humans, northeastern United States. Vector Borne Zoonotic Dis. 2002, 2, 145–155. [Google Scholar] [CrossRef]
- Nielsen, C.F.; Reisen, W.K.; Armijos, V.; MacLachlan, N.J.; Scott, T.W. High subclinical West Nile virus incidence among unvaccinated horses in Northern California associated with low vector abundance. Am. J. Trop Med. Hyg. 2008, 78, 45–52. [Google Scholar]
- Kilpatrick, A.M.; Meola, M.A.; Moudy, R.M.; Kramer, L.D. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. Plos Pathog. 2008, 4, e1000092. [Google Scholar] [CrossRef]
- Moudy, R.M.; Meola, M.A.; Morin, L.L.; Ebel, G.D.; Kramer, L.D. A newly emergent genotype of West Nile virus is transmitted earlier and more efficiently by Culex mosquitoes. Am. J. Trop Med. Hyg. 2007, 77, 365–370. [Google Scholar]
- McMullen, A.R.; May, F.J.; Li, L.; Guzman, H.; Bueno, R., Jr.; Dennett, J.A.; Tesh, R.B.; Barrett, A.D. Evolution of new genotype of West Nile virus in North America. Emerg. Infect. Dis. 2011, 17, 785–793. [Google Scholar] [CrossRef]
- Reisen, W.K.; Fang, Y.; Martinez, V.M. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae). J. Med. Entomol. 2006, 43, 309–317. [Google Scholar] [CrossRef]
- Su, T.; Webb, J.P.; Meyer, R.P.; Mulla, M.S. Spatial and temporal distribution of mosquitoes in underground storm drain systems in Orange County, California. J. Vector Ecol. 2003, 28, 79–89. [Google Scholar]
- Oke, T.R. The energetic basis of the urban heat island. Quart J. R. Meterol. Soc. 1982, 108, 1–24. [Google Scholar]
- Meyer, R.P.; Hardy, J.L.; Reisen, W.K. Diel changes in adult mosquito microhabitat temperatures and their relationship to the extrinsic incubation of arboviruses in mosquitoes in Kern County, California, U.S.A. J. Med. Entomol. 1990, 27, 607–614. [Google Scholar]
- Reisen, W.K.; Lothrop, H.D.; Meyer, R.P. Time of host-seeking by Culex tarsalis (Diptera: Culicidae) in California. J. Med. Entomol. 1997, 34, 430–437. [Google Scholar]
- Reisen, W.K. Effect of temperature on Culex tarsalis (Diptera: Culicidae) from the Coachella and San Joaquin Valleys of California. J. Med. Entomol. 1995, 32, 636–645. [Google Scholar]
- Reisen, W.K.; Milby, M.M.; Presser, S.B.; Hardy, J.L. Ecology of mosquitoes and St. Louis encephalitis virus in the Los Angeles Basin of California, 1987–1990. J. Med. Entomol. 1992, 29, 582–598. [Google Scholar]
- Reisen, W.K.; Fang, Y.; Martinez, V.M. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae). J. Med. Entomol. 2006, 43, 309–317. [Google Scholar] [CrossRef]
- Hartley, D.M.; Barker, C.M.; le Menac'h, A.; Niu, T.; Gaff, H.D.; Reisen, W.K. The effects of temperature on the emergence and seasonality of West Nile virus in California. Am. J. Trop Med. Hyg. 2012, 86, 884–894. [Google Scholar] [CrossRef]
- Reeves, W.C.; Hardy, J.L.; Reisen, W.K.; Milby, M.M. Potential effect of global warming on mosquito-borne arboviruses. J. Med. Entomol. 1994, 31, 323–332. [Google Scholar]
- Johnson, G.D.; Eidson, M.; Schmit, K.; Ellis, A.; Kulldorff, M. Geographic prediction of human onset of West Nile virus using dead crow clusters: an evaluation of year 2002 data in New York State. Am. J. Epidemiol. 2006, 163, 171–180. [Google Scholar]
- Reisen, W.K.; Fang, Y.; Martinez, V.M. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. J. Med. Entomol. 2005, 42, 367–375. [Google Scholar] [CrossRef]
- Nielsen, C.F.; Reisen, W.K. Dead birds increase the risk of West Nile Virus infection in Culex mosquitoes (Diptera: Culicidae) in Domestic Landscapes. J. Med. Entomol. 2007, 44, 1007–1013. [Google Scholar]
- Reisen, W.K.; Barker, C.M.; Carney, R.; Lothrop, H.D.; Wheeler, S.S.; Wilson, J.L.; Madon, M.B.; Takahashi, R.; Carroll, B.; Garcia, S.; et al. Role of corvids in epidemiology of West Nile virus in southern California. J. Med. Entomol. 2006, 43, 356–367. [Google Scholar] [CrossRef]
- Holloway, M. Outbreak not contained. West Nile virus triggers a reevaluation of public health surveillance. Sci. Am. 2000, 282, 20–22. [Google Scholar] [CrossRef]
- Dusek, R.J.; McLean, R.G.; Kramer, L.D.; Ubico, S.R.; DuPuis, A.P.; Ebel, G.D.; Guptill, S.C. Prevalence of West Nile Virus in migratory birds during spring and fall migration. Am. J. Trop Med. Hyg. 2009, 81, 1151–1158. [Google Scholar] [CrossRef]
- Reisen, W.K.; Wheeler, S.S.; Garcia, S.; Fang, Y. Migratory birds and the dispersal of arboviruses in California. Am. J. Trop Med. Hyg. 2010, 83, 808–815. [Google Scholar] [CrossRef]
- Rappole, J.H.; Compton, B.W.; Leimgruber, P.; Robertson, J.; King, D.I.; Renner, S.C. Modeling movement of West Nile virus in the Western hemisphere. Vector Borne Zoonotic Dis. 2006, 6, 128–139. [Google Scholar] [CrossRef]
- Hom, A.; Marcus, L.; Kramer, V.L.; Cahoon, B.; Glaser, C.; Cossen, C.; Baylis, E.; Jean, C.; Tu, E.; Eldridge, B.F.; et al. Surveillance for mosquito-borne encephalitis virus activity and human disease, including West Nile virus, in California, 2004. Proc. Mosq. Vector Control. Assoc. Calif. 2005, 73, 66–77. [Google Scholar]
- Sellers, R.F. Weather, host and vector--their interplay in the spread of insect-borne animal virus diseases. J. Hyg. (Lond) 1980, 85, 65–102. [Google Scholar] [CrossRef]
- Hubalek, Z. European experience with the West Nile virus ecology and epidemiology: Could it be relevant for the New World? Viral Immunol. 2000, 13, 415–426. [Google Scholar] [CrossRef]
- Barnard, B. Outbreak! Plagues That Changed History; Crown Publishers: New York, NY, USA, 2005. [Google Scholar]
- Baqar, S.; Hayes, C.G.; Murphy, J.R.; Watts, D.M. Vertical transmission of West Nile virus by Culex and Aedes species mosquitoes. Am. J. Trop. Med. Hyg. 1993, 48, 757–762. [Google Scholar]
- Nasci, R.S.; Savage, H.M.; White, D.J.; Miller, J.R.; Cropp, B.C.; Godsey, M.S.; Kerst, A.J.; Bennett, P.; Gottfried, K.; Lanciotti, R.S. West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg. Infect. Dis. 2001, 7, 742–744. [Google Scholar]
- Farajollahi, A.; Crans, W.J.; Bryant, P.; Wolf, B.; Burkhalter, K.L.; Godsey, M.S.; Aspen, S.E.; Nasci, R.S. Dectection of West Nile viral RNA from an overwintering pool of Culex pipiens pipiens (Diptera: Culicidae) in New Jersey, 2003. J. Med. Entomol. 2005, 42, 490–494. [Google Scholar] [CrossRef]
- Bugbee, L.M.; Forte, L.R. The discovery of West Nile virus in overwintering Culex pipiens (Diptera: Culicidae) mosquitoes in Lehigh County, Pennsylvania. J. Am. Mosq. Control. Assoc. 2004, 20, 326–327. [Google Scholar]
- Nelms, B.M.; Macedo, P.A.; Kothera, L.; Savage, H.M.; Reisen, W.K. Overwintering biology of Culex mosquitoes (Diptera: Culicidae) in the Sacramento Valley, California. J. Med. Entomol. 2013, in press. [Google Scholar]
- Anderson, J.F.; Main, A.J. Importance of vertical and horizontal transmission of West Nile virus by Culex pipiens in the Northeastern United States. J. Infect. Dis. 2006, 194, 1577–1579. [Google Scholar] [CrossRef]
- Anderson, J.F.; Main, A.J.; Delroux, K.; Fikrig, E. Extrinsic incubation periods for horizontal and vertical transmission of West Nile virus by Culex pipiens pipiens (Diptera: Culicidae). J. Med. Entomol. 2008, 45, 445–451. [Google Scholar] [CrossRef]
- Nelms, B.M.; Fechter-Leggett, E.; Carroll, B.D.; Macedo, P.A.; Kluh, S.; Reisen, W.K. Experimental and natural vertical transmission of West Nile Virus by California Culex mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2013, in press. [Google Scholar]
- Reisen, W.K.; Fang, Y.; Lothrop, H.D.; Martinez, V.M.; Wilson, J.; O'Connor, P.; Carney, R.; Cahoon-Young, B.; Shafii, M.; Brault, A.C. Overwintering of West Nile virus in Southern California. J. Med. Entomol. 2006, 43, 344–355. [Google Scholar] [CrossRef]
- Wheeler, S.S.; Langevin, S.A.; Brault, A.C.; Woods, L.; Carroll, B.D.; Reisen, W.K. Detection of persistent West Nile virus RNA in experimentally and naturally infected avian hosts. Am. J. Trop Med. Hyg. 2012, 87, 559–564. [Google Scholar] [CrossRef]
- Nemeth, N.; Young, G.; Ndaluka, C.; Bielefeldt-Ohmann, H.; Komar, N.; Bowen, R. Persistent West Nile virus infection in the house sparrow (Passer domesticus). Arch. Virol. 2009, 154, 783–789. [Google Scholar] [CrossRef]
- Nemeth, N.M.; Oesterle, P.T.; Bowen, R.A. Humoral immunity to West Nile virus is long-lasting and protective in the house sparrow (Passer domesticus). Am. J. Trop Med. Hyg. 2009, 80, 864–869. [Google Scholar]
- Wheeler, S.S.; Vineyard, M.P.; Woods, L.W.; Reisen, W.K. Dynamics of West Nile Virus Persistence in House Sparrows (Passer domesticus). PLoS Negl. Trop Dis. 2012, 6, e1860. [Google Scholar] [CrossRef]
- Reisen, W.K.; Kramer, L.D.; Chiles, R.E.; Green, E.-.G.N.; Martinez, V.M. Encephalitis virus persistence in California birds: Preliminary studies with house finches (Carpodacus mexicanus). J. Med. Entomol. 2001, 38, 393–399. [Google Scholar] [CrossRef]
- Reisen, W.K.; Chiles, R.E.; Martinez, V.M.; Fang, Y.; Green, E.N.; Clark, S. Effect of dose on house finch (Carpodacus mexicanus) infection with western equine encephalomyelitis and St. Louis encephalitis viruses. J. Med. Entomol. 2004, 41, 978–981. [Google Scholar] [CrossRef]
- Reisen, W.K.; Padgett, K.; Fang, Y.; Woods, L.; Foss, L.; Anderson, J.; Kramer, V.L. Chronic infections of West Nile virus detected in California dead birds. Vector Borne Zoonotic Dis. 2013, 13, 401–405. [Google Scholar] [CrossRef]
- Semenov, B.F.; Chunikhin, S.P.; Karmysheva, V.I.; Iakovleva, N.I. Study of chronic forms of arbovirus infections in birds. 1. Experiments with West Nile, Sindbis, Bhandja and Sicilian mosquito fever viruses. Vestn Akad Med. Nauk SSSR 1973, 28, 79–83. [Google Scholar]
- Applegate, J.E. Spring relapse of Plasmodium relictum infections in an experimental field population of English sparrows (Passer domesticus). J. Wildl. Dis. 1971, 7, 37–42. [Google Scholar]
- Dawson, J.R.; Stone, W.B.; Ebel, G.D.; Young, D.S.; Galinski, D.S.; Pensabene, J.P.; Franke, M.A.; Eidson, M.; Kramer, L.D. Crow deaths caused by West Nile virus during winter. Emerg. Infect. Dis. 2007, 13, 1912–1914. [Google Scholar] [CrossRef]
- Anderson, J.F.; Andreadis, T.G.; Vossbrinck, C.R.; Tirrell, S.; Wakem, E.M.; French, R.A.; Garmendia, A.E.; van Kruiningen, H.J. Isolation of West Nile virus from mosquitoes, crows, and a Cooper's hawk in Connecticut. Science 1999, 286, 2331–2333. [Google Scholar] [CrossRef]
- Garmendia, A.E.; Van Kruiningen, H.J.; French, R.A.; Anderson, J.F.; Andreadis, T.G.; Kumar, A.; West, A.B. Recovery and identification of West Nile virus from a hawk in winter. J. Clin. Microbiol. 2000, 38, 3110–3111. [Google Scholar]
- Tesh, R.B.; Parsons, R.; Siirin, M.; Randle, Y.; Sargent, C.; Guzman, H.; Wuithiranyagool, T.; Higgs, S.; Vanlandingham, D.L.; Bala, A.A.; et al. Year-round West Nile virus activity, Gulf Coast region, Texas and Louisiana. Emerg. Infect. Dis. 2004, 10, 1649–1652. [Google Scholar] [CrossRef]
- Wheeler, S.S.; Armijos, M.V.; Garcia, S.; Fang, Y.; Reisen, W.K. Migratory birds and the spread of encephalitis viruses in California: 10 years of data from the Coachella Valley. Proc. Mosq. Vector Control. Assoc. Calif. 2007, 75, 4–6. [Google Scholar]
- Lord, R.D.; Calisher, C.H. Further evidence of southward transport of arboviruses by migratory birds. Am. J. Epidemiol. 1970, 92, 73–78. [Google Scholar]
- Stamm, D.D.; Newman, R.J. Evidence of southward transport of arboviruses from the U.S. by migratory birds. Ann. Microbiol. 1963, 11, 123–133. [Google Scholar]
- Reisen, W.K.; Meyer, R.P.; Milby, M.M. Overwintering studies on Culex tarsalis (Diptera:Culicidae) in Kern County, California: Survival and the experimental induction and termination of diapause. Ann. Entomol. Soc. Am. 1986, 79, 664–673. [Google Scholar]
- Eldridge, B.F. Diapause and related phenomena in Culex mosquitoes: Their relation to arbovirus disease ecology. Curr. Topics Vector Res. 1987, 4, 1–28. [Google Scholar] [CrossRef]
- Reisen, W.K.; Smith, P.T.; Lothrop, H.D. Short term reproductive diapause by Culex tarsalis (Diptera: Culicidae) in the Coachella Valley of California. J. Med. Entomol. 1995, 32, 654–662. [Google Scholar]
- Reisen, W.K.; Thiemann, T.; Barker, C.M.; Lu, H.; Carroll, B.; Fang, Y.; Lothrop, H.D. Effects of warm winter temperature on the abundance and gonotrophic activity of Culex (Diptera: Culicidae) in California. J. Med. Entomol. 2010, 47, 230–237. [Google Scholar] [CrossRef]
- Nielsen, C.; Reisen, W.K.; Armijos, V.; Wheeler, S.S.; Kelley, K.; Brown, D.A. Impact of climate variation and adult mosquito control on the West Nile virus epidemic in Davis, California during 2006. Proc. Mosq. Vector Control. Assoc. Calif. 2007, 75, 125–130. [Google Scholar]
- Kwan, J.L.; Kluh, S.; Madon, M.B.; Reisen, W.K. West Nile virus emergence and persistence in Los Angeles, California, 2003–2008. Am. J. Trop. Med. Hyg. 2010, 83, 400–412. [Google Scholar] [CrossRef]
- Snapinn, K.W.; Holmes, E.C.; Young, D.S.; Bernard, K.A.; Kramer, L.D.; Ebel, G.D. Declining growth rate of West Nile virus in North America. J. Virol. 2007, 81, 2531–2534. [Google Scholar] [CrossRef]
- Tempelis, C.H.; Reeves, W.C.; Bellamy, R.E.; Lofy, M.F. A three-year study of the feeding habits of Culex tarsalis in Kern County, California. Am. J. Trop. Med. Hyg. 1965, 14, 170–177. [Google Scholar]
- Tempelis, C.H. Host-feeding patterns of mosquitoes, with a review of advances in analysis of blood meals by serology. J. Med. Entomol. 1975, 11, 635–653. [Google Scholar]
- Loss, S.R.; Hamer, G.L.; Goldberg, T.L.; Ruiz, M.O.; Kitron, U.D.; Walker, E.D.; Brawn, J.D. Nestling passerines are not important hosts for amplification of west nile virus in Chicago, Illinois. Vector Borne Zoonotic Dis. 2008, 9, 13–17. [Google Scholar]
- Hamer, G.L.; Walker, E.D.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Goldberg, T.L.; Schotthoefer, A.M.; Brown, W.M.; Wheeler, E.; Kitron, U.D. Rapid amplification of West Nile virus: The role of hatch-year birds. Vector Borne Zoonotic Dis. 2008, 8, 57–68. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Kramer, L.D.; Jones, M.J.; Marra, P.P.; Daszak, P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006, 4, e82. [Google Scholar] [CrossRef]
- Cope, S.E.; Barr, A.R.; Bangs, M.J.; Morrison, A.C.; Guptavanij, P. Human bait collections of mosquitoes in a southern California freshwater marsh. Calif. Mosq. Vector Contr. Assoc. 1986, 54, 110–112. [Google Scholar]
- Cope, S.E.; Hazelrigg, J.E. Evaluation of trapping methods, biting behavior, and parity rates for mosquitoes--Harbor Lakes, Wilmington, California, U.S.A. Bull. Soc. Vector Ecol. 1989, 14, 277–281. [Google Scholar]
- Gahlinger, P.M.; Reeves, W.C.; Milby, M.M. Air conditioning and television as protective factors in arboviral encephalitis risk. Am. J. Trop. Med. Hyg. 1986, 35, 601–610. [Google Scholar]
- Reisen, W.K.; Takahashi, R.M.; Carroll, B.D.; Quiring, R. Delinquent mortgages, neglected swimming pools, and West Nile virus, California. Emerg. Infect. Dis. 2008, 14, 1747–1749. [Google Scholar] [CrossRef]
- Kim, M.; Holt, J.B.; Eisen, R.J.; Padgett, K.; Reisen, W.K.; Crom, R.L.; Croft, J.B. Detection of swimming pools by geographic object-based image analysis to support West Nile Virus control efforts. Photogramm. Eng. Remote Sens. 2011, 77, 1169–1179. [Google Scholar]
- Carney, R.M.; Husted, S.; Jean, C.; Glaser, C.; Kramer, V. Efficacy of aerial spraying of mosquito adulticide in reducing incidence of West Nile Virus, California, 2005. Emerg. Infect. Dis. 2008, 14, 747–754. [Google Scholar] [CrossRef]
- Elnaiem, D.E.; Kelley, K.; Wright, S.; Laffey, R.; Yoshimura, G.; Reed, M.; Goodman, G.; Thiemann, T.; Reimer, L.; Reisen, W.K.; et al. Impact of aerial spraying of pyrethrin insecticide on Culex pipiens and Culex tarsalis (Diptera: Culicidae) abundance and West Nile virus infection rates in an urban/suburban area of Sacramento County, California. J. Med. Entomol. 2008, 45, 751–757. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Meola, M.A.; Moudy, R.M.; Kramer, L.D. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008, 4, e1000092. [Google Scholar] [CrossRef]
- Anderson, J.F.; Main, A.J.; Cheng, G.; Ferrandino, F.J.; Fikrig, E. Horizontal and vertical transmission of West Nile Virus Genotype NY99 by Culex salinarius and Genotypes NY99 and WN02 by Culex tarsalis. Am. J. Trop. Med. Hyg. 2012, 86, 134–139. [Google Scholar] [CrossRef]
- Andrade, C.C.; Maharaj, P.D.; Reisen, W.K.; Brault, A.C. North American West Nile virus genotype isolates demonstrate differential replicative capacities in response to temperature. J. Gen. Virol. 2011, 92, 2523–2533. [Google Scholar] [CrossRef]
- Reisen, W.K.; Barker, C.M.; Fang, Y.; Martinez, V.M. Does variation in Culex (Diptera: Culicidae) vector competence enable outbreaks of West Nile virus in California? J. Med. Entomol. 2008, 45, 1126–1138. [Google Scholar] [CrossRef]
- Langevin, S.A.; Brault, A.C.; Panella, N.A.; Bowen, R.A.; Komar, N. Variation in virulence of West Nile virus strains for house sparrows (Passer domesticus). Am. J. Trop. Med. Hyg. 2005, 72, 99–102. [Google Scholar]
- Hom, A.; Bonilla, D.; Kjemtrup, A.; Kramer, V.L.; Cahoon-Young, B.; Barker, C.M.; Marcus, L.; Glaser, C.; Cossen, C.; Baylis, E.; et al. Surveillance for mosquito-borne encephalitis virus activity and human disease, including West Nile virus, in California, 2005. Proc. Mosq. Vector Control. Assoc. Calif. 2006, 74, 43–55. [Google Scholar]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Panella, A.J.; Velez, J.O.; Lambert, A.J.; Campbell, G.L. Chikungunya virus in US travelers returning from India, 2006. Emerg. Infect. Dis. 2007, 13, 764–767. [Google Scholar] [CrossRef]
- Reiter, P.; Lathrop, S.; Bunning, M.; Biggerstaff, B.; Singer, D.; Tiwari, T.; Baber, L.; Amador, M.; Thirion, J.; Hayes, J.; et al. Texas lifestyle limits transmission of dengue virus. Emerg. Infect. Dis. 2003, 9, 86–89. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Reisen, W.K. Ecology of West Nile Virus in North America. Viruses 2013, 5, 2079-2105. https://doi.org/10.3390/v5092079
Reisen WK. Ecology of West Nile Virus in North America. Viruses. 2013; 5(9):2079-2105. https://doi.org/10.3390/v5092079
Chicago/Turabian StyleReisen, William K. 2013. "Ecology of West Nile Virus in North America" Viruses 5, no. 9: 2079-2105. https://doi.org/10.3390/v5092079
APA StyleReisen, W. K. (2013). Ecology of West Nile Virus in North America. Viruses, 5(9), 2079-2105. https://doi.org/10.3390/v5092079



