Abstract
Prion diseases or transmissible spongiform encephalopathies (TSEs) are fatal neurodegenerative disorders in mammals that are caused by unconventional agents predominantly composed of aggregated misfolded prion protein (PrP). Prions self-propagate by recruitment of host-encoded PrP into highly ordered β-sheet rich aggregates. Prion strains differ in their clinical, pathological and biochemical characteristics and are likely to be the consequence of distinct abnormal prion protein conformers that stably replicate their alternate states in the host cell. Understanding prion cell biology is fundamental for identifying potential drug targets for disease intervention. The development of permissive cell culture models has greatly enhanced our knowledge on entry, propagation and dissemination of TSE agents. However, despite extensive research, the precise mechanism of prion infection and potential strain effects remain enigmatic. This review summarizes our current knowledge of the cell biology and propagation of prions derived from cell culture experiments. We discuss recent findings on the trafficking of cellular and pathologic PrP, the potential sites of abnormal prion protein synthesis and potential co-factors involved in prion entry and propagation.
1. Introduction
Prion diseases or transmissible spongiform encephalopathies (TSEs) are neurodegenerative disorders that affect many mammalian species. TSEs include Creutzfeldt-Jakob disease, fatal familial insomnia and Gerstmann-Sträussler-Scheinker syndrome in humans, scrapie in sheep and goats, chronic wasting disease in deer and elk and bovine spongiform encephalopathy. In humans, prion diseases can be sporadic, infectious or of genetic origin. Natural genetic prion diseases in animals have not been reported until now. In animals, infection occurs mainly through the intestinal tract due to ingestion of prions present in the food or the natural environment [1]. During TSE disease, an abnormally folded conformer (PrPSc) of the cellular prion protein (PrPC) accumulates in the central nervous and lymphoreticular system of the infected host. According to the prion hypothesis, PrPSc constitutes the major, if not only, component of the proteinaceous infectious particles [2,3]. The conversion of the host-encoded PrPC to PrPSc is a post-translational process that involves a conformational change from a predominantly α-helical structure to a protein fold increased in ß-sheet. PrPSc is likely generated by a seeded polymerization reaction in which it serves as a template that binds to normal PrPC and catalyzes its conformational conversion to an abnormal, aggregated isoform. PrP aggregates consist of fibrils with a cross-ß-structure that is characteristic of amyloid. As the amyloid fibril elongates and matures, it acquires an increase in conformational stability that is resistant to denaturation by heating, detergents and proteases. Amyloid fibrils are associated with many other neurodegenerative protein misfolding disorders, notably Alzheimer’s and Parkinson’s disease [4]. However, prion diseases are unconventional protein misfolding disorders because they constitute infectious diseases that are often naturally transmitted within species and sometimes even across species barriers.
The first prion disease studied was scrapie of sheep and goats. Seminal work on scrapie by Pattison and Millson in 1961 laid the foundations for the hypothesis that prions exist as different strains [5]. At least 20 different prion strains have been isolated from scrapie that can be propagated in the same inbred mouse line. Prion strains are distinguished by several semi-quantitative factors including incubation time before disease onset, lesion profiles in the brain and the areas of deposition of aggregated PrP [6,7]. PrPSc molecules associated with prion strains differ in their biochemical and biophysical properties. For example, PrPSc molecules exhibit strain-specific glycosylation profiles, and differ in their resistance to proteases as well as in their binding to conformation-specific antibodies [8,9]. This led to the proposal that prion strains are enciphered by the specific fold of PrPSc [3,10]. According to this theory, strain-specific PrPSc conformations would be adopted and amplified by the binding and subsequent conversion of PrPC, thereby preserving the strain-specific information enciphered by the respective quaternary structures of PrPSc.
2. The Cellular Prion Protein PrPC: Structure, Biosynthesis and Intracellular Trafficking
In 1985 researchers identified the Prnp gene encoding the prion protein [11,12] on chromosome 20 in humans and chromosome 2 in mice [13,14]. The Prnp gene is evolutionary highly conserved, exhibiting a sequence homology of approximately 80% from amphibia to mammals [15,16,17]. The Prnp gene contains two to three exons depending on the species, with the last exon encoding the open reading frame [12]. Cellular prion protein is constitutively expressed in many tissues, including the central and peripheral nervous system as well as the immune, lymphoreticular and intestinal system [18]. A particularly high expression is found in neurons localized both at pre- and post-synaptic sites [19] and in glial cells [20].
PrPC is synthesized on the rough endoplasmic reticulum (ER) and transits through the Golgi apparatus to the cell surface (Figure 1A). Within the ER and Golgi, PrPC becomes glycosylated at two asparagine residues [21]. Further post-translational modifications include the formation of a disulfide bond between two cysteine residues (amino acid residues 179 and 214 in human PrP) [22] and the attachment of a glycosyl-phosphatidyl-inositol (GPI) moiety at the carboxy-terminus of the protein [23]. At the plasma membrane, PrPC is incorporated into lipid rafts and caveolae (raft structures with caveolin-1), which are regions of the membrane enriched in cholesterol and sphingolipids [24,25]. Targeting to these lipid rafts is mediated by the amino-terminus of PrPC [26,27]. An early association of PrPC with lipid rafts during its biosynthesis appears to be necessary for its correct folding [28]. Although PrPC is normally translocated to the plasma membrane, high concentrations have been detected within multivesicular bodies [29]. Once on the plasma membrane, PrPC can undergo proteolytic processing by metalloproteases, resulting in a membrane-attached carboxyterminal (C1) and an extracellularly released amino-terminal fragment [30,31,32,33]. In addition, it has been observed that a small percentage of full-length PrPC molecules is secreted, either in a soluble form [34,35] or in association with exosomes [36,37]. Within the cell there is a minor sub-population of PrPC molecules present in the cytosol [38]. Interestingly, using an inducible cell line, PrP23-230 was found in the nucleus of these cells and in association with chromatin [39]. The physiological relevance of such intranuclear localization so far is unclear.
Extensive research into the biological function of PrPC has resulted in a plethora of different possible functions. So far, these include involvement in signaling cascades, neuronal survival, apoptosis, oxidative stress, cell adhesion, differentiation, immunomodulation and more recently, microRNA metabolism [40,41]. PrPC has a high affinity for metals such as copper, zinc and manganese through binding at its amino-terminus. Binding to PrPC mediates neuronal uptake of these metal ions potentially through interaction with other receptors [42,43]. PrPC has also been proposed to act as a cell surface scaffold protein that interacts with different partners. These mediate the activation of a range of diverse signaling pathways that modulate neuritogenesis and synapse formation [40]. Interactions of PrPC with the neural cell adhesion molecule NCAM or with the laminin receptor precursor LRP/LR have been reported to elicit specific signaling cascades in neurons [44,45,46]. In non-neuronal cells, PrPC also plays an important role during embryogenesis or during stem-cell proliferation and differentiation [47,48]. Interestingly, PrP has also been shown to bind both RNA and DNA in vitro [49,50,51,52]. Evidence for a physiological role of these nucleic acid-protein associations [41] is accumulating but needs further clarification.
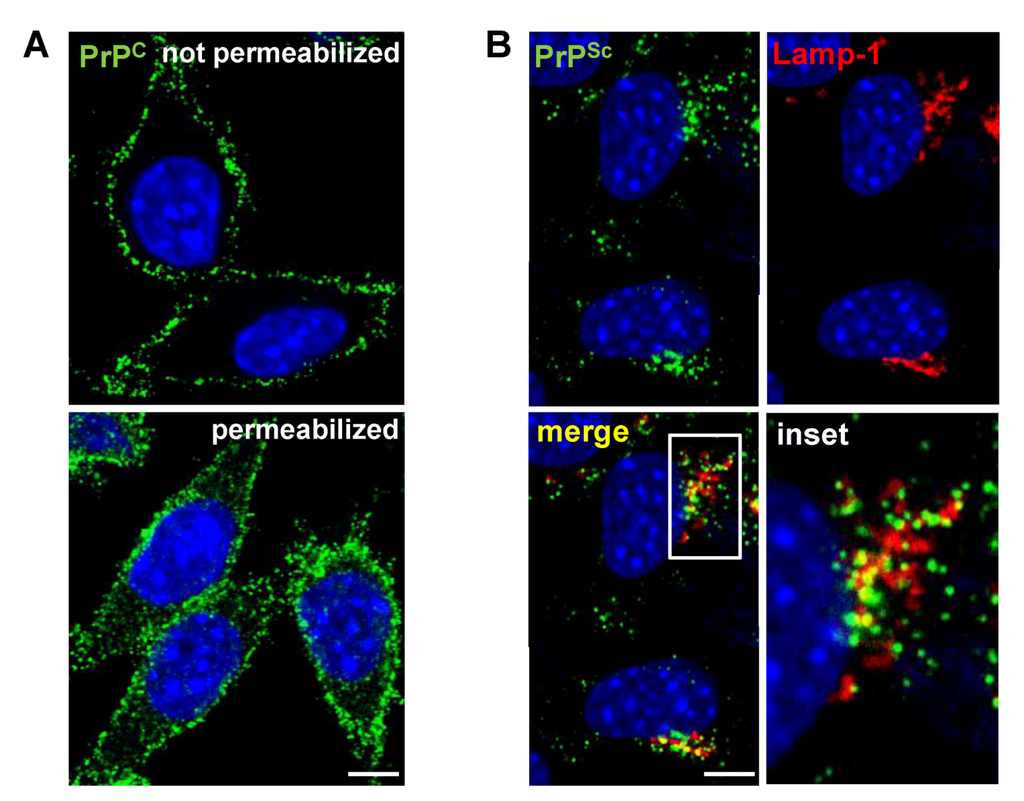
Figure 1.
Localization of PrPC and PrPSc in L929 fibroblast cells. (A) Indirect immunofluorescence (IF) staining of cellular PrP (green) in uninfected L929 cells. PrPC predominantly resides at the cell surface with some intracellular localization. (B) Detection of PrPSc in L929 cells persistently infected with prion strain 22L by IF. In contrast to PrPC, PrPSc (green) primarily localizes intracellularly and partially co-localizes with the lysosomal marker Lamp-1 (red). (A,B) Nuclei were counterstained with Hoechst (blue). Scale bar: 5 µm.
PrPC is rapidly and constitutively endocytosed from the plasma membrane [53,54]. External stimuli such as the binding of copper or stress-inducible protein 1 (STI1) to PrPC can stimulate its internalization [55]. Endocytosis occurs via a dynamin-dependent pathway. PrPC transits through Rab5 positive early endosomes (EEs) before it is degraded via the endosomal/lysosomal pathway [54,56,57,58,59]. Alternatively, endocytosed PrPC can transfer rapidly and directly to recycling endosomes (RE) and back to the cell surface [54,56,60,61]. It has been proposed that the dynamin-dependent endocytosis of PrPC is a GPI-anchor independent event mediated by the interaction of other proteins with specific domains within PrPC [56].
Both, clathrin-dependent and -independent pathways have been described for PrPC internalization [54,59,62,63,64] (Figure 2). Although PrPC may be endocytosed through rafts in some cells [62,64,65], most studies demonstrate that PrPC translocates out of rafts prior to its internalization via clathrin-coated pits in permanent cell cultures and primary neurons [54,60,66,67,68,69]. An amino-terminal, positively charged domain of PrPC is important for its endocytosis by clathrin-coated vesicles [54,66]. PrPC has been detected in clathrin-coated vesicles using electron microscopy [54,60,70]. Still, PrPC internalization in mature primary hippocampal neurons appears to depend on rafts and cholesterol [71]. In agreement with this, Sarnataro et al. showed that lipid rafts and clathrin-coated vesicles can co-operate in the internalization of PrPC [72]. The conflicting results obtained in different cell culture models argue that the internalization of PrPC is a complex event that may involve different receptors and co-receptors and more than one endocytic route depending on the cell type or stimulus.
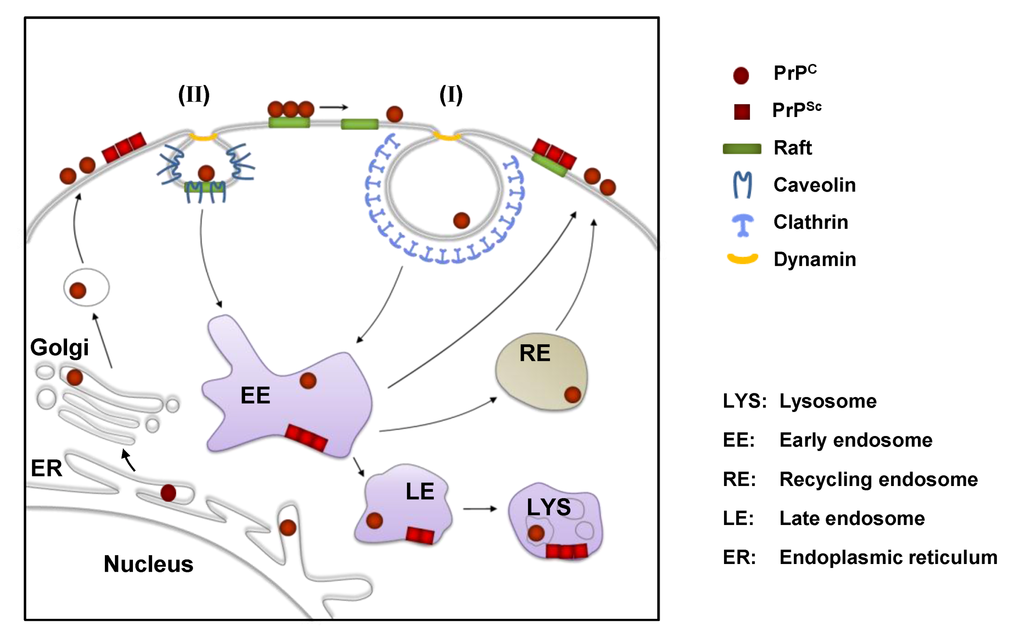
Figure 2.
Cell biology of PrP in scrapie-infected cells. PrPC is synthesized in the endoplasmic reticulum (ER) and passes through the secretory pathway to the cell surface, where it resides in lipid rafts. In many cells, PrPC leaves lipid rafts prior to being internalized by clathrin-dependent endocytosis (I). Clathrin-independent raft/caveolae-dependent internalization (II) of PrPC has also been proposed for some cells. PrPC can be degraded by lysosomes or rapidly recycled back to the cell surface by recycling endosomes (RE). In cultured scrapie-infected cells the conversion of PrPC to PrPSc is believed to take place on the cell surface and/or in vesicles along the endolysosomal pathway. After conversion PrPSc can accumulate at the cell surface or in intracellular vesicles (e.g. lysosomes).
3. Cell Surface Receptors for PrPC
Clathrin-coated vesicles mediate internalization of transmembrane proteins by interaction with accessory proteins [73]. Since PrPC lacks a transmembrane domain capable of interacting with adaptor proteins, co-internalization of PrPC with other proteins has been suggested. Several potential receptors for co-internalization have been proposed, including the laminin receptor precursor LRP/LR, the low-density lipoprotein receptor-related protein 1 (LRP1) and glycosaminoglycans (GAGs). Comparative studies on the involvement of these receptors for PrPC endocytosis are lacking, so it is still unclear which role these receptors play in certain cell types. Of note, also other so far unidentified receptors might be involved.
The membrane-associated form of the ribosomal protein SA (RPSA), termed laminin receptor precursor LRP/LR, has been characterized as a potential binding partner of PrP [74,75,76,77]. RPSA is a multifunctional protein present on the cell surface or associated with cytosolic ribosomes. The 67 kDa membrane-bound form LRP/LR is a high affinity receptor for laminin derived from a 37 kDa polypeptide (37LRP) precursor by homo- or heterodimerization through fatty acid acylation. LRP/LR is expressed in a variety of tissues and cells, including neurons [78], and also binds growth factors, toxins and pathogens. LRP/LR was first identified in a yeast two-hybrid system using a HeLa cDNA library as prey and PrP as bait [76]. Interaction of PrP23-231 and LRP/LR, both ectopically expressed, was confirmed in insect cells and N2a cells. Recombinant human PrP also bound to N2a and BHK cells expressing LRP/LR [74]. Direct binding of recombinant PrP to LRP/LR is mediated through a region in PrP encompassing amino-acid residues 144-179 in human PrP [77]. Recombinant PrP also indirectly associates with LRP/LR on the surface of CHO cells through the interaction of both molecules with the GAG heparan sulfate proteoglycan (HSPG). Although an association of PrP and LRP/LR was confirmed in an interactome analysis of tagged-PrP expressed in neuroblastoma cells, co-internalization of LRP/LR and endogenously expressed GPI-anchored PrPC has not been formally demonstrated [79].
Another putative PrPC co-receptor, LRP1, belongs to the low-density lipoprotein (LDL) receptor family and is abundantly expressed in neurons and hepatocytes [80]. LRP1 acts as a scavenger receptor with two clusters of complement-type repeats with high affinity for at least 24 different ligands [80]. Ligands either directly adhere to LRP1 or initially bind to HSPG before being scavenged by LRP1 for endocytosis. Thus, HSPGs serve as a primary docking site for ligands acting as a ligand reservoir and thereby regulating LRP1 activity. Recognition sites for cytosolic adaptor proteins in the cytoplasmic tail of the light chain regulate receptor-mediated endocytosis by clathrin-coated pits (CCPs) [80]. LRP1 transiently associates with rafts before it undergoes rapid endocytosis by CCPs. Partial lipid raft localization has been demonstrated in some, but not all cell lines tested [81]. LDL receptor family members also physically and functionally interact with other cell surface proteins, including GPI-anchored proteins and thereby modulate their activity [80]. Bridging of LRP1 to other cell surface receptors by ligands or cytosolic adaptor proteins has been shown to promote their co-internalization [81]. PrPC and LRP1 have been shown to co-localize on the cell surface of SH-SY5Y cells [82]. In neurons, association of PrPC with LRP1 in the secretory pathway assists in trafficking of PrPC to the cell membrane [83]. Both, knock-down of LRP1 or the use of LRP antagonists, inhibit endocytosis of PrPC [82,83]. A stretch of basic amino acid residues in the amino-terminus of PrPC mediates binding to LRP1. HSPGs have been shown to be required for LRP1-protein complex internalization in some cases [84].
The metabolism and trafficking of PrPC is influenced by the interaction with cell surface bound GAGs. These are long unbranched polysaccharides, composed of repeating disaccharide units that are highly sulfated. GAGs are either secreted or linked to core proteins to make an entity known as a proteoglycan. Proteoglycans are abundantly expressed on cell surfaces and differ by their polysaccharide backbone and the degree of sulfation. Heparin is a GAG mainly produced and secreted by mast cells that is structurally closely related to heparan sulfate (HS). The most common disaccharide unit within HS consists of glucuronic acid and N-acetylglucosamine. HS side chains are covalently linked to either transmembrane syndecans or GPI-anchored glypicans. The basic amino acid motif KKRPKP present in the amino-terminus of PrP (residues 23-28) and two additional regions (residues 53-93 and 110-120) are important for the association of PrPC with HS [85,86,87]. Experimentally, brain-derived PrPC as well as recombinant PrP have been shown to bind to heparin or another polyanion, Congo red, in vitro [88,89]. Recombinant PrP has also been shown to bind to HSPG on the plasma membrane of CHO cells [90].
Whether HSPGs are important for the cell surface localization of PrPC remains controversial. In N2a cells, degradation of HS by heparinases neither affected the cell surface expression of PrPC nor influenced its raft association [91]. GAG mimetics could potentially modulate cell surface expression of PrPC by competing for the binding site of endogenously expressed HSPGs, as exogenously added soluble GAGs have been previously shown to influence cellular PrPC levels. Early studies demonstrated that treatment of N2a cells with HS increased total cellular levels of PrPC [88]. While pentosan polysulfate (PPS), a GAG analogue, had no apparent effect on PrPC cell surface expression in N2a cells at concentrations of 0.1 μg/mL [89], a concentration of 100 μg/mL drastically reduced PrPC cell membrane localization [90]. Likewise, other polyanionic compounds such as Congo red and dextran sulfate 500 kDa (DS500) at concentrations of 10 μg/mL reduced the amount of cell surface PrPC in N2a cells [90]. PPS treatment did not affect biosynthesis or trafficking through the secretory pathway but instead enhanced the endocytosis rate of PrPC, resulting in a redistribution of a proportion of PrPC into late endosomal vesicles. The amino-terminus, comprising residues 25-91, was shown to be important for this. In contrast, GAG analogue suramin was shown to impair PrPC folding in the secretory pathway, resulting in the re-routing of PrPC to acidic compartments [92].
4. Cellular Models for Studying PrPSc Formation
Cell culture models replicating prion infectivity were already established in 1970 [93], even before PrPSc was identified as a surrogate marker and potential TSE agent. PrPSc formation was first detected in the murine neuroblastoma cell line N2a when exposed to mouse-adapted scrapie [94,95]. Subsequent infection experiments demonstrated susceptibility of N2a cells to several different mouse-adapted scrapie strains [96]. Since then, several cell lines of neuronal and non-neuronal origin have been identified to be susceptible to a stable infection with prions (Table 1). Once prions have successfully infected a cell line, they can replicate persistently over multiple cell passages, with very few exceptions [97], without any overt cytopathic effect. Cell lines that have been successfully infected include microglial cells as well as epithelial cells, fibroblasts and myoblasts, which have all been demonstrated to persistently replicate an array of prion strains in vitro [98,99,100,101,102,103,104,105,106,107,108]. Curiously, a rabbit kidney epithelial cell line genetically engineered to express PrPC of different species was shown to be susceptible to a variety of prion strains isolated from different sources [99,100,101,102,103,104,105,109]. Several primary cell culture models for prion replication have been reported, some of which show cytopathic effects upon infection [110,111,112,113,114,115].
Despite recent success with prion cell culture models (Table 1), prion infection of cells in vitro has been notoriously difficult and often unsuccessful. Most cell lines expressing PrPC are resistant to prion infection, and for many prion strains, suitable cell culture models have not been established [95,116,117,118]. Importantly, ex vivo models for the propagation of prion strains of human origin have only been reported once [119]. Infections with human strains were more successful when prions had been previously adapted to mice [99,120]. Whilst expression of PrPC is necessary for prion infection in vitro [121,122], the expression level of PrPC does not generally appear to influence susceptibility [107,118,123]. Importantly, infection rates and prion titers in cell culture are usually low and subsequent cloning of infected cells or pre-selection of clones is a necessary process to increase the percentage of infected cells within a cell population [95,118,124,125,126,127]. Remarkably, persistent prion infection is often lost over continuous passage. Changes in growth medium composition and culture conditions can account for prion loss in cell culture [128,129]. Additionally, genetic heterogeneity and chromosomal instability have been proposed to affect susceptibility of cell populations over time [118,130].
Cell lines that are susceptible to some prion strains demonstrate a remarkable resistance to other strains [107,118,125]. The mouse derived fibroblast cell line NIH/3T3 for example has been shown to be susceptible only to mouse-adapted scrapie strain 22L, whilst the murine fibroblast cell line L929 is capable of replicating the strains 22L, RML and ME7 [107,125]. The reason for the differences in susceptibility to prion infection is unclear but points to substantial differences in the cell biology of prion strain replication. So far, susceptibility of a cell line to any given prion strain can only be determined empirically.
A major restriction in the analysis and understanding of prion cell biology is the specific detection of the disease-associated isoform PrPSc over the host-encoded isoform. There is a shortage of antibodies that are suitable for the convincing and specific detection of PrPSc by western blot or immunofluorescence. Therefore, it is extremely difficult to investigate the uptake of PrPSc, the subcellular distribution and location of de novo synthesis. Presently, the protocols for the specific detection of the misfolded isoform take advantage of the unique biochemical features of PrPSc and include treatments with denaturants to enhance immunoreactivity [131]. Moreover, newly generated PrPSc cannot be discriminated from the inoculated PrPSc used, unless either the substrate PrPC or template PrPSc are tagged by antibody-specific epitopes or fluorescent labels. In most studies done so far, cells overexpressed tagged PrPC [132,133,134]. Thus, either the presence of the tag or overexpression of PrPC could influence the conversion process. Amino-acid residue substitutions in PrP often create complications such as a transmission barrier. Tagging of PrPC at the amino-terminus with GFP has been shown to compromise prion infection and PrPSc formation in vivo and in vitro [135]. Alternatively, fluorescent labeling of purified prion preparations has been successfully used to study prion uptake and intraneuronal transport in vitro. However, the uptake characteristics of labeled fibrils show striking differences compared to those of untagged PrPSc from crude brain homogenate preparations [133,136]. More recently, 3F4-tagged PrPSc derived from transgenic mice that were infected with prions proved effective in studying prion uptake [133]. Of note, changes in the PrP amino acid substitutions could affect prion strain characteristics and might thus not be suited to study the cell biology of different prion strains.
Previously, prion cell culture systems relied on the detection of PrPSc as a marker for infection and prion titers were determined by inoculation of cell lysates into panels of mice [107]. A major breakthrough came in determining the titers of standard prion strains with the development of the “Standard Scrapie Cell Assay” (SSCA) [118]. The SSCA incorporates a highly susceptible N2a subclone that is inoculated with serial dilutions of the prion strain RML as a standard. These infected N2a cells are propagated in a microtiter format until de novo formed PrPSc accumulates to detectable levels. After three cell passages, defined numbers of cells are filtered onto nitrocellulose membranes and PrPSc positive cells are detected by immunoblot using an ELISPOT reader. The SSCA can also be used as an endpoint assay (SCEPA) to quantify prion titers of individual samples by comparison with the standard titration curve [137]. The SSCA was subsequently adapted to a panel of cell lines exhibiting selective susceptibility to different strains [125,138].

Table 1.
Cell culture models susceptible to transmissible spongiform encephalopathy (TSE) agents.
| Cell designation | Tissue of origin or cell type | Species of origin | Prion strain | References |
|---|---|---|---|---|
| 1. Neuronal or brain-derived cells | ||||
| N2a | neuroblastoma cell line* | mouse | Chandler, RML, 139A, 22L, C506, Fukuoka-1, FU CJD | [95,96,127,139,140,141,142,143,144] |
| GT1 | hypothalamic cell line | mouse | Chandler, RML, 139A, 22L, kCJD, FU CJD , M1000 | [96,97,99,120,139,145] |
| SN56 | cholinergic septal cell line | mouse | Chandler, ME7, 22L | [146] |
| HpL3-4 | hippocampal PrP-deficient cell line, upon ectopic expression of moPrP* | mouse | 22L | [121,147] |
| CF10 | brain derived PrP-deficient cell line, upon ectopic expression of moPrP | mouse | 22L | [122] |
| SMB | prion-infected brain cell | mouse | Chandler, 139A, 22F, 79A | [93,148,149] |
| CAD | catecholaminergic cell line | mouse | RML, 22L, 22F, 79A, 139A, ME7 | [125,150,151,152] |
| MG20 | microglial cell line overexpressing PrPC | tg20 mouse | Chandler, ME7, Obihiro, mouse-adapted BSE | [98] |
| PC12 | pheochromocytoma cell line | rat | 139A, ME7 | [153,154,155] |
| HaB | brain-derived cell line | hamster | Sc237 | [131] |
| SH-SY5Y | neuroblastoma cell line | human | sCJD brain material | [119] |
| MDB | primary brain cells, SV40 transformed | mule deer | CWD | [129] |
| 2. Primary neuronal or brain-derived cells | ||||
| CGN | cerebellar granule neurons overexpressing ovine PrPC | tgov mouse | mo 127S | [111] |
| CAS | cerebellar astrocytes overexpressing ovine PrPC | tgov mouse | mo 127S | [111] |
| NSC | neural stem cells | mouse | 22L, RML | [112,113,115] |
| 3. Non-neuronal cells | ||||
| C2C12 | skeletal myoblast cell line | mouse | 22L | [108] |
| L fibroblasts | fibroblast cell line | mouse | ME7, Chandler | [106] |
| L929 | fibroblast cell line | mouse | 22L, RML, ME7 | [107] |
| NIH/3T3 | fibroblast cell line | mouse | 22L | [107] |
| MSC-80 | Schwann cell line | mouse | Chandler | [156] |
| MovS | Schwann cell-like from dorsal root ganglia | tgov mouse | PG127, SSBP/1, scrapie field isolates | [104,157] |
| moRK13 | epithelial cell line expressing mouse PrPC | rabbit | Fukuoka-1, 22L, Chandler, M1000, mo sCJD | [99,100,101,120] |
| voRK13 | epithelial cell line expressing vole PrPC | rabbit | vo BSE | [100] |
| ovRK13/ RoV9 | epithelial cell line expressing ovine PrPC | rabbit | PG127, LA404, SSBP/1, scrapie field isolates | [102,103,104] |
| elkRK13 | epithelial cell line expressing elk PrPC | rabbit | CWD | [105,109] |
| 4. Primary non-neuronal cells | ||||
| BM-derived MSC | bone marrow derived mesenchymal stem cell | mouse | Fukuoka-1 | [110] |
| BM-derived MSC-like | bone marrow derived mesenchymal stem cell like | mouse | Fukuoka-1 | [114] |
* some cells overexpress moPrPC-A or 3F4 antibody-epitope tagged moPrPC
5. PrPSc Uptake During the Infection Process
The prion infection process in vitro can be divided into four main steps: 1) Attachment of PrPSc to the cell; 2) uptake; 3) initiation of PrPSc formation and establishment of productive infection; and 4) persistent propagation. Most of the steps have been studied separately. The use of different prion preparations, strains, and cell lines has complicated direct comparison of results. Consequently, the following paragraphs can only give an overview of the possible infection processes.
Most cell lines in vitro are capable of taking up PrPSc (Figure 3). Uptake of prion strains was reported to be neither cell type nor strain dependent [133]. However, even within a cell population exposed to scrapie brain homogenate, uptake is evident only in a subset of cells [133]. The observed differences in the speed of internalization are at least in part due to variations in the PrPSc sample preparation [133,158]. Detergent extraction of PrPSc prior to fluorescence labeling resulted in a slow uptake over a number of days [136]. However, PrPSc from crude brain homogenate preparations was taken up rapidly within minutes to hours post prion exposure [132,133,159,160,161,162,163,164]. Several studies have demonstrated that PrPSc is readily taken up by cells known to be resistant to prion infection [159,160,165,166], arguing that potential receptors and uptake mechanisms for PrPSc are also present in non-permissive cells.
As physical interaction between PrPC and PrPSc is required for the conversion of cellular prion protein to its pathological isoform, PrPC might also serve as a receptor for PrPSc uptake. Interestingly, overexpression of PrPC did not affect initial binding of PrPSc to CHO cells [159]. It was later shown that cells devoid of PrPC also take up PrPSc, demonstrating that PrPC is not generally required for PrPSc uptake (Figure 3) [133,136,159,162]. But how does PrPSc bind to the cell and how does it enter? Three putative cell surface receptors have been characterized that could be involved in PrPSc uptake. LRP/LR has been found expressed in human small intestinal mucosa [167], suggesting that it could mediate the initial PrPSc uptake in the gut when the animal is first exposed to prions by food contaminants. Importantly, PrPSc uptake in human intestinal enterocytes in culture depended on both prion preparations and strains [158]. Uptake of PrPSc present in brain homogenate from mice infected with bovine spongiform encephalopathy was reduced upon preincubation of cells with anti-LRP/LR antibodies, suggesting that LRP/LR is involved in this process. Likewise, uptake of proteinase K treated mouse-adapted scrapie prions into non-permissive BHK cells was dependent on the LRP/LR receptor and HS [166]. Of note, establishment of prion infection in these systems has not been shown.
Jen and colleagues recently demonstrated that a specific inhibitor of LRP1 receptors and siRNA-mediated knock-down both drastically impaired binding and uptake of both recombinant PrP fibrils and PrPSc in wildtype and PrP knock-out neurons [164]. Interestingly, addition of PrPSc to the cells slowed down endocytosis of endogenous PrPC, suggesting that PrPSc and PrPC were competing for the same binding site on LRP1. Further studies demonstrated that the binding of PrPSc to LRP1 was mediated by cluster 4 of LRP1 that is also implicated in endocytosis of PrPC [82,83,164].
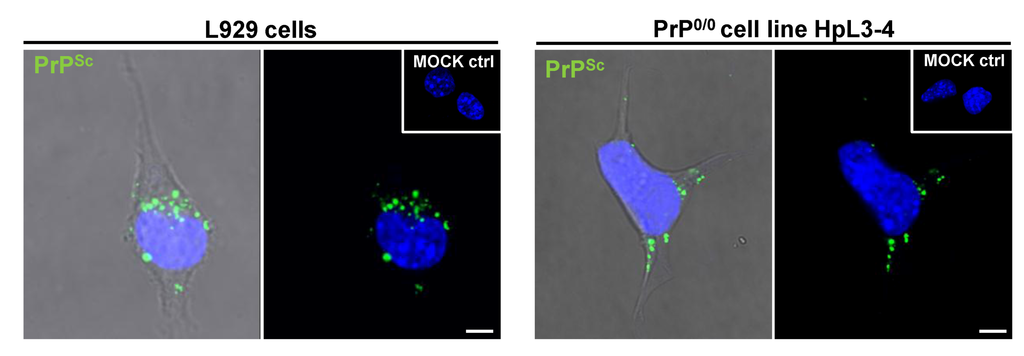
Figure 3.
Non-neuronal cells and PrP-deficient cells take up PrPSc. Brain homogenate from mice infected with the 22L prion strain is taken up by L929 fibroblast cells (left panel) and PrP-deficient HpL3-4 cells (right panel). Cells were incubated with infected brain homogenate for 18 hours prior to fixation, permeabilization, guanidine hydrochloride treatment and immunofluorescence staining. Cells incubated with uninfected brain homogenate (MOCK ctrl) served as control for specific detection of PrPSc. PrPSc uptake is observed in both fibroblast cells and PrP-deficient cells. Monoclonal anti-PrP antibody: 4H11. Nuclei were counterstained with Hoechst (blue). Scale bar: 5 µm.
Proteoglycans could represent the third class of PrPSc receptors necessary for binding and early uptake of exogenous PrPSc. PrPSc binds to the HS analog heparin and disulfonated Congo red in vitro [159,168]. HS serves as a binding partner for PrPSc in vivo, as proteinase K digested PrPSc (hamster scrapie Sc237) poorly bound to mutant CHO cells lacking HS or GAGs [159]. Addition of heparin, a natural HS analog, competitively inhibited binding of PrPSc to N2a and wildtype CHO cells [159]. GAG mimetics have also been shown to inhibit uptake of PrPSc in cell culture. Incubation of non-permissive CHO cells with heparan mimetic HM2602 drastically impaired uptake of hamster prion rods (strain Sc237) [165]. Likewise, DS500 and HM2602 impaired entry of hamster prion rods in N2a cells [160]. Of note, concentrations sufficient to inhibit PrPSc accumulation in RML infected N2a cells were inefficient in inhibiting PrPSc uptake [160]. A candidate proteoglycan for PrPSc binding and uptake is glypican-1 [169,170,171].
Although several putative receptors for PrPSc endocytosis have been identified, the exact mechanism of uptake has not been elucidated. Besides the classical endocytosis pathways of clathrin-mediated endocytosis or raft-mediated endocytosis, PrPSc could also be taken up by macropinocytosis. Macropinocytosis is a relatively non-selective process that delivers its cargo to late endosomal and lysosomal compartments. Studies on the uptake of fluorescently labeled detergent extracted, proteinase K treated PrPSc (Chandler scrapie strain) by SN56 cells revealed no co-localization with raft marker choleratoxin [136]. Instead, extensive co-localization was observed with fluorescent dextran, a marker for internalization by macropinocytosis. Addition of amiloride, an inhibitor of macropinocytosis, to Rov cells (RK13 cells expressing ovine PrP) did not inhibit uptake of exogenous PrPSc, arguing that this internalization process is not involved in PrPSc uptake at least in these cells [162]. However, productive infection with RML prions was prevented in N2a cells upon addition of macropinocytosis inhibitor EIPA for 48 hours during the infection process [172]. Whether this treatment influenced external PrPSc uptake or impaired de novo PrPSc production has not been shown. Future studies will need to clarify the role of macropinocytosis for PrPSc uptake and establishment of persistent infections.
In summary, the mechanism of PrPSc internalization is not fully understood. PrPSc uptake might not be restricted to one pathway but could occur through multiple pathways [173] and host factors are likely to influence the outcome of the infection process [133,174,175]. One important question that needs to be addressed further is if the proposed uptake pathways also lead to a productive prion infection. So far, it cannot be excluded that productive infection requires a distinct internalization route and alternative uptake mechanisms might prevent chronic infection. Furthermore, it is unclear if different prion strains utilize the same entry pathways for establishing chronic infections.
6. Early Steps of Prion Infection
The aforementioned studies so far demonstrated that PrPSc can be taken up by a vast majority of cells in vitro, independent of PrPC expression and receptors such as LRP/LR, LPR1 and proteoglycans might contribute to PrPSc internalization. But where exactly is PrPSc formed, and is the uptake of PrPSc necessary for a productive prion infection? Recent progress in studying the earliest events of prion infection has been made by expressing tagged PrPC [132,134]. According to these studies, de novo PrPSc formation is a fast process, initiated within minutes [134] to hours post-exposure [132]. Remarkably, initial PrPSc formation was independent of the scrapie strain and was even apparent in cells that do not become persistently infected or with strains previously not shown to propagate in cell culture. However, PrPSc formation was often transient and did not result in a productive infection [132]. These data demonstrate that (1) non-permissive cells can transiently produce PrPSc, (2) the establishment of a prion infection is initiated after the first round of PrPSc formation and (3) restricted susceptibility to certain strains is controlled by processes that take place after the initial PrPSc formation. Studies using myc-tagged PrPC expressed in N2a cells demonstrated that PrPSc was formed on the plasma membrane within 2 minutes post prion exposure and was then rapidly trafficked to the perinuclear region [134]. Lipid rafts appeared to be important for PrPSc formation, as treatment with the cholesterol sequestering drug filipin, abolished this process [134]. Neither de novo PrPSc formation nor PrPSc accumulation in perinuclear compartments was abolished by inhibitors of dynamin-dependent endocytosis, CCPs or macropinocytosis. Thus, these endocytic pathways are either not involved in de novo formation and trafficking of PrPSc or multiple pathways can be utilized for PrPSc uptake [134]. The involvement of the LRP1 receptor for the establishment of a productive infection is unclear. Knock-down of LRP1 in sensory neurons during the acute infection step appeared to decrease uptake of PrPSc but had no influence on overall PrPSc levels four weeks post infection, a time point at which PrPSc replication is usually not observed in untreated sensory neurons [164]. Further experiments will be necessary to prove if the LRP1 receptor is also contributing to the establishment of a productive prion infection.
7. PrPSc Formation in Persistently Infected Cells
The cellular compartments involved in PrPSc formation and accumulation are still ill-defined. In cell culture, PrPSc accumulation has been reported mainly on the cell surface and within endocytic compartments [176,177,178], but also within vesicles of the secretory pathway [179,180,181], and even in the nucleus [182]. PrPSc formation is a post-translational event that requires physical interaction between PrPSc and PrPC (Figure 2). Although both PrPC and PrPSc are present on the plasma membrane of infected N2a cells [180,183], PrPSc localizes primarily intracellularly, with only minor amounts on the cell surface (Figure 1B) [131]. Still, transport of PrPC to the plasma membrane is required for conversion into the abnormal isoform [92,134,177,178,181,184]. Removal of PrPC from the plasma membrane by phospholipase C diminishes PrPSc accumulation in N2a cells [140,177,184]. Likewise, impaired transport of PrPC to the cell surface by suramin cures chronically infected N2a cells and prevents PrPSc formation [92]. Lipid rafts appear to play an important role in the formation of PrPSc [58]. Detergent-resistant microdomains isolated from persistently infected N2a cells contain both PrPC and PrPSc [25,179,181,184]. Inhibition of cellular cholesterol synthesis drastically impairs raft formation and also influences cellular PrPSc levels [24,179]. Filipin extraction of membrane cholesterol also affects cellular PrPSc levels in persistently infected N2a cells [65]. Mutant PrPC with a transmembrane anchor that redistributes into non-raft regions is not converted to its abnormal isoform, suggesting that raft association is required for conversion. Of note, changing the PrP amino acid sequence by addition of a transmembrane anchor to PrP could also impair the conversion process per se, and the convertibility of such PrP molecule has not been formally proven in vitro [24]. Cells expressing PrPC lacking the GPI moiety do not support sustained prion infection in vitro, arguing that the anchor is necessary for efficient PrPSc formation in cell culture [122].
The role of the secretory pathway for PrPSc formation is unclear. Early studies reported that PrPSc co-localized with Golgi markers [131]. It has been speculated that either PrPC or PrPSc are directly translocated from the cell membrane to the ER by a Rab6 controlled retrograde pathway [185]. Interestingly, PrP mutants that are retained in the ER or Golgi apparatus can drastically interfere with PrPSc accumulation in RML infected N2a cells, suggesting that the mutants competitively inhibited binding or conversion of wildtype PrPC in these compartments [186]. Alternatively, minute amounts of PrPC trafficked correctly through the secretory pathway to the cell surface are capable of dominant negative interference with the conversion of PrPC.
An important role in the conversion of PrPC to PrPSc in persistently infected cells is assigned to the endocytic pathway [176,177,178]. In primary hippocampal neurons, PrPSc was found at the cell surface and in early as well as recycling endosomes [61]. The early recycling compartment was suggested to be the primary location of prion conversion [187]. Recently, Zurzolo and co-workers studied the intracellular localization of PrPSc in three cell lines persistently infected with different prion strains and detected more than 25% of the protein co-localized with a marker for the early recycling compartment [187]. Others found that in chronically infected cell lines N2a and GT1, the majority of PrPSc accumulates intracellularly mainly localized within late endosomes and lysosomal compartments [131,177,181,184,188,189]. In endosomal or lysosomal compartments, PrPSc undergoes an initial proteolytic cleavage, leading to PrPSc lacking its amino-terminus [178,181,189,190]. Importantly, inhibition of amino-terminal trimming does not inhibit PrPSc accumulation, arguing that this step is not essential for PrPSc biogenesis [176,178]. In conclusion, while it is unclear if PrPSc replication mechanisms are the same for different strains and in different cell types, most studies argue that PrPSc formation in persistently infected cells takes place either on the cell surface or along the endocytic pathway, with the majority of PrPSc eventually accumulating in the lysosomal compartment (Figure 2B).
8. GAGs As Co-Factors for PrPSc Formation
The interaction of PrPC and PrPSc with receptors for binding and uptake is closely linked to the conversion process. GAGs are not only involved in the binding and uptake of PrP, but also play an important role for PrPSc formation or stabilization. In vivo HS is a prominent component of cerebral prion amyloid plaques and diffuse PrPSc deposits [191]. Treatment of uninfected cells with lyases that cleave GAG chains from endogenous proteoglycans prevents prion infection, arguing that GAGs are essential for initiation of a productive prion infection [160]. However, GAGs also play an essential role in PrPSc accumulation in cells chronically infected with prions. Enzymatic digestion of cellular HS, but not cellular chondroitin or dermatan sulfate, reduced PrPSc levels in N2a cells chronically infected with RML prions, suggesting that HS is a major co-factor necessary during PrPSc biogenesis [91]. In line with this, sodium chlorate and xyloside EDX, inhibitors for sulfation and proteoglycan glycosylation, drastically reduced PrPSc levels in N2a cells chronically infected with RML [88,91]. Most exogenously added sulfated glycans interfere with PrPSc accumulation in a variety of persistently infected cell culture models, likely by binding to PrPSc or PrPC and by competing for the interaction with endogenous sulfated glycans required for PrPSc formation and/or stabilization [88,89,192]. The degree of sulfation, but also other properties such as the glycan backbone, positioning of sulfates, non-sulfate substituents and glycan chain size are important for the anti-PrPSc activity of GAG analogs [89,165]. Disulfonated Congo red and sulfated glycans such as low molecular weight heparin, dextran sulfate, suramin and PPS all reduced PrPSc accumulation in N2a cells persistently infected with RML or Chandler [88,89,92,192]. Less sulfated HS, high molecular weight heparin, or other GAGs such as dermatan sulfate, chondroitin sulfate and hyaluronic acid exerted no anti-PrPSc activity [88]. HS side chains on glypican-1 are likely important for facilitating PrP conversion, as siRNA knock-down of glypican-1 significantly reduces total PrPSc levels in N2a cells [170]. In conclusion, in vivo and in vitro data argue that endogenous GAGs stimulate prion conversion, potentially by providing a scaffold for PrPC/PrPSc clustering and interaction [193,194,195]. Exogenous GAGs competitively inhibit the interaction of PrPSc and PrPC with endogenous GAGs and thereby interfere with the conversion process.
9. Cell-To-Cell Transmission of Prions
Under the right culturing conditions, prion-infected cells retain stable PrPSc levels over multiple cell divisions. PrPSc accumulation in dividing cells is strongly influenced by the rate of PrPSc synthesis, degradation and cell division [196]. In persistently infected cells, prion infectivity is primarily transmitted from mother to daughter cells [196]. Interestingly, an increase of infected cells during cell propagation was observed in some [118,197] but not all cell cultures [107], arguing that at least in some cultures, prions spread to neighboring cells. Two major routes have been described for intercellular spread of prions in vitro. Several studies have reported release of PrPSc and/or infectivity into the cell culture medium (Table 2). Prions have also been found to be associated with exosomes released from infected cells [36,37,99,198]. In NIH/3T3 cells, retroviral co-infection enhanced the release of PrPSc and prion infectivity into the cell culture supernatant. Prion proteins were released in association with exosomes and viral particles, suggesting that retroviral co-infection could contribute to prion spreading [198]. Kanu and colleagues showed that in SMB cells infected with Chandler scrapie, cell-to-cell infection was dependent on close proximity or direct cell contact between donor and recipient cell [149]. Culturing infected and uninfected cell populations separated by transwells abolished infection of target cells. Likewise, conditioned medium was ineffective at transmitting prions to recipient cells. For some cell lines, secretion of infectivity has been reported, but prions were preferentially transmitted to nearby cells, suggesting that direct cell proximity promoted efficient infection [197]. The fact that living cells were far more effective in transmitting infectivity than dead cells argues that cell biological processes are involved in prion transmission. The exact mechanism of direct cell-to-cell spread in SMB, Mov and Rov cells needs to be determined, but recent studies argue that cytoplasmic bridges, so called tunneling nanotubes (TNTs), are involved in this process in CAD cells persistently infected with 139A prions [199]. TNTs are actin and/or microtubule containing cytoplasmic bridges that allow intercellular communication. These sometimes contradictory results might be explained by the use of different cell types and prion strains. Indeed, the intercellular transmission efficiency can differ significantly in different cell lines bearing comparable titers of the same prion strain, arguing that the ability to propagate and to disseminate prions are distinct phenomena [197].

Table 2.
Routes of prion dissemination in cell culture.
| Prion-infected donor cell line | Prion strain | Intercellular prion spreading | PrPSc secreted | References |
|---|---|---|---|---|
| N2a | 22L | Yes, via conditioned medium | Yes, associated with exosomes | [36] |
| N2a | RML | No or inefficient | Not determined | [97,196] |
| SMB | Chandler | Yes, via direct cell contact | Not determined | [149] |
| HpL3-4* | 22L | Yes, via conditioned medium | Not determined | [121] |
| NIH/3T3 | 22L | Yes, via conditioned medium | Yes, associated with exosomes | [198] |
| CAD | 139A | Yes, via TNTs | Not determined | [200] |
| GT1 | RML | Yes, via conditioned medium | Not determined | [97] |
| GT1 | FU CJD | Yes, via conditioned medium | Not determined | [201] |
| GT1 | M1000 | Yes | Yes, associated with exosomes | [99] |
| ovRK13/ RoV9 | PG127 | Yes (inefficiently) | Yes, associated with exosomes | [37,197] |
| moRK13 | M1000 | Yes | Yes, associated with exosomes | [99] |
| Mov | PG127 | Yes, via close proximity of cells | Yes, associated with exosomes | [37,111,197] |
| SN56 | Chandler | Yes, via conditioned medium | Yes | [202] |
* cells ectopically express 3F4 antibody-epitope tagged moPrPC
10. Other Protein Aggregates Can Spread and Propagate in Cell Culture
Over the last few years an increasing number of studies have shown that non-prion protein aggregates associated with other neurodegenerative diseases can spread from cell to cell in a prion-like manner [203]. The most studied amyloid proteins are Aβ and tau in Alzheimer`s disease (AD), α-synuclein in Parkinson`s disease (PD), superoxide dismutase 1 (SOD1) in amyotrophic lateral sclerosis (ALS), and polyglutamine-rich huntingtin fragments in Huntington`s disease (HD). These proteins differ from PrP in their amino acid sequences, functions and cellular locations, but all share the cross β-sheet conformation in their aggregated states. Although not infectious from a classical point of view, protein aggregates accumulating during those diseases have been shown to spread in tissues in vivo [204,205,206,207] and infect neighboring cells in vitro [208,209,210,211]. Spreading of protein misfolding along interconnected brain regions argues for direct cell contact as a potential route of transmission [212]. Co-cultures of donor and recipient cell lines demonstrated that tau, α-synuclein and SOD1 could be transmitted via conditioned medium, sometimes in association with exosomes [208,209,210,213,214]. A prerequisite of aggregate spreading is the presence of multiple seeds that can be transmitted in the infection process. The high spreading efficiency of prions compared to other amyloidogenic protein aggregates might, at least in part, be due to a more efficient aggregate fragmentation process that produces new seeds [203]. Using a model system of mammalian cells expressing the yeast prion protein Sup35 we have recently shown that the cytosol of mammalian cells provides an environment for efficient aggregate replication (Figure 4) [215]. The efficiency at which aggregate seeds are formed might differ depending on the protein aggregate, as Sup35 and SOD1 aggregates could be stably propagated over serial passages, while polyQ aggregates were diluted out over time [211,215,216].
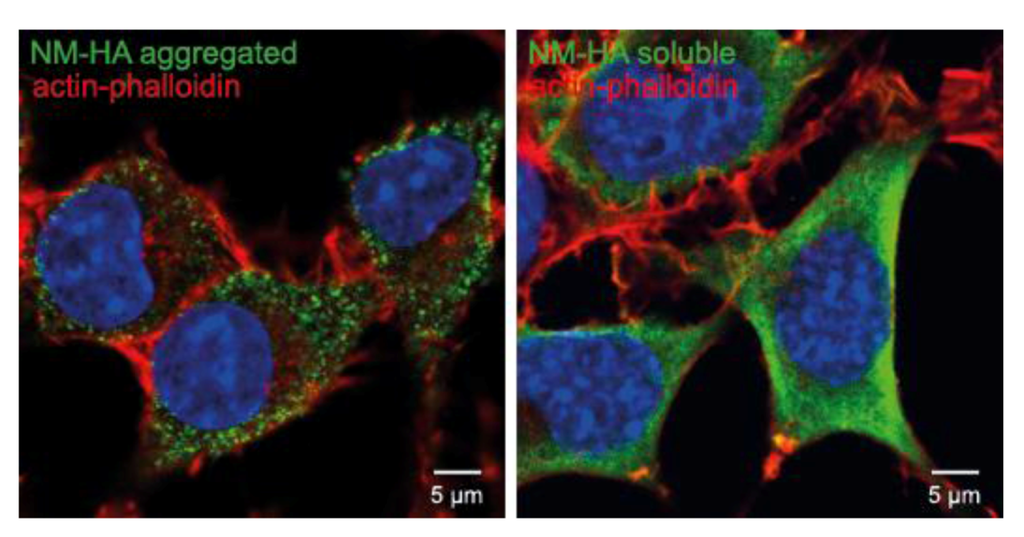
Figure 4.
Propagation of cytosolic prions derived from the S. cerevisiae Sup35 prion domain NM. N2a cells ectopically express the HA-tagged prion domain NM of Sup35, which is the most well characterized yeast prion. The left image shows aggregated NM-HA (green) after induction with recombinant NM fibrils, the right image shows the soluble NM-HA (green). NM was stained with anti-HA antibody. F-Actin was stained with fluorescently conjugated phalloidin (red). Nuclei were stained with Hoechst (blue). Scale bar: 5 µm.
11. Concluding Remarks
Almost 25 years after the discovery of prion susceptible neuroblastoma cells, persistently infected N2a cells still constitute the prototype cell-culture system for studying prions. Consequently, prion cell biology has been mostly studied in permanent cell lines chronically infected with prion strains RML, Chandler or 22L. Still, it is unclear if the identified pathways and co-factors are required for all prion strains, or if different prion strains utilize different subcellular compartments for efficient propagation. Clearly, pharmacological studies revealed significant differences in the anti-prion efficacy of compounds tested against various prion strains in permanent cells and primary neurons [111,217]. Whilst some of the anti-prion effects might be directly attributed to their special binding properties to PrPC or PrPSc [193], some might exert their effect more indirectly by affecting cellular metabolism. Thus, thorough investigations with different strains propagated in the same cell line are necessary to determine if prion strains utilize the same cellular pathways and co-factors for initial infection and sustained propagation.
Acknowledgments
This work was funded by the DFG grant VO1277/1-3. We thank Hans Fried and Ireen König for sharing expertise in microscopy and Donato DiMonte and Sybille Krauss for discussions and careful review of this manuscript.
Conflict of Interest
The authors declare no conflict of interest.
References
- Beekes, M.; McBride, P.A. The spread of prions through the body in naturally acquired transmissible spongiform encephalopathies. FEBS J. 2007, 274, 588–605. [Google Scholar] [CrossRef]
- Bolton, D.C.; McKinley, M.P.; Prusiner, S.B. Identification of a protein that purifies with the scrapie prion. Science 1982, 218, 1309–1311. [Google Scholar]
- Prusiner, S.B. Novel proteinaceous infectious particles cause scrapie. Science 1982, 216, 136–144. [Google Scholar]
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef]
- Pattison, I.H.; Millson, G.C. Scrapie produced experimentally in goats with special reference to the clinical syndrome. J. Comp. Pathol. 1961, 71, 101–109. [Google Scholar] [CrossRef]
- Bessen, R.A.; Marsh, R.F. Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J. Virol. 1992, 66, 2096–2101. [Google Scholar]
- Safar, J.; Wille, H.; Itri, V.; Groth, D.; Serban, H.; Torchia, M.; Cohen, F.E.; Prusiner, S.B. Eight prion strains have prp(sc) molecules with different conformations. Nat. Med. 1998, 4, 1157–1165. [Google Scholar]
- Asante, E.A.; Linehan, J.M.; Desbruslais, M.; Joiner, S.; Gowland, I.; Wood, A.L.; Welch, J.; Hill, A.F.; Lloyd, S.E.; Wadsworth, J.D.; et al. Bse prions propagate as either variant cjd-like or sporadic cjd-like prion strains in transgenic mice expressing human prion protein. EMBO J. 2002, 21, 6358–6366. [Google Scholar] [CrossRef]
- Cali, I.; Castellani, R.; Alshekhlee, A.; Cohen, Y.; Blevins, J.; Yuan, J.; Langeveld, J.P.; Parchi, P.; Safar, J.G.; Zou, W.Q.; et al. Co-existence of scrapie prion protein types 1 and 2 in sporadic creutzfeldt-jakob disease: Its effect on the phenotype and prion-type characteristics. Brain 2009, 132, 2643–2658. [Google Scholar] [CrossRef]
- Weissmann, C. Birth of a prion: Spontaneous generation revisited. Cell. 2005, 122, 165–168. [Google Scholar] [CrossRef]
- Chesebro, B.; Race, R.; Wehrly, K.; Nishio, J.; Bloom, M.; Lechner, D.; Bergstrom, S.; Robbins, K.; Mayer, L.; Keith, J.M.; et al. Identification of scrapie prion protein-specific mrna in scrapie-infected and uninfected brain. Nature 1985, 315, 331–333. [Google Scholar] [CrossRef]
- Basler, K.; Oesch, B.; Scott, M.; Westaway, D.; Walchli, M.; Groth, D.F.; McKinley, M.P.; Prusiner, S.B.; Weissmann, C. Scrapie and cellular prp isoforms are encoded by the same chromosomal gene. Cell. 1986, 46, 417–428. [Google Scholar] [CrossRef]
- Robakis, N.K.; Sawh, P.R.; Wolfe, G.C.; Rubenstein, R.; Carp, R.I.; Innis, M.A. Isolation of a cdna clone encoding the leader peptide of prion protein and expression of the homologous gene in various tissues. Proc. Natl. Acad. Sci. USA 1986, 83, 6377–6381. [Google Scholar] [CrossRef]
- Sparkes, R.S.; Simon, M.; Cohn, V.H.; Fournier, R.E.; Lem, J.; Klisak, I.; Heinzmann, C.; Blatt, C.; Lucero, M.; Mohandas, T.; et al. Assignment of the human and mouse prion protein genes to homologous chromosomes. Proc. Natl. Acad. Sci. USA 1986, 83, 7358–7362. [Google Scholar]
- Oesch, B.; Westaway, D.; Prusiner, S.B. Prion protein genes: Evolutionary and functional aspects. Curr. Top. Microbiol. Immunol. 1991, 172, 109–124. [Google Scholar]
- Schatzl, H.M.; Da Costa, M.; Taylor, L.; Cohen, F.E.; Prusiner, S.B. Prion protein gene variation among primates. J. Mol. Biol 1995, 245, 362–374. [Google Scholar] [CrossRef]
- Wopfner, F.; Weidenhofer, G.; Schneider, R.; von Brunn, A.; Gilch, S.; Schwarz, T.F.; Werner, T.; Schatzl, H.M. Analysis of 27 mammalian and 9 avian prps reveals high conservation of flexible regions of the prion protein. J. Mol. Biol. 1999, 289, 1163–1178. [Google Scholar] [CrossRef]
- Linden, R.; Martins, V.R.; Prado, M.A.; Cammarota, M.; Izquierdo, I.; Brentani, R.R. Physiology of the prion protein. Physiol. Rev. 2008, 88, 673–728. [Google Scholar] [CrossRef]
- Brown, D.R.; Schmidt, B.; Kretzschmar, H.A. Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature 1996, 380, 345–347. [Google Scholar] [CrossRef]
- Moser, M.; Colello, R.J.; Pott, U.; Oesch, B. Developmental expression of the prion protein gene in glial cells. Neuron 1995, 14, 509–517. [Google Scholar] [CrossRef]
- Haraguchi, T.; Fisher, S.; Olofsson, S.; Endo, T.; Groth, D.; Tarentino, A.; Borchelt, D.R.; Teplow, D.; Hood, L.; Burlingame, A.; et al. Asparagine-linked glycosylation of the scrapie and cellular prion proteins. Arch. Biochem. Biophys. 1989, 274, 1–13. [Google Scholar] [CrossRef]
- Turk, E.; Teplow, D.B.; Hood, L.E.; Prusiner, S.B. Purification and properties of the cellular and scrapie hamster prion proteins. Eur. J. Biochem. 1988, 176, 21–30. [Google Scholar] [CrossRef]
- Stahl, N.; Baldwin, M.A.; Hecker, R.; Pan, K.M.; Burlingame, A.L.; Prusiner, S.B. Glycosylinositol phospholipid anchors of the scrapie and cellular prion proteins contain sialic acid. Biochemistry 1992, 31, 5043–5053. [Google Scholar]
- Taraboulos, A.; Scott, M.; Semenov, A.; Avrahami, D.; Laszlo, L.; Prusiner, S.B. Cholesterol depletion and modification of cooh-terminal targeting sequence of the prion protein inhibit formation of the scrapie isoform. J. Cell. Biol 1995, 129, 121–132. [Google Scholar] [CrossRef]
- Vey, M.; Pilkuhn, S.; Wille, H.; Nixon, R.; DeArmond, S.J.; Smart, E.J.; Anderson, R.G.; Taraboulos, A.; Prusiner, S.B. Subcellular colocalization of the cellular and scrapie prion proteins in caveolae-like membranous domains. Proc. Natl. Acad. Sci. USA 1996, 93, 14945–14949. [Google Scholar]
- Baron, G.S.; Wehrly, K.; Dorward, D.W.; Chesebro, B.; Caughey, B. Conversion of raft associated prion protein to the protease-resistant state requires insertion of prp-res (prp(sc)) into contiguous membranes. EMBO J. 2002, 21, 1031–1040. [Google Scholar] [CrossRef]
- Walmsley, A.R.; Watt, N.T.; Taylor, D.R.; Perera, W.S.; Hooper, N.M. Alpha-cleavage of the prion protein occurs in a late compartment of the secretory pathway and is independent of lipid rafts. Mol. Cell. Neurosci. 2009, 40, 242–248. [Google Scholar] [CrossRef]
- Sarnataro, D.; Campana, V.; Paladino, S.; Stornaiuolo, M.; Nitsch, L.; Zurzolo, C. Prp(c) association with lipid rafts in the early secretory pathway stabilizes its cellular conformation. Mol. Biol. Cell. 2004, 15, 4031–4042. [Google Scholar] [CrossRef]
- Mironov, A., Jr.; Latawiec, D.; Wille, H.; Bouzamondo-Bernstein, E.; Legname, G.; Williamson, R.A.; Burton, D.; DeArmond, S.J.; Prusiner, S.B.; Peters, P.J. Cytosolic prion protein in neurons. J. Neurosci. 2003, 23, 7183–7193. [Google Scholar]
- Vincent, B.; Paitel, E.; Frobert, Y.; Lehmann, S.; Grassi, J.; Checler, F. Phorbol ester-regulated cleavage of normal prion protein in hek293 human cells and murine neurons. J. Biol. Chem. 2000, 275, 35612–35616. [Google Scholar]
- Vincent, B.; Paitel, E.; Saftig, P.; Frobert, Y.; Hartmann, D.; De Strooper, B.; Grassi, J.; Lopez-Perez, E.; Checler, F. The disintegrins adam10 and tace contribute to the constitutive and phorbol ester-regulated normal cleavage of the cellular prion protein. J. Biol. Chem. 2001, 276, 37743–37746. [Google Scholar]
- Parkin, E.T.; Watt, N.T.; Turner, A.J.; Hooper, N.M. Dual mechanisms for shedding of the cellular prion protein. J. Biol. Chem. 2004, 279, 11170–11178. [Google Scholar]
- Alfa Cisse, M.; Sunyach, C.; Slack, B.E.; Fisher, A.; Vincent, B.; Checler, F. M1 and m3 muscarinic receptors control physiological processing of cellular prion by modulating adam17 phosphorylation and activity. J. Neurosci. 2007, 27, 4083–4092. [Google Scholar] [CrossRef]
- Borchelt, D.R.; Rogers, M.; Stahl, N.; Telling, G.; Prusiner, S.B. Release of the cellular prion protein from cultured cells after loss of its glycoinositol phospholipid anchor. Glycobiology 1993, 3, 319–329. [Google Scholar] [CrossRef]
- Starke, R.; Harrison, P.; Drummond, O.; Macgregor, I.; Mackie, I.; Machin, S. The majority of cellular prion protein released from endothelial cells is soluble. Transfusion 2003, 43, 677–678, author reply 678.. [Google Scholar] [CrossRef]
- Alais, S.; Simoes, S.; Baas, D.; Lehmann, S.; Raposo, G.; Darlix, J.L.; Leblanc, P. Mouse neuroblastoma cells release prion infectivity associated with exosomal vesicles. Biol. Cell 2008, 100, 603–615. [Google Scholar] [CrossRef]
- Fevrier, B.; Vilette, D.; Archer, F.; Loew, D.; Faigle, W.; Vidal, M.; Laude, H.; Raposo, G. Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. USA 2004, 101, 9683–9688. [Google Scholar]
- Rane, N.S.; Yonkovich, J.L.; Hegde, R.S. Protection from cytosolic prion protein toxicity by modulation of protein translocation. EMBO J. 2004, 23, 4550–4559. [Google Scholar] [CrossRef]
- Crozet, C.; Vezilier, J.; Delfieu, V.; Nishimura, T.; Onodera, T.; Casanova, D.; Lehmann, S.; Beranger, F. The truncated 23–230 form of the prion protein localizes to the nuclei of inducible cell lines independently of its nuclear localization signals and is not cytotoxic. Mol. Cell. Neurosci. 2006, 32, 315–323. [Google Scholar] [CrossRef]
- Linden, R.; Cordeiro, Y.; Lima, L.M. Allosteric function and dysfunction of the prion protein. Cell. Mol. Life Sci. 2012, 69, 1105–1124. [Google Scholar] [CrossRef]
- Gibbings, D.; Leblanc, P.; Jay, F.; Pontier, D.; Michel, F.; Schwab, Y.; Alais, S.; Lagrange, T.; Voinnet, O. Human prion protein binds argonaute and promotes accumulation of microrna effector complexes. Nat. Struct. Mol. Biol. 2012, 19, 517–524, S511. [Google Scholar] [CrossRef]
- Cheng, F.; Lindqvist, J.; Haigh, C.L.; Brown, D.R.; Mani, K. Copper-dependent co-internalization of the prion protein and glypican-1. J. Neurochem. 2006, 98, 1445–1457. [Google Scholar] [CrossRef]
- Watt, N.T.; Taylor, D.R.; Kerrigan, T.L.; Griffiths, H.H.; Rushworth, J.V.; Whitehouse, I.J.; Hooper, N.M. Prion protein facilitates uptake of zinc into neuronal cells. Nat. Commun. 2012, 3, 1134. [Google Scholar] [CrossRef]
- Graner, E.; Mercadante, A.F.; Zanata, S.M.; Forlenza, O.V.; Cabral, A.L.; Veiga, S.S.; Juliano, M.A.; Roesler, R.; Walz, R.; Minetti, A.; et al. Cellular prion protein binds laminin and mediates neuritogenesis. Brain Res. Mol. 2000, 76, 85–92. [Google Scholar] [CrossRef]
- Zanata, S.M.; Lopes, M.H.; Mercadante, A.F.; Hajj, G.N.; Chiarini, L.B.; Nomizo, R.; Freitas, A.R.; Cabral, A.L.; Lee, K.S.; Juliano, M.A.; et al. Stress-inducible protein 1 is a cell surface ligand for cellular prion that triggers neuroprotection. EMBO J. 2002, 21, 3307–3316. [Google Scholar]
- Santuccione, A.; Sytnyk, V.; Leshchyns'ka, I.; Schachner, M. Prion protein recruits its neuronal receptor ncam to lipid rafts to activate p59fyn and to enhance neurite outgrowth. J. Cell. Biol. 2005, 169, 341–354. [Google Scholar] [CrossRef]
- Zhang, C.C.; Steele, A.D.; Lindquist, S.; Lodish, H.F. Prion protein is expressed on long-term repopulating hematopoietic stem cells and is important for their self-renewal. Proc. Natl. Acad. Sci. USA 2006, 103, 2184–2189. [Google Scholar] [CrossRef]
- Steele, A.D.; Emsley, J.G.; Ozdinler, P.H.; Lindquist, S.; Macklis, J.D. Prion protein (prpc) positively regulates neural precursor proliferation during developmental and adult mammalian neurogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 3416–3421. [Google Scholar]
- Gabus, C.; Auxilien, S.; Pechoux, C.; Dormont, D.; Swietnicki, W.; Morillas, M.; Surewicz, W.; Nandi, P.; Darlix, J.L. The prion protein has DNA strand transfer properties similar to retroviral nucleocapsid protein. J. Mol. Biol. 2001, 307, 1011–1021. [Google Scholar] [CrossRef]
- Gabus, C.; Derrington, E.; Leblanc, P.; Chnaiderman, J.; Dormont, D.; Swietnicki, W.; Morillas, M.; Surewicz, W.K.; Marc, D.; Nandi, P.; et al. The prion protein has rna binding and chaperoning properties characteristic of nucleocapsid protein ncp7 of hiv-1. J. Biol. Chem. 2001, 276, 19301–19309. [Google Scholar]
- Cordeiro, Y.; Machado, F.; Juliano, L.; Juliano, M.A.; Brentani, R.R.; Foguel, D.; Silva, J.L. DNA converts cellular prion protein into the beta-sheet conformation and inhibits prion peptide aggregation. J. Biol. Chem. 2001, 276, 49400–49409. [Google Scholar]
- Lima, L.M.; Cordeiro, Y.; Tinoco, L.W.; Marques, A.F.; Oliveira, C.L.; Sampath, S.; Kodali, R.; Choi, G.; Foguel, D.; Torriani, I.; et al. Structural insights into the interaction between prion protein and nucleic acid. Biochemistry 2006, 45, 9180–9187. [Google Scholar]
- Morris, R.J.; Parkyn, C.J.; Jen, A. Traffic of prion protein between different compartments on the neuronal surface, and the propagation of prion disease. FEBS Lett. 2006, 580, 5565–5571. [Google Scholar] [CrossRef]
- Sunyach, C.; Jen, A.; Deng, J.; Fitzgerald, K.T.; Frobert, Y.; Grassi, J.; McCaffrey, M.W.; Morris, R. The mechanism of internalization of glycosylphosphatidylinositol-anchored prion protein. EMBO J. 2003, 22, 3591–3601. [Google Scholar] [CrossRef]
- Caetano, F.A.; Lopes, M.H.; Hajj, G.N.; Machado, C.F.; Pinto Arantes, C.; Magalhaes, A.C.; Vieira Mde, P.; Americo, T.A.; Massensini, A.R.; Priola, S.A.; et al. Endocytosis of prion protein is required for erk1/2 signaling induced by stress-inducible protein 1. J. Neurosci 2008, 28, 6691–6702. [Google Scholar]
- Magalhaes, A.C.; Silva, J.A.; Lee, K.S.; Martins, V.R.; Prado, V.F.; Ferguson, S.S.; Gomez, M.V.; Brentani, R.R.; Prado, M.A. Endocytic intermediates involved with the intracellular trafficking of a fluorescent cellular prion protein. J. Biol. Chem. 2002, 277, 33311–33318. [Google Scholar]
- Prado, M.A.; Alves-Silva, J.; Magalhaes, A.C.; Prado, V.F.; Linden, R.; Martins, V.R.; Brentani, R.R. Prpc on the road: Trafficking of the cellular prion protein. J. Neurochem. 2004, 88, 769–781. [Google Scholar] [CrossRef]
- Campana, V.; Sarnataro, D.; Zurzolo, C. The highways and byways of prion protein trafficking. Trends Cell. Biol. 2005, 15, 102–111. [Google Scholar] [CrossRef]
- Stuermer, C.A.; Langhorst, M.F.; Wiechers, M.F.; Legler, D.F.; Von Hanwehr, S.H.; Guse, A.H.; Plattner, H. Prpc capping in t cells promotes its association with the lipid raft proteins reggie-1 and reggie-2 and leads to signal transduction. FASEB J. 2004, 18, 1731–1733. [Google Scholar]
- Shyng, S.L.; Huber, M.T.; Harris, D.A. A prion protein cycles between the cell surface and an endocytic compartment in cultured neuroblastoma cells. J. Biol. Chem. 1993, 268, 15922–15928. [Google Scholar]
- Godsave, S.F.; Wille, H.; Kujala, P.; Latawiec, D.; DeArmond, S.J.; Serban, A.; Prusiner, S.B.; Peters, P.J. Cryo-immunogold electron microscopy for prions: Toward identification of a conversion site. J. Neurosci. 2008, 28, 12489–12499. [Google Scholar]
- Peters, P.J.; Mironov, A.., Jr.; Peretz, D.; van Donselaar, E.; Leclerc, E.; Erpel, S.; DeArmond, S.J.; Burton, D.R.; Williamson, R.A.; Vey, M.; et al. Trafficking of prion proteins through a caveolae-mediated endosomal pathway. J. Cell. Biol. 2003, 162, 703–717. [Google Scholar] [CrossRef]
- Baumann, M.H.; Kallijarvi, J.; Lankinen, H.; Soto, C.; Haltia, M. Apolipoprotein e includes a binding site which is recognized by several amyloidogenic polypeptides. Biochem. J. 2000, 349, 77–84. [Google Scholar] [CrossRef]
- Kang, Y.S.; Zhao, X.; Lovaas, J.; Eisenberg, E.; Greene, L.E. Clathrin-independent internalization of normal cellular prion protein in neuroblastoma cells is associated with the arf6 pathway. J. Cell. Sci. 2009, 122, 4062–4069. [Google Scholar] [CrossRef]
- Marella, M.; Lehmann, S.; Grassi, J.; Chabry, J. Filipin prevents pathological prion protein accumulation by reducing endocytosis and inducing cellular prp release. J. Biol. Chem. 2002, 277, 25457–25464. [Google Scholar]
- Taylor, D.R.; Watt, N.T.; Perera, W.S.; Hooper, N.M. Assigning functions to distinct regions of the n-terminus of the prion protein that are involved in its copper-stimulated, clathrin-dependent endocytosis. J. Cell Sci. 2005, 118, 5141–5153. [Google Scholar] [CrossRef]
- Watt, N.T.; Hooper, N.M. Reactive oxygen species (ros)-mediated beta-cleavage of the prion protein in the mechanism of the cellular response to oxidative stress. Biochem. Soc. Trans. 2005, 33, 1123–1125. [Google Scholar] [CrossRef]
- Martins, V.R.; Graner, E.; Garcia-Abreu, J.; de Souza, S.J.; Mercadante, A.F.; Veiga, S.S.; Zanata, S.M.; Neto, V.M.; Brentani, R.R. Complementary hydropathy identifies a cellular prion protein receptor. Nat. Med. 1997, 3, 1376–1382. [Google Scholar] [CrossRef]
- Pauly, P.C.; Harris, D.A. Copper stimulates endocytosis of the prion protein. J. Biol. Chem. 1998, 273, 33107–33110. [Google Scholar] [CrossRef]
- Madore, N.; Smith, K.L.; Graham, C.H.; Jen, A.; Brady, K.; Hall, S.; Morris, R. Functionally different gpi proteins are organized in different domains on the neuronal surface. EMBO J. 1999, 18, 6917–6926. [Google Scholar] [CrossRef]
- Galvan, C.; Camoletto, P.G.; Dotti, C.G.; Aguzzi, A.; Ledesma, M.D. Proper axonal distribution of prp(c) depends on cholesterol-sphingomyelin-enriched membrane domains and is developmentally regulated in hippocampal neurons. Mol. Cell. Neurosci. 2005, 30, 304–315. [Google Scholar] [CrossRef]
- Sarnataro, D.; Caputo, A.; Casanova, P.; Puri, C.; Paladino, S.; Tivodar, S.S.; Campana, V.; Tacchetti, C.; Zurzolo, C. Lipid rafts and clathrin cooperate in the internalization of prp in epithelial frt cells. PLoS One 2009, 4, e5829. [Google Scholar]
- Kirchhausen, T. Clathrin. Annu Rev. Biochem. 2000, 69, 699–727. [Google Scholar] [CrossRef]
- Gauczynski, S.; Peyrin, J.M.; Haik, S.; Leucht, C.; Hundt, C.; Rieger, R.; Krasemann, S.; Deslys, J.P.; Dormont, D.; Lasmezas, C.I.; et al. The 37-kda/67-kda laminin receptor acts as the cell-surface receptor for the cellular prion protein. EMBO J. 2001, 20, 5863–5875. [Google Scholar] [CrossRef]
- Gauczynski, S.; Hundt, C.; Leucht, C.; Weiss, S. Interaction of prion proteins with cell surface receptors, molecular chaperones, and other molecules. Adv. Protein. Chem. 2001, 57, 229–272. [Google Scholar] [CrossRef]
- Rieger, R.; Edenhofer, F.; Lasmezas, C.I.; Weiss, S. The human 37-kda laminin receptor precursor interacts with the prion protein in eukaryotic cells. Nat. Med. 1997, 3, 1383–1388. [Google Scholar] [CrossRef]
- Hundt, C.; Peyrin, J.M.; Haik, S.; Gauczynski, S.; Leucht, C.; Rieger, R.; Riley, M.L.; Deslys, J.P.; Dormont, D.; Lasmezas, C.I.; et al. Identification of interaction domains of the prion protein with its 37-kda/67-kda laminin receptor. EMBO J. 2001, 20, 5876–5886. [Google Scholar] [CrossRef]
- Douville, P.J.; Harvey, W.J.; Carbonetto, S. Isolation and partial characterization of high affinity laminin receptors in neural cells. J. Biol. Chem. 1988, 263, 14964–14969. [Google Scholar]
- Watts, J.C.; Huo, H.; Bai, Y.; Ehsani, S.; Jeon, A.H.; Shi, T.; Daude, N.; Lau, A.; Young, R.; Xu, L.; et al. Interactome analyses identify ties of prp and its mammalian paralogs to oligomannosidic n-glycans and endoplasmic reticulum-derived chaperones. PLoS Pathog. 2009, 5, e1000608. [Google Scholar] [CrossRef]
- Nykjaer, A.; Willnow, T.E. The low-density lipoprotein receptor gene family: A cellular swiss army knife? Trends Cell. Biol. 2002, 12, 273–280. [Google Scholar] [CrossRef]
- Wu, L.; Gonias, S.L. The low-density lipoprotein receptor-related protein-1 associates transiently with lipid rafts. J. Cell. Biochem. 2005, 96, 1021–1033. [Google Scholar] [CrossRef]
- Taylor, D.R.; Hooper, N.M. The low-density lipoprotein receptor-related protein 1 (lrp1) mediates the endocytosis of the cellular prion protein. Biochem. J. 2007, 402, 17–23. [Google Scholar] [CrossRef]
- Parkyn, C.J.; Vermeulen, E.G.; Mootoosamy, R.C.; Sunyach, C.; Jacobsen, C.; Oxvig, C.; Moestrup, S.; Liu, Q.; Bu, G.; Jen, A.; et al. Lrp1 controls biosynthetic and endocytic trafficking of neuronal prion protein. J. Cell. Sci. 2008, 121, 773–783. [Google Scholar] [CrossRef]
- Wang, S.; Herndon, M.E.; Ranganathan, S.; Godyna, S.; Lawler, J.; Argraves, W.S.; Liau, G. Internalization but not binding of thrombospondin-1 to low density lipoprotein receptor-related protein-1 requires heparan sulfate proteoglycans. J. Cell. Biochem. 2004, 91, 766–776. [Google Scholar] [CrossRef]
- Yin, S.; Yu, S.; Li, C.; Wong, P.; Chang, B.; Xiao, F.; Kang, S.C.; Yan, H.; Xiao, G.; Grassi, J.; et al. Prion proteins with insertion mutations have altered n-terminal conformation and increased ligand binding activity and are more susceptible to oxidative attack. J. Biol. Chem. 2006, 281, 10698–10705. [Google Scholar]
- Pan, T.; Wong, B.S.; Liu, T.; Li, R.; Petersen, R.B.; Sy, M.S. Cell-surface prion protein interacts with glycosaminoglycans. Biochem. J. 2002, 368, 81–90. [Google Scholar] [CrossRef]
- Warner, R.G.; Hundt, C.; Weiss, S.; Turnbull, J.E. Identification of the heparan sulfate binding sites in the cellular prion protein. J. Biol. Chem. 2002, 277, 18421–18430. [Google Scholar]
- Gabizon, R.; Meiner, Z.; Halimi, M.; Ben-Sasson, S.A. Heparin-like molecules bind differentially to prion-proteins and change their intracellular metabolic fate. J. Cell. Physiol. 1993, 157, 319–325. [Google Scholar] [CrossRef]
- Caughey, B.; Raymond, G.J. Sulfated polyanion inhibition of scrapie-associated prp accumulation in cultured cells. J. Virol. 1993, 67, 643–650. [Google Scholar]
- Shyng, S.L.; Lehmann, S.; Moulder, K.L.; Harris, D.A. Sulfated glycans stimulate endocytosis of the cellular isoform of the prion protein, prpc, in cultured cells. J. Biol. Chem. 1995, 270, 30221–30229. [Google Scholar]
- Ben-Zaken, O.; Tzaban, S.; Tal, Y.; Horonchik, L.; Esko, J.D.; Vlodavsky, I.; Taraboulos, A. Cellular heparan sulfate participates in the metabolism of prions. J. Biol. Chem. 2003, 278, 40041–40049. [Google Scholar]
- Gilch, S.; Winklhofer, K.F.; Groschup, M.H.; Nunziante, M.; Lucassen, R.; Spielhaupter, C.; Muranyi, W.; Riesner, D.; Tatzelt, J.; Schatzl, H.M. Intracellular re-routing of prion protein prevents propagation of prp(sc) and delays onset of prion disease. EMBO J. 2001, 20, 3957–3966. [Google Scholar] [CrossRef]
- Clarke, M.C.; Haig, D.A. Multiplication of scrapie agent in cell culture. Res. Vet. Sci 1970, 11, 500–501. [Google Scholar]
- Caughey, B.; Race, R.E.; Ernst, D.; Buchmeier, M.J.; Chesebro, B. Prion protein biosynthesis in scrapie-infected and uninfected neuroblastoma cells. J. Virol. 1989, 63, 175–181. [Google Scholar]
- Butler, D.A.; Scott, M.R.; Bockman, J.M.; Borchelt, D.R.; Taraboulos, A.; Hsiao, K.K.; Kingsbury, D.T.; Prusiner, S.B. Scrapie-infected murine neuroblastoma cells produce protease-resistant prion proteins. J. Virol 1988, 62, 1558–1564. [Google Scholar]
- Nishida, N.; Harris, D.A.; Vilette, D.; Laude, H.; Frobert, Y.; Grassi, J.; Casanova, D.; Milhavet, O.; Lehmann, S. Successful transmission of three mouse-adapted scrapie strains to murine neuroblastoma cell lines overexpressing wild-type mouse prion protein. J. Virol. 2000, 74, 320–325. [Google Scholar] [CrossRef]
- Schatzl, H.M.; Laszlo, L.; Holtzman, D.M.; Tatzelt, J.; DeArmond, S.J.; Weiner, R.I.; Mobley, W.C.; Prusiner, S.B. A hypothalamic neuronal cell line persistently infected with scrapie prions exhibits apoptosis. J. Virol. 1997, 71, 8821–8831. [Google Scholar]
- Iwamaru, Y.; Takenouchi, T.; Ogihara, K.; Hoshino, M.; Takata, M.; Imamura, M.; Tagawa, Y.; Hayashi-Kato, H.; Ushiki-Kaku, Y.; Shimizu, Y.; et al. Microglial cell line established from prion protein-overexpressing mice is susceptible to various murine prion strains. J. Virol. 2007, 81, 1524–1527. [Google Scholar]
- Vella, L.J.; Sharples, R.A.; Lawson, V.A.; Masters, C.L.; Cappai, R.; Hill, A.F. Packaging of prions into exosomes is associated with a novel pathway of prp processing. J. Pathol. 2007, 211, 582–590. [Google Scholar] [CrossRef]
- Courageot, M.P.; Daude, N.; Nonno, R.; Paquet, S.; Di Bari, M.A.; Le Dur, A.; Chapuis, J.; Hill, A.F.; Agrimi, U.; Laude, H.; et al. A cell line infectible by prion strains from different species. J. Gen. Virol. 2008, 89, 341–347. [Google Scholar] [CrossRef]
- Lawson, V.A.; Vella, L.J.; Stewart, J.D.; Sharples, R.A.; Klemm, H.; Machalek, D.M.; Masters, C.L.; Cappai, R.; Collins, S.J.; Hill, A.F. Mouse-adapted sporadic human creutzfeldt-jakob disease prions propagate in cell culture. Int. J. Biochem. Cell. Biol. 2008, 40, 2793–2801. [Google Scholar] [CrossRef]
- Vilette, D.; Andreoletti, O.; Archer, F.; Madelaine, M.F.; Vilotte, J.L.; Lehmann, S.; Laude, H. Ex vivo propagation of infectious sheep scrapie agent in heterologous epithelial cells expressing ovine prion protein. Proc. Natl. Acad. Sci. USA 2001, 98, 4055–4059. [Google Scholar]
- Sabuncu, E.; Petit, S.; Le Dur, A.; Lan Lai, T.; Vilotte, J.L.; Laude, H.; Vilette, D. Prp polymorphisms tightly control sheep prion replication in cultured cells. J. Virol. 2003, 77, 2696–2700. [Google Scholar]
- Neale, M.H.; Mountjoy, S.J.; Edwards, J.C.; Vilette, D.; Laude, H.; Windl, O.; Saunders, G.C. Infection of cell lines with experimental and natural ovine scrapie agents. J. Virol. 2010, 84, 2444–2452. [Google Scholar]
- Bian, J.; Napier, D.; Khaychuck, V.; Angers, R.; Graham, C.; Telling, G. Cell-based quantification of chronic wasting disease prions. J. Virol. 2010, 84, 8322–8326. [Google Scholar] [CrossRef]
- Clarke, M.C.; Millson, G.C. Infection of a cell line of mouse l fibroblasts with scrapie agent. Nature 1976, 261, 144–145. [Google Scholar] [CrossRef]
- Vorberg, I.; Raines, A.; Story, B.; Priola, S.A. Susceptibility of common fibroblast cell lines to transmissible spongiform encephalopathy agents. J. Infect. Dis. 2004, 189, 431–439. [Google Scholar] [CrossRef]
- Dlakic, W.M.; Grigg, E.; Bessen, R.A. Prion infection of muscle cells in vitro. J. Virol. 2007, 81, 4615–4624. [Google Scholar] [CrossRef]
- Kim, H.J.; Tark, D.S.; Lee, Y.H.; Kim, M.J.; Lee, W.Y.; Cho, I.S.; Sohn, H.J.; Yokoyama, T. Establishment of a cell line persistently infected with chronic wasting disease prions. J. Vet. Med. Sci. 2012, 74, 1377–1380. [Google Scholar] [CrossRef]
- Akimov, S.; Vasilyeva, I.; Yakovleva, O.; McKenzie, C.; Cervenakova, L. Murine bone marrow stromal cell culture with features of mesenchymal stem cells susceptible to mouse-adapted human tse agent, fukuoka-1. Folia. Neuropathol. 2009, 47, 205–214. [Google Scholar]
- Cronier, S.; Laude, H.; Peyrin, J.M. Prions can infect primary cultured neurons and astrocytes and promote neuronal cell death. Proc. Natl. Acad. Sci. USA 2004, 101, 12271–12276. [Google Scholar] [CrossRef]
- Giri, R.K.; Young, R.; Pitstick, R.; DeArmond, S.J.; Prusiner, S.B.; Carlson, G.A. Prion infection of mouse neurospheres. Proc. Natl. Acad. Sci. USA 2006, 103, 3875–3880. [Google Scholar]
- Milhavet, O.; Casanova, D.; Chevallier, N.; McKay, R.D.; Lehmann, S. Neural stem cell model for prion propagation. Stem. Cell. 2006, 24, 2284–2291. [Google Scholar] [CrossRef]
- Cervenakova, L.; Akimov, S.; Vasilyeva, I.; Yakovleva, O.; McKenzie, C.; Cervenak, J.; Piccardo, P.; Asher, D.M. Fukuoka-1 strain of transmissible spongiform encephalopathy agent infects murine bone marrow-derived cells with features of mesenchymal stem cells. Transfusion 2011, 51, 1755–1768. [Google Scholar] [CrossRef]
- Herva, M.E.; Relano-Gines, A.; Villa, A.; Torres, J.M. Prion infection of differentiated neurospheres. J. Neurosci. Meth. 2010, 188, 270–275. [Google Scholar] [CrossRef]
- Polymenidou, M.; Trusheim, H.; Stallmach, L.; Moos, R.; Julius, C.; Miele, G.; Lenz-Bauer, C.; Aguzzi, A. Canine mdck cell lines are refractory to infection with human and mouse prions. Vaccine 2008, 26, 2601–2614. [Google Scholar] [CrossRef]
- Gibson, P.E.; Bell, T.M.; Field, E.J. Failure of the scrapie agent to replicate in l5178y mouse leukaemic cells. Res. Vet. Sci. 1972, 13, 95–96. [Google Scholar]
- Klohn, P.C.; Stoltze, L.; Flechsig, E.; Enari, M.; Weissmann, C. A quantitative, highly sensitive cell-based infectivity assay for mouse scrapie prions. Proc. Natl. Acad. Sci. USA 2003, 100, 11666–11671. [Google Scholar]
- Ladogana, A.; Liu, Q.; Xi, Y.G.; Pocchiari, M. Proteinase-resistant protein in human neuroblastoma cells infected with brain material from creutzfeldt-jakob patient. Lancet 1995, 345, 594–595. [Google Scholar]
- Lewis, V.; Hill, A.F.; Haigh, C.L.; Klug, G.M.; Masters, C.L.; Lawson, V.A.; Collins, S.J. Increased proportions of c1 truncated prion protein protect against cellular m1000 prion infection. J. Neuropathol. Exp. Neurol. 2009, 68, 1125–1135. [Google Scholar] [CrossRef]
- Maas, E.; Geissen, M.; Groschup, M.H.; Rost, R.; Onodera, T.; Schatzl, H.; Vorberg, I.M. Scrapie infection of prion protein-deficient cell line upon ectopic expression of mutant prion proteins. J. Biol. Chem. 2007, 282, 18702–18710. [Google Scholar]
- McNally, K.L.; Ward, A.E.; Priola, S.A. Cells expressing anchorless prion protein are resistant to scrapie infection. J. Virol. 2009, 83, 4469–4475. [Google Scholar] [CrossRef]
- Bosque, P.J.; Ryou, C.; Telling, G.; Peretz, D.; Legname, G.; DeArmond, S.J.; Prusiner, S.B. Prions in skeletal muscle. Proc. Natl. Acad. Sci. USA 2002, 99, 3812–3817. [Google Scholar]
- Bosque, P.J.; Prusiner, S.B. Cultured cell sublines highly susceptible to prion infection. J. Virol. 2000, 74, 4377–4386. [Google Scholar] [CrossRef]
- Mahal, S.P.; Baker, C.A.; Demczyk, C.A.; Smith, E.W.; Julius, C.; Weissmann, C. Prion strain discrimination in cell culture: The cell panel assay. Proc. Natl. Acad. Sci. USA 2007, 104, 20908–20913. [Google Scholar]
- Bach, C.; Gilch, S.; Rost, R.; Greenwood, A.D.; Horsch, M.; Hajj, G.N.; Brodesser, S.; Facius, A.; Schadler, S.; Sandhoff, K.; et al. Prion-induced activation of cholesterogenic gene expression by srebp2 in neuronal cells. J. Biol. Chem. 2009, 284, 31260–31269. [Google Scholar]
- Race, R.E.; Fadness, L.H.; Chesebro, B. Characterization of scrapie infection in mouse neuroblastoma cells. J. Gen. Virol 1987, 68, 1391–1399. [Google Scholar]
- Kocisko, D.A.; Caughey, B. Searching for anti-prion compounds: Cell-based high-throughput in vitro assays and animal testing strategies. Methods Enzymol. 2006, 412, 223–234. [Google Scholar] [CrossRef]
- Raymond, G.J.; Olsen, E.A.; Lee, K.S.; Raymond, L.D.; Bryant, P.K., 3rd; Baron, G.S.; Caughey, W.S.; Kocisko, D.A.; McHolland, L.E.; Favara, C.; et al. Inhibition of protease-resistant prion protein formation in a transformed deer cell line infected with chronic wasting disease. J. Virol. 2006, 80, 596–604. [Google Scholar] [CrossRef]
- Chasseigneaux, S.; Pastore, M.; Britton-Davidian, J.; Manie, E.; Stern, M.H.; Callebert, J.; Catalan, J.; Casanova, D.; Belondrade, M.; Provansal, M.; et al. Genetic heterogeneity versus molecular analysis of prion susceptibility in neuroblasma n2a sublines. Arch. Virol. 2008, 153, 1693–1702. [Google Scholar] [CrossRef]
- Taraboulos, A.; Serban, D.; Prusiner, S.B. Scrapie prion proteins accumulate in the cytoplasm of persistently infected cultured cells. J. Cell. Biol. 1990, 110, 2117–2132. [Google Scholar]
- Vorberg, I.; Raines, A.; Priola, S.A. Acute formation of protease-resistant prion protein does not always lead to persistent scrapie infection in vitro. J. Biol. Chem. 2004, 279, 29218–29225. [Google Scholar]
- Greil, C.S.; Vorberg, I.M.; Ward, A.E.; Meade-White, K.D.; Harris, D.A.; Priola, S.A. Acute cellular uptake of abnormal prion protein is cell type and scrapie-strain independent. Virology 2008, 379, 284–293. [Google Scholar] [CrossRef]
- Goold, R.; Rabbanian, S.; Sutton, L.; Andre, R.; Arora, P.; Moonga, J.; Clarke, A.R.; Schiavo, G.; Jat, P.; Collinge, J.; et al. Rapid cell-surface prion protein conversion revealed using a novel cell system. Nat. Commun. 2011, 2, 281. [Google Scholar] [CrossRef]
- Bian, J.; Nazor, K.E.; Angers, R.; Jernigan, M.; Seward, T.; Centers, A.; Green, M.; Telling, G.C. Gfp-tagged prp supports compromised prion replication in transgenic mice. Biochem. Biophys. Res. Comm. 2006, 340, 894–900. [Google Scholar] [CrossRef]
- Magalhaes, A.C.; Baron, G.S.; Lee, K.S.; Steele-Mortimer, O.; Dorward, D.; Prado, M.A.; Caughey, B. Uptake and neuritic transport of scrapie prion protein coincident with infection of neuronal cells. J. Neurosci. 2005, 25, 5207–5216. [Google Scholar] [CrossRef]
- Mahal, S.P.; Demczyk, C.A.; Smith, E.W., Jr.; Klohn, P.C.; Weissmann, C. Assaying prions in cell culture: The standard scrapie cell assay (ssca) and the scrapie cell assay in end point format (scepa). Methods Mol. Biol. 2008, 459, 49–68. [Google Scholar] [CrossRef]
- Arellano-Anaya, Z.E.; Savistchenko, J.; Mathey, J.; Huor, A.; Lacroux, C.; Andreoletti, O.; Vilette, D. A simple, versatile and sensitive cell-based assay for prions from various species. PLoS One 2011, 6, e20563. [Google Scholar]
- Arjona, A.; Simarro, L.; Islinger, F.; Nishida, N.; Manuelidis, L. Two creutzfeldt-jakob disease agents reproduce prion protein-independent identities in cell cultures. Proc. Natl. Acad. Sci. USA 2004, 101, 8768–8773. [Google Scholar] [CrossRef]
- Enari, M.; Flechsig, E.; Weissmann, C. Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc. Natl. Acad. Sci. USA 2001, 98, 9295–9299. [Google Scholar]
- Markovits, P.; Dautheville, C.; Dormont, D.; Dianoux, L.; Latarjet, R. In vitro propagation of the scrapie agent. I. Transformation of mouse glia and neuroblastoma cells after infection with the mouse-adapted scrapie strain c-506. Acta. Neuropathol. 1983, 60, 75–80. [Google Scholar] [CrossRef]
- Ostlund, P.; Lindegren, H.; Pettersson, C.; Bedecs, K. Up-regulation of functionally impaired insulin-like growth factor-1 receptor in scrapie-infected neuroblastoma cells. J. Biol. Chem. 2001, 276, 36110–36115. [Google Scholar]
- Race, R. The scrapie agent in vitro. Curr Top. Microbiol. Immunol. 1991, 172, 181–193. [Google Scholar] [CrossRef]
- Scott, M.R.; Kohler, R.; Foster, D.; Prusiner, S.B. Chimeric prion protein expression in cultured cells and transgenic mice. Protein. Sci. 1992, 1, 986–997. [Google Scholar] [CrossRef]
- Miyazawa, K.; Emmerling, K.; Manuelidis, L. High cjd infectivity remains after prion protein is destroyed. J. Cell. Biochem. 2011, 112, 3630–3637. [Google Scholar] [CrossRef]
- Baron, T.G.; Biacabe, A.G.; Bencsik, A.; Langeveld, J.P. Transmission of new bovine prion to mice. Emerg. Infect. Dis. 2006, 12, 1125–1128. [Google Scholar] [CrossRef]
- Nunziante, M.; Ackermann, K.; Dietrich, K.; Wolf, H.; Gadtke, L.; Gilch, S.; Vorberg, I.; Groschup, M.; Schatzl, H.M. Proteasomal dysfunction and endoplasmic reticulum stress enhance trafficking of prion protein aggregates through the secretory pathway and increase accumulation of pathologic prion protein. J. Biol. Chem. 2011, 286, 33942–33953. [Google Scholar]
- Birkett, C.R.; Hennion, R.M.; Bembridge, D.A.; Clarke, M.C.; Chree, A.; Bruce, M.E.; Bostock, C.J. Scrapie strains maintain biological phenotypes on propagation in a cell line in culture. EMBO J. 2001, 20, 3351–3358. [Google Scholar] [CrossRef]
- Kanu, N.; Imokawa, Y.; Drechsel, D.N.; Williamson, R.A.; Birkett, C.R.; Bostock, C.J.; Brockes, J.P. Transfer of scrapie prion infectivity by cell contact in culture. Curr. Biol. 2002, 12, 523–530. [Google Scholar] [CrossRef]
- Dron, M.; Dandoy-Dron, F.; Farooq Salamat, M.K.; Laude, H. Proteasome inhibitors promote the sequestration of prpsc into aggresomes within the cytosol of prion-infected cad neuronal cells. J. Gen. Virol. 2009, 90, 2050–2060. [Google Scholar] [CrossRef]
- Browning, S.; Baker, C.A.; Smith, E.; Mahal, S.P.; Herva, M.E.; Demczyk, C.A.; Li, J.; Weissmann, C. Abrogation of complex glycosylation by swainsonine results in strain- and cell-specific inhibition of prion replication. J. Biol. Chem. 2011, 286, 40962–40973. [Google Scholar]
- Julius, C.; Hutter, G.; Wagner, U.; Seeger, H.; Kana, V.; Kranich, J.; Klohn, P.C.; Weissmann, C.; Miele, G.; Aguzzi, A. Transcriptional stability of cultured cells upon prion infection. J. Mol. Biol. 2008, 375, 1222–1233. [Google Scholar] [CrossRef]
- Rubenstein, R.; Deng, H.; Race, R.E.; Ju, W.; Scalici, C.L.; Papini, M.C.; Kascsak, R.J.; Carp, R.I. Demonstration of scrapie strain diversity in infected pc12 cells. J. Gen. Virol 1992, 73, 3027–3031. [Google Scholar]
- Rubenstein, R.; Carp, R.I.; Callahan, S.M. In vitro replication of scrapie agent in a neuronal model: Infection of pc12 cells. J. Gen. Virol. 1984, 65, 2191–2198. [Google Scholar] [CrossRef]
- Rubenstein, R.; Deng, H.; Scalici, C.L.; Papini, M.C. Alterations in neurotransmitter-related enzyme activity in scrapie-infected pc12 cells. J. Gen. Virol. 1991, 72, 1279–1285. [Google Scholar] [CrossRef]
- Follet, J.; Lemaire-Vieille, C.; Blanquet-Grossard, F.; Podevin-Dimster, V.; Lehmann, S.; Chauvin, J.P.; Decavel, J.P.; Varea, R.; Grassi, J.; Fontes, M.; et al. Prp expression and replication by schwann cells: Implications in prion spreading. J. Virol. 2002, 76, 2434–2439. [Google Scholar]
- Archer, F.; Bachelin, C.; Andreoletti, O.; Besnard, N.; Perrot, G.; Langevin, C.; Le Dur, A.; Vilette, D.; Baron-Van Evercooren, A.; Vilotte, J.L.; et al. Cultured peripheral neuroglial cells are highly permissive to sheep prion infection. J. Virol. 2004, 78, 482–490. [Google Scholar]
- Morel, E.; Andrieu, T.; Casagrande, F.; Gauczynski, S.; Weiss, S.; Grassi, J.; Rousset, M.; Dormont, D.; Chambaz, J. Bovine prion is endocytosed by human enterocytes via the 37 kda/67 kda laminin receptor. Am. J. Pathol. 2005, 167, 1033–1042. [Google Scholar] [CrossRef]
- Hijazi, N.; Kariv-Inbal, Z.; Gasset, M.; Gabizon, R. Prpsc incorporation to cells requires endogenous glycosaminoglycan expression. J. Biol. Chem. 2005, 280, 17057–17061. [Google Scholar]
- Horonchik, L.; Tzaban, S.; Ben-Zaken, O.; Yedidia, Y.; Rouvinski, A.; Papy-Garcia, D.; Barritault, D.; Vlodavsky, I.; Taraboulos, A. Heparan sulfate is a cellular receptor for purified infectious prions. J. Biol. Chem. 2005, 280, 17062–17067. [Google Scholar]
- Mohan, J.; Hopkins, J.; Mabbott, N.A. Skin-derived dendritic cells acquire and degrade the scrapie agent following in vitro exposure. Immunology 2005, 116, 122–133. [Google Scholar] [CrossRef]
- Paquet, S.; Daude, N.; Courageot, M.P.; Chapuis, J.; Laude, H.; Vilette, D. Prpc does not mediate internalization of prpsc but is required at an early stage for de novo prion infection of rov cells. J. Virol. 2007, 81, 10786–10791. [Google Scholar] [CrossRef]
- Langevin, C.; Gousset, K.; Costanzo, M.; Richard-Le Goff, O.; Zurzolo, C. Characterization of the role of dendritic cells in prion transfer to primary neurons. Biochem. J. 2010, 431, 189–198. [Google Scholar] [CrossRef]
- Jen, A.; Parkyn, C.J.; Mootoosamy, R.C.; Ford, M.J.; Warley, A.; Liu, Q.; Bu, G.; Baskakov, I.V.; Moestrup, S.; McGuinness, L.; et al. Neuronal low-density lipoprotein receptor-related protein 1 binds and endocytoses prion fibrils via receptor cluster 4. J. Cell. Sci. 2010, 123, 246–255. [Google Scholar] [CrossRef]
- Schonberger, O.; Horonchik, L.; Gabizon, R.; Papy-Garcia, D.; Barritault, D.; Taraboulos, A. Novel heparan mimetics potently inhibit the scrapie prion protein and its endocytosis. Biochem. Biophys. Res. Commun. 2003, 312, 473–479. [Google Scholar] [CrossRef]
- Gauczynski, S.; Nikles, D.; El-Gogo, S.; Papy-Garcia, D.; Rey, C.; Alban, S.; Barritault, D.; Lasmezas, C.I.; Weiss, S. The 37-kda/67-kda laminin receptor acts as a receptor for infectious prions and is inhibited by polysulfated glycanes. J. Infect. Dis. 2006, 194, 702–709. [Google Scholar] [CrossRef]
- Shmakov, A.N.; Bode, J.; Kilshaw, P.J.; Ghosh, S. Diverse patterns of expression of the 67-kd laminin receptor in human small intestinal mucosa: Potential binding sites for prion proteins? J. Pathol. 2000, 191, 318–322. [Google Scholar] [CrossRef]
- Prusiner, S.B.; McKinley, M.P.; Bowman, K.A.; Bolton, D.C.; Bendheim, P.E.; Groth, D.F.; Glenner, G.G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell 1983, 35, 349–358. [Google Scholar]
- Lofgren, K.; Wahlstrom, A.; Lundberg, P.; Langel, U.; Graslund, A.; Bedecs, K. Antiprion properties of prion protein-derived cell-penetrating peptides. FASEB J. 2008, 22, 2177–2184. [Google Scholar] [CrossRef]
- Taylor, D.R.; Whitehouse, I.J.; Hooper, N.M. Glypican-1 mediates both prion protein lipid raft association and disease isoform formation. PLoS Pathog. 2009, 5, e1000666. [Google Scholar] [CrossRef]
- Hooper, N.M. Glypican-1 facilitates prion conversion in lipid rafts. J. Neurochem. 2011, 116, 721–725. [Google Scholar] [CrossRef]
- Wadia, J.S.; Schaller, M.; Williamson, R.A.; Dowdy, S.F. Pathologic prion protein infects cells by lipid-raft dependent macropinocytosis. PLoS One 2008, 3, e3314. [Google Scholar] [CrossRef]
- Taylor, D.R.; Hooper, N.M. The prion protein and lipid rafts. Mol. Membr. Biol. 2006, 23, 89–99. [Google Scholar]
- Deleault, N.R.; Harris, B.T.; Rees, J.R.; Supattapone, S. Formation of native prions from minimal components in vitro. Proc. Natl. Acad. Sci. USA 2007, 104, 9741–9746. [Google Scholar]
- Abid, K.; Morales, R.; Soto, C. Cellular factors implicated in prion replication. FEBS Lett. 2010, 584, 2409–2414. [Google Scholar] [CrossRef]
- Caughey, B.; Raymond, G.J.; Ernst, D.; Race, R.E. N-terminal truncation of the scrapie-associated form of prp by lysosomal protease(s): Implications regarding the site of conversion of prp to the protease-resistant state. J. Virol. 1991, 65, 6597–6603. [Google Scholar]
- Borchelt, D.R.; Taraboulos, A.; Prusiner, S.B. Evidence for synthesis of scrapie prion proteins in the endocytic pathway. J. Biol. Chem. 1992, 267, 16188–16199. [Google Scholar]
- Taraboulos, A.; Raeber, A.J.; Borchelt, D.R.; Serban, D.; Prusiner, S.B. Synthesis and trafficking of prion proteins in cultured cells. Mol. Biol. Cell. 1992, 3, 851–863. [Google Scholar]
- Naslavsky, N.; Stein, R.; Yanai, A.; Friedlander, G.; Taraboulos, A. Characterization of detergent-insoluble complexes containing the cellular prion protein and its scrapie isoform. J. Biol. Chem. 1997, 272, 6324–6331. [Google Scholar]
- Stahl, N.; Baldwin, M.A.; Burlingame, A.L.; Prusiner, S.B. Identification of glycoinositol phospholipid linked and truncated forms of the scrapie prion protein. Biochemistry 1990, 29, 8879–8884. [Google Scholar] [CrossRef]
- Veith, N.M.; Plattner, H.; Stuermer, C.A.; Schulz-Schaeffer, W.J.; Burkle, A. Immunolocalisation of prpsc in scrapie-infected n2a mouse neuroblastoma cells by light and electron microscopy. Eur. J. Cell Biol. 2009, 88, 45–63. [Google Scholar] [CrossRef]
- Mange, A.; Crozet, C.; Lehmann, S.; Beranger, F. Scrapie-like prion protein is translocated to the nuclei of infected cells independently of proteasome inhibition and interacts with chromatin. J. Cell Sci. 2004, 117, 2411–2416. [Google Scholar] [CrossRef]
- Lehmann, S.; Harris, D.A. Mutant and infectious prion proteins display common biochemical properties in cultured cells. J. Biol. Chem. 1996, 271, 1633–1637. [Google Scholar] [CrossRef]
- Caughey, B.; Raymond, G.J. The scrapie-associated form of prp is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J. Biol. Chem. 1991, 266, 18217–18223. [Google Scholar]
- Beranger, F.; Mange, A.; Goud, B.; Lehmann, S. Stimulation of prp(c) retrograde transport toward the endoplasmic reticulum increases accumulation of prp(sc) in prion-infected cells. J. Biol. Chem. 2002, 277, 38972–38977. [Google Scholar]
- Vorberg, I.; Chan, K.; Priola, S.A. Deletion of beta-strand and alpha-helix secondary structure in normal prion protein inhibits formation of its protease-resistant isoform. J. Virol. 2001, 75, 10024–10032. [Google Scholar] [CrossRef]
- Marijanovic, Z.; Caputo, A.; Campana, V.; Zurzolo, C. Identification of an intracellular site of prion conversion. PLoS Pathog. 2009, 5, e1000426. [Google Scholar] [CrossRef]
- McKinley, M.P.; Taraboulos, A.; Kenaga, L.; Serban, D.; Stieber, A.; DeArmond, S.J.; Prusiner, S.B.; Gonatas, N. Ultrastructural localization of scrapie prion proteins in cytoplasmic vesicles of infected cultured cells. Lab. Invest. 1991, 65, 622–630. [Google Scholar]
- Pimpinelli, F.; Lehmann, S.; Maridonneau-Parini, I. The scrapie prion protein is present in flotillin-1-positive vesicles in central- but not peripheral-derived neuronal cell lines. Eur. J. Neurosci. 2005, 21, 2063–2072. [Google Scholar] [CrossRef]
- McKinley, M.P.; Meyer, R.K.; Kenaga, L.; Rahbar, F.; Cotter, R.; Serban, A.; Prusiner, S.B. Scrapie prion rod formation in vitro requires both detergent extraction and limited proteolysis. J. Virol. 1991, 65, 1340–1351. [Google Scholar]
- Snow, A.D.; Kisilevsky, R.; Willmer, J.; Prusiner, S.B.; DeArmond, S.J. Sulfated glycosaminoglycans in amyloid plaques of prion diseases. Acta. Neuropathol. 1989, 77, 337–342. [Google Scholar] [CrossRef]
- Adjou, K.T.; Simoneau, S.; Sales, N.; Lamoury, F.; Dormont, D.; Papy-Garcia, D.; Barritault, D.; Deslys, J.P.; Lasmezas, C.I. A novel generation of heparan sulfate mimetics for the treatment of prion diseases. J. Gen. Virol. 2003, 84, 2595–2603. [Google Scholar] [CrossRef]
- Wong, C.; Xiong, L.W.; Horiuchi, M.; Raymond, L.; Wehrly, K.; Chesebro, B.; Caughey, B. Sulfated glycans and elevated temperature stimulate prp(sc)-dependent cell-free formation of protease-resistant prion protein. EMBO J. 2001, 20, 377–386. [Google Scholar] [CrossRef]
- Murayama, Y.; Yoshioka, M.; Masujin, K.; Okada, H.; Iwamaru, Y.; Imamura, M.; Matsuura, Y.; Fukuda, S.; Onoe, S.; Yokoyama, T.; et al. Sulfated dextrans enhance in vitro amplification of bovine spongiform encephalopathy prp(sc) and enable ultrasensitive detection of bovine prp(sc). PLoS One 2010, 5. [Google Scholar]
- Yokoyama, T.; Takeuchi, A.; Yamamoto, M.; Kitamoto, T.; Ironside, J.W.; Morita, M. Heparin enhances the cell-protein misfolding cyclic amplification efficiency of variant creutzfeldt-jakob disease. Neurosci. Lett. 2011, 498, 119–123. [Google Scholar] [CrossRef]
- Ghaemmaghami, S.; Phuan, P.W.; Perkins, B.; Ullman, J.; May, B.C.; Cohen, F.E.; Prusiner, S.B. Cell division modulates prion accumulation in cultured cells. Proc. Natl. Acad. Sci. USA 2007, 104, 17971–17976. [Google Scholar]
- Paquet, S.; Langevin, C.; Chapuis, J.; Jackson, G.S.; Laude, H.; Vilette, D. Efficient dissemination of prions through preferential transmission to nearby cells. J. Gen. Virol. 2007, 88, 706–713. [Google Scholar]
- Leblanc, P.; Alais, S.; Porto-Carreiro, I.; Lehmann, S.; Grassi, J.; Raposo, G.; Darlix, J.L. Retrovirus infection strongly enhances scrapie infectivity release in cell culture. EMBO J. 2006, 25, 2674–2685. [Google Scholar] [CrossRef]
- Gousset, K.; Zurzolo, C. Tunnelling nanotubes: A highway for prion spreading? Prion 2009, 3, 94–98. [Google Scholar] [CrossRef]
- Gousset, K.; Schiff, E.; Langevin, C.; Marijanovic, Z.; Caputo, A.; Browman, D.T.; Chenouard, N.; de Chaumont, F.; Martino, A.; Enninga, J.; et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat. Cell. Biol. 2009, 11, 328–336. [Google Scholar] [CrossRef]
- Nishida, N.; Katamine, S.; Manuelidis, L. Reciprocal interference between specific cjd and scrapie agents in neural cell cultures. Science 2005, 310, 493–496. [Google Scholar] [CrossRef]
- Baron, G.S.; Magalhaes, A.C.; Prado, M.A.; Caughey, B. Mouse-adapted scrapie infection of sn56 cells: Greater efficiency with microsome-associated versus purified prp-res. J. Virol. 2006, 80, 2106–2117. [Google Scholar]
- Krammer, C.; Schatzl, H.M.; Vorberg, I. Prion-like propagation of cytosolic protein aggregates: Insights from cell culture models. Prion 2009, 3, 206–212. [Google Scholar] [CrossRef]
- Luk, K.C.; Kehm, V.M.; Zhang, B.; O'Brien, P.; Trojanowski, J.Q.; Lee, V.M. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J. Exp. Med. 2012, 209, 975–986. [Google Scholar]
- Meyer-Luehmann, M.; Coomaraswamy, J.; Bolmont, T.; Kaeser, S.; Schaefer, C.; Kilger, E.; Neuenschwander, A.; Abramowski, D.; Frey, P.; Jaton, A.L.; et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science 2006, 313, 1781–1784. [Google Scholar]
- Desplats, P.; Lee, H.J.; Bae, E.J.; Patrick, C.; Rockenstein, E.; Crews, L.; Spencer, B.; Masliah, E.; Lee, S.J. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. USA 2009, 106, 13010–13015. [Google Scholar]
- Clavaguera, F.; Bolmont, T.; Crowther, R.A.; Abramowski, D.; Frank, S.; Probst, A.; Fraser, G.; Stalder, A.K.; Beibel, M.; Staufenbiel, M.; et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell. Biol 2009, 11, 909–913. [Google Scholar] [CrossRef]
- Munch, C.; O'Brien, J.; Bertolotti, A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc. Natl. Acad. Sci. USA 2011, 108, 3548–3553. [Google Scholar] [CrossRef]
- Kfoury, N.; Holmes, B.B.; Jiang, H.; Holtzman, D.M.; Diamond, M.I. Trans-cellular propagation of tau aggregation by fibrillar species. J. Biol. Chem. 2012, 287, 19440–19451. [Google Scholar]
- Hansen, C.; Angot, E.; Bergstrom, A.L.; Steiner, J.A.; Pieri, L.; Paul, G.; Outeiro, T.F.; Melki, R.; Kallunki, P.; Fog, K.; et al. Alpha-synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J. Clin. Invest. 2011, 121, 715–725. [Google Scholar] [CrossRef]
- Ren, P.H.; Lauckner, J.E.; Kachirskaia, I.; Heuser, J.E.; Melki, R.; Kopito, R.R. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat. Cell. Biol. 2009, 11, 219–225. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rub, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic parkinson's disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Lee, H.J.; Patel, S.; Lee, S.J. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J. Neurosci. 2005, 25, 6016–6024. [Google Scholar] [CrossRef]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O'Brien, P.; Trojanowski, J.Q.; Lee, V.M. Pathological alpha-synuclein transmission initiates parkinson-like neurodegeneration in nontransgenic mice. Science 2012, 338, 949–953. [Google Scholar]
- Krammer, C.; Kryndushkin, D.; Suhre, M.H.; Kremmer, E.; Hofmann, A.; Pfeifer, A.; Scheibel, T.; Wickner, R.B.; Schatzl, H.M.; Vorberg, I. The yeast sup35nm domain propagates as a prion in mammalian cells. Proc. Natl. Acad. Sci. USA 2009, 106, 462–467. [Google Scholar]
- Munch, C.; Bertolotti, A. Self-propagation and transmission of misfolded mutant sod1: Prion or prion-like phenomenon? Cell. Cycle. 2011, 10, 1711. [Google Scholar] [CrossRef]
- Kocisko, D.A.; Engel, A.L.; Harbuck, K.; Arnold, K.M.; Olsen, E.A.; Raymond, L.D.; Vilette, D.; Caughey, B. Comparison of protease-resistant prion protein inhibitors in cell cultures infected with two strains of mouse and sheep scrapie. Neurosci. Lett. 2005, 388, 106–111. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).