Renal Alterations in Feline Immunodeficiency Virus (FIV)-Infected Cats: A Natural Model of Lentivirus-Induced Renal Disease Changes
Abstract
:1. Introduction
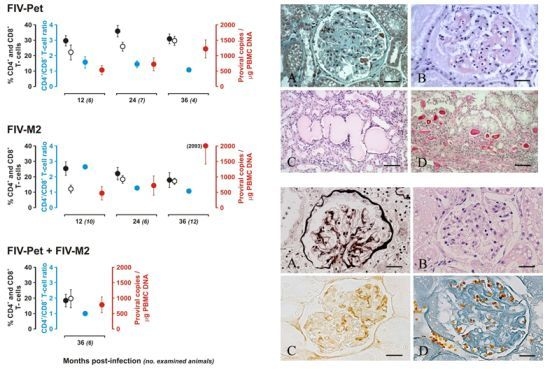
2. Results and Discussion
| Characteristic | Naturally Infected (n = 21) | Experimentally Infected | Controls (n = 4) | ||
|---|---|---|---|---|---|
| FIV-Pet (n = 17) | FIV-M2 (n = 28) | FIV-Pet + FIV-M2 (n = 6) | |||
| Median (Range) | Median (Range) | Median (Range) | Median (Range) | Median (Range) | |
| Age (years) | 8.0 (4.0–13.0) | 3.0 (2.0–6.0) | 4.0 (2.0–6.0) | 4.5 (4.0–5.0) | 4.5 (4.0–6.0) |
| Sex | |||||
| Male | 15 | ||||
| Female | 6 | 17 | 28 | 6 | |
| Neuter status | |||||
| Intact | 17 | 17 | 28 | 6 | |
| Altered | 4 | ||||
| Clinical status | |||||
| Sick | 11 | 1 | |||
| Healthy | 10 | 17 | 27 | 6 | |
| Creatinine concentration | 120 (73–709) | 123 (100–131) | 118 (93–144) | 121 (103–139) | 107 (95–125) |
| UPC | 1.17 (0,18–14.00) | 0.69 (0.31–7.00) | 0.52 (0.25–18.00) | 0.92 (0.55–13.00) | 0.25 (0.17–0.31) |
| Renal Alterations | Naturally Infected n = 21 (%) | Controls | 12 Months pi | 24 Months pi | ≥36 Months pi | ||||
|---|---|---|---|---|---|---|---|---|---|
| FIV-Pet n = 6 (%) | FIV-M2 n = 10 (%) | FIV-Pet n = 7 (%) | FIV-M2 n = 6 (%) | FIV-Pet n = 4 (%) | FIV-M2 n = 12 (%) | FIV-Pet + FIV-M2 n = 6 (%) | |||
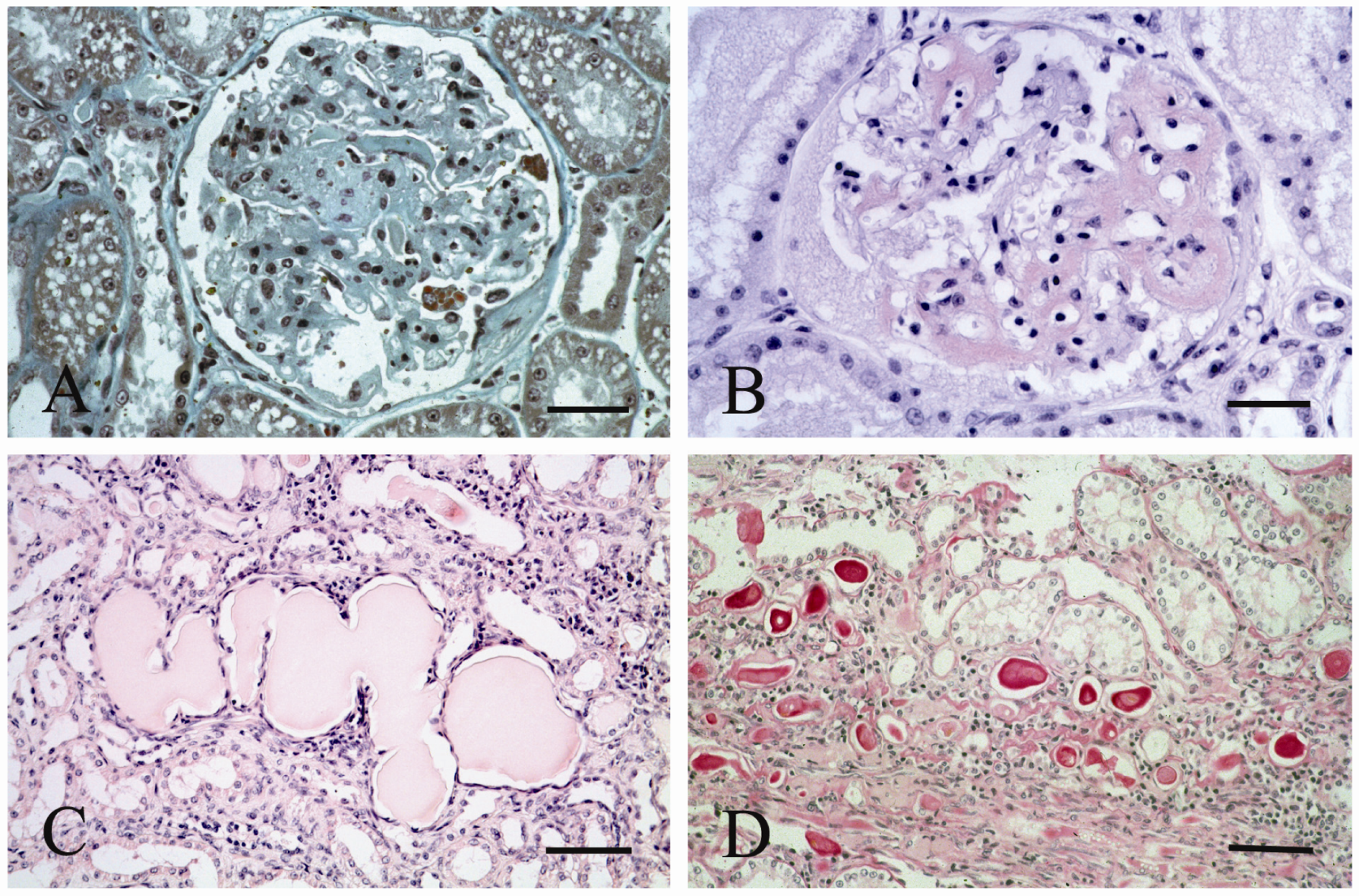
| Mesangial widening | 9 (42.9) | 0 | 2 (33.3) | 2 (20.0) | 1 (14.3) | 1 (16.6) | 3 (75.0) | 3 (25.0) | 2 (33.3) |
| Glomerulo-nephritis | 3 (14.3) | 0 | 1 (16.6) | 1 (10.0) | 2 (28.6) | 1 (16.6) | 0 | 4 (33.3) | 3 (50.0) |
| Glomerular amyloidosis | 8 (30.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tubular changes | 10 (47.6) | 0 | 2 (33.3) | 2 (20.0) | 1 (14.3) | 2 (33.3) | 3 (75.0) | 7 (58.3) | 3 (50.0) |
| Interstitial lesions | 17 (81–0) | 0 | 0 | 0 | 1 (14.3) | 1 (16.6) | 0 | 2 (16.7) | 2 (33.3) |
| Interstitial amyloidosis | 7 (33.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| No alterations | 3 (14.3) | 4 (100) | 3 (50.0) | 7 (70.0) | 4 (57.1) | 4 (66.6) | 1 (25.0) | 5 (41.6) | 1 (16.6) |
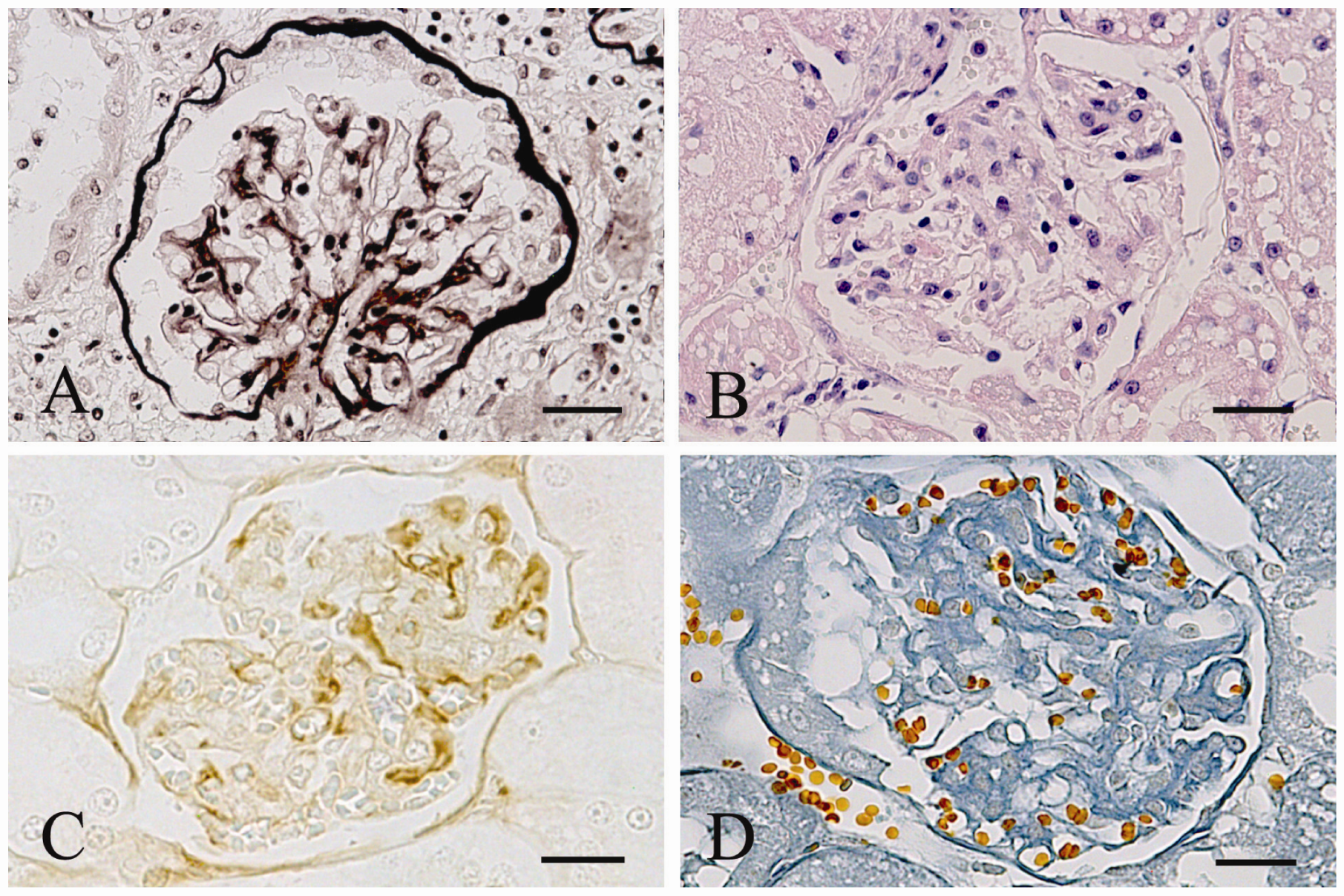
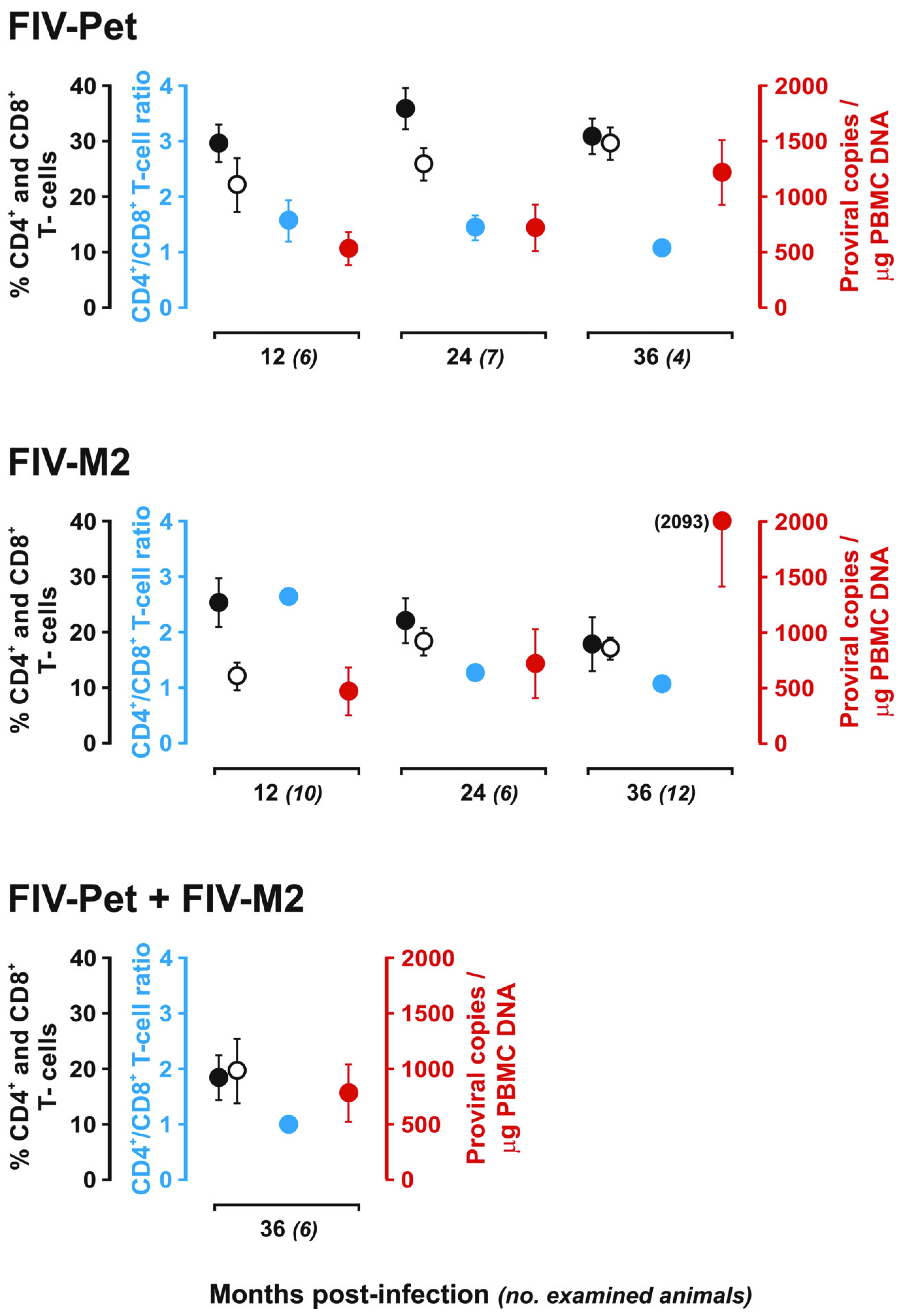
| Immunohistochemistry | Control Cats n = 4 (%) | Naturally Infected cats n = 21 (%) | Experimentally Infected Cats | ||
|---|---|---|---|---|---|
| FIV-Pet n = 17 (%) | FIV M2 n = 28 (%) | FIV-Pet + FIV M2 n = 6 (%) | |||
| IgG deposits in mesangium | 0 | 3 (14.3) | 3 (17.6) | 5 (17.9) | 3 (50.0) |
| IgG deposits in capillary loops | 0 | 1 (04.8) | 1 (05.9) | 2 (07.1) | 2 (33.3) |
| IgM deposits | 0 | 14 (66.7) | 6 (35.3) | 6 (21.3) | 3 (50.0) |
| IgA deposits | 0 | 0 | 0 | 0 | 0 |
| C3 deposits | 0 | 14 (66.7) | 6 (35.3) | 6 (21.3) | 3 (50.0) |
| Mouse monoclonal anti-human AA amyloid | 0 | 8 (38.1) | 0 | 0 | 0 |
| Rabbit polyclonal anti-feline AA amyloid | 0 | 8 (38.1) | 0 | 0 | 0 |
| Rabbit polyclonal anti-feline AL amyloid | 0 | 0 | 0 | 0 | 0 |
3. Experimental Section
3.1. Studied Subjects and Experimental Design
3.2. Biochemistry and Urine Analysis
3.3. Molecular Analysis
3.4. Flow Cytometry
3.5. Histological Investigations
3.6. Immunohistochemical Investigations
3.7. Statistics
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Kimmel, P.L. The nephropathies of HIV infection: Pathogenesis and treatment. Curr. Opin. Nephrol. Hypertens 2000, 9, 117–122. [Google Scholar] [CrossRef]
- Rosenstiel, P.; Gharavi, A.; D’Agati, V.; Klotman, P. Transgenic and infectious animal model of HIV-associated nephropathy. J. Am. Soc. Nephrol. 2009, 20, 2296–2304. [Google Scholar] [CrossRef]
- Ackley, C.D.; Yamamoto, J.K.; Levy, N. Immunologic abnormalities in pathogen-free cats experimentally infected with feline immunodeficiency virus. J. Virol. 1990, 64, 5652–5655. [Google Scholar]
- Hosie, M.J.; Addie, D.; Belak, S. Feline immunodeficiency ABCD guideline on prevention and management. J. Fel. Med. Surg. 2009, 11, 575–584. [Google Scholar] [CrossRef]
- Pistello, M.; Matteucci, D.; Cammarota, G.; Mazzetti, P.; Giannecchini, S.; del Mauro, D.; Macchi, S.; Zaccaro, L.; Bendinelli, M. Kinetics of replication of a partially attenuated virus and of the challenge virus during a three-year intersubtype feline immunodeficiency virus superinfection experiment in cats. J. Virol. 1999, 73, 1518–1527. [Google Scholar]
- Poli, A.; Abramo, F.; Taccini, E. Renal involvement in feline immunodeficiency virus infection: A clinicopathological study. Nephron 1993, 64, 282–288. [Google Scholar] [CrossRef]
- Poli, A.; Abramo, F.; Matteucci, D. Renal involvement in feline immunodeficiency virus: p24 antigen detection, virus isolation and PCR analysis. Vet. Immunol. Immunopathol. 1995, 46, 13–20. [Google Scholar] [CrossRef]
- Thomas, J.B.; Robinson, F.; Chadwick, G.J. Association of renal disease indicators with feline immunodeficiency virus infection. J. Am. Anim. Hosp. Assoc. 1993, 29, 320–326. [Google Scholar]
- Levy, J. FIV causes nephropathy in cats. San Antonio, TX, USA, 1996; p. 56.
- Belford, C.J.; Miller, R.I.; Mitchell, G. Evidence of feline immunodeficiency virus in Queensland cats: Preliminary observations. Aust. Vet. Pract. 1989, 29, 320–326. [Google Scholar]
- Brown, P.J.; Cherida, D.; Hopper, D. Pathological features of lymphoid tissues in cats with natural feline immunodeficiency virus infection. J. Comp. Path. 1991, 104, 345–355. [Google Scholar] [CrossRef]
- Swinney, G.R.; Pauli, J.V.; Jones, B.R. Feline T-lyphotropic virus (FTLV) (feline immunodeficiency virus infection) in cats in New Zealand. N. Z. Vet. J. 1989, 37, 41–43. [Google Scholar]
- Ishida, T.; Washizu, T.; Toriyabe, K. Feline immunodeficiency virus infection in cats of Japan. J. Am. Vet. Med. Assoc. 1989, 194, 221–225. [Google Scholar]
- Bo, S.; Garetto, M.; Latti, D. Indagine epidemiologica e quadri clinici di FIV e FeLV nell’Italia nord-occidentale in una popolazione di 850 gatti (in Italian). Veterinaria 1992, 4, 105–112. [Google Scholar]
- Poli, A.; Falcone, L.; Bigalli, L.; Massi, C.; Hofmann-Lehmann, R.; Lombardi, S.; Bendinelli, M.; Lutz, H. Circulating immune complex and analysis of renal immune deposits in feline immunodeficiency virus-infected cats. Clin. Exp. Immunol. 1995, 101, 254–258. [Google Scholar]
- Matsumoto, H.; Takemura, N.; Sako, T. Serum concentration circulating immune complexes in cats infected with feline immunodeficiency virus detected by immune adherence hemagglutination method. J. Vet. Med. Sci. 1997, 59, 395–396. [Google Scholar] [CrossRef]
- Marin, R.; Gorostidi, M.; Fernandez-Vega, F. Systemic and glomerular hypertension and progression of chronic renal disease: The dilemma of nephrosclerosis. Kidney Int. Suppl. 2005, 52–56. [Google Scholar]
- Ishida, T.; Taniguchi, A.; Matsumura, S. Long-term clinical observations on feline immuno-deficiency virus infected asymptomatic carries. Vet. Immunol. Immunopathol. 1992, 1-2, 15–22. [Google Scholar]
- Shibata, T.; Sawashima, Y.; Sawashima, K. Two cases of systemic amyloidosis in FIV-infected cats. J. Jpn. Vet. Med. Assoc. 1995, 48, 268–270. [Google Scholar]
- Baxter, K.J.; Levy, J.K.; Edinboro, S.L.; Vaden, S.L.; Tompkins, M.B. Renal disease in cats infected with feline immunodeficiency virus. J. Vet. Internal Med. 2012, 26, 238–243. [Google Scholar] [CrossRef]
- International Renal Interest Society (IRIS). IRIS Staging of CKD. 2009. Available online: http://www.iris-kidney.com/guidelines/en/index.shtml (accessed on 10 April 2012).
- Monga, G.; Mazzucco, G.; Boldorini, R.; Cristina, S.; Giacalone, A.; Fortunato, M.; Motta, M.; Campobasso, O. Renal changes in patients with acquired immunodeficiency syndrome: A post-mortem study on an unselected population in northwestern Italy. Mod. Pathol. 1997, 10, 159–167. [Google Scholar]
- Kimmel, P.L.; Ferreira-Centeno, A.; Farkas-Szallasi, T.; Abraham, A.A.; Garrett, C.T. Viral DNA in microdissected renal biopsy tissue from HIV infected patients with nephrotic syndrome. Kidney Int. 1993, 43, 1347–1352. [Google Scholar] [CrossRef]
- Connolly, J.O.; Weston, C.E.; Hendry, B.M. HIV-associated renal disease in London hospitals. QJM 1995, 88, 627–634. [Google Scholar]
- Williams, D.I.; Williams, D.J.; Williams, I.G.; Unwin, R.J.; Griffiths, M.H.; Miller, R.F. Presentation, pathology, and outcome of HIV associated renal disease in a specialist centre for HIV/AIDS. Sex. Transm. Infect. 1998, 74, 179–184. [Google Scholar] [CrossRef]
- Kimmel, P.L.; Phillips, T.M. Immune complex glomerulonephritis associated with HIV infection. In Renal and Urologic Aspects of HIV Infection; Kimmel, P.L., Berns, J.S., Stein, J.H., Eds.; Churchill Livingstone: New York, NY, USA, 1995; pp. 77–110. [Google Scholar]
- Cheng, J.T.; Anderson, H.L., Jr.; Markowitz, G.S.; Appel, G.B.; Pogue, V.A.; D’Agati, V.D. Hepatitis C virus-associated glomerular disease in patients with human immunodeficiency virus coinfection. J. Am. Soc. Nephrol. 1999, 10, 1566–1574. [Google Scholar]
- Stokes, M.B.; Chawla, H.; Brody, R.I.; Kumar, A.; Gertner, R.; Goldfarb, D.S. Immune complex glomerulonephritis in patients coinfected with human immunodeficiency virus and hepatitis C virus. Am. J. Kidney Dis. 1997, 29, 514–525. [Google Scholar] [CrossRef]
- Guerra, I.L.; Abraham, A.A.; Kimmel, P.L.; Sabnis, S.G.; Antonovych, T.T. Nephrotic syndrome associated with chronic persistent hepatitis B in an HIV antibody positive patient. Am. J. Kidney Dis. 1987, 10, 385–388. [Google Scholar]
- Korbet, S.M.; Schwartz, M.M. Human immunodeficiency virus infection and nephrotic syndrome. Am. J. Kidney Dis. 1992, 20, 97–103. [Google Scholar]
- Kimmel, P.L.; Phillips, T.M.; Ferreira-Centeno, A.; Farkas-Szallasi, T.; Abraham, A.A.; Garrett, C.T. HIV-associated immune-mediated renal disease. Kidney Int. 1993, 44, 1327–1340. [Google Scholar] [CrossRef]
- Thomas, J.B.; Robinson, F.; Chadwick, G.J. Association of renal disease indicators with feline immunodeficiency virus infection. J. Am. Anim. Hosp. Assoc. 1993, 29, 320–326. [Google Scholar]
- Cohen, A.H.; Nast, C.C. HIV-associated nephropathy. A unique combined glomerular, tubular, and interstitial lesion. Mod. Pathol. 1988, 1, 87–97. [Google Scholar]
- D’Agati, V.; Appel, G.B. Renal pathology of human immunodeficiency virus infection. Semin. Nephrol. 1998, 18, 406–421. [Google Scholar]
- Kopp, J.B.; Klotman, M.E.; Adler, S.H.; Bruggeman, L.A.; Dickie, P.; Marinos, N.J. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc. Natl. Acad. Sci. USA 1992, 89, 1577–1581. [Google Scholar]
- Reid, W.; Sadowska, M.; Denaro, F.; Rao, S.; Foulke, J., Jr.; Hayes, N.; Jones, O.; Doodnauth, D.; Davis, H.; Sill, A.; et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc. Natl. Acad. Sci. USA 2001, 98, 9271–9276. [Google Scholar]
- Alpers, C.E.; Tsai, C.C.; Hudkins, K.L.; Cui, Y.; Kuller, L.; Benveniste, R.E.; Ward, J.M.; Morton, W.R. Focal segmental glomerulosclerosis in primates infected with a simian immunodeficiency virus. AIDS Res. Hum. Retrovir. 1997, 13, 413–424. [Google Scholar] [CrossRef]
- Stephens, E.B.; Tian, C.; Dalton, S.B.; Gattone, V.H., 2nd. Simian-human immunodeficiency virus-associated nephropathy in macaques. AIDS Res. Hum. Retrovir. 2000, 16, 1295–1306. [Google Scholar] [CrossRef]
- Wali, R.K.; Drachenberg, C.I.; Papadimitriou, J.C.; Keay, S.; Ramos, E. HIV-1-associated nephropathy and response to highly-active antiretroviral therapy. Lancet 1998, 352, 783–784. [Google Scholar]
- Winston, J.A.; Bruggeman, L.A.; Ross, M.D.; Jacobson, J.; Ross, L.; D’Agati, V.D. Nephropathy and establishment of a renal reservoir of HIV type 1 during primary infection. N. Engl. J. Med. 2001, 344, 1979–1984. [Google Scholar] [CrossRef]
- Marras, D.; Bruggeman, L.A.; Gao, F.; Tanji, N.; Mansukhani, M.M.; Cara, A.; Ross, M.D.; Gusella, G.L.; Benson, G.; D’Agati, V.D.; et al. Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nat. Med. 2002, 8, 522–526. [Google Scholar]
- Bruggeman, L.A.; Ross, M.D.; Tanji, N.; Cara, A.; Dikman, S.; Gordon, R.E.; Burns, G.C.; D’Agati, V.D.; Winston, J.A.; Klotman, M.E.; et al. Renal epithelium is a previously unrecognized site of HIV-1 infection. J. Am. Soc. Nephrol. 2000, 11, 2079–2087. [Google Scholar]
- Ross, M.J.; Klotman, P.E. Recent progress in HIV-associated nephropathy. J. Am. Soc. Nephrol. 2002, 13, 2997–3004. [Google Scholar] [CrossRef]
- Kenyon, J.C.; Lever, A.M. The molecular biology of feline immunodeficiency virus (FIV). Viruses 2011, 11, 2192–2213. [Google Scholar] [CrossRef]
- Wang, J.F.; Shackelford, J.M.; Casella, C.R.; Shivers, D.K.; Rapaport, E.L.; Liu, B.; Yu, X.F.; Finkel, T.H. The Vif accessory protein alters the cell cycle of human immunodeficiency virus type 1 infected cells. Virology 2007, 359, 243–252. [Google Scholar] [CrossRef]
- Sakai, K.; Dimas, J.; Lenardo, M.J. The Vif and Vpr accessory proteins independently cause HIV-1-induced T cytopathicity and cell cycle arrest. Proc. Natl. Acad. Sci. USA 2006, 103, 3369–3374. [Google Scholar]
- Yamamoto, J.K.; Ackley, C.D.; Zochlinski, H. Development of IL-2 independent feline lymphoid cell lines chronically infected with feline immunodeficiency virus: Importance for diagnostic reagents and vaccines. Intervirology 1991, 32, 361–375. [Google Scholar]
- Pistello, M.; Matteucci, D.; Bonci, F.; Isola, P.; Mazzetti, P.; Zaccaro, L.; Merico, A.; del Mauro, D.; Flynn, N.; Bendinelli, M. AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: Protection from an intraclade challenge administered systemically or mucosally by an attenuated vaccine. J. Virol. 2003, 77, 10740–10750. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1990, 227, 680–685. [Google Scholar]
- Pistello, M.; Menzo, S.; Giorgi, M.; da Prato, L.; Cammarota, G.; Clementi, M.; Bendinelli, M. Competitive polymerase chain reaction for quantitating feline immunodeficiency virus load in infected cat tissues. Mol. Cell. Probes 1994, 8, 229–234. [Google Scholar] [CrossRef]
- Torten, M.; Franchini, M.; Barlough, J.E. Progressive immune dysfunction in cats experimentally infected with feline immunodeficiency virus. J. Virol. 1991, 5, 2225–2230. [Google Scholar]
- Puchtler, H.; Waldrop, F.S.; Meloan, S.N. A review of light, polarization, and fluorescence microscopic methods for amyloid. Appl. Pathol. 1985, 3, 5–17. [Google Scholar]
- Wright, J.R.; Colkins, E.; Humphrey, R.L. Potassium permanganate reaction in amyloidosis. A histologic method to assist in differntiating forms of this disease. Lab. Invest. 1977, 36, 274–275. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Poli, A.; Tozon, N.; Guidi, G.; Pistello, M. Renal Alterations in Feline Immunodeficiency Virus (FIV)-Infected Cats: A Natural Model of Lentivirus-Induced Renal Disease Changes. Viruses 2012, 4, 1372-1389. https://doi.org/10.3390/v4091372
Poli A, Tozon N, Guidi G, Pistello M. Renal Alterations in Feline Immunodeficiency Virus (FIV)-Infected Cats: A Natural Model of Lentivirus-Induced Renal Disease Changes. Viruses. 2012; 4(9):1372-1389. https://doi.org/10.3390/v4091372
Chicago/Turabian StylePoli, Alessandro, Natasa Tozon, Grazia Guidi, and Mauro Pistello. 2012. "Renal Alterations in Feline Immunodeficiency Virus (FIV)-Infected Cats: A Natural Model of Lentivirus-Induced Renal Disease Changes" Viruses 4, no. 9: 1372-1389. https://doi.org/10.3390/v4091372
APA StylePoli, A., Tozon, N., Guidi, G., & Pistello, M. (2012). Renal Alterations in Feline Immunodeficiency Virus (FIV)-Infected Cats: A Natural Model of Lentivirus-Induced Renal Disease Changes. Viruses, 4(9), 1372-1389. https://doi.org/10.3390/v4091372