Tropism-Modification Strategies for Targeted Gene Delivery Using Adenoviral Vectors
Abstract
1. Introduction
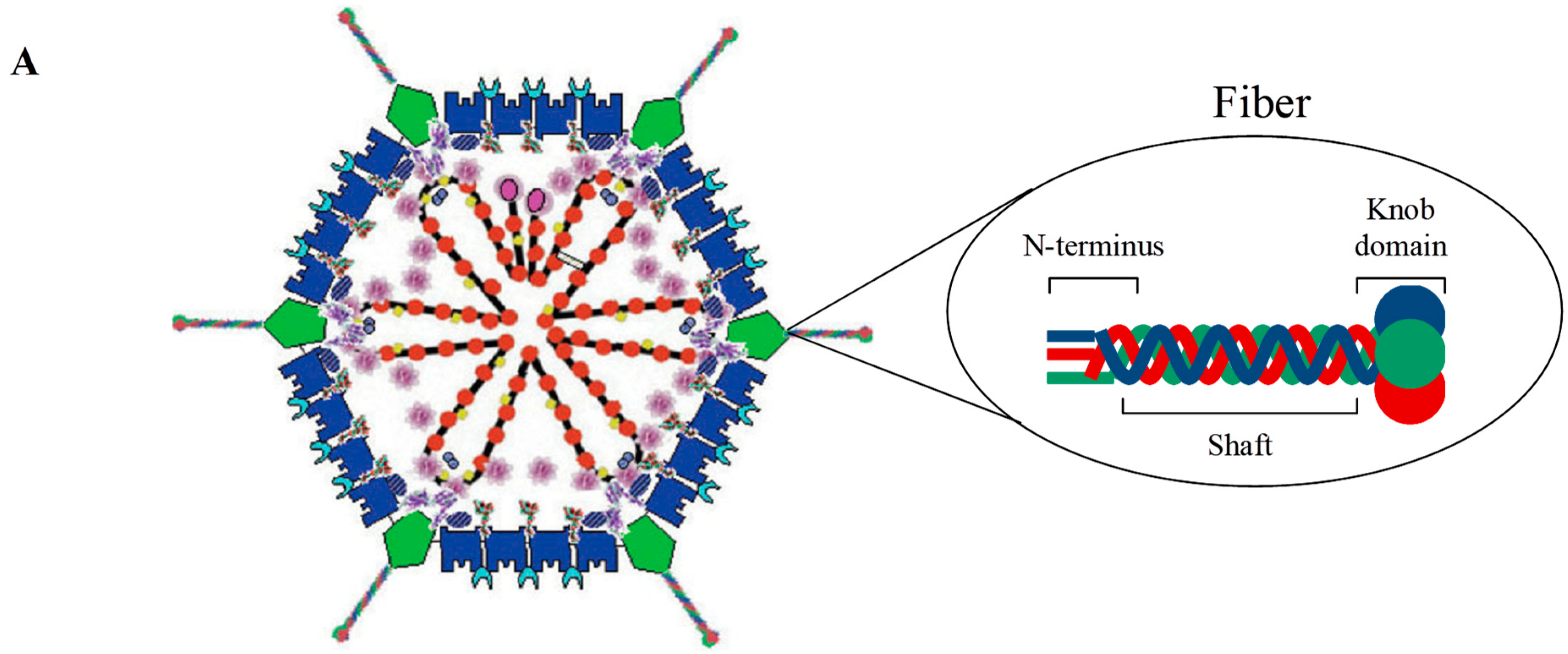
1.1. Adenovirus Structure
1.2. In Vitro Entry Pathway of Ad5
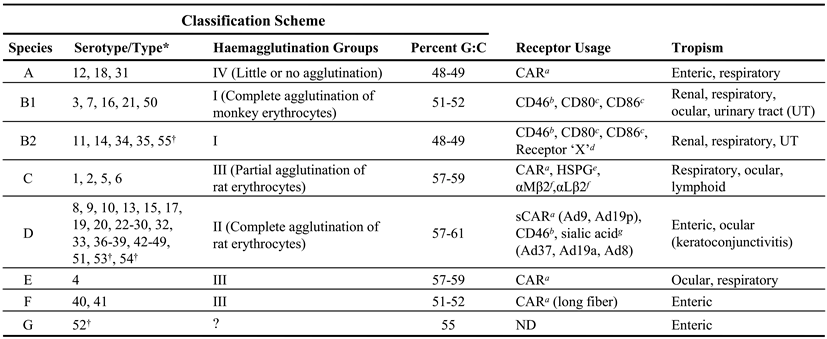
1.3. Bridging Receptors for Adenovirus Entry
 |
2. Retargeting Adenoviral Vectors
2.1. Transductional Retargeting by Genetic Incorporation of Ligands
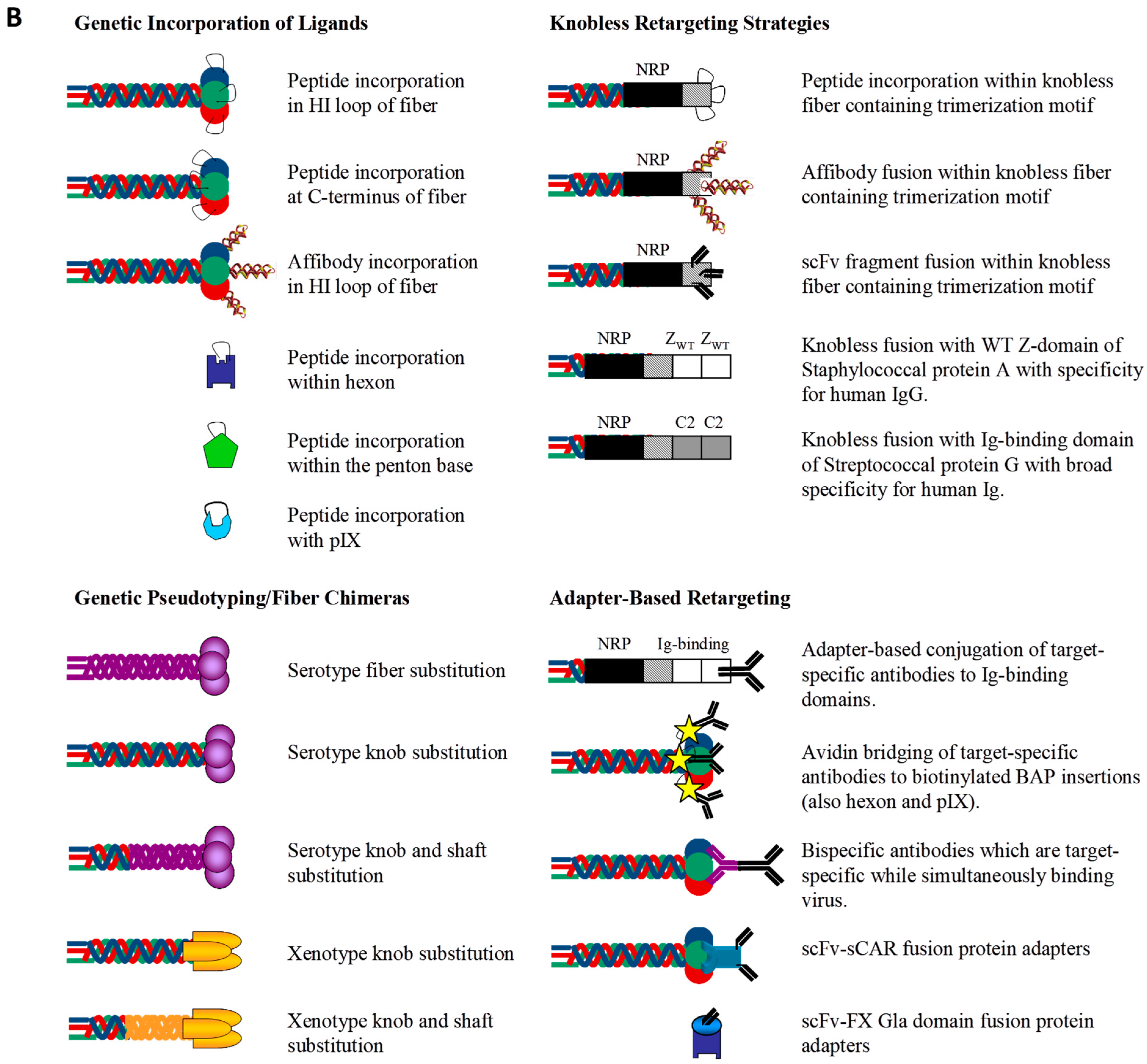
2.1.1. Fiber Retargeting Strategies: Peptide Incorporation
2.1.2. Fiber Retargeting Strategies: Generation of Knobless Fiber Shaft Fusions
2.1.3. Hexon Retargeting Strategies
2.1.4. Alternative Capsid Retargeting Strategies: Penton Base and pIX
2.2. Transductional Retargeting by Genetic Pseudotyping
2.3. Transductional Retargeting by Conjugation of Ligands: Adapter Ligand Complexes
2.4. Summary of Retargeting Efforts
3. Transductional Detargeting Strategies
 |
3.1. Transductional Detargeting by Ablation of Native Tropism
3.2. Transductional Detargeting by Ablation of “Bridging” Interactions
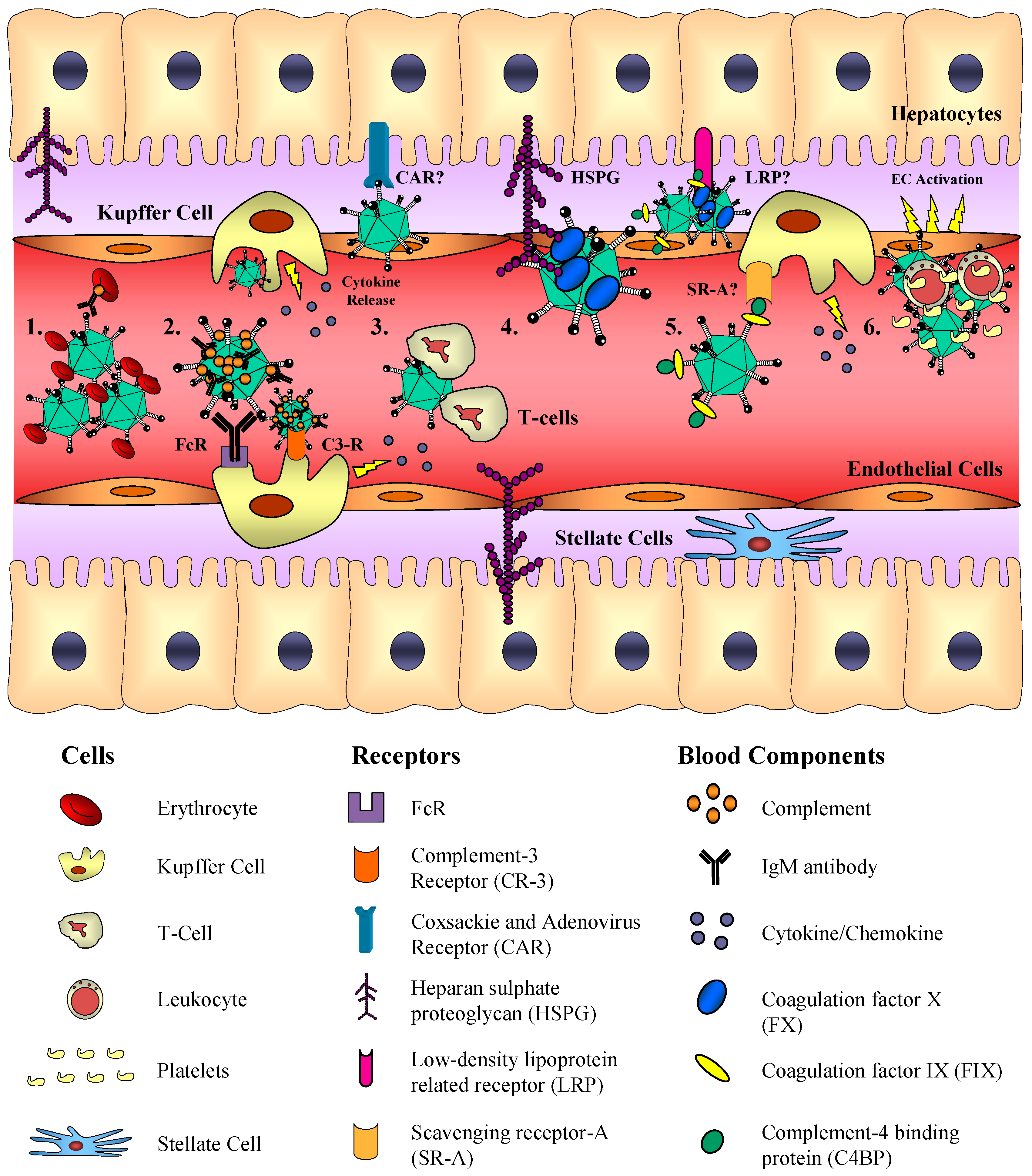
3.3. Detargeting from the Reticuloendothelial System
 |
3.4. Transductional Detargeting and Retargeting by Chemical Modification
3.4.1. Tropism Detargeting Adenovirus by Chemical Modification
3.4.2. Tropism Retargeting Adenovirus by Chemical Modification
3.4.3. Avoidance of Immune Responses Following Chemical Modification of Adenovirus
3.4.4. Summary of Chemical Modification Strategies
3.5. Summary of Detargeting Strategies
4. Final Concluding Remarks
Acknowledgments
References and Notes
- Crawford-Miksza, L.; Schnurr, D.P. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J. Virol. 1996, 70, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Seto, J.; Walsh, M.P.; Mahadevan, P.; Purkayastha, A.; Clark, J.M.; Tibbetts, C.; Seto, D. Genomic and bioinformatics analyses of HAdV-14p, reference strain of a re-emerging respiratory pathogen and analysis of B1/B2. Virus Res. 2009, 143, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.A.K.; Knipe, D.M.; Fields, B.N.; Howley, P.M.; Griffin, D.; Lamb, R. Adenoviridae: The Viruses and Their Replication. In Fields’ Virology, 5th ed.; Knipe, D.M., Ed.; Lippincott Williams and Wilkins Publishers: Philadelphia, USA, 2007; Volume II, pp. 2355–2395. [Google Scholar]
- Virus Taxonomy, VIIIth Report of the ICTV; Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U., Ball, L.A., Eds.; Elsevier/Academic Press Publishers: London, UK, 2005. [Google Scholar]
- Green, M.; Mackey, J.K.; Wold, W.S.; Rigden, P. Thirty-one human adenovirus serotypes (Ad1-Ad31) form five groups (A-E) based upon DNA genome homologies. Virology 1979, 93, 481–492. [Google Scholar] [CrossRef]
- Rosen, L. A hemagglutination-inhibition technique for typing adenoviruses. Am. J. Hyg. 1960, 71, 120–128. [Google Scholar] [PubMed]
- Wadell, G. Molecular epidemiology of human adenoviruses. Curr. Top. Microbiol. Immunol. 1984, 110, 191–220. [Google Scholar] [PubMed]
- Wadell, G.; Hammarskjold, M.L.; Winberg, G.; Varsanyi, T.M.; Sundell, G. Genetic variability of adenoviruses. Ann. N. Y. Acad. Sci. 1980, 354, 16–42. [Google Scholar] [CrossRef]
- Walsh, M.P.; Chintakuntlawar, A.; Robinson, C.M.; Madisch, I.; Harrach, B.; Hudson, N.R.; Schnurr, D.; Heim, A.; Chodosh, J.; Seto, D.; Jones, M.S. Evidence of molecular evolution driven by recombination events influencing tropism in a novel human adenovirus that causes epidemic keratoconjunctivitis. PLoS One 2009, 4, e5635. [Google Scholar] [CrossRef]
- Kaneko, H.; Suzutani, T.; Aoki, K.; Kitaichi, N.; Ishida, S.; Ishiko, H.; Ohashi, T.; Okamoto, S.; Nakagawa, H.; Hinokuma, R.; Asato, Y.; Oniki, S.; Hashimoto, T.; Iida, T.; Ohno, S. Epidemiological and virological features of epidemic keratoconjunctivitis due to new human adenovirus type 54 in Japan. Br. J. Ophthalmol. 2010. [Google Scholar] [CrossRef]
- Walsh, M.P.; Seto, J.; Jones, M.S.; Chodosh, J.; Xu, W.; Seto, D. Computational analysis identifies human adenovirus type 55 as a re-emergent acute respiratory disease pathogen. J. Clin. Microbiol. 2010, 48, 991–993. [Google Scholar] [CrossRef]
- Christensen, J.B.; Byrd, S.A.; Walker, A.K.; Strahler, J.R.; Andrews, P.C.; Imperiale, M.J. Presence of the adenovirus IVa2 protein at a single vertex of the mature virion. J. Virol. 2008, 82, 9086–9093. [Google Scholar] [CrossRef]
- Russell, W.C. Adenoviruses: update on structure and function. J. Gen. Virol. 2009, 90, 1–20. [Google Scholar] [CrossRef]
- Hasson, T.B.; Ornelles, D.A.; Shenk, T. Adenovirus L1 52- and 55-kilodalton proteins are present within assembling virions and colocalize with nuclear structures distinct from replication centers. J. Virol. 1992, 66, 6133–6142. [Google Scholar] [PubMed]
- Hasson, T.B.; Soloway, P.D.; Ornelles, D.A.; Doerfler, W.; Shenk, T. Adenovirus L1 52- and 55-kilodalton proteins are required for assembly of virions. J. Virol. 1989, 63, 3612–3621. [Google Scholar] [CrossRef] [PubMed]
- Silvestry, M.; Lindert, S.; Smith, J.G.; Maier, O.; Wiethoff, C.M.; Nemerow, G.R.; Stewart, P.L. Cryo-electron microscopy structure of adenovirus type 2 temperature-sensitive mutant 1 reveals insight into the cell entry defect. J. Virol. 2009, 83, 7375–7383. [Google Scholar] [CrossRef]
- Rekosh, D.M.; Russell, W.C.; Bellet, A.J.; Robinson, A.J. Identification of a protein linked to the ends of adenovirus DNA. Cell 1977, 11, 283–295. [Google Scholar] [CrossRef]
- Robinson, A.J.; Younghusband, H.B.; Bellett, A.J. A circula DNA-protein complex from adenoviruses. Virology 1973, 56, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Everitt, E.; Lutter, L.; Philipson, L. Structural proteins of adenoviruses. XII. Location and neighbor relationship among proteins of adenovirion type 2 as revealed by enzymatic iodination, immunoprecipitation and chemical cross-linking. Virology 1975, 67, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Everitt, E.; Sundquist, B.; Pettersson, U.; Philipson, L. Structural proteins of adenoviruses. X. Isolation and topography of low molecular weight antigens from the virion of adenovirus type 2. Virology 1973, 52, 130–147. [Google Scholar] [CrossRef]
- Chroboczek, J.; Ruigrok, R.W.; Cusack, S. Adenovirus fiber. Curr. Top. Microbiol. Immunol. 1995, 199 Pt 1, 163–200. [Google Scholar]
- Green, N.M.; Wrigley, N.G.; Russell, W.C.; Martin, S.R.; McLachlan, A.D. Evidence for a repeating cross-beta sheet structure in the adenovirus fibre. EMBO J. 1983, 2, 1357–1365. [Google Scholar] [CrossRef]
- van Raaij, M.J.; Mitraki, A.; Lavigne, G.; Cusack, S. A triple beta-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature 1999, 401, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Mautner, V.; Pereira, H.G. Crystallization of a second adenovirus protein (the fibre). Nature 1971, 230, 456–457. [Google Scholar] [CrossRef] [PubMed]
- van Oostrum, J.; Burnett, R.M. Molecular composition of the adenovirus type 2 virion. J. Virol. 1985, 56, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Bergelson, J.M.; Cunningham, J.A.; Droguett, G.; Kurt-Jones, E.A.; Krithivas, A.; Hong, J.S.; Horwitz, M.S.; Crowell, R.L.; Finberg, R.W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 1997, 275, 1320–1323. [Google Scholar] [CrossRef]
- Bergelson, J.M.; Krithivas, A.; Celi, L.; Droguett, G.; Horwitz, M.S.; Wickham, T.; Crowell, R.L.; Finberg, R.W. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J. Virol. 1998, 72, 415–419. [Google Scholar]
- Kirby, I.; Davison, E.; Beavil, A.J.; Soh, C.P.; Wickham, T.J.; Roelvink, P.W.; Kovesdi, I.; Sutton, B.J.; Santis, G. Identification of contact residues and definition of the CAR-binding site of adenovirus type 5 fiber protein. J. Virol. 2000, 74, 2804–2813. [Google Scholar] [CrossRef]
- Roelvink, P.W.; Lizonova, A.; Lee, J.G.; Li, Y.; Bergelson, J.M.; Finberg, R.W.; Brough, D.E.; Kovesdi, I.; Wickham, T.J. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 1998, 72, 7909–7915. [Google Scholar] [CrossRef]
- Roelvink, P.W.; Mi Lee, G.; Einfeld, D.A.; Kovesdi, I.; Wickham, T.J. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science 1999, 286, 1568–1571. [Google Scholar] [CrossRef]
- Santis, G.; Legrand, V.; Hong, S.S.; Davison, E.; Kirby, I.; Imler, J.L.; Finberg, R.W.; Bergelson, J.M.; Mehtali, M.; Boulanger, P. Molecular determinants of adenovirus serotype 5 fibre binding to its cellular receptor CAR. J. Gen. Virol. 1999, 80 Pt 6, 1519–1527. [Google Scholar] [CrossRef]
- Tomko, R.P.; Xu, R.; Philipson, L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. U. S. A. 1997, 94, 3352–3356. [Google Scholar] [CrossRef]
- Wickham, T.J.; Mathias, P.; Cheresh, D.A.; Nemerow, G.R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 1993, 73, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Stupack, D.; Klemke, R.; Cheresh, D.A.; Nemerow, G.R. Adenovirus endocytosis via alpha(v) integrins requires phosphoinositide-3-OH kinase. J. Virol. 1998, 72, 2055–2061. [Google Scholar] [CrossRef]
- Li, E.; Stupack, D.; Bokoch, G.M.; Nemerow, G.R. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J. Virol. 1998, 72, 8806–8812. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.R.; Fan, F. Adenovirus infection induces microglial activation: involvement of mitogen-activated protein kinase pathways. Brain Res. 2002, 948, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Tibbles, L.A.; Spurrell, J.C.; Bowen, G.P.; Liu, Q.; Lam, M.; Zaiss, A.K.; Robbins, S.M.; Hollenberg, M.D.; Wickham, T.J.; Muruve, D.A. Activation of p38 and ERK signaling during adenovirus vector cell entry lead to expression of the C-X-C chemokine IP-10. J. Virol. 2002, 76, 1559–1568. [Google Scholar] [CrossRef]
- Suomalainen, M.; Nakano, M.Y.; Keller, S.; Boucke, K.; Stidwill, R.P.; Greber, U.F. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J. Cell Biol. 1999, 144, 657–672. [Google Scholar] [CrossRef]
- Farmer, C.; Morton, P.E.; Snippe, M.; Santis, G.; Parsons, M. Coxsackie adenovirus receptor (CAR) regulates integrin function through activation of p44/42 MAPK. Exp. Cell Res. 2009, 315, 2637–2647. [Google Scholar] [CrossRef]
- Meier, O.; Boucke, K.; Hammer, S.V.; Keller, S.; Stidwill, R.P.; Hemmi, S.; Greber, U.F. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 2002, 158, 1119–1131. [Google Scholar] [CrossRef]
- Wang, K.; Huang, S.; Kapoor-Munshi, A.; Nemerow, G. Adenovirus internalization and infection require dynamin. J. Virol. 1998, 72, 3455–3458. [Google Scholar] [CrossRef]
- Greber, U.F.; Willetts, M.; Webster, P.; Helenius, A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell 1993, 75, 477–486. [Google Scholar] [CrossRef]
- Dales, S.; Chardonnet, Y. Early events in the interaction of adenoviruses with HeLa cells. IV. Association with microtubules and the nuclear pore complex during vectorial movement of the inoculum. Virology 1973, 56, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Wiethoff, C.M.; Wodrich, H.; Gerace, L.; Nemerow, G.R. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J. Virol. 2005, 79, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, S.A.; Pfister, K.K.; Crystal, R.G.; Leopold, P.L. Cytoplasmic dynein mediates adenovirus binding to microtubules. J. Virol. 2004, 78, 10122–10132. [Google Scholar] [CrossRef] [PubMed]
- Leopold, P.L.; Kreitzer, G.; Miyazawa, N.; Rempel, S.; Pfister, K.K.; Rodriguez-Boulan, E.; Crystal, R.G. Dynein- and microtubule-mediated translocation of adenovirus serotype 5 occurs after endosomal lysis. Hum. Gene Ther. 2000, 11, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Greber, U.F.; Suomalainen, M.; Stidwill, R.P.; Boucke, K.; Ebersold, M.W.; Helenius, A. The role of the nuclear pore complex in adenovirus DNA entry. EMBO J. 1997, 16, 5998–6007. [Google Scholar] [CrossRef]
- Trotman, L.C.; Mosberger, N.; Fornerod, M.; Stidwill, R.P.; Greber, U.F. Import of adenovirus DNA involves the nuclear pore complex receptor CAN/Nup214 and histone H1. Nat. Cell Biol. 2001, 3, 1092–1100. [Google Scholar] [CrossRef]
- Dechecchi, M.C.; Melotti, P.; Bonizzato, A.; Santacatterina, M.; Chilosi, M.; Cabrini, G. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J. Virol. 2001, 75, 8772–8780. [Google Scholar] [CrossRef]
- Dechecchi, M.C.; Tamanini, A.; Bonizzato, A.; Cabrini, G. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology 2000, 268, 382–390. [Google Scholar] [CrossRef]
- Chu, Y.; Heistad, D.; Cybulsky, M.I.; Davidson, B.L. Vascular cell adhesion molecule-1 augments adenovirus-mediated gene transfer. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 238–242. [Google Scholar] [CrossRef]
- Hong, S.S.; Karayan, L.; Tournier, J.; Curiel, D.T.; Boulanger, P.A. Adenovirus type 5 fiber knob binds to MHC class I alpha2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 1997, 16, 2294–2306. [Google Scholar] [CrossRef]
- Davison, E.; Kirby, I.; Elliott, T.; Santis, G. The human HLA-A*0201 allele, expressed in hamster cells, is not a high-affinity receptor for adenovirus type 5 fiber. J. Virol. 1999, 73, 4513–4517. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Stockwin, L.; Matzow, T.; Blair Zajdel, M.E.; Blair, G.E. Coxsackie and adenovirus receptor (CAR)-dependent and major histocompatibility complex (MHC) class I-independent uptake of recombinant adenoviruses into human tumour cells. Gene Ther. 1999, 6, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Kamata, T.; Takada, Y.; Ruggeri, Z.M.; Nemerow, G.R. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J. Virol. 1996, 70, 4502–4508. [Google Scholar] [CrossRef]
- Davison, E.; Kirby, I.; Whitehouse, J.; Hart, I.; Marshall, J.F.; Santis, G. Adenovirus type 5 uptake by lung adenocarcinoma cells in culture correlates with Ad5 fibre binding is mediated by alpha(v)beta1 integrin and can be modulated by changes in beta1 integrin function. J. Gene. Med. 2001, 3, 550–559. [Google Scholar] [CrossRef]
- Davison, E.; Diaz, R.M.; Hart, I.R.; Santis, G.; Marshall, J.F. Integrin alpha5beta1-mediated adenovirus infection is enhanced by the integrin-activating antibody TS2/16. J. Virol. 1997, 71, 6204–6207. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Brown, S.L.; Stupack, D.G.; Puente, X.S.; Cheresh, D.A.; Nemerow, G.R. Integrin alpha(v)beta1 is an adenovirus coreceptor. J. Virol. 2001, 75, 5405–5409. [Google Scholar] [CrossRef] [PubMed]
- Salone, B.; Martina, Y.; Piersanti, S.; Cundari, E.; Cherubini, G.; Franqueville, L.; Failla, C.M.; Boulanger, P.; Saggio, I. Integrin alpha3beta1 is an alternative cellular receptor for adenovirus serotype 5. J. Virol. 2003, 77, 13448–13454. [Google Scholar] [CrossRef]
- Alba, R.; Bradshaw, A.C.; Parker, A.L.; Bhella, D.; Waddington, S.N.; Nicklin, S.A.; van Rooijen, N.; Custers, J.; Goudsmit, J.; Barouch, D.H.; McVey, J.H.; Baker, A.H. Identification of coagulation factor (F)X binding sites on the adenovirus serotype 5 hexon: effect of mutagenesis on FX interactions and gene transfer. Blood 2009, 114, 965–971. [Google Scholar] [CrossRef]
- Kalyuzhniy, O.; Di Paolo, N.C.; Silvestry, M.; Hofherr, S.E.; Barry, M.A.; Stewart, P.L.; Shayakhmetov, D.M. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 5483–5488. [Google Scholar] [CrossRef]
- Parker, A.L.; McVey, J.H.; Doctor, J.H.; Lopez-Franco, O.; Waddington, S.N.; Havenga, M.J.; Nicklin, S.A.; Baker, A.H. Influence of coagulation factor zymogens on the infectivity of adenoviruses pseudotyped with fibers from subgroup D. J. Virol. 2007, 81, 3627–3631. [Google Scholar] [CrossRef]
- Parker, A.L.; Waddington, S.N.; Nicol, C.G.; Shayakhmetov, D.M.; Buckley, S.M.; Denby, L.; Kemball-Cook, G.; Ni, S.; Lieber, A.; McVey, J.H.; Nicklin, S.A.; Baker, A.H. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 2006, 108, 2554–2561. [Google Scholar] [CrossRef]
- Vigant, F.; Descamps, D.; Jullienne, B.; Esselin, S.; Connault, E.; Opolon, P.; Tordjmann, T.; Vigne, E.; Perricaudet, M.; Benihoud, K. Substitution of hexon hypervariable region 5 of adenovirus serotype 5 abrogates blood factor binding and limits gene transfer to liver. Mol. Ther. 2008, 16, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Shayakhmetov, D.M.; Gaggar, A.; Ni, S.; Li, Z.Y.; Lieber, A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 2005, 79, 7478–7491. [Google Scholar] [CrossRef] [PubMed]
- Waddington, S.; McVey, J.; Bhella, D.; Parker, A.; Barker, K.; Atoda, H.; Pink, R.; Buckley, S.; Greig, J.; Denby, L.; Custers, J.; Morita, T.; Francischetti, I.; Monteiro, R.; Barouch, D.; Van Rooijen, N.; Napoli, C.; Havenga, M.; Nicklin, S.; Baker, A. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 2008, 132, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, A.C.; Parker, A.L.; Duffy, M.R.; Coughlan, L.; Van Rooijen, N.; Kähäri, V.M.; Nicklin, S.A.; Baker, A.H. Requirements for Receptor Engagement during Infection by Adenovirus Complexed with Blood Coagulation Factor X. PLoS Pathog. 2010. [Google Scholar] [CrossRef]
- Jonsson, M.I.; Lenman, A.E.; Frangsmyr, L.; Nyberg, C.; Abdullahi, M.; Arnberg, N. Coagulation factors IX and X enhance binding and infection of adenovirus types 5 and 31 in human epithelial cells. J. Virol. 2009, 83, 3816–3825. [Google Scholar] [CrossRef]
- Johansson, C.; Jonsson, M.; Marttila, M.; Persson, D.; Fan, X.L.; Skog, J.; Frangsmyr, L.; Wadell, G.; Arnberg, N. Adenoviruses use lactoferrin as a bridge for CAR-independent binding to and infection of epithelial cells. J. Virol. 2007, 81, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.C.; Bond, E.; Havenga, M.J.; Holterman, L.; Goudsmit, J.; Karlsson Hedestam, G.B.; Koup, R.A.; Lore, K. Adenovirus serotype 5 infects human dendritic cells via a coxsackievirus-adenovirus receptor-independent receptor pathway mediated by lactoferrin and DC-SIGN. J. Gen. Virol. 2009, 90, 1600–1610. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Muruve, D.A. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003, 10, 935–940. [Google Scholar] [CrossRef]
- Liu, Q.; Zaiss, A.K.; Colarusso, P.; Patel, K.; Haljan, G.; Wickham, T.J.; Muruve, D.A. The role of capsid-endothelial interactions in the innate immune response to adenovirus vectors. Hum. Gene Ther. 2003, 14, 627–643. [Google Scholar] [CrossRef]
- Lieber, A.; He, C.Y.; Meuse, L.; Schowalter, D.; Kirillova, I.; Winther, B.; Kay, M.A. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 1997, 71, 8798–8807. [Google Scholar] [CrossRef]
- Alemany, R.; Suzuki, K.; Curiel, D.T. Blood clearance rates of adenovirus type 5 in mice. J. Gen. Virol. 2000, 81, 2605–2609. [Google Scholar] [CrossRef]
- Nicol, C.G.; Graham, D.; Miller, W.H.; White, S.J.; Smith, T.A.; Nicklin, S.A.; Stevenson, S.C.; Baker, A.H. Effect of adenovirus serotype 5 fiber and penton modifications on in vivo tropism in rats. Mol. Ther. 2004, 10, 344–354. [Google Scholar] [CrossRef]
- Worgall, S.; Wolff, G.; Falck-Pedersen, E.; Crystal, R.G. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum. Gene Ther. 1997, 8, 37–44. [Google Scholar] [CrossRef]
- Di Paolo, N.C.; van Rooijen, N.; Shayakhmetov, D.M. Redundant and synergistic mechanisms control the sequestration of blood-born adenovirus in the liver. Mol. Ther. 2009, 17, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Haisma, H.J.; Boesjes, M.; Beerens, A.M.; van der Strate, B.W.; Curiel, D.T.; Pluddemann, A.; Gordon, S.; Bellu, A.R. Scavenger Receptor A: A New Route for Adenovirus 5. Mol. Pharmacol. 2009, 6, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.; Liu, Y.; Shayakhmetov, D.; Li, Z.Y.; Ni, S.; Lieber, A. Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J. Virol. 2007, 81, 4866–4871. [Google Scholar] [CrossRef] [PubMed]
- Shayakhmetov, D.M.; Li, Z.Y.; Ni, S.; Lieber, A. Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J. Virol. 2004, 78, 5368–5381. [Google Scholar] [CrossRef]
- Carlisle, R.C.; Di, Y.; Cerny, A.M.; Sonnen, A.F.; Sim, R.B.; Green, N.K.; Subr, V.; Ulbrich, K.; Gilbert, R.J.; Fisher, K.D.; Finberg, R.W.; Seymour, L.W. Human erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1. Blood 2009, 113, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Seiradake, E.; Henaff, D.; Wodrich, H.; Billet, O.; Perreau, M.; Hippert, C.; Mennechet, F.; Schoehn, G.; Lortat-Jacob, H.; Dreja, H.; Ibanes, S.; Kalatzis, V.; Wang, J.P.; Finberg, R.W.; Cusack, S.; Kremer, E.J. The cell adhesion molecule "CAR" and sialic acid on human erythrocytes influence adenovirus in vivo biodistribution. PLoS Pathog. 2009, 5, e1000277. [Google Scholar] [CrossRef]
- Xu, Z.; Tian, J.; Smith, J.S.; Byrnes, A.P. Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J. Virol. 2008, 82, 11705–11713. [Google Scholar] [CrossRef] [PubMed]
- Onion, D.; Crompton, L.J.; Milligan, D.W.; Moss, P.A.; Lee, S.P.; Mautner, V. The CD4+ T-cell response to adenovirus is focused against conserved residues within the hexon protein. J. Gen. Virol. 2007, 88, 2417–2425. [Google Scholar] [CrossRef]
- Othman, M.; Labelle, A.; Mazzetti, I.; Elbatarny, H.S.; Lillicrap, D. Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood 2007, 109, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Wolff, G.; Worgall, S.; van Rooijen, N.; Song, W.R.; Harvey, B.G.; Crystal, R.G. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J. Virol. 1997, 71, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, A.I.; Finegold, M.J.; Eisensmith, R.C. Macrophage depletion increases the safety, efficacy and persistence of adenovirus-mediated gene transfer in vivo. Gene Ther. 1997, 4, 309–316. [Google Scholar] [CrossRef]
- Schiedner, G.; Hertel, S.; Johnston, M.; Dries, V.; van Rooijen, N.; Kochanek, S. Selective depletion or blockade of Kupffer cells leads to enhanced and prolonged hepatic transgene expression using high-capacity adenoviral vectors. Mol. Ther. 2003, 7, 35–43. [Google Scholar] [CrossRef]
- Kiang, A.; Hartman, Z.C.; Everett, R.S.; Serra, D.; Jiang, H.; Frank, M.M.; Amalfitano, A. Multiple innate inflammatory responses induced after systemic adenovirus vector delivery depend on a functional complement system. Mol. Ther. 2006, 14, 588–598. [Google Scholar] [CrossRef]
- Cichon, G.; Schmidt, H.H.; Benhidjeb, T.; Loser, P.; Ziemer, S.; Haas, R.; Grewe, N.; Schnieders, F.; Heeren, J.; Manns, M.P.; Schlag, P.M.; Strauss, M. Intravenous administration of recombinant adenoviruses causes thrombocytopenia, anemia and erythroblastosis in rabbits. J. Gene Med. 1999, 1, 360–371. [Google Scholar] [CrossRef]
- Wolins, N.; Lozier, J.; Eggerman, T.L.; Jones, E.; Aguilar-Cordova, E.; Vostal, J.G. Intravenous administration of replication-incompetent adenovirus to rhesus monkeys induces thrombocytopenia by increasing in vivo platelet clearance. Br. J. Haematol. 2003, 123, 903–905. [Google Scholar] [CrossRef]
- Cotter, M. J.; Zaiss, A. K.; Muruve, D. A. Neutrophils interact with adenovirus vectors via Fc receptors and complement receptor 1. J. Virol. 2005, 79, 14622–14631. [Google Scholar] [CrossRef]
- Lyons, M.; Onion, D.; Green, N.K.; Aslan, K.; Rajaratnam, R.; Bazan-Peregrino, M.; Phipps, S.; Hale, S.; Mautner, V.; Seymour, L.W.; Fisher, K.D. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol. Ther. 2006, 14, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Schiedner, G.; Bloch, W.; Hertel, S.; Johnston, M.; Molojavyi, A.; Dries, V.; Varga, G.; Van Rooijen, N.; Kochanek, S. A hemodynamic response to intravenous adenovirus vector particles is caused by systemic Kupffer cell-mediated activation of endothelial cells. Hum. Gene Ther. 2003, 14, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, C.C.; Johnson, L.; Bagus, B.; Choi, S.; Nicholas, C.; Shen, A.; Boyle, L.; Pandey, K.; Soria, C.; Kunich, J.; Shen, Y.; Habets, G.; Ginzinger, D.; McCormick, F. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell 2004, 6, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Fueyo, J.; Gomez-Manzano, C.; Alemany, R.; Lee, P.S.; McDonnell, T.J.; Mitlianga, P.; Shi, Y.X.; Levin, V.A.; Yung, W.K.; Kyritsis, A.P. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 2000, 19, 2–12. [Google Scholar] [CrossRef]
- Bauerschmitz, G.J.; Guse, K.; Kanerva, A.; Menzel, A.; Herrmann, I.; Desmond, R.A.; Yamamoto, M.; Nettelbeck, D.M.; Hakkarainen, T.; Dall, P.; Curiel, D.T.; Hemminki, A. Triple-targeted oncolytic adenoviruses featuring the cox2 promoter, E1A transcomplementation, and serotype chimerism for enhanced selectivity for ovarian cancer cells. Mol. Ther. 2006, 14, 164–174. [Google Scholar] [CrossRef]
- Hernandez-Alcoceba, R.; Pihalja, M.; Wicha, M.S.; Clarke, M.F. A novel, conditionally replicative adenovirus for the treatment of breast cancer that allows controlled replication of E1a-deleted adenoviral vectors. Hum. Gene Ther. 2000, 11, 2009–2024. [Google Scholar] [CrossRef]
- Tsukuda, K.; Wiewrodt, R.; Molnar-Kimber, K.; Jovanovic, V.P.; Amin, K.M. An E2F-responsive replication-selective adenovirus targeted to the defective cell cycle in cancer cells: potent antitumoral efficacy but no toxicity to normal cell. Cancer Res. 2002, 62, 3438–3447. [Google Scholar]
- Lockley, M.; Fernandez, M.; Wang, Y.; Li, N.F.; Conroy, S.; Lemoine, N.; McNeish, I. Activity of the adenoviral E1A deletion mutant dl922-947 in ovarian cancer: comparison with E1A wild-type viruses, bioluminescence monitoring, and intraperitoneal delivery in icodextrin. Cancer Res. 2006, 66, 989–998. [Google Scholar] [CrossRef]
- Sherr, C.J.; McCormick, F. The RB and p53 pathways in cancer. Cancer Cell 2002, 2, 103–112. [Google Scholar] [CrossRef]
- Morelli, A.E.; Larregina, A.T.; Smith-Arica, J.; Dewey, R.A.; Southgate, T.D.; Ambar, B.; Fontana, A.; Castro, M.G.; Lowenstein, P.R. Neuronal and glial cell type-specific promoters within adenovirus recombinants restrict the expression of the apoptosis-inducing molecule Fas ligand to predetermined brain cell types, and abolish peripheral liver toxicity. J. Gen. Virol. 1999, 80 Pt 3, 571–583. [Google Scholar] [CrossRef]
- Gou, D.; Narasaraju, T.; Chintagari, N.R.; Jin, N.; Wang, P.; Liu, L. Gene silencing in alveolar type II cells using cell-specific promoter in vitro and in vivo. Nucleic Acids Res. 2004, 32, e134. [Google Scholar] [CrossRef] [PubMed]
- Nicklin, S.A.; Reynolds, P.N.; Brosnan, M.J.; White, S.J.; Curiel, D.T.; Dominiczak, A.F.; Baker, A.H. Analysis of cell-specific promoters for viral gene therapy targeted at the vascular endothelium. Hypertension 2001, 38, 65–70. [Google Scholar] [CrossRef]
- Work, L.M.; Ritchie, N.; Nicklin, S.A.; Reynolds, P.N.; Baker, A.H. Dual targeting of gene delivery by genetic modification of adenovirus serotype 5 fibers and cell-selective transcriptional control. Gene Ther. 2004, 11, 1296–1300. [Google Scholar] [CrossRef]
- Anders, M.; Vieth, M.; Rocken, C.; Ebert, M.; Pross, M.; Gretschel, S.; Schlag, P.M.; Wiedenmann, B.; Kemmner, W.; Hocker, M. Loss of the coxsackie and adenovirus receptor contributes to gastric cancer progression. Br. J. Cancer. 2009, 100, 352–359. [Google Scholar] [CrossRef]
- Jee, Y.S.; Lee, S.G.; Lee, J.C.; Kim, M.J.; Lee, J.J.; Kim, D.Y.; Park, S.W.; Sung, M.W.; Heo, D.S. Reduced expression of coxsackievirus and adenovirus receptor (CAR) in tumor tissue compared to normal epithelium in head and neck squamous cell carcinoma patients. Anti-Cancer Res. 2002, 22, 2629–2634. [Google Scholar]
- Matsumoto, K.; Shariat, S.F.; Ayala, G.E.; Rauen, K.A.; Lerner, S.P. Loss of coxsackie and adenovirus receptor expression is associated with features of aggressive bladder cancer. Urology 2005, 66, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Ookawa, K.; Shimoyama, T.; Fukuda, S.; Saito, H.; Munakata, A. KAI1, CAR, and Smad4 expression in the progression of colorectal tumor. J. Gastroenterol. 2001, 36, 465–469. [Google Scholar] [CrossRef]
- Rauen, K.A.; Sudilovsky, D.; Le, J.L.; Chew, K.L.; Hann, B.; Weinberg, V.; Schmitt, L.D.; McCormick, F. Expression of the coxsackie adenovirus receptor in normal prostate and in primary and metastatic prostate carcinoma: potential relevance to gene therapy. Cancer Res. 2002, 62, 3812–3818. [Google Scholar]
- Sachs, M.D.; Rauen, K.A.; Ramamurthy, M.; Dodson, J.L.; De Marzo, A.M.; Putzi, M.J.; Schoenberg, M.P.; Rodriguez, R. Integrin alpha(v) and coxsackie adenovirus receptor expression in clinical bladder cancer. Urology 2002, 60, 531–536. [Google Scholar] [CrossRef]
- Vincent, T.; Neve, E.P.; Johnson, J.R.; Kukalev, A.; Rojo, F.; Albanell, J.; Pietras, K.; Virtanen, I.; Philipson, L.; Leopold, P.L.; Crystal, R.G.; de Herreros, A.G.; Moustakas, A.; Pettersson, R.F.; Fuxe, J. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat. Cell Biol. 2009, 11, 943–950. [Google Scholar] [CrossRef]
- Douglas, J.T.; Kim, M.; Sumerel, L.A.; Carey, D.E.; Curiel, D.T. Efficient oncolysis by a replicating adenovirus (Ad) in vivo is critically dependent on tumor expression of primary Ad receptors. Cancer Res. 2001, 61, 813–817. [Google Scholar] [PubMed]
- Li, D.; Duan, L.; Freimuth, P.; O’Malley, B.W., Jr. Variability of adenovirus receptor density influences gene transfer efficiency and therapeutic response in head and neck cancer. Clin. Cancer Res. 1999, 5, 4175–4181. [Google Scholar] [PubMed]
- Li, Y.; Pong, R.C.; Bergelson, J.M.; Hall, M.C.; Sagalowsky, A.I.; Tseng, C.P.; Wang, Z.; Hsieh, J.T. Loss of adenoviral receptor expression in human bladder cancer cells: a potential impact on the efficacy of gene therapy. Cancer Res. 1999, 59, 325–330. [Google Scholar] [PubMed]
- Abbink, P.; Lemckert, A.A.; Ewald, B.A.; Lynch, D.M.; Denholtz, M.; Smits, S.; Holterman, L.; Damen, I.; Vogels, R.; Thorner, A.R.; O’Brien, K.L.; Carville, A.; Mansfield, K.G.; Goudsmit, J.; Havenga, M.J.; Barouch, D.H. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 2007, 81, 4654–4663. [Google Scholar] [CrossRef]
- Gaggar, A.; Shayakhmetov, D.M.; Lieber, A. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 2003, 9, 1408–1412. [Google Scholar] [CrossRef]
- Short, J.J.; Vasu, C.; Holterman, M.J.; Curiel, D.T.; Pereboev, A. Members of adenovirus species B utilize CD80 and CD86 as cellular attachment receptors. Virus Res. 2006, 122, 144–153. [Google Scholar] [CrossRef]
- Tuve, S.; Wang, H.; Ware, C.; Liu, Y.; Gaggar, A.; Bernt, K.; Shayakhmetov, D.; Li, Z.; Strauss, R.; Stone, D.; Lieber, A. A new group B adenovirus receptor is expressed at high levels on human stem and tumor cells. J. Virol. 2006, 80, 12109–12120. [Google Scholar] [CrossRef]
- Arnberg, N.; Edlund, K.; Kidd, A.H.; Wadell, G. Adenovirus type 37 uses sialic acid as a cellular receptor. J. Virol. 2000, 74, 42–48. [Google Scholar] [CrossRef]
- Burmeister, W.P.; Guilligay, D.; Cusack, S.; Wadell, G.; Arnberg, N. Crystal structure of species D adenovirus fiber knobs and their sialic acid binding sites. J. Virol. 2004, 78, 7727–7736. [Google Scholar] [CrossRef]
- Arnberg, N.; Kidd, A.H.; Edlund, K.; Olfat, F.; Wadell, G. Initial interactions of subgenus D adenoviruses with A549 cellular receptors: sialic acid versus alpha(v) integrins. J. Virol. 2000, 74, 7691–7693. [Google Scholar] [CrossRef]
- Mathis, J.M.; Stoff-Khalili, M.A.; Curiel, D.T. Oncolytic adenoviruses - selective retargeting to tumor cells. Oncogene 2005, 24, 7775–7791. [Google Scholar] [CrossRef] [PubMed]
- Kurachi, S.; Tashiro, K.; Sakurai, F.; Sakurai, H.; Kawabata, K.; Yayama, K.; Okamoto, H.; Nakagawa, S.; Mizuguchi, H. Fiber-modified adenovirus vectors containing the TAT peptide derived from HIV-1 in the fiber knob have efficient gene transfer activity. Gene Ther. 2007, 14, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Kurachi, S.; Koizumi, N.; Sakurai, F.; Kawabata, K.; Sakurai, H.; Nakagawa, S.; Hayakawa, T.; Mizuguchi, H. Characterization of capsid-modified adenovirus vectors containing heterologous peptides in the fiber knob, protein IX, or hexon. Gene Ther. 2007, 14, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Belousova, N.; Krendelchtchikova, V.; Curiel, D.T.; Krasnykh, V. Modulation of adenovirus vector tropism via incorporation of polypeptide ligands into the fiber protein. J. Virol. 2002, 76, 8621–8631. [Google Scholar] [CrossRef] [PubMed]
- Krasnykh, V.; Dmitriev, I.; Mikheeva, G.; Miller, C.R.; Belousova, N.; Curiel, D.T. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J. Virol. 1998, 72, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, I.; Krasnykh, V.; Miller, C.R.; Wang, M.; Kashentseva, E.; Mikheeva, G.; Belousova, N.; Curiel, D.T. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J. Virol. 1998, 72, 9706–9713. [Google Scholar] [CrossRef]
- Wickham, T.J.; Segal, D.M.; Roelvink, P.W.; Carrion, M.E.; Lizonova, A.; Lee, G.M.; Kovesdi, I. Targeted adenovirus gene transfer to endothelial and smooth muscle cells by using bispecific antibodies. J. Virol. 1996, 70, 6831–6838. [Google Scholar] [CrossRef]
- Einfeld, D.A.; Brough, D.E.; Roelvink, P.W.; Kovesdi, I.; Wickham, T.J. Construction of a pseudoreceptor that mediates transduction by adenoviruses expressing a ligand in fiber or penton base. J. Virol. 1999, 73, 9130–9136. [Google Scholar] [CrossRef]
- Wu, H.; Han, T.; Belousova, N.; Krasnykh, V.; Kashentseva, E.; Dmitriev, I.; Kataram, M.; Mahasreshti, P.J.; Curiel, D.T. Identification of sites in adenovirus hexon for foreign peptide incorporation. J. Virol. 2005, 79, 3382–3390. [Google Scholar] [CrossRef]
- Campos, S.K.; Parrott, M.B.; Barry, M.A. Avidin-based targeting and purification of a protein IX-modified, metabolically biotinylated adenoviral vector. Mol. Ther. 2004, 9, 942–954. [Google Scholar] [CrossRef]
- Le, L.P.; Everts, M.; Dmitriev, I.P.; Davydova, J.G.; Yamamoto, M.; Curiel, D.T. Fluorescently labeled adenovirus with pIX-EGFP for vector detection. Mol. Imaging. 2004, 3, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Le, L.P.; Li, J.; Ternovoi, V.V.; Siegal, G.P.; Curiel, D.T. Fluorescently tagged canine adenovirus via modification with protein IX-enhanced green fluorescent protein. J. Gen. Virol. 2005, 86, 3201–3208. [Google Scholar] [CrossRef] [PubMed]
- Meulenbroek, R.A.; Sargent, K.L.; Lunde, J.; Jasmin, B.J.; Parks, R.J. Use of adenovirus protein IX (pIX) to display large polypeptides on the virion-generation of fluorescent virus through the incorporation of pIX-GFP. Mol. Ther. 2004, 9, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Krasnykh, V.; Belousova, N.; Korokhov, N.; Mikheeva, G.; Curiel, D.T. Genetic targeting of an adenovirus vector via replacement of the fiber protein with the phage T4 fibritin. J. Virol. 2001, 75, 4176–4183. [Google Scholar] [CrossRef] [PubMed]
- Belousova, N.; Mikheeva, G.; Gelovani, J.; Krasnykh, V. Modification of adenovirus capsid with a designed protein ligand yields a gene vector targeted to a major molecular marker of cancer. J. Virol. 2008, 82, 630–637. [Google Scholar] [PubMed]
- Magnusson, M.K.; Hong, S.S.; Henning, P.; Boulanger, P.; Lindholm, L. Genetic retargeting of adenovirus vectors: functionality of targeting ligands and their influence on virus viability. J. Gene Med. 2002, 4, 356–370. [Google Scholar] [CrossRef]
- Michael, S.I.; Hong, J.S.; Curiel, D.T.; Engler, J.A. Addition of a short peptide ligand to the adenovirus fiber protein. Gene Ther. 1995, 2, 660–668. [Google Scholar]
- Gaden, F.; Franqueville, L.; Hong, S.S.; Legrand, V.; Figarella, C.; Boulanger, P. Mechanism of restriction of normal and cystic fibrosis transmembrane conductance regulator-deficient human tracheal gland cells to adenovirus infection and ad-mediated gene transfer. Am. J. Respir. Cell Mol. Biol. 2002, 27, 628–640. [Google Scholar]
- Wickham, T.J.; Roelvink, P.W.; Brough, D.E.; Kovesdi, I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat. Biotechnol. 1996, 14, 1570–1573. [Google Scholar] [CrossRef]
- Wickham, T.J.; Tzeng, E.; Shears, L.L., II; Roelvink, P.W.; Li, Y.; Lee, G.M.; Brough, D.E.; Lizonova, A.; Kovesdi, I. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J. Virol. 1997, 71, 8221–8229. [Google Scholar] [CrossRef]
- Yoshida, Y.; Sadata, A.; Zhang, W.; Saito, K.; Shinoura, N.; Hamada, H. Generation of fiber-mutant recombinant adenoviruses for gene therapy of malignant glioma. Hum. Gene Ther. 1998, 9, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Tang, Y.; Ugai, H.; Perry, L.E.; Siegal, G.P.; Contreras, J.L.; Wu, H. Genetic incorporation of the protein transduction domain of Tat into Ad5 fiber enhances gene transfer efficacy. Virol. J. 2007, 4, 103. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.S.; Engler, J.A. Domains required for assembly of adenovirus type 2 fiber trimers. J. Virol. 1996, 70, 7071–7078. [Google Scholar] [CrossRef] [PubMed]
- Parrott, M.B.; Adams, K.E.; Mercier, G.T.; Mok, H.; Campos, S.K.; Barry, M.A. Metabolically biotinylated adenovirus for cell targeting, ligand screening, and vector purification. Mol. Ther. 2003, 8, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Henry, L.J.; Gerard, R.D.; Deisenhofer, J. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 A resolution. Structure 1994, 2, 1259–1270. [Google Scholar] [CrossRef]
- Einfeld, D.A.; Schroeder, R.; Roelvink, P.W.; Lizonova, A.; King, C.R.; Kovesdi, I.; Wickham, T. J. Reducing the native tropism of adenovirus vectors requires removal of both CAR and integrin interactions. J. Virol. 2001, 75, 11284–11291. [Google Scholar] [CrossRef]
- Coughlan, L.; Vallath, S.; Saha, A.; Flak, M.; McNeish, I.A.; Vassaux, G.; Marshall, J.F.; Hart, I.R.; Thomas, G.J. In vivo retargeting of adenovirus type 5 to alphavbeta6 integrin results in reduced hepatotoxicity and improved tumor uptake following systemic delivery. J. Virol. 2009, 83, 6416–6428. [Google Scholar] [CrossRef]
- van Geer, M.A.; Bakker, C.T.; Koizumi, N.; Mizuguchi, H.; Wesseling, J.G.; Oude Elferink, R.P.; Bosma, P.J. Ephrin A2 receptor targeting does not increase adenoviral pancreatic cancer transduction in vivo. World J. Gastroenterol. 2009, 15, 2754–2762. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 2010, 188, 759–768. [Google Scholar] [CrossRef]
- Majhen, D.; Gabrilovac, J.; Eloit, M.; Richardson, J.; Ambriovic-Ristov, A. Disulfide bond formation in NGR fiber-modified adenovirus is essential for retargeting to aminopeptidase N. Biochem. Biophys. Res. Commun. 2006, 348, 278–287. [Google Scholar] [CrossRef]
- Gaden, F.; Franqueville, L.; Magnusson, M.K.; Hong, S.S.; Merten, M.D.; Lindholm, L.; Boulanger, P. Gene transduction and cell entry pathway of fiber-modified adenovirus type 5 vectors carrying novel endocytic peptide ligands selected on human tracheal glandular cells. J. Virol. 2004, 78, 7227–7247. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Anderson, B.; Mao, Q.; Davidson, B.L. Recombinant human adenovirus: targeting to the human transferrin receptor improves gene transfer to brain microcapillary endothelium. J. Virol. 2000, 74, 11359–11366. [Google Scholar] [CrossRef] [PubMed]
- Joung, I.; Harber, G.; Gerecke, K.M.; Carroll, S.L.; Collawn, J.F.; Engler, J.A. Improved gene delivery into neuroglial cells using a fiber-modified adenovirus vector. Biochem. Biophys. Res. Commun. 2005, 328, 1182–1187. [Google Scholar] [CrossRef]
- Nicklin, S.A.; Von Seggern, D.J.; Work, L.M.; Pek, D.C.; Dominiczak, A.F.; Nemerow, G.R.; Baker, A.H. Ablating adenovirus type 5 fiber-CAR binding and HI loop insertion of the SIGYPLP peptide generate an endothelial cell-selective adenovirus. Mol. Ther. 2001, 4, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Work, L.M.; Nicklin, S.A.; Brain, N.J.; Dishart, K.L.; Von Seggern, D.J.; Hallek, M.; Buning, H.; Baker, A.H. Development of efficient viral vectors selective for vascular smooth muscle cells. Mol. Ther. 2004, 9, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Denby, L.; Work, L.M.; Seggern, D.J.; Wu, E.; McVey, J.H.; Nicklin, S.A.; Baker, A.H. Development of renal-targeted vectors through combined in vivo phage display and capsid engineering of adenoviral fibers from serotype 19p. Mol. Ther. 2007, 15, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Cripe, T.P.; Dunphy, E.J.; Holub, A.D.; Saini, A.; Vasi, N.H.; Mahller, Y.Y.; Collins, M.H.; Snyder, J.D.; Krasnykh, V.; Curiel, D.T.; Wickham, T.J.; DeGregori, J.; Bergelson, J.M.; Currier, M.A. Fiber knob modifications overcome low, heterogeneous expression of the coxsackievirus-adenovirus receptor that limits adenovirus gene transfer and oncolysis for human rhabdomyosarcoma cells. Cancer Res. 2001, 61, 2953–2960. [Google Scholar]
- Grill, J.; Van Beusechem, V.W.; Van Der Valk, P.; Dirven, C.M.; Leonhart, A.; Pherai, D.S.; Haisma, H.J.; Pinedo, H.M.; Curiel, D.T.; Gerritsen, W.R. Combined targeting of adenoviruses to integrins and epidermal growth factor receptors increases gene transfer into primary glioma cells and spheroids. Clin. Cancer Res. 2001, 7, 641–650. [Google Scholar]
- Nagel, H.; Maag, S.; Tassis, A.; Nestle, F.O.; Greber, U.F.; Hemmi, S. The alphavbeta5 integrin of hematopoietic and nonhematopoietic cells is a transduction receptor of RGD-4C fiber-modified adenoviruses. Gene Ther. 2003, 10, 1643–1653. [Google Scholar] [CrossRef]
- Wesseling, J.G.; Bosma, P.J.; Krasnykh, V.; Kashentseva, E.A.; Blackwell, J.L.; Reynolds, P.N.; Li, H.; Parameshwar, M.; Vickers, S.M.; Jaffee, E.M.; Huibregtse, K.; Curiel, D.T.; Dmitriev, I. Improved gene transfer efficiency to primary and established human pancreatic carcinoma target cells via epidermal growth factor receptor and integrin-targeted adenoviral vectors. Gene Ther. 2001, 8, 969–976. [Google Scholar] [CrossRef]
- Reynolds, P.; Dmitriev, I.; Curiel, D. Insertion of an RGD motif into the HI loop of adenovirus fiber protein alters the distribution of transgene expression of the systemically administered vector. Gene Ther. 1999, 6, 1336–1339. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Vereecque, R.; Wickham, T.J.; Facon, T.; Hetuin, D.; Kovesdi, I.; Bauters, F.; Fenaux, P.; Quesnel, B. Transduction of bone marrow cells by the AdZ.F(pK7) modified adenovirus demonstrates preferential gene transfer in myeloma cells. Hum. Gene Ther. 1999, 10, 2709–2717. [Google Scholar] [CrossRef] [PubMed]
- Ranki, T.; Kanerva, A.; Ristimaki, A.; Hakkarainen, T.; Sarkioja, M.; Kangasniemi, L.; Raki, M.; Laakkonen, P.; Goodison, S.; Hemminki, A. A heparan sulfate-targeted conditionally replicative adenovirus, Ad5.pk7-Delta24, for the treatment of advanced breast cancer. Gene Ther. 2007, 14, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.T.; Breidenbach, M.; Wu, H.; Han, T.; Haviv, Y.S.; Wang, M.; Kirby, T.O.; Kawakami, Y.; Dall, P.; Alvarez, R.D.; Curiel, D.T. Gene transfer to cervical cancer with fiber-modified adenoviruses. Int. J. Cancer 2004, 111, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Staba, M.J.; Wickham, T.J.; Kovesdi, I.; Hallahan, D.E. Modifications of the fiber in adenovirus vectors increase tropism for malignant glioma models. Cancer Gene Ther. 2000, 7, 13–19. [Google Scholar] [CrossRef]
- Wu, H.; Seki, T.; Dmitriev, I.; Uil, T.; Kashentseva, E.; Han, T.; Curiel, D.T. Double modification of adenovirus fiber with RGD and polylysine motifs improves coxsackievirus-adenovirus receptor-independent gene transfer efficiency. Hum. Gene Ther. 2002, 13, 1647–1653. [Google Scholar] [CrossRef]
- Alderson, M.R.; Armitage, R.J.; Tough, T.W.; Strockbine, L.; Fanslow, W.C.; Spriggs, M.K. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J. Exp. Med. 1993, 178, 669–674. [Google Scholar] [CrossRef]
- Galy, A.H.; Spits, H. CD40 is functionally expressed on human thymic epithelial cells. J. Immunol. 1992, 149, 775–782. [Google Scholar] [CrossRef]
- Hsu, Y.M.; Lucci, J.; Su, L.; Ehrenfels, B.; Garber, E.; Thomas, D. Heteromultimeric complexes of CD40 ligand are present on the cell surface of human T lymphocytes. J. Biol. Chem. 1997, 272, 911–915. [Google Scholar] [CrossRef]
- Sasisekharan, R.; Shriver, Z.; Venkataraman, G.; Narayanasami, U. Roles of heparan-sulphate glycosaminoglycans in cancer. Nat. Rev. Cancer 2002, 2, 521–528. [Google Scholar] [CrossRef]
- Ahmed, N.; Riley, C.; Rice, G.E.; Quinn, M.A.; Baker, M.S. Alpha(v)beta(6) integrin-A marker for the malignant potential of epithelial ovarian cancer. J. Histochem. Cytochem. 2002, 50, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Bates, R.C.; Bellovin, D.I.; Brown, C.; Maynard, E.; Wu, B.; Kawakatsu, H.; Sheppard, D.; Oettgen, P.; Mercurio, A.M. Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J. Clin. Invest. 2005, 115, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Elayadi, A.N.; Samli, K.N.; Prudkin, L.; Liu, Y.H.; Bian, A.; Xie, X.J.; Wistuba, II; Roth, J.A.; McGuire, M.J.; Brown, K.C. A peptide selected by biopanning identifies the integrin alphavbeta6 as a prognostic biomarker for nonsmall cell lung cancer. Cancer Res. 2007, 67, 5889–5895. [Google Scholar] [CrossRef] [PubMed]
- Hazelbag, S.; Kenter, G.G.; Gorter, A.; Dreef, E.J.; Koopman, L.A.; Violette, S.M.; Weinreb, P.H.; Fleuren, G.J. Overexpression of the alphavbeta6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J. Pathol. 2007, 212, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Mizuguchi, H.; Utoguchi, N.; Watanabe, Y.; Hayakawa, T. Generation of fiber-modified adenovirus vectors containing heterologous peptides in both the HI loop and C terminus of the fiber knob. J. Gene Med. 2003, 5, 267–276. [Google Scholar] [CrossRef]
- Mizuguchi, H.; Koizumi, N.; Hosono, T.; Utoguchi, N.; Watanabe, Y.; Kay, M.A.; Hayakawa, T. A simplified system for constructing recombinant adenoviral vectors containing heterologous peptides in the HI loop of their fiber knob. Gene Ther. 2001, 8, 730–735. [Google Scholar] [CrossRef]
- Magnusson, M.K.; Henning, P.; Myhre, S.; Wikman, M.; Uil, T.G.; Friedman, M.; Andersson, K. M.; Hong, S.S.; Hoeben, R.C.; Habib, N.A.; Stahl, S.; Boulanger, P.; Lindholm, L. Adenovirus 5 vector genetically re-targeted by an Affibody molecule with specificity for tumor antigen HER2/neu. Cancer Gene Ther. 2007, 14, 468–479. [Google Scholar] [CrossRef]
- Binz, H.K.; Amstutz, P.; Pluckthun, A. Engineering novel binding proteins from non-immunoglobulin domains. Nat. Biotechnol. 2005, 23, 1257–1268. [Google Scholar] [CrossRef]
- Henning, P.; Magnusson, M.K.; Gunneriusson, E.; Hong, S.S.; Boulanger, P.; Nygren, P.A.; Lindholm, L. Genetic modification of adenovirus 5 tropism by a novel class of ligands based on a three-helix bundle scaffold derived from staphylococcal protein A. Hum. Gene Ther. 2002, 13, 1427–1439. [Google Scholar] [CrossRef]
- Myhre, S.; Henning, P.; Friedman, M.; Stahl, S.; Lindholm, L.; Magnusson, M.K. Re-targeted adenovirus vectors with dual specificity; binding specificities conferred by two different Affibody molecules in the fiber. Gene Ther. 2009, 16, 252–261. [Google Scholar] [CrossRef]
- Nord, K.; Gunneriusson, E.; Ringdahl, J.; Stahl, S.; Uhlen, M.; Nygren, P.A. Binding proteins selected from combinatorial libraries of an alpha-helical bacterial receptor domain. Nat. Biotechnol. 1997, 15, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Menard, S.; Pupa, S.M.; Campiglio, M.; Tagliabue, E. Biologic and therapeutic role of HER2 in cancer. Oncogene 2003, 22, 6570–6578. [Google Scholar] [CrossRef] [PubMed]
- Legrand, V.; Spehner, D.; Schlesinger, Y.; Settelen, N.; Pavirani, A.; Mehtali, M. Fiberless recombinant adenoviruses: virus maturation and infectivity in the absence of fiber. J. Virol. 1999, 73, 907–919. [Google Scholar] [CrossRef]
- Miyazawa, N.; Leopold, P.L.; Hackett, N.R.; Ferris, B.; Worgall, S.; Falck-Pedersen, E.; Crystal, R.G. Fiber swap between adenovirus subgroups B and C alters intracellular trafficking of adenovirus gene transfer vectors. J. Virol. 1999, 73, 6056–6065. [Google Scholar] [CrossRef]
- Magnusson, M.K.; Hong, S.S.; Boulanger, P.; Lindholm, L. Genetic retargeting of adenovirus: novel strategy employing "deknobbing" of the fiber. J. Virol. 2001, 75, 7280–7289. [Google Scholar] [CrossRef] [PubMed]
- Shayakhmetov, D.M.; Li, Z.Y.; Ternovoi, V.; Gaggar, A.; Gharwan, H.; Lieber, A. The interaction between the fiber knob domain and the cellular attachment receptor determines the intracellular trafficking route of adenoviruses. J. Virol. 2003, 77, 3712–3723. [Google Scholar] [CrossRef]
- van Beusechem, V.W.; van Rijswijk, A.L.; van Es, H.H.; Haisma, H.J.; Pinedo, H.M.; Gerritsen, W.R. Recombinant adenovirus vectors with knobless fibers for targeted gene transfer. Gene Ther. 2000, 7, 1940–1946. [Google Scholar] [CrossRef]
- Schagen, F.H.; Wensveen, F.M.; Carette, J.E.; Dermody, T.S.; Gerritsen, W.R.; van Beusechem, V.W. Genetic targeting of adenovirus vectors using a reovirus sigma1-based attachment protein. Mol. Ther. 2006, 13, 997–1005. [Google Scholar] [CrossRef]
- Hedley, S.J.; Auf der Maur, A.; Hohn, S.; Escher, D.; Barberis, A.; Glasgow, J.N.; Douglas, J.T.; Korokhov, N.; Curiel, D.T. An adenovirus vector with a chimeric fiber incorporating stabilized single chain antibody achieves targeted gene delivery. Gene Ther. 2006, 13, 88–94. [Google Scholar] [CrossRef]
- Belousova, N.; Korokhov, N.; Krendelshchikova, V.; Simonenko, V.; Mikheeva, G.; Triozzi, P. L.; Aldrich, W.A.; Banerjee, P.T.; Gillies, S.D.; Curiel, D.T.; Krasnykh, V. Genetically targeted adenovirus vector directed to CD40-expressing cells. J. Virol. 2003, 77, 11367–11377. [Google Scholar] [CrossRef]
- Izumi, M.; Kawakami, Y.; Glasgow, J.N.; Belousova, N.; Everts, M.; Kim-Park, S.; Yamamoto, S.; Wang, M.; Le, L.P.; Reynolds, P.N.; Curiel, D.T. In vivo analysis of a genetically modified adenoviral vector targeted to human CD40 using a novel transient transgenic model. J. Gene Med. 2005, 7, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lad, S.; Yang, G.; Luo, Y.; Iacobelli-Martinez, M.; Primus, F. J.; Reisfeld, R.A.; Li, E. Adenovirus fiber shaft contains a trimerization element that supports peptide fusion for targeted gene delivery. J. Virol. 2006, 80, 12324–12331. [Google Scholar] [CrossRef] [PubMed]
- Henning, P.; Lundgren, E.; Carlsson, M.; Frykholm, K.; Johannisson, J.; Magnusson, M.K.; Tang, E.; Franqueville, L.; Hong, S.S.; Lindholm, L.; Boulanger, P. Adenovirus type 5 fiber knob domain has a critical role in fiber protein synthesis and encapsidation. J. Gen. Virol. 2006, 87, 3151–3160. [Google Scholar] [CrossRef] [PubMed]
- Franqueville, L.; Henning, P.; Magnusson, M.; Vigne, E.; Schoehn, G.; Blair-Zajdel, M.E.; Habib, N.; Lindholm, L.; Blair, G.E.; Hong, S.S.; Boulanger, P. Protein crystals in Adenovirus type 5-infected cells: requirements for intranuclear crystallogenesis, structural and functional analysis. PLoS One 2008, 3, e2894. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.W.; Freimuth, P.; Moninger, T.O.; Ganske, I.; Zabner, J.; Welsh, M.J. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell 2002, 110, 789–799. [Google Scholar] [CrossRef]
- Hong, S.S.; Magnusson, M.K.; Henning, P.; Lindholm, L.; Boulanger, P.A. Adenovirus stripping: a versatile method to generate adenovirus vectors with new cell target specificity. Mol. Ther. 2003, 7, 692–699. [Google Scholar] [CrossRef]
- Bayo-Puxan, N.; Gimenez-Alejandre, M.; Lavilla-Alonso, S.; Gros, A.; Cascallo, M.; Hemminki, A.; Alemany, R. Replacement of adenovirus type 5 fiber shaft heparan sulfate proteoglycan-binding domain with RGD for improved tumor infectivity and targeting. Hum. Gene Ther. 2009, 20, 1214–1221. [Google Scholar] [CrossRef]
- Athappilly, F.K.; Murali, R.; Rux, J.J.; Cai, Z.; Burnett, R.M. The refined crystal structure of hexon, the major coat protein of adenovirus type 2, at 2.9 A resolution. J. Mol. Biol. 1994, 242, 430–455. [Google Scholar] [CrossRef]
- Vigne, E.; Mahfouz, I.; Dedieu, J.F.; Brie, A.; Perricaudet, M.; Yeh, P. RGD inclusion in the hexon monomer provides adenovirus type 5-based vectors with a fiber knob-independent pathway for infection. J. Virol. 1999, 73, 5156–5161. [Google Scholar] [CrossRef]
- Crompton, J.; Toogood, C.I.; Wallis, N.; Hay, R.T. Expression of a foreign epitope on the surface of the adenovirus hexon. J. Gen. Virol. 1994, 75 Pt 1, 133–139. [Google Scholar] [CrossRef]
- McConnell, M.J.; Danthinne, X.; Imperiale, M.J. Characterization of a permissive epitope insertion site in adenovirus hexon. J. Virol. 2006, 80, 5361–5370. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.K.; Barry, M.A. Rapid construction of capsid-modified adenoviral vectors through bacteriophage lambda Red recombination. Hum. Gene Ther. 2004, 15, 1125–1130. [Google Scholar] [CrossRef]
- Kurachi, S.; Koizumi, N.; Tashiro, K.; Sakurai, H.; Sakurai, F.; Kawabata, K.; Nakagawa, S.; Mizuguchi, H. Modification of pIX or hexon based on fiberless Ad vectors is not effective for targeted Ad vectors. J. Controlled Release 2008, 127, 88–95. [Google Scholar] [CrossRef]
- Sumida, S.M.; Truitt, D.M.; Lemckert, A.A.; Vogels, R.; Custers, J.H.; Addo, M.M.; Lockman, S.; Peter, T.; Peyerl, F.W.; Kishko, M.G.; Jackson, S.S.; Gorgone, D.A.; Lifton, M.A.; Essex, M.; Walker, B.D.; Goudsmit, J.; Havenga, M.J.; Barouch, D.H. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol. 2005, 174, 7179–7185. [Google Scholar] [CrossRef] [PubMed]
- Vlachaki, M.T.; Hernandez-Garcia, A.; Ittmann, M.; Chhikara, M.; Aguilar, L.K.; Zhu, X.; Teh, B.S.; Butler, E.B.; Woo, S.; Thompson, T.C.; Barrera-Saldana, H.; Aguilar-Cordova, E. Impact of preimmunization on adenoviral vector expression and toxicity in a subcutaneous mouse cancer model. Mol. Ther. 2002, 6, 342–348. [Google Scholar] [CrossRef]
- Varnavski, A.N.; Calcedo, R.; Bove, M.; Gao, G.; Wilson, J.M. Evaluation of toxicity from high-dose systemic administration of recombinant adenovirus vector in vector-naive and pre-immunized mice. Gene Ther. 2005, 12, 427–436. [Google Scholar] [CrossRef]
- Zubieta, C.; Schoehn, G.; Chroboczek, J.; Cusack, S. The structure of the human adenovirus 2 penton. Mol. Cell 2005, 17, 121–135. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Mathias, P.; Nemerow, G.R.; Stewart, P.L. Structure of adenovirus complexed with its internalization receptor, alphavbeta5 integrin. J. Virol. 1999, 73, 6759–6768. [Google Scholar] [CrossRef] [PubMed]
- Wickham, T.J.; Carrion, M.E.; Kovesdi, I. Targeting of adenovirus penton base to new receptors through replacement of its RGD motif with other receptor-specific peptide motifs. Gene Ther. 1995, 2, 750–756. [Google Scholar]
- Boulanger, P.; Lemay, P.; Blair, G.E.; Russell, W.C. Characterization of adenovirus protein IX. J. Gen. Virol. 1979, 44, 783–800. [Google Scholar] [CrossRef]
- Stewart, P.L.; Burnett, R.M.; Cyrklaff, M.; Fuller, S.D. Image reconstruction reveals the complex molecular organization of adenovirus. Cell 1991, 67, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Furcinitti, P.S.; van Oostrum, J.; Burnett, R.M. Adenovirus polypeptide IX revealed as capsid cement by difference images from electron microscopy and crystallography. EMBO J. 1989, 8, 3563–3570. [Google Scholar] [CrossRef] [PubMed]
- Ghosh-Choudhury, G.; Haj-Ahmad, Y.; Graham, F.L. Protein IX, a minor component of the human adenovirus capsid, is essential for the packaging of full length genomes. EMBO J. 1987, 6, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Calatrava, M.; Grave, L.; Puvion-Dutilleul, F.; Chatton, B.; Kedinger, C. Functional analysis of adenovirus protein IX identifies domains involved in capsid stability, transcriptional activity, and nuclear reorganization. J. Virol. 2001, 75, 7131–7141. [Google Scholar] [CrossRef]
- Sargent, K.L.; Meulenbroek, R.A.; Parks, R.J. Activation of adenoviral gene expression by protein IX is not required for efficient virus replication. J. Virol. 2004, 78, 5032–5037. [Google Scholar] [CrossRef]
- Saban, S.D.; Silvestry, M.; Nemerow, G.R.; Stewart, P.L. Visualization of alpha-helices in a 6-angstrom resolution cryoelectron microscopy structure of adenovirus allows refinement of capsid protein assignments. J. Virol. 2006, 80, 12049–12059. [Google Scholar] [CrossRef]
- Marsh, M.P.; Campos, S.K.; Baker, M.L.; Chen, C.Y.; Chiu, W.; Barry, M.A. Cryoelectron microscopy of protein IX-modified adenoviruses suggests a new position for the C terminus of protein IX. J. Virol. 2006, 80, 11881–11886. [Google Scholar] [CrossRef]
- Dmitriev, I.P.; Kashentseva, E.A.; Curiel, D.T. Engineering of adenovirus vectors containing heterologous peptide sequences in the C terminus of capsid protein IX. J. Virol. 2002, 76, 6893–6899. [Google Scholar] [CrossRef]
- Vellinga, J.; Rabelink, M.J.; Cramer, S.J.; van den Wollenberg, D.J.; Van der Meulen, H.; Leppard, K.N.; Fallaux, F.J.; Hoeben, R.C. Spacers increase the accessibility of peptide ligands linked to the carboxyl terminus of adenovirus minor capsid protein IX. J. Virol. 2004, 78, 3470–3479. [Google Scholar] [CrossRef]
- Campos, S.K.; Barry, M.A. Comparison of adenovirus fiber, protein IX, and hexon capsomeres as scaffolds for vector purification and cell targeting. Virology 2006, 349, 453–462. [Google Scholar] [CrossRef]
- Li, J.; Le, L.; Sibley, D.A.; Mathis, J.M.; Curiel, D.T. Genetic incorporation of HSV-1 thymidine kinase into the adenovirus protein IX for functional display on the virion. Virology 2005, 338, 247–258. [Google Scholar] [CrossRef]
- Vellinga, J.; van den Wollenberg, D.J.; van der Heijdt, S.; Rabelink, M.J.; Hoeben, R.C. The coiled-coil domain of the adenovirus type 5 protein IX is dispensable for capsid incorporation and thermostability. J. Virol. 2005, 79, 3206–3210. [Google Scholar] [CrossRef] [PubMed]
- Barnett, B.G.; Crews, C.J.; Douglas, J.T. Targeted adenoviral vectors. Biochim. Biophys. Acta. 2002, 1575, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tarassishin, L.; Szawlowski, P.; Kidd, A.H.; Russell, W.C. An epitope on the adenovirus fibre tail is common to all human subgroups. Arch. Virol. 2000, 145, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.; Liu, Y.; Li, Z.Y.; Tuve, S.; Strauss, R.; Lieber, A. Comparison of adenoviruses from species B, C, E, and F after intravenous delivery. Mol. Ther. 2007, 15, 2146–2153. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.; Ni, S.; Li, Z.Y.; Gaggar, A.; DiPaolo, N.; Feng, Q.; Sandig, V.; Lieber, A. Development and assessment of human adenovirus type 11 as a gene transfer vector. J. Virol. 2005, 79, 5090–5104. [Google Scholar] [CrossRef]
- Segerman, A.; Atkinson, J.P.; Marttila, M.; Dennerquist, V.; Wadell, G.; Arnberg, N. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 2003, 77, 9183–9191. [Google Scholar] [CrossRef]
- Tuve, S.; Wang, H.; Jacobs, J.D.; Yumul, R.C.; Smith, D.F.; Lieber, A. Role of cellular heparan sulfate proteoglycans in infection of human adenovirus serotype 3 and 35. PLoS Pathog. 2008, 4, e1000189. [Google Scholar] [CrossRef]
- Rea, D.; Havenga, M.J.; van Den Assem, M.; Sutmuller, R.P.; Lemckert, A.; Hoeben, R.C.; Bout, A.; Melief, C.J.; Offringa, R. Highly efficient transduction of human monocyte-derived dendritic cells with subgroup B fiber-modified adenovirus vectors enhances transgene-encoded antigen presentation to cytotoxic T cells. J. Immunol. 2001, 166, 5236–5244. [Google Scholar] [CrossRef]
- Havenga, M.J.; Lemckert, A.A.; Grimbergen, J.M.; Vogels, R.; Huisman, L.G.; Valerio, D.; Bout, A.; Quax, P.H. Improved adenovirus vectors for infection of cardiovascular tissues. J. Virol. 2001, 75, 3335–3342. [Google Scholar] [CrossRef]
- Shayakhmetov, D.M.; Papayannopoulou, T.; Stamatoyannopoulos, G.; Lieber, A. Efficient gene transfer into human CD34(+) cells by a retargeted adenovirus vector. J. Virol. 2000, 74, 2567–2583. [Google Scholar] [CrossRef] [PubMed]
- Granio, O.; Ashbourne Excoffon, K.J.; Henning, P.; Melin, P.; Norez, C.; Gonzalez, G.; Karp, P.H.; Magnusson, M.K.; Habib, N.; Lindholm, L.; Becq, F.; Boulanger, P.; Zabner, J.; Hong, S.S. Adenovirus 5-fiber 35 chimeric vector mediates efficient apical correction of the cystic fibrosis transmembrane conductance regulator defect in cystic fibrosis primary airway epithelia. Hum. Gene Ther. 2010, 21, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.W.; Grunst, T.; Bergelson, J.M.; Finberg, R.W.; Welsh, M.J.; Zabner, J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J. Biol. Chem. 1999, 274, 10219–10226. [Google Scholar] [CrossRef] [PubMed]
- Sinn, P.L.; Williams, G.; Vongpunsawad, S.; Cattaneo, R.; McCray, P.B., Jr. Measles virus preferentially transduces the basolateral surface of well-differentiated human airway epithelia. J. Virol. 2002, 76, 2403–2409. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Yumul, R.; Gao, W.; Gambotto, A.; Morita, T.; Baker, A.; Shayakhmetov, D.; Lieber, A. Transduction of liver metastases after intravenous injection of Ad5/35 or Ad35 vectors with and without factor X-binding protein pretreatment. Hum. Gene Ther. 2009, 20, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, E.; Doi, R.; Kami, K.; Mori, T.; Ito, D.; Koizumi, M.; Kida, A.; Nagai, K.; Ito, T.; Masui, T.; Wada, M.; Tagawa, M.; Uemoto, S. Adenovirus vectors with chimeric type 5 and 35 fiber proteins exhibit enhanced transfection of human pancreatic cancer cells. Int J Oncol 2008, 33, 1141–1147. [Google Scholar]
- Shayakhmetov, D.M.; Li, Z.Y.; Ni, S.; Lieber, A. Targeting of adenovirus vectors to tumor cells does not enable efficient transduction of breast cancer metastases. Cancer Res. 2002, 62, 1063–1068. [Google Scholar]
- Wang, H.; Liu, Y.; Li, Z.; Tuve, S.; Stone, D.; Kalyushniy, O.; Shayakhmetov, D.; Verlinde, C.L.; Stehle, T.; McVey, J.; Baker, A.; Peng, K.W.; Roffler, S.; Lieber, A. In vitro and in vivo properties of adenovirus vectors with increased affinity to CD46. J. Virol. 2008, 82, 10567–10579. [Google Scholar] [CrossRef]
- Wang, G.; Li, G.; Liu, H.; Yang, C.; Yang, X.; Jin, J.; Liu, X.; Qian, Q.; Qian, W. E1B 55-kDa deleted, Ad5/F35 fiber chimeric adenovirus, a potential oncolytic agent for B-lymphocytic malignancies. J. Gene Med. 2009, 11, 477–485. [Google Scholar] [CrossRef]
- Alba, R.; Bradshaw, A.C.; Coughlan, L.; Denby, L.; McDonald, R.A.; Waddington, S.N.; Buckley, S.M.K.; Greig, J.A.; Parker, A.L.; Miller, A.M.; Wang, H.; Lieber, A.; van Rooijen, N.; McVey, J.H.; Nicklin, S.A.; Baker, A.H. Biodistribution and retargeting of FX-binding ablated adenovirus serotype 5 vectors. Blood 2010. [Google Scholar] [CrossRef]
- Haviv, Y.S.; Blackwell, J.L.; Kanerva, A.; Nagi, P.; Krasnykh, V.; Dmitriev, I.; Wang, M.; Naito, S.; Lei, X.; Hemminki, A.; Carey, D.; Curiel, D.T. Adenoviral gene therapy for renal cancer requires retargeting to alternative cellular receptors. Cancer Res. 2002, 62, 4273–4281. [Google Scholar] [PubMed]
- Kanerva, A.; Mikheeva, G.V.; Krasnykh, V.; Coolidge, C.J.; Lam, J.T.; Mahasreshti, P.J.; Barker, S.D.; Straughn, M.; Barnes, M.N.; Alvarez, R.D.; Hemminki, A.; Curiel, D.T. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin. Cancer Res. 2002, 8, 275–280. [Google Scholar] [PubMed]
- Ulasov, I.V.; Rivera, A.A.; Han, Y.; Curiel, D.T.; Zhu, Z.B.; Lesniak, M.S. Targeting adenovirus to CD80 and CD86 receptors increases gene transfer efficiency to malignant glioma cells. J. Neurosurg. 2007, 107, 617–627. [Google Scholar] [CrossRef]
- Volk, A.L.; Rivera, A.A.; Kanerva, A.; Bauerschmitz, G.; Dmitriev, I.; Nettelbeck, D.M.; Curiel, D.T. Enhanced adenovirus infection of melanoma cells by fiber-modification: incorporation of RGD peptide or Ad5/3 chimerism. Cancer Biol Ther 2003, 2, 511–515. [Google Scholar] [CrossRef]
- Von Seggern, D.J.; Huang, S.; Fleck, S.K.; Stevenson, S.C.; Nemerow, G.R. Adenovirus vector pseudotyping in fiber-expressing cell lines: improved transduction of Epstein-Barr virus-transformed B cells. J. Virol. 2000, 74, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Raki, M.; Sarkioja, M.; Desmond, R.A.; Chen, D.T.; Butzow, R.; Hemminki, A.; Kanerva, A. Oncolytic adenovirus Ad5/3-delta24 and chemotherapy for treatment of orthotopic ovarian cancer. Gynecol. Oncol. 2008, 108, 166–172. [Google Scholar] [CrossRef]
- Guse, K.; Ranki, T.; Ala-Opas, M.; Bono, P.; Sarkioja, M.; Rajecki, M.; Kanerva, A.; Hakkarainen, T.; Hemminki, A. Treatment of metastatic renal cancer with capsid-modified oncolytic adenoviruses. Mol. Cancer Ther. 2007, 6, 2728–2736. [Google Scholar] [CrossRef]
- Nandi, S.; Ulasov, I.V.; Rolle, C.E.; Han, Y.; Lesniak, M.S. A chimeric adenovirus with an Ad3 fiber knob modification augments glioma virotherapy. J. Gene Med. 2009, 11, 1005–1011. [Google Scholar] [CrossRef]
- Rajecki, M.; Kanerva, A.; Stenman, U.H.; Tenhunen, M.; Kangasniemi, L.; Sarkioja, M.; Ala-Opas, M.Y.; Alfthan, H.; Sankila, A.; Rintala, E.; Desmond, R.A.; Hakkarainen, T.; Hemminki, A. Treatment of prostate cancer with Ad5/3Delta24hCG allows non-invasive detection of the magnitude and persistence of virus replication in vivo. Mol. Cancer Ther. 2007, 6, 742–751. [Google Scholar] [CrossRef]
- Ni, S.; Bernt, K.; Gaggar, A.; Li, Z.Y.; Kiem, H.P.; Lieber, A. Evaluation of biodistribution and safety of adenovirus vectors containing group B fibers after intravenous injection into baboons. Hum. Gene Ther. 2005, 16, 664–677. [Google Scholar] [CrossRef]
- Hsu, C.; Boysen, M.; Gritton, L.D.; Frosst, P.D.; Nemerow, G.R.; Von Seggern, D.J. In vitro dendritic cell infection by pseudotyped adenoviral vectors does not correlate with their in vivo immunogenicity. Virology 2005, 332, 1–7. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Wu, E.; Brown, S.L.; Von Seggern, D.J.; Nemerow, G.R.; Stewart, P.L. Structural analysis of a fiber-pseudotyped adenovirus with ocular tropism suggests differential modes of cell receptor interactions. J. Virol. 2001, 75, 5375–5380. [Google Scholar] [CrossRef]
- Denby, L.; Work, L.M.; Graham, D.; Hsu, C.; von Seggern, D.J.; Nicklin, S.A.; Baker, A.H. Adenoviral serotype 5 vectors pseudotyped with fibers from subgroup D show modified tropism in vitro and in vivo. Hum. Gene Ther. 2004, 15, 1054–1064. [Google Scholar] [CrossRef]
- Diaconu, I.; Denby, L.; Pesonen, S.; Cerullo, V.; Bauerschmitz, G.J.; Guse, K.; Rajecki, M.; Dias, J.D.; Taari, K.; Kanerva, A.; Baker, A.H.; Hemminki, A. Serotype chimeric and fiber-mutated adenovirus Ad5/19p-HIT for targeting renal cancer and untargeting the liver. Hum. Gene Ther. 2009, 20, 611–620. [Google Scholar] [CrossRef]
- Hesse, A.; Kosmides, D.; Kontermann, R.E.; Nettelbeck, D.M. Tropism modification of adenovirus vectors by peptide ligand insertion into various positions of the adenovirus serotype 41 short-fiber knob domain. J. Virol. 2007, 81, 2688–2699. [Google Scholar] [CrossRef]
- Stoff-Khalili, M.A.; Rivera, A.A.; Glasgow, J.N.; Le, L.P.; Stoff, A.; Everts, M.; Tsuruta, Y.; Kawakami, Y.; Bauerschmitz, G.J.; Mathis, J.M.; Pereboeva, L.; Seigal, G.P.; Dall, P.; Curiel, D.T. A human adenoviral vector with a chimeric fiber from canine adenovirus type 1 results in novel expanded tropism for cancer gene therapy. Gene Ther. 2005, 12, 1696–1706. [Google Scholar] [CrossRef]
- Nakayama, M.; Both, G.W.; Banizs, B.; Tsuruta, Y.; Yamamoto, S.; Kawakami, Y.; Douglas, J. T.; Tani, K.; Curiel, D.T.; Glasgow, J.N. An adenovirus serotype 5 vector with fibers derived from ovine atadenovirus demonstrates CAR-independent tropism and unique biodistribution in mice. Virology 2006, 350, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Rogee, S.; Grellier, E.; Bernard, C.; Jouy, N.; Loyens, A.; Beauvillain, J.C.; Fender, P.; Corjon, S.; Hong, S.S.; Boulanger, P.; Quesnel, B.; D’Halluin, J.C.; Colin, M. Influence of chimeric human-bovine fibers on adenoviral uptake by liver cells and the antiviral immune response. Gene Ther. 2010. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, Y.; Pereboeva, L.; Glasgow, J.N.; Luongo, C.L.; Komarova, S.; Kawakami, Y.; Curiel, D.T. Reovirus sigma1 fiber incorporated into adenovirus serotype 5 enhances infectivity via a CAR-independent pathway. Biochem. Biophys. Res. Commun. 2005, 335, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Mercier, G.T.; Campbell, J.A.; Chappell, J.D.; Stehle, T.; Dermody, T.S.; Barry, M.A. A chimeric adenovirus vector encoding reovirus attachment protein sigma1 targets cells expressing junctional adhesion molecule 1. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 6188–6193. [Google Scholar] [CrossRef] [PubMed]
- Barton, E.S.; Forrest, J.C.; Connolly, J.L.; Chappell, J.D.; Liu, Y.; Schnell, F.J.; Nusrat, A.; Parkos, C.A.; Dermody, T.S. Junction adhesion molecule is a receptor for reovirus. Cell 2001, 104, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Chappell, J.D.; Gunn, V.L.; Wetzel, J.D.; Baer, G.S.; Dermody, T.S. Mutations in type 3 reovirus that determine binding to sialic acid are contained in the fibrous tail domain of viral attachment protein sigma1. J. Virol. 1997, 71, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, N.; Crystal, R.G.; Leopold, P.L. Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein. J. Virol. 2001, 75, 1387–1400. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, J.N.; Everts, M.; Curiel, D.T. Transductional targeting of adenovirus vectors for gene therapy. Cancer Gene Ther. 2006, 13, 830–844. [Google Scholar] [CrossRef]
- Korokhov, N.; Mikheeva, G.; Krendelshchikov, A.; Belousova, N.; Simonenko, V.; Krendelshchikova, V.; Pereboev, A.; Kotov, A.; Kotova, O.; Triozzi, P.L.; Aldrich, W.A.; Douglas, J.T.; Lo, K.M.; Banerjee, P.T.; Gillies, S.D.; Curiel, D.T.; Krasnykh, V. Targeting of adenovirus via genetic modification of the viral capsid combined with a protein bridge. J. Virol. 2003, 77, 12931–12940. [Google Scholar] [CrossRef]
- Nettelbeck, D.M.; Miller, D.W.; Jerome, V.; Zuzarte, M.; Watkins, S.J.; Hawkins, R.E.; Muller, R.; Kontermann, R.E. Targeting of adenovirus to endothelial cells by a bispecific single-chain diabody directed against the adenovirus fiber knob domain and human endoglin (CD105). Mol. Ther. 2001, 3, 882–891. [Google Scholar] [CrossRef]
- Miller, C.R.; Buchsbaum, D.J.; Reynolds, P.N.; Douglas, J.T.; Gillespie, G.Y.; Mayo, M.S.; Raben, D.; Curiel, D.T. Differential susceptibility of primary and established human glioma cells to adenovirus infection: targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer Res. 1998, 58, 5738–5748. [Google Scholar]
- Nettelbeck, D.M.; Rivera, A.A.; Kupsch, J.; Dieckmann, D.; Douglas, J.T.; Kontermann, R.E.; Alemany, R.; Curiel, D.T. Retargeting of adenoviral infection to melanoma: combining genetic ablation of native tropism with a recombinant bispecific single-chain diabody (scDb) adapter that binds to fiber knob and HMWMAA. Int. J. Cancer 2004, 108, 136–145. [Google Scholar] [CrossRef]
- Harvey, T.J.; Burdon, D.; Steele, L.; Ingram, N.; Hall, G.D.; Selby, P.J.; Vile, R.G.; Cooper, P.A.; Shnyder, S.D.; Chester, J.D. Retargeted adenoviral cancer gene therapy for tumour cells overexpressing epidermal growth factor receptor or urokinase-type plasminogen activator receptor. Gene Ther. 2010, 17, 1000–1010. [Google Scholar] [CrossRef]
- Li, H.J.; Everts, M.; Pereboeva, L.; Komarova, S.; Idan, A.; Curiel, D.T.; Herschman, H.R. Adenovirus tumor targeting and hepatic untargeting by a coxsackie/adenovirus receptor ectodomain anti-carcinoembryonic antigen bispecific adapter. Cancer Res. 2007, 67, 5354–5361. [Google Scholar] [CrossRef]
- Chen, C.Y.; May, S.M.; Barry, M.A. Targeting adenoviruses with factor X-single-chain antibody fusion proteins. Hum. Gene Ther. 2010, 21, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Volpers, C.; Thirion, C.; Biermann, V.; Hussmann, S.; Kewes, H.; Dunant, P.; von der Mark, H.; Herrmann, A.; Kochanek, S.; Lochmuller, H. Antibody-mediated targeting of an adenovirus vector modified to contain a synthetic immunoglobulin g-binding domain in the capsid. J. Virol. 2003, 77, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Hudson, P.J.; Souriau, C. Engineered antibodies. Nat. Med. 2003, 9, 129–134. [Google Scholar] [CrossRef]
- Kontermann, R.E. Recombinant bispecific antibodies for cancer therapy. Acta Pharmacol. Sin. 2005, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Henning, P.; Andersson, K.M.; Frykholm, K.; Ali, A.; Magnusson, M.K.; Nygren, P.A.; Granio, O.; Hong, S.S.; Boulanger, P.; Lindholm, L. Tumor cell targeted gene delivery by adenovirus 5 vectors carrying knobless fibers with antibody-binding domains. Gene Ther. 2005, 12, 211–224. [Google Scholar] [CrossRef]
- Pereboeva, L.; Komarova, S.; Roth, J.; Ponnazhagan, S.; Curiel, D.T. Targeting EGFR with metabolically biotinylated fiber-mosaic adenovirus. Gene Ther. 2007, 14, 627–637. [Google Scholar] [CrossRef]
- Watkins, S.J.; Mesyanzhinov, V.V.; Kurochkina, L.P.; Hawkins, R.E. The ‘adenobody’ approach to viral targeting: specific and enhanced adenoviral gene delivery. Gene Ther. 1997, 4, 1004–1012. [Google Scholar] [CrossRef]
- Kraaij, R.; van Rijswijk, A.L.; Oomen, M.H.; Haisma, H.J.; Bangma, C.H. Prostate specific membrane antigen (PSMA) is a tissue-specific target for adenoviral transduction of prostate cancer in vitro. Prostate 2005, 62, 253–259. [Google Scholar] [CrossRef]
- Korn, T.; Nettelbeck, D.M.; Volkel, T.; Muller, R.; Kontermann, R.E. Recombinant bispecific antibodies for the targeting of adenoviruses to CEA-expressing tumour cells: a comparative analysis of bacterially expressed single-chain diabody and tandem scFv. J. Gene Med. 2004, 6, 642–651. [Google Scholar] [CrossRef]
- van Beusechem, V.W.; Mastenbroek, D.C.; van den Doel, P.B.; Lamfers, M.L.; Grill, J.; Wurdinger, T.; Haisma, H.J.; Pinedo, H.M.; Gerritsen, W.R. Conditionally replicative adenovirus expressing a targeting adapter molecule exhibits enhanced oncolytic potency on CAR-deficient tumors. Gene Ther. 2003, 10, 1982–1991. [Google Scholar] [CrossRef]
- van Zeeburg, H.J.; van Beusechem, V.W.; Huizenga, A.; Haisma, H.J.; Korokhov, N.; Gibbs, S.; Leemans, C.R.; Brakenhoff, R.H. Adenovirus retargeting to surface expressed antigens on oral mucosa. J. Gene Med. 2010, 12, 365–376. [Google Scholar] [CrossRef]
- Miller, W.H.; Brosnan, M.J.; Graham, D.; Nicol, C.G.; Morecroft, I.; Channon, K.M.; Danilov, S. M.; Reynolds, P.N.; Baker, A.H.; Dominiczak, A.F. Targeting endothelial cells with adenovirus expressing nitric oxide synthase prevents elevation of blood pressure in stroke-prone spontaneously hypertensive rats. Mol. Ther. 2005, 12, 321–327. [Google Scholar] [CrossRef]
- Reynolds, P.N.; Zinn, K.R.; Gavrilyuk, V.D.; Balyasnikova, I.V.; Rogers, B.E.; Buchsbaum, D.J.; Wang, M.H.; Miletich, D.J.; Grizzle, W.E.; Douglas, J.T.; Danilov, S.M.; Curiel, D.T. A targetable, injectable adenoviral vector for selective gene delivery to pulmonary endothelium in vivo. Mol. Ther. 2000, 2, 562–578. [Google Scholar] [CrossRef]
- Reynolds, P.N.; Nicklin, S.A.; Kaliberova, L.; Boatman, B.G.; Grizzle, W.E.; Balyasnikova, I.V.; Baker, A.H.; Danilov, S.M.; Curiel, D.T. Combined transductional and transcriptional targeting improves the specificity of transgene expression in vivo. Nat. Biotechnol. 2001, 19, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Kashentseva, E.A.; Seki, T.; Curiel, D.T.; Dmitriev, I.P. Adenovirus targeting to c-erbB-2 oncoprotein by single-chain antibody fused to trimeric form of adenovirus receptor ectodomain. Cancer Res. 2002, 62, 609–616. [Google Scholar] [PubMed]
- Dmitriev, I.; Kashentseva, E.; Rogers, B.E.; Krasnykh, V.; Curiel, D.T. Ectodomain of coxsackievirus and adenovirus receptor genetically fused to epidermal growth factor mediates adenovirus targeting to epidermal growth factor receptor-positive cells. J. Virol. 2000, 74, 6875–6884. [Google Scholar] [CrossRef]
- Pereboev, A.V.; Asiedu, C.K.; Kawakami, Y.; Dong, S.S.; Blackwell, J.L.; Kashentseva, E.A.; Triozzi, P.L.; Aldrich, W.A.; Curiel, D.T.; Thomas, J.M.; Dmitriev, I.P. Coxsackievirus-adenovirus receptor genetically fused to anti-human CD40 scFv enhances adenoviral transduction of dendritic cells. Gene Ther. 2002, 9, 1189–1193. [Google Scholar] [CrossRef]
- Matthews, K.; Noker, P.E.; Tian, B.; Grimes, S.D.; Fulton, R.; Schweikart, K.; Harris, R.; Aurigemma, R.; Wang, M.; Barnes, M.N.; Siegal, G.P.; Hemminki, A.; Zinn, K.; Curiel, D.T.; Alvarez, R.D. Identifying the safety profile of Ad5.SSTR/TK.RGD, a novel infectivity-enhanced bicistronic adenovirus, in anticipation of a phase I clinical trial in patients with recurrent ovarian cancer. Clin. Cancer Res. 2009, 15, 4131–4137. [Google Scholar] [CrossRef]
- Cascallo, M.; Alonso, M.M.; Rojas, J.J.; Perez-Gimenez, A.; Fueyo, J.; Alemany, R. Systemic toxicity-efficacy profile of ICOVIR-5, a potent and selective oncolytic adenovirus based on the pRB pathway. Mol. Ther. 2007, 15, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.J.; Cascallo, M.; Guedan, S.; Gros, A.; Martinez-Quintanilla, J.; Hemminki, A.; Alemany, R. A modified E2F-1 promoter improves the efficacy to toxicity ratio of oncolytic adenoviruses. Gene Ther. 2009, 16, 1441–1451. [Google Scholar] [CrossRef]
- Pesonen, S.; Helin, H.; Nokisalmi, P.; Escutenaire, S.; Ribacka, C.; Sarkioja, M.; Cerullo, V.; Guse, K.; Bauerschmitz, G.; Laasonen, L.; Kantola, T.; Ristimaki, A.; Rajecki, M.; Oksanen, M.; Haavisto, E.; Kanerva, A.; Joensuu, T.; Hemminki, A. Oncolytic adenovirus treatment of a patient with refractory neuroblastoma. Acta Oncol. 2010, 49, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Nokisalmi, P.; Pesonen, S.; Escutenaire, S.; Sarkioja, M.; Raki, M.; Cerullo, V.; Laasonen, L.; Alemany, R.; Rojas, J.; Cascallo, M.; Guse, K.; Rajecki, M.; Kangasniemi, L.; Haavisto, E.; Karioja-Kallio, A.; Hannuksela, P.; Oksanen, M.; Kanerva, A.; Joensuu, T.; Ahtiainen, L.; Hemminki, A. Oncolytic adenovirus ICOVIR-7 in patients with advanced and refractory solid tumors. Clin. Cancer Res. 2010, 16, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, S.; Nokisalmi, P.; Escutenaire, S.; Sarkioja, M.; Raki, M.; Cerullo, V.; Kangasniemi, L.; Laasonen, L.; Ribacka, C.; Guse, K.; Haavisto, E.; Oksanen, M.; Rajecki, M.; Helminen, A.; Ristimaki, A.; Karioja-Kallio, A.; Karli, E.; Kantola, T.; Bauerschmitz, G.; Kanerva, A.; Joensuu, T.; Hemminki, A. Prolonged systemic circulation of chimeric oncolytic adenovirus Ad5/3-Cox2L-D24 in patients with metastatic and refractory solid tumors. Gene Ther. 2010, 17, 892–904. [Google Scholar] [CrossRef]
- Tao, N.; Gao, G.P.; Parr, M.; Johnston, J.; Baradet, T.; Wilson, J.M.; Barsoum, J.; Fawell, S.E. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol. Ther. 2001, 3, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Worgall, S.; Leopold, P.L.; Wolff, G.; Ferris, B.; Van Roijen, N.; Crystal, R.G. Role of alveolar macrophages in rapid elimination of adenovirus vectors administered to the epithelial surface of the respiratory tract. Hum. Gene Ther. 1997, 8, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Z.; Serra, D.; Frank, M.M.; Amalfitano, A. Recombinant adenovirus vectors activate the alternative complement pathway, leading to the binding of human complement protein C3 independent of anti-ad antibodies. Mol. Ther. 2004, 10, 1140–1142. [Google Scholar] [CrossRef]
- Parker, A.L.; Waddington, S.N.; Buckley, S.M.; Custers, J.; Havenga, M.J.; van Rooijen, N.; Goudsmit, J.; McVey, J.H.; Nicklin, S.A.; Baker, A.H. Effect of neutralizing sera on factor X-mediated adenovirus serotype 5 gene transfer. J. Virol. 2009, 83, 479–483. [Google Scholar] [CrossRef]
- Alemany, R.; Curiel, D.T. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 2001, 8, 1347–1353. [Google Scholar] [CrossRef]
- Fechner, H.; Haack, A.; Wang, H.; Wang, X.; Eizema, K.; Pauschinger, M.; Schoemaker, R.; Veghel, R.; Houtsmuller, A.; Schultheiss, H.P.; Lamers, J.; Poller, W. Expression of coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 1999, 6, 1520–1535. [Google Scholar] [CrossRef]
- Leissner, P.; Legrand, V.; Schlesinger, Y.; Hadji, D. A.; van Raaij, M.; Cusack, S.; Pavirani, A.; Mehtali, M. Influence of adenoviral fiber mutations on viral encapsidation, infectivity and in vivo tropism. Gene Ther. 2001, 8, 49–57. [Google Scholar] [CrossRef]
- Martin, K.; Brie, A.; Saulnier, P.; Perricaudet, M.; Yeh, P.; Vigne, E. Simultaneous CAR- and alpha V integrin-binding ablation fails to reduce Ad5 liver tropism. Mol. Ther. 2003, 8, 485–494. [Google Scholar] [CrossRef]
- Mizuguchi, H.; Koizumi, N.; Hosono, T.; Ishii-Watabe, A.; Uchida, E.; Utoguchi, N.; Watanabe, Y.; Hayakawa, T. CAR- or alphav integrin-binding ablated adenovirus vectors, but not fiber-modified vectors containing RGD peptide, do not change the systemic gene transfer properties in mice. Gene Ther. 2002, 9, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Idamakanti, N.; Kylefjord, H.; Rollence, M.; King, L.; Kaloss, M.; Kaleko, M.; Stevenson, S.C. In vivo hepatic adenoviral gene delivery occurs independently of the coxsackievirus-adenovirus receptor. Mol. Ther. 2002, 5, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Tamanini, A.; Nicolis, E.; Bonizzato, A.; Bezzerri, V.; Melotti, P.; Assael, B.M.; Cabrini, G. Interaction of adenovirus type 5 fiber with the coxsackievirus and adenovirus receptor activates inflammatory response in human respiratory cells. J. Virol. 2006, 80, 11241–11254. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; Nociari, M.; Philpott, N.; Falck-Pedersen, E. Influence of fiber detargeting on adenovirus-mediated innate and adaptive immune activation. J. Virol. 2005, 79, 11627–11637. [Google Scholar] [CrossRef]
- Shaw, C.A.; Holland, P.C.; Sinnreich, M.; Allen, C.; Sollerbrant, K.; Karpati, G.; Nalbantoglu, J. Isoform-specific expression of the Coxsackie and adenovirus receptor (CAR) in neuromuscular junction and cardiac intercalated discs. BMC Cell Biol. 2004, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, C.; Lisewski, U.; Wrackmeyer, U.; Radke, M.; Westermann, D.; Sauter, M.; Tschope, C.; Poller, W.; Klingel, K.; Gotthardt, M. Cardiac deletion of the Coxsackievirus-adenovirus receptor abolishes Coxsackievirus B3 infection and prevents myocarditis in vivo. J. Am. Coll. Cardiol. 2009, 53, 1219–1226. [Google Scholar] [CrossRef]
- Bowles, N.E.; Ni, J.; Kearney, D.L.; Pauschinger, M.; Schultheiss, H.P.; McCarthy, R.; Hare, J.; Bricker, J.T.; Bowles, K.R.; Towbin, J.A. Detection of viruses in myocardial tissues by polymerase chain reaction; evidence of adenovirus as a common cause of myocarditis in children and adults. J. Am. Coll. Cardiol. 2003, 42, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Excoffon, K.J.; Gansemer, N.D.; Mobily, M.E.; Karp, P.H.; Parekh, K.R.; Zabner, J. Isoform-specific regulation and localization of the coxsackie and adenovirus receptor in human airway epithelia. PLoS One 2010, 5, e9909. [Google Scholar] [CrossRef]
- Zhang, Y.; Bergelson, J.M. Adenovirus receptors. J. Virol. 2005, 79, 12125–12131. [Google Scholar] [CrossRef]
- Xia, D.; Henry, L.; Gerard, R.D.; Deisenhofer, J. Structure of the receptor binding domain of adenovirus type 5 fiber protein. Curr. Top. Microbiol. Immunol. 1995, 199 Pt 1, 39–46. [Google Scholar] [PubMed]
- Smith, T.A.; Idamakanti, N.; Marshall-Neff, J.; Rollence, M.L.; Wright, P.; Kaloss, M.; King, L.; Mech, C.; Dinges, L.; Iverson, W.O.; Sherer, A.D.; Markovits, J.E.; Lyons, R.M.; Kaleko, M.; Stevenson, S.C. Receptor interactions involved in adenoviral-mediated gene delivery after systemic administration in non-human primates. Hum. Gene Ther. 2003, 14, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.; Deakin, J.A.; Mizuno, K.; Nakamura, T.; Gallagher, J.T. Interaction of hepatocyte growth factor with heparan sulfate. Elucidation of the major heparan sulfate structural determinants. J. Biol. Chem. 1994, 269, 11216–11223. [Google Scholar] [CrossRef]
- MacArthur, J.M.; Bishop, J.R.; Stanford, K.I.; Wang, L.; Bensadoun, A.; Witztum, J.L.; Esko, J.D. Liver heparan sulfate proteoglycans mediate clearance of triglyceride-rich lipoproteins independently of LDL receptor family members. J. Clin. Invest. 2007, 117, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Vongchan, P.; Warda, M.; Toyoda, H.; Toida, T.; Marks, R.M.; Linhardt, R.J. Structural characterization of human liver heparan sulfate. Biochim. Biophys. Acta. 2005, 1721, 1–8. [Google Scholar] [CrossRef]
- Bayo-Puxan, N.; Cascallo, M.; Gros, A.; Huch, M.; Fillat, C.; Alemany, R. Role of the putative heparan sulfate glycosaminoglycan-binding site of the adenovirus type 5 fiber shaft on liver detargeting and knob-mediated retargeting. J. Gen. Virol. 2006, 87, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.A.; Idamakanti, N.; Rollence, M.L.; Marshall-Neff, J.; Kim, J.; Mulgrew, K.; Nemerow, G. R.; Kaleko, M.; Stevenson, S.C. Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Hum. Gene Ther. 2003, 14, 777–787. [Google Scholar] [CrossRef]
- Kritz, A.B.; Nicol, C.G.; Dishart, K.L.; Nelson, R.; Holbeck, S.; Von Seggern, D.J.; Work, L.M.; McVey, J.H.; Nicklin, S.A.; Baker, A.H. Adenovirus 5 fibers mutated at the putative HSPG-binding site show restricted retargeting with targeting peptides in the HI loop. Mol. Ther. 2007, 15, 741–749. [Google Scholar] [CrossRef]
- Di Paolo, N.C.; Kalyuzhniy, O.; Shayakhmetov, D.M. Fiber shaft-chimeric adenovirus vectors lacking the KKTK motif efficiently infect liver cells in vivo. J. Virol. 2007, 81, 12249–12259. [Google Scholar] [CrossRef]
- Wu, E.; Pache, L.; Von Seggern, D.J.; Mullen, T.M.; Mikyas, Y.; Stewart, P.L.; Nemerow, G.R. Flexibility of the adenovirus fiber is required for efficient receptor interaction. J. Virol. 2003, 77, 7225–7235. [Google Scholar] [CrossRef]
- Terashima, T.; Oka, K.; Kritz, A.B.; Kojima, H.; Baker, A.H.; Chan, L. DRG-targeted helper-dependent adenoviruses mediate selective gene delivery for therapeutic rescue of sensory neuronopathies in mice. J. Clin. Invest. 2009, 119, 2100–2112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Waddington, S.N.; Parker, A.L.; Havenga, M.; Nicklin, S.A.; Buckley, S.M.; McVey, J.H.; Baker, A.H. Targeting of adenovirus serotype 5 (Ad5) and 5/47 pseudotyped vectors in vivo: fundamental involvement of coagulation factors and redundancy of CAR binding by Ad5. J. Virol. 2007, 81, 9568–9571. [Google Scholar] [CrossRef] [PubMed]
- Greig, J.A.; Buckley, S.M.; Waddington, S.N.; Parker, A.L.; Bhella, D.; Pink, R.; Rahim, A.A.; Morita, T.; Nicklin, S.A.; McVey, J.H.; Baker, A.H. Influence of coagulation factor X on in vitro and in vivo gene delivery by adenovirus (Ad) 5, Ad35, and chimeric Ad5/Ad35 vectors. Mol. Ther. 2009, 17, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Shashkova, E.V.; Doronin, K.; Senac, J.S.; Barry, M.A. Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res. 2008, 68, 5896–5904. [Google Scholar] [CrossRef]
- Koski, A.; Rajecki, M.; Guse, K.; Kanerva, A.; Ristimaki, A.; Pesonen, S.; Escutenaire, S.; Hemminki, A. Systemic adenoviral gene delivery to orthotopic murine breast tumors with ablation of coagulation factors, thrombocytes and Kupffer cells. J. Gene Med. 2009, 11, 966–977. [Google Scholar] [CrossRef]
- Gimenez-Alejandre, M.; Cascallo, M.; Bayo-Puxan, N.; Alemany, R. Coagulation factors determine tumor transduction in vivo. Hum. Gene Ther. 2008, 19, 1415–1419. [Google Scholar] [CrossRef]
- Shashkova, E.V.; May, S.M.; Doronin, K.; Barry, M.A. Expanded anticancer therapeutic window of hexon-modified oncolytic adenovirus. Mol. Ther. 2009, 17, 2121–2130. [Google Scholar] [CrossRef]
- Roberts, D.M.; Nanda, A.; Havenga, M.J.; Abbink, P.; Lynch, D.M.; Ewald, B.A.; Liu, J.; Thorner, A.R.; Swanson, P.E.; Gorgone, D.A.; Lifton, M.A.; Lemckert, A.A.; Holterman, L.; Chen, B.; Dilraj, A.; Carville, A.; Mansfield, K.G.; Goudsmit, J.; Barouch, D.H. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 2006, 441, 239–243. [Google Scholar] [CrossRef]
- Roy, S.; Shirley, P.S.; McClelland, A.; Kaleko, M. Circumvention of immunity to the adenovirus major coat protein hexon. J. Virol. 1998, 72, 6875–6879. [Google Scholar] [CrossRef]
- Gall, J.G.; Crystal, R.G.; Falck-Pedersen, E. Construction and characterization of hexon-chimeric adenoviruses: specification of adenovirus serotype. J. Virol. 1998, 72, 10260–10264. [Google Scholar] [CrossRef]
- Ostapchuk, P.; Hearing, P. Pseudopackaging of adenovirus type 5 genomes into capsids containing the hexon proteins of adenovirus serotypes B, D, or E. J. Virol. 2001, 75, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dmitriev, I.; Kashentseva, E.; Seki, T.; Wang, M.; Curiel, D.T. Construction and characterization of adenovirus serotype 5 packaged by serotype 3 hexon. J. Virol. 2002, 76, 12775–12782. [Google Scholar] [CrossRef] [PubMed]
- Youil, R.; Toner, T.J.; Su, Q.; Chen, M.; Tang, A.; Bett, A.J.; Casimiro, D. Hexon gene switch strategy for the generation of chimeric recombinant adenovirus. Hum. Gene Ther. 2002, 13, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Furumoto, K.; Nagayama, S.; Ogawara, K.; Takakura, Y.; Hashida, M.; Higaki, K.; Kimura, T. Hepatic uptake of negatively charged particles in rats: possible involvement of serum proteins in recognition by scavenger receptor. J. Controlled Release 2004, 97, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Haisma, H.J.; Kamps, J.A.; Kamps, G.K.; Plantinga, J.A.; Rots, M.G.; Bellu, A.R. Polyinosinic acid enhances delivery of adenovirus vectors in vivo by preventing sequestration in liver macrophages. J. Gen. Virol. 2008, 89, 1097–1105. [Google Scholar] [CrossRef]
- Chonn, A.; Cullis, P.R.; Devine, D.V. The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes. J. Immunol. 1991, 146, 4234–4241. [Google Scholar] [CrossRef]
- Chillon, M.; Lee, J.H.; Fasbender, A.; Welsh, M.J. Adenovirus complexed with polyethylene glycol and cationic lipid is shielded from neutralizing antibodies in vitro. Gene Ther. 1998, 5, 995–1002. [Google Scholar] [CrossRef]
- De Geest, B.; Snoeys, J.; Van Linthout, S.; Lievens, J.; Collen, D. Elimination of innate immune responses and liver inflammation by PEGylation of adenoviral vectors and methylprednisolone. Hum. Gene Ther. 2005, 16, 1439–1451. [Google Scholar] [CrossRef]
- O’Riordan, C.R.; Lachapelle, A.; Delgado, C.; Parkes, V.; Wadsworth, S.C.; Smith, A.E.; Francis, G.E. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum. Gene Ther. 1999, 10, 1349–1358. [Google Scholar] [CrossRef]
- Corjon, S.; Wortmann, A.; Engler, T.; van Rooijen, N.; Kochanek, S.; Kreppel, F. Targeting of adenovirus vectors to the LRP receptor family with the high-affinity ligand RAP via combined genetic and chemical modification of the pIX capsomere. Mol. Ther. 2008, 16, 1813–1824. [Google Scholar] [CrossRef]
- Kreppel, F.; Gackowski, J.; Schmidt, E.; Kochanek, S. Combined genetic and chemical capsid modifications enable flexible and efficient de- and retargeting of adenovirus vectors. Mol. Ther. 2005, 12, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Abuchowski, A.; McCoy, J.R.; Palczuk, N.C.; van Es, T.; Davis, F.F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J. Biol. Chem. 1977, 252, 3582–3586. [Google Scholar] [CrossRef] [PubMed]
- Abuchowski, A.; Kazo, G.M.; Verhoest, C.R., Jr.; Van Es, T.; Kafkewitz, D.; Nucci, M.L.; Viau, A.T.; Davis, F.F. Cancer therapy with chemically modified enzymes. I. Antitumor properties of polyethylene glycol-asparaginase conjugates. Cancer Biochem Biophys 1984, 7, 175–186. [Google Scholar] [PubMed]
- Green, N.K.; Herbert, C.W.; Hale, S.J.; Hale, A.B.; Mautner, V.; Harkins, R.; Hermiston, T.; Ulbrich, K.; Fisher, K.D.; Seymour, L.W. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 2004, 11, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Fasbender, A.; Zabner, J.; Chillon, M.; Moninger, T.O.; Puga, A.P.; Davidson, B.L.; Welsh, M.J. Complexes of adenovirus with polycationic polymers and cationic lipids increase the efficiency of gene transfer in vitro and in vivo. J. Biol. Chem. 1997, 272, 6479–6489. [Google Scholar] [CrossRef]
- Croyle, M.A.; Yu, Q.C.; Wilson, J.M. Development of a rapid method for the PEGylation of adenoviruses with enhanced transduction and improved stability under harsh storage conditions. Hum. Gene Ther. 2000, 11, 1713–1722. [Google Scholar] [CrossRef]
- Mok, H.; Palmer, D.J.; Ng, P.; Barry, M.A. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol. Ther. 2005, 11, 66–79. [Google Scholar] [CrossRef]
- Wortmann, A.; Vohringer, S.; Engler, T.; Corjon, S.; Schirmbeck, R.; Reimann, J.; Kochanek, S.; Kreppel, F. Fully detargeted polyethylene glycol-coated adenovirus vectors are potent genetic vaccines and escape from pre-existing anti-adenovirus antibodies. Mol. Ther. 2008, 16, 154–162. [Google Scholar] [CrossRef]
- Hofherr, S.E.; Shashkova, E.V.; Weaver, E.A.; Khare, R.; Barry, M.A. Modification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol. Ther. 2008, 16, 1276–1282. [Google Scholar] [CrossRef]
- Fisher, K.D.; Stallwood, Y.; Green, N.K.; Ulbrich, K.; Mautner, V.; Seymour, L.W. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 2001, 8, 341–348. [Google Scholar] [CrossRef]
- Huang, S.K.; Lee, K.D.; Hong, K.; Friend, D.S.; Papahadjopoulos, D. Microscopic localization of sterically stabilized liposomes in colon carcinoma-bearing mice. Cancer Res. 1992, 52, 5135–5143. [Google Scholar]
- Huang, S.K.; Mayhew, E.; Gilani, S.; Lasic, D.D.; Martin, F.J.; Papahadjopoulos, D. Pharmacokinetics and therapeutics of sterically stabilized liposomes in mice bearing C-26 colon carcinoma. Cancer Res. 1992, 52, 6774–6781. [Google Scholar] [PubMed]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Torchilin, V.P.; Jain, R.K. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995, 55, 3752–3756. [Google Scholar]
- Romanczuk, H.; Galer, C.E.; Zabner, J.; Barsomian, G.; Wadsworth, S.C.; O’Riordan, C.R. Modification of an adenoviral vector with biologically selected peptides: a novel strategy for gene delivery to cells of choice. Hum. Gene Ther. 1999, 10, 2615–2626. [Google Scholar] [CrossRef]
- Lanciotti, J.; Song, A.; Doukas, J.; Sosnowski, B.; Pierce, G.; Gregory, R.; Wadsworth, S.; O’Riordan, C. Targeting adenoviral vectors using heterofunctional polyethylene glycol FGF2 conjugates. Mol. Ther. 2003, 8, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.L.; Gonzalez, A.M.; Printz, M.A.; Doukas, J.; Ying, W.; D’Andrea, M.; Hoganson, D.K.; Curiel, D.T.; Douglas, J.T.; Sosnowski, B.A.; Baird, A.; Aukerman, S.L.; Pierce, G.F. Fibroblast growth factor 2 retargeted adenovirus has redirected cellular tropism: evidence for reduced toxicity and enhanced antitumor activity in mice. Cancer Res. 1999, 59, 2608–2614. [Google Scholar] [PubMed]
- Bikfalvi, A.; Sauzeau, C.; Moukadiri, H.; Maclouf, J.; Busso, N.; Bryckaert, M.; Plouet, J.; Tobelem, G. Interaction of vasculotropin/vascular endothelial cell growth factor with human umbilical vein endothelial cells: binding, internalization, degradation, and biological effects. J. Cell. Physiol. 1991, 149, 50–59. [Google Scholar] [CrossRef]
- Parker, A.L.; Fisher, K.D.; Oupicky, D.; Read, M.L.; Nicklin, S.A.; Baker, A.H.; Seymour, L.W. Enhanced gene transfer activity of peptide-targeted gene-delivery vectors. J. Drug Targeting 2005, 13, 39–51. [Google Scholar] [CrossRef]
- Stevenson, M.; Hale, A.B.; Hale, S.J.; Green, N.K.; Black, G.; Fisher, K.D.; Ulbrich, K.; Fabra, A.; Seymour, L.W. Incorporation of a laminin-derived peptide (SIKVAV) on polymer-modified adenovirus permits tumor-specific targeting via alpha6-integrins. Cancer Gene Ther. 2007, 14, 335–345. [Google Scholar] [CrossRef]
- Ramos, D.M.; Berston, E.D.; Kramer, R.H. Analysis of integrin receptors for laminin and type IV collagen on metastatic B16 melanoma cells. Cancer Res. 1990, 50, 728–734. [Google Scholar] [PubMed]
- Lin, C. S.; Zhang, K.; Kramer, R. Alpha 6 integrin is up-regulated in step increments accompanying neoplastic transformation and tumorigenic conversion of human fibroblasts. Cancer Res. 1993, 53, 2950–2953. [Google Scholar] [PubMed]
- Vogelmann, R.; Kreuser, E.D.; Adler, G.; Lutz, M.P. Integrin alpha6beta1 role in metastatic behavior of human pancreatic carcinoma cells. Int. J. Cancer 1999, 80, 791–795. [Google Scholar] [CrossRef]
- Morrison, J.; Briggs, S.S.; Green, N.; Fisher, K.; Subr, V.; Ulbrich, K.; Kehoe, S.; Seymour, L.W. Virotherapy of ovarian cancer with polymer-cloaked adenovirus retargeted to the epidermal growth factor receptor. Mol. Ther. 2008, 16, 244–251. [Google Scholar] [CrossRef]
- Morrison, J.; Briggs, S.S.; Green, N.K.; Thoma, C.; Fisher, K.D.; Kehoe, S.; Seymour, L.W. Cetuximab retargeting of adenovirus via the epidermal growth factor receptor for treatment of intraperitoneal ovarian cancer. Hum. Gene Ther. 2009, 20, 239–251. [Google Scholar] [CrossRef]
- Willemsen, R.A.; Pechar, M.; Carlisle, R.C.; Schooten, E.; Pola, R.; Thompson, A.J.; Seymour, L.W.; Ulbrich, K. Multi-component Polymeric System for Tumour Cell-Specific Gene Delivery Using a Universal Bungarotoxin Linker. Pharm. Res. 2010. [Google Scholar] [CrossRef]
- Wang, I.J.; Jhuang, M.C.; Chen, Y.H.; Yeh, L.K.; Liu, C.Y.; Young, T.H. Chitosan modification of adenovirus to modify transfection efficiency in bovine corneal epithelial cells. PLoS One 2010, 5, e12085. [Google Scholar] [CrossRef]
- Harding, S.E. Mucoadhesive interactions. Biochem. Soc. Trans. 2003, 31, 1036–1041. [Google Scholar] [CrossRef]
- Green, N.K.; Morrison, J.; Hale, S.; Briggs, S.S.; Stevenson, M.; Subr, V.; Ulbrich, K.; Chandler, L.; Mautner, V.; Seymour, L.W.; Fisher, K.D. Retargeting polymer-coated adenovirus to the FGF receptor allows productive infection and mediates efficacy in a peritoneal model of human ovarian cancer. J. Gene Med. 2008, 10, 280–289. [Google Scholar] [CrossRef]
- Pearce, O.M.; Fisher, K.D.; Humphries, J.; Seymour, L.W.; Smith, A.; Davis, B.G. Glycoviruses: chemical glycosylation retargets adenoviral gene transfer. Angew. Chem. Int. Ed. Engl. 2005, 44, 1057–1061. [Google Scholar] [CrossRef]
- Koizumi, N.; Yamaguchi, T.; Kawabata, K.; Sakurai, F.; Sasaki, T.; Watanabe, Y.; Hayakawa, T.; Mizuguchi, H. Fiber-modified adenovirus vectors decrease liver toxicity through reduced IL-6 production. J. Immunol. 2007, 178, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.F.; Fella, C.; Pelisek, J.; Fahrmeir, J.; Boeckle, S.; Ogris, M.; Wagner, E. Toward synthetic viruses: endosomal pH-triggered deshielding of targeted polyplexes greatly enhances gene transfer in vitro and in vivo. Mol. Ther. 2005, 11, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.L.; Spencer, J.F.; Doronin, K.; Patra, D.; Meyer, J.M.; Shashkova, E.V.; Kuppuswamy, M.; Dhar, D.; Thomas, M.A.; Tollefson, A.E.; Zumstein, L.A.; Wold, W.S.; Toth, K. An acute toxicology study with INGN 007, an oncolytic adenovirus vector, in mice and permissive Syrian hamsters; comparisons with wild-type Ad5 and a replication-defective adenovirus vector. Cancer Gene Ther. 2009, 16, 644–654. [Google Scholar] [CrossRef]
- Ying, B.; Toth, K.; Spencer, J.F.; Meyer, J.; Tollefson, A.E.; Patra, D.; Dhar, D.; Shashkova, E.V.; Kuppuswamy, M.; Doronin, K.; Thomas, M.A.; Zumstein, L. A.; Wold, W.S.; Lichtenstein, D. L. INGN 007, an oncolytic adenovirus vector, replicates in Syrian hamsters but not mice: comparison of biodistribution studies. Cancer Gene Ther. 2009, 16, 625–637. [Google Scholar] [CrossRef]
- Lievens, J.; Snoeys, J.; Vekemans, K.; Van Linthout, S.; de Zanger, R.; Collen, D.; Wisse, E.; De Geest, B. The size of sinusoidal fenestrae is a critical determinant of hepatocyte transduction after adenoviral gene transfer. Gene Ther. 2004, 11, 1523–1531. [Google Scholar] [CrossRef]
- Wisse, E.; Jacobs, F.; Topal, B.; Frederik, P.; De Geest, B. The size of endothelial fenestrae in human liver sinusoids: implications for hepatocyte-directed gene transfer. Gene Ther. 2008, 15, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, F.; Wisse, E.; De Geest, B. The role of liver sinusoidal cells in hepatocyte-directed gene transfer. Am. J. Pathol. 2010, 176, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Cooper, A.; DeFord, M.E.; Carmeliet, P.; Collen, D.; Castellino, F.J.; Rosen, E.D. Cloning and characterization of a cDNA encoding murine coagulation factor X. Thromb. Haemost. 1998, 80, 87–91. [Google Scholar] [CrossRef]
- Lenaerts, L.; McVey, J.H.; Baker, A.H.; Denby, L.; Nicklin, S.; Verbeken, E.; Naesens, L. Mouse adenovirus type 1 and human adenovirus type 5 differ in endothelial cell tropism and liver targeting. J. Gene Med. 2009, 11, 119–127. [Google Scholar] [CrossRef]
- Falanga, A. Thrombophilia in cancer. Semin. Thromb. Hemost. 2005, 31, 104–110. [Google Scholar] [CrossRef]
- Ferrigno, D.; Buccheri, G.; Ricca, I. Prognostic significance of blood coagulation tests in lung cancer. Eur. Respir. J. 2001, 17, 667–673. [Google Scholar] [CrossRef]
- Iversen, N.; Lindahl, A.K.; Abildgaard, U. Elevated plasma levels of the factor Xa-TFPI complex in cancer patients. Thromb. Res. 2002, 105, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Atencio, I.A.; Grace, M.; Bordens, R.; Fritz, M.; Horowitz, J.A.; Hutchins, B.; Indelicato, S.; Jacobs, S.; Kolz, K.; Maneval, D.; Musco, M.L.; Shinoda, J.; Venook, A.; Wen, S.; Warren, R. Biological activities of a recombinant adenovirus p53 (SCH 58500) administered by hepatic arterial infusion in a Phase 1 colorectal cancer trial. Cancer Gene Ther. 2006, 13, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Reid, T.; Galanis, E.; Abbruzzese, J.; Sze, D.; Wein, L.M.; Andrews, J.; Randlev, B.; Heise, C.; Uprichard, M.; Hatfield, M.; Rome, L.; Rubin, J.; Kirn, D. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002, 62, 6070–6079. [Google Scholar]
- Reid, T.; Warren, R.; Kirn, D. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther. 2002, 9, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Raper, S.E.; Yudkoff, M.; Chirmule, N.; Gao, G.P.; Nunes, F.; Haskal, Z.J.; Furth, E.E.; Propert, K.J.; Robinson, M.B.; Magosin, S.; Simoes, H.; Speicher, L.; Hughes, J.; Tazelaar, J.; Wivel, N.A.; Wilson, J.M.; Batshaw, M.L. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum. Gene Ther. 2002, 13, 163–175. [Google Scholar] [CrossRef]
- Di Paolo, N.C.; Miao, E.A.; Iwakura, Y.; Murali-Krishna, K.; Aderem, A.; Flavell, R.A.; Papayannopoulou, T.; Shayakhmetov, D.M. Virus Binding to a Plasma Membrane Receptor Triggers Interleukin-1alpha-Mediated Proinflammatory Macrophage Response. Immunity 2009, 31, 110–121. [Google Scholar] [CrossRef]
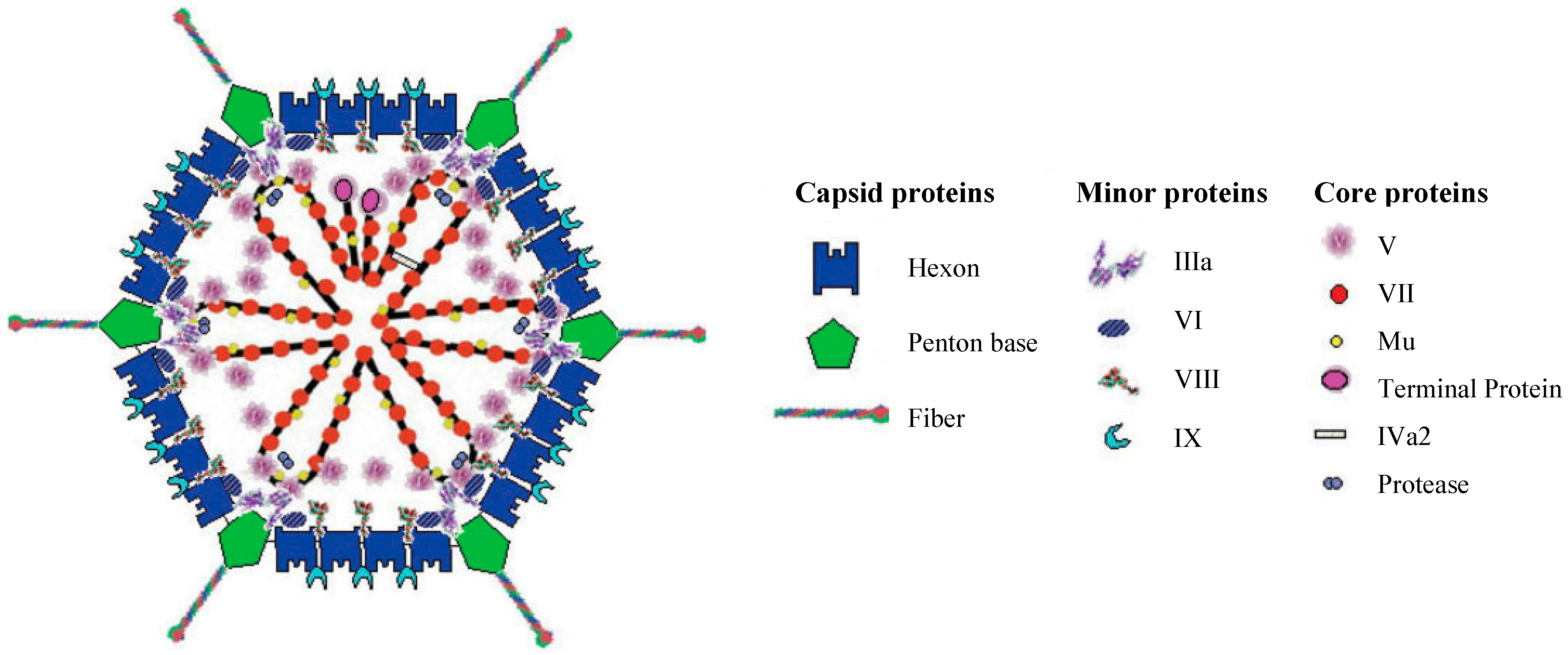
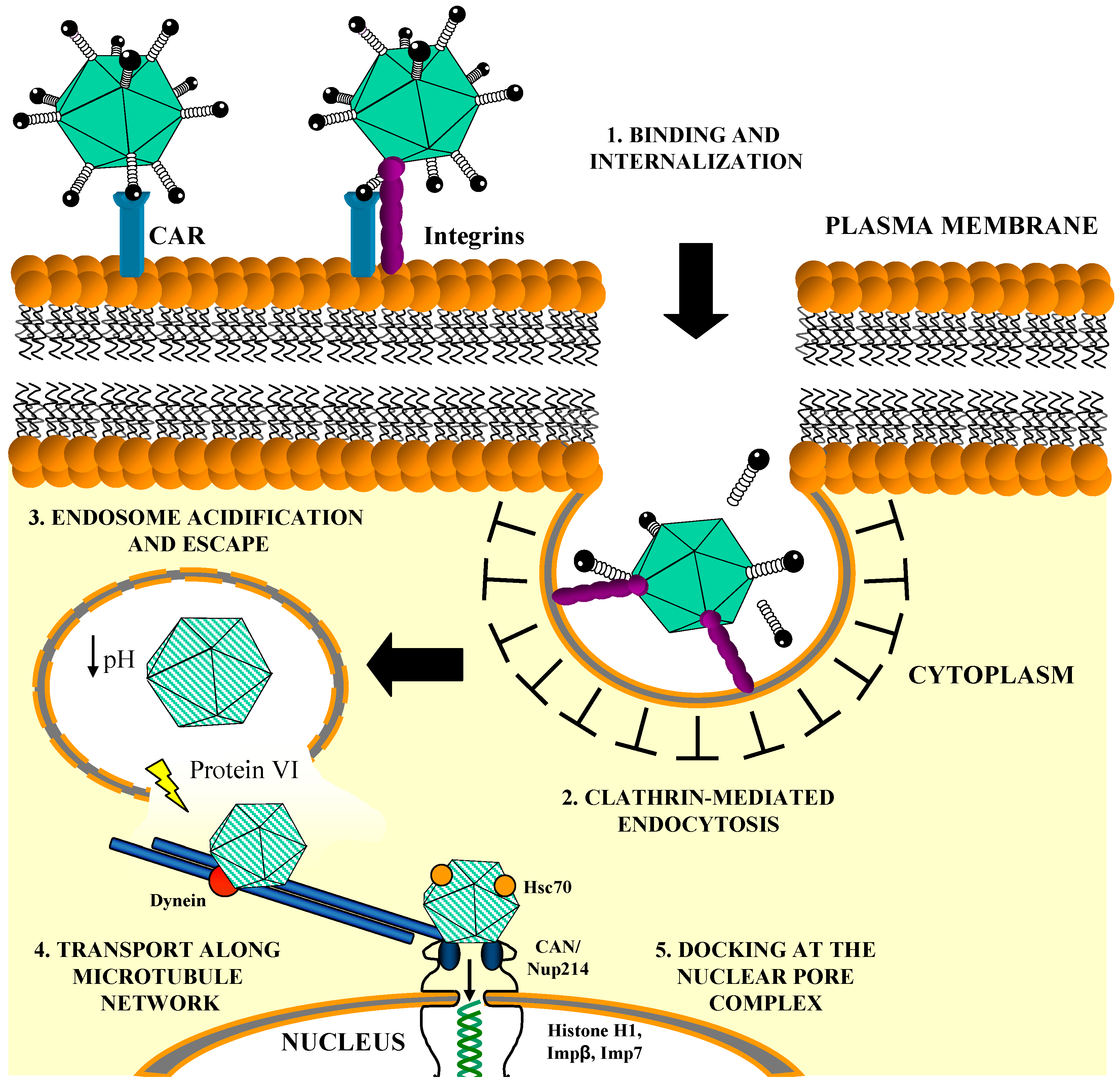
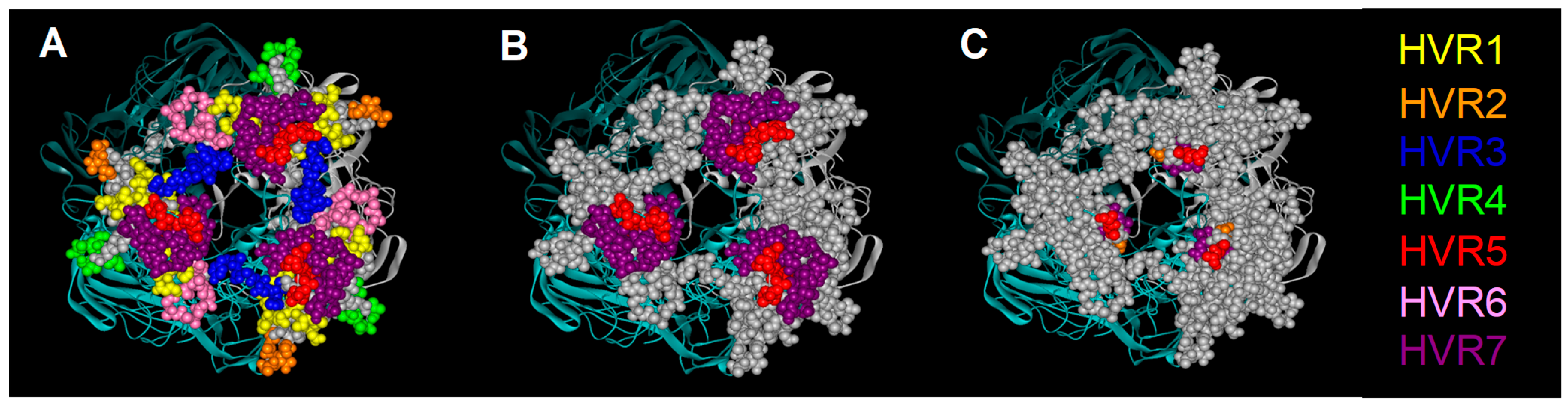
© 2010 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Coughlan, L.; Alba, R.; Parker, A.L.; Bradshaw, A.C.; McNeish, I.A.; Nicklin, S.A.; Baker, A.H. Tropism-Modification Strategies for Targeted Gene Delivery Using Adenoviral Vectors. Viruses 2010, 2, 2290-2355. https://doi.org/10.3390/v2102290
Coughlan L, Alba R, Parker AL, Bradshaw AC, McNeish IA, Nicklin SA, Baker AH. Tropism-Modification Strategies for Targeted Gene Delivery Using Adenoviral Vectors. Viruses. 2010; 2(10):2290-2355. https://doi.org/10.3390/v2102290
Chicago/Turabian StyleCoughlan, Lynda, Raul Alba, Alan L. Parker, Angela C. Bradshaw, Iain A. McNeish, Stuart A. Nicklin, and Andrew H. Baker. 2010. "Tropism-Modification Strategies for Targeted Gene Delivery Using Adenoviral Vectors" Viruses 2, no. 10: 2290-2355. https://doi.org/10.3390/v2102290
APA StyleCoughlan, L., Alba, R., Parker, A. L., Bradshaw, A. C., McNeish, I. A., Nicklin, S. A., & Baker, A. H. (2010). Tropism-Modification Strategies for Targeted Gene Delivery Using Adenoviral Vectors. Viruses, 2(10), 2290-2355. https://doi.org/10.3390/v2102290




