Anti-HIV-1 Activity of the Integrase Strand Transfer Inhibitor ACC017
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Compounds
2.3. Cells and Viruses
2.4. Cytotoxicity Assays
2.5. Anti-HIV-1 Activities Assay
2.6. Combined Antiviral Activity Assay
2.7. In Vitro HIV-1 Integrase Inhibitory Assay
2.8. Molecular Docking Assay
2.9. Determination of Late-RT, 2LTR and Alu-LTR
2.10. In Vitro Selection of HIV-1 Mutant Strains and Analysis of Drug Resistance
2.11. Data Statistics
3. Results and Discussion
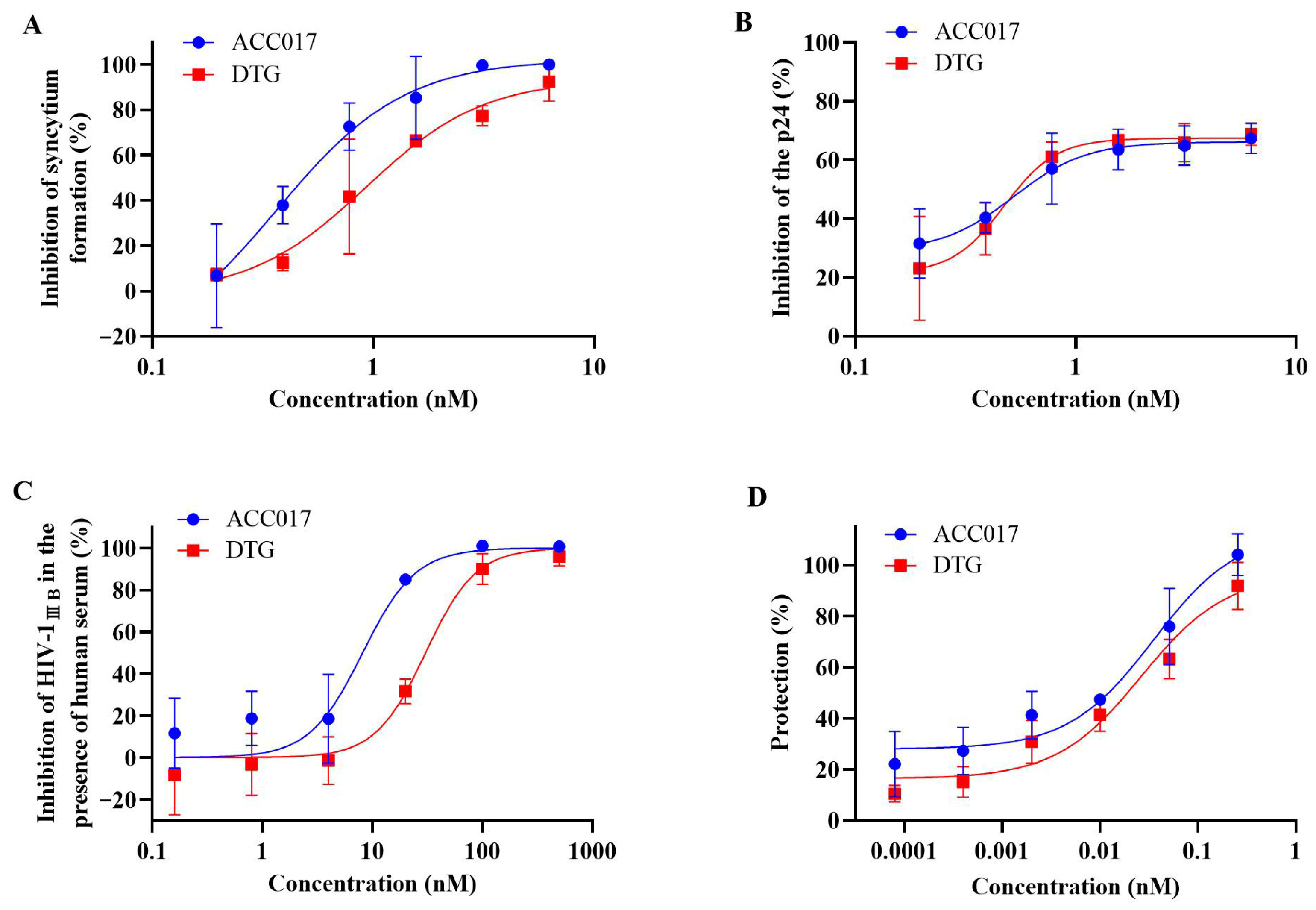
3.1. Activity of ACC017 Against HIV-1
3.2. Inhibitory Activity Against HIV-1 Integrase
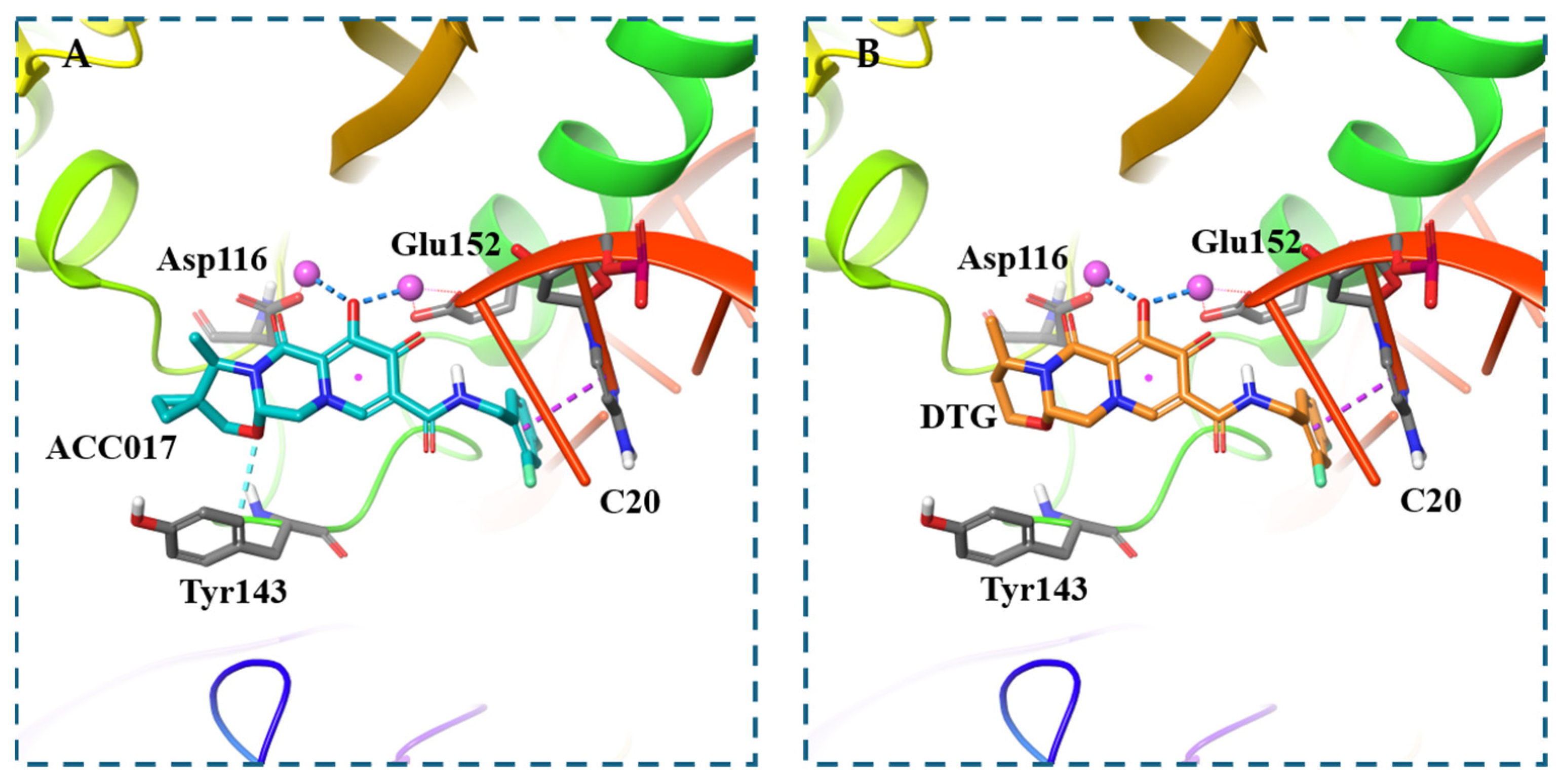
3.3. Molecular Docking Study
3.4. Antiviral Activity of ACC017 in Combination with Anti-HIV-1 Drugs
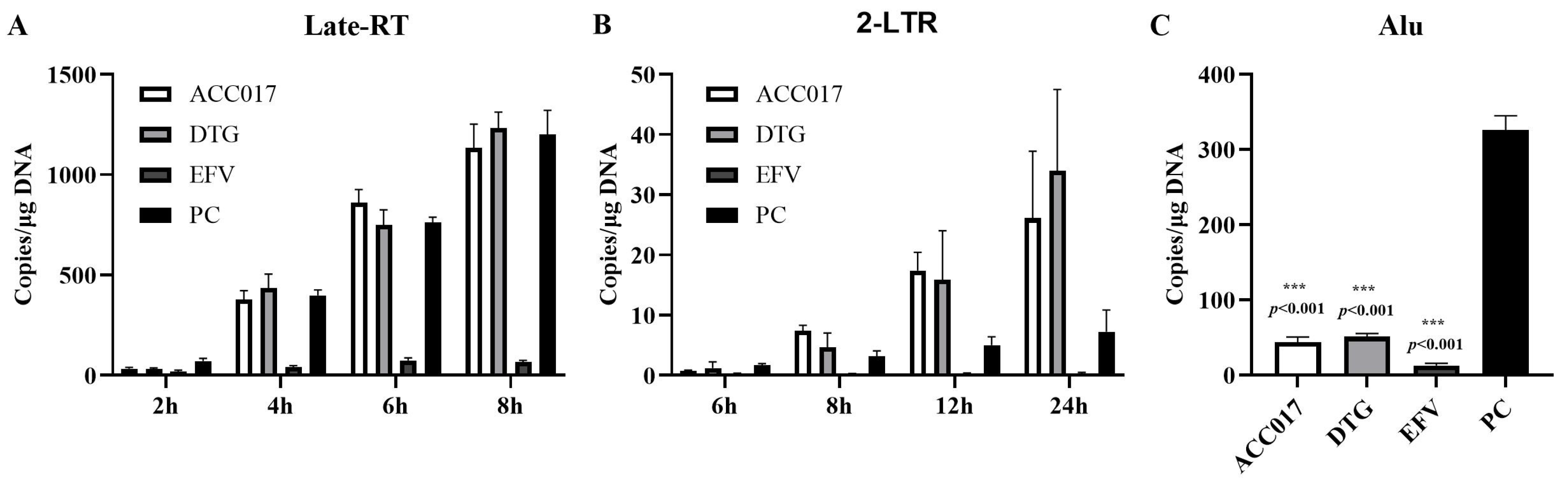
3.5. Effects of ACC017 on Formation of Late-RT, 2-LTR and Alu-LTR
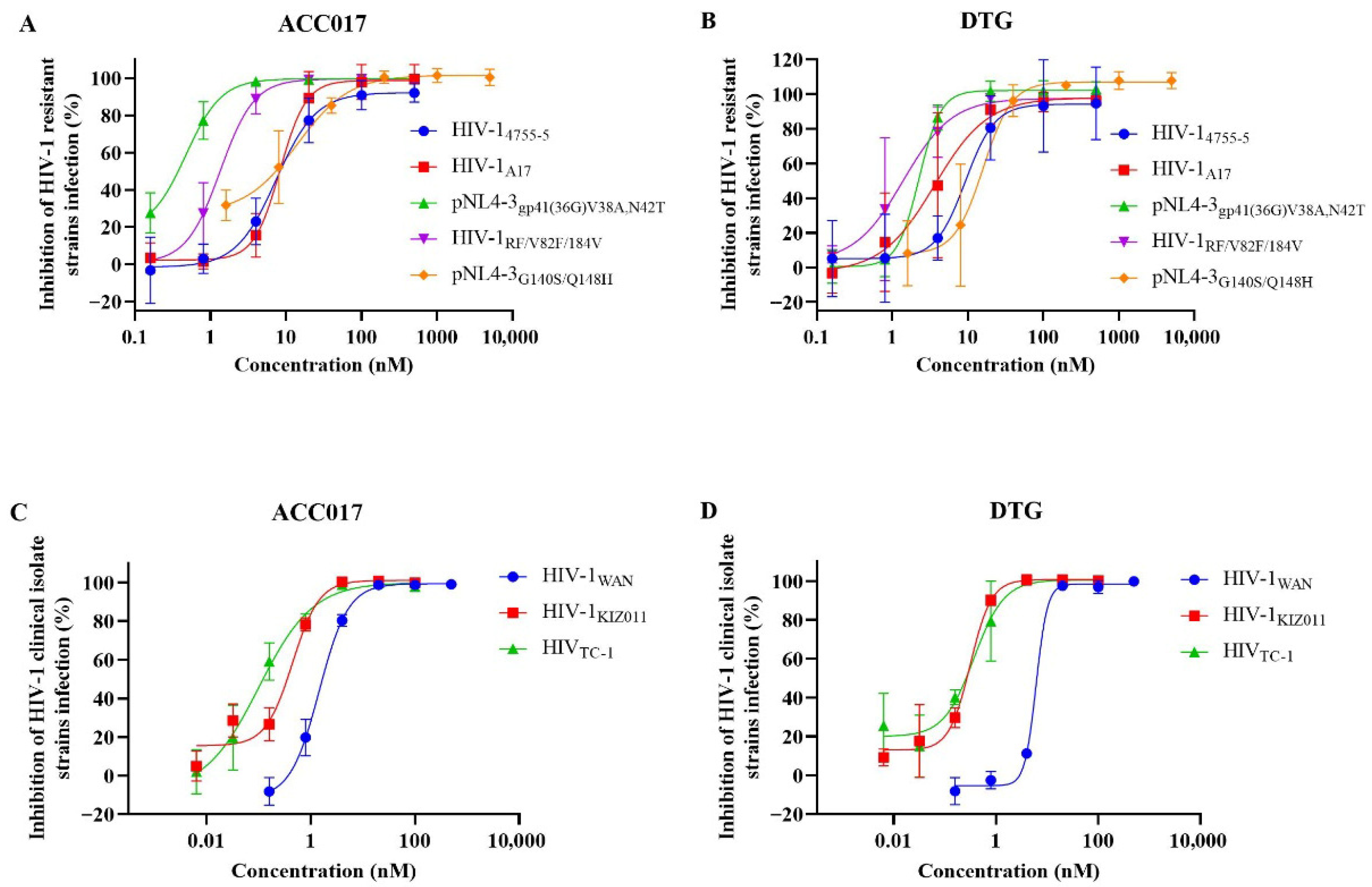
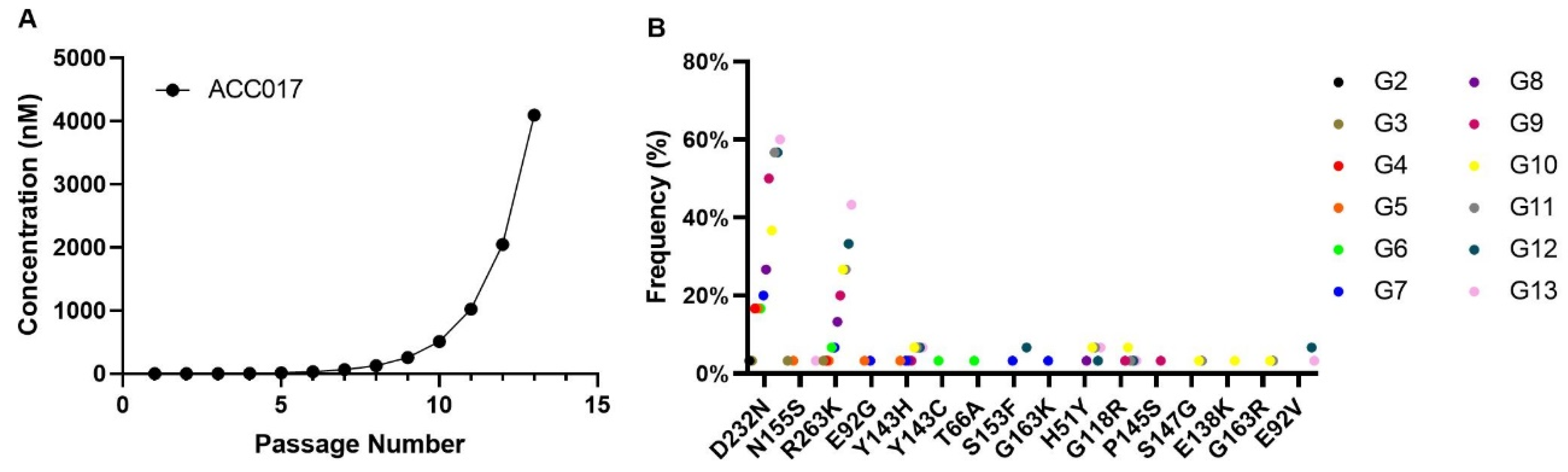
3.6. In Vitro Induction of ACC017-Resistant Strains and Analysis of Drug Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- HIV Data and Statistics. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics (accessed on 14 September 2025).
- Sever, B.; Otsuka, M.; Fujita, M.; Ciftci, H. A Review of FDA-Approved Anti-HIV-1 Drugs, Anti-Gag Compounds, and Potential Strategies for HIV-1 Eradication. Int. J. Mol. Sci. 2024, 25, 3659. [Google Scholar] [CrossRef]
- Bekker, L.G.; Beyrer, C.; Mgodi, N.; Lewin, S.R.; Delany-Moretlwe, S.; Taiwo, B.; Masters, M.C.; Lazarus, J.V. HIV infection. Nat. Rev. Dis. Primers 2023, 9, 42. [Google Scholar] [CrossRef]
- Cahn, P.; Sued, O. Raltegravir: A new antiretroviral class for salvage therapy. Lancet 2007, 369, 1235–1236. [Google Scholar] [CrossRef]
- Delelis, O.; Carayon, K.; Saïb, A.; Deprez, E.; Mouscadet, J.F. Integrase and integration: Biochemical activities of HIV-1 integrase. Retrovirology 2008, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Maertens, G.N.; Engelman, A.N.; Cherepanov, P. Structure and function of retroviral integrase. Nat. Rev. Microbiol. 2022, 20, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Shalbi, F.; Ali, A.R. A mini-review on integrase inhibitors: The cornerstone of next-generation HIV treatment. Eur. J. Med. Chem. 2024, 279, 116900. [Google Scholar] [CrossRef]
- Jóźwik, I.K.; Passos, D.O.; Lyumkis, D. Structural Biology of HIV Integrase Strand Transfer Inhibitors. Trends Pharmacol. Sci. 2020, 41, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Scarsi, K.K.; Havens, J.P.; Podany, A.T.; Avedissian, S.N.; Fletcher, C.V. HIV-1 Integrase Inhibitors: A Comparative Review of Efficacy and Safety. Drugs 2020, 80, 1649–1676. [Google Scholar] [CrossRef]
- Wensing, A.M.; Calvez, V.; Ceccherini-Silberstein, F.; Charpentier, C.; Günthard, H.F.; Jacobsen, D.M.; Paredes, R.; Shafer, R.W.; Richman, D.D. 2025 update of the drug resistance mutations in HIV-1. Top. Antivir. Med. 2025, 33, 457–473. [Google Scholar]
- Blanco, J.L.; Marcelin, A.G.; Katlama, C.; Martinez, E. Dolutegravir resistance mutations: Lessons from monotherapy studies. Curr. Opin. Infect. Dis. 2018, 31, 237–245. [Google Scholar] [CrossRef]
- Lübke, N.; Jensen, B.; Hüttig, F.; Feldt, T.; Walker, A.; Thielen, A.; Däumer, M.; Obermeier, M.; Kaiser, R.; Knops, E.; et al. Failure of Dolutegravir First-Line ART with Selection of Virus Carrying R263K and G118R. N. Engl. J. Med. 2019, 381, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.Y.; Grant, P.M.; Tzou, P.L.; Barrow, G.; Harrigan, P.R.; Ioannidis, J.P.A.; Shafer, R.W. A systematic review of the genetic mechanisms of dolutegravir resistance. J. Antimicrob. Chemother. 2019, 74, 3135–3149. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Zhao, X.Z.; Passos, D.O.; Lyumkis, D.; Burke, T.R., Jr.; Hughes, S.H. HIV-1 Integrase Inhibitors That Are Active against Drug-Resistant Integrase Mutants. Antimicrob. Agents Chemother. 2020, 64, e00611–e00620. [Google Scholar] [CrossRef]
- Parikh, U.M.; Koss, C.A.; Mellors, J.W. Long-Acting Injectable Cabotegravir for HIV Prevention: What Do We Know and Need to Know about the Risks and Consequences of Cabotegravir Resistance? Curr. HIV/AIDS Rep. 2022, 19, 384–393. [Google Scholar] [CrossRef]
- Tsiang, M.; Jones, G.S.; Goldsmith, J.; Mulato, A.; Hansen, D.; Kan, E.; Tsai, L.; Bam, R.A.; Stepan, G.; Stray, K.M.; et al. Antiviral Activity of Bictegravir (GS-9883), a Novel Potent HIV-1 Integrase Strand Transfer Inhibitor with an Improved Resistance Profile. Antimicrob. Agents Chemother. 2016, 60, 7086–7097. [Google Scholar] [CrossRef]
- Blanco, J.L.; Varghese, V.; Rhee, S.Y.; Gatell, J.M.; Shafer, R.W. HIV-1 integrase inhibitor resistance and its clinical implications. J. Infect. Dis. 2011, 203, 1204–1214. [Google Scholar] [CrossRef]
- Malet, I.; Delelis, O.; Valantin, M.A.; Montes, B.; Soulie, C.; Wirden, M.; Tchertanov, L.; Peytavin, G.; Reynes, J.; Mouscadet, J.F.; et al. Mutations associated with failure of raltegravir treatment affect integrase sensitivity to the inhibitor in vitro. Antimicrob. Agents Chemother. 2008, 52, 1351–1358. [Google Scholar] [CrossRef]
- Evering, T.H.; Markowitz, M. Raltegravir: An integrase inhibitor for HIV-1. Expert Opin. Investig. Drugs 2008, 17, 413–422. [Google Scholar] [CrossRef]
- Quashie, P.K.; Mesplède, T.; Wainberg, M.A. Evolution of HIV integrase resistance mutations. Curr. Opin. Infect. Dis. 2013, 26, 43–49. [Google Scholar] [CrossRef]
- Deeks, E.D. Elvitegravir: A review of its use in adults with HIV-1 infection. Drugs 2014, 74, 687–697. [Google Scholar] [CrossRef] [PubMed]
- White, K.; Kulkarni, R.; Miller, M.D. Analysis of early resistance development at the first failure timepoint in elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate-treated patients. J. Antimicrob. Chemother. 2015, 70, 2632–2638. [Google Scholar] [CrossRef]
- Mbhele, N.; Chimukangara, B.; Gordon, M. HIV-1 integrase strand transfer inhibitors: A review of current drugs, recent advances and drug resistance. Int. J. Antimicrob. Agents 2021, 57, 106343. [Google Scholar] [CrossRef]
- Cid-Silva, P.; Llibre, J.M.; Fernández-Bargiela, N.; Margusino-Framiñán, L.; Balboa-Barreiro, V.; Pernas-Souto, B.; Martín-Herranz, I.; Castro-Iglesias, Á.; Poveda, E. Clinical Experience with the Integrase Inhibitors Dolutegravir and Elvitegravir in HIV-infected Patients: Efficacy, Safety and Tolerance. Basic Clin. Pharmacol. Toxicol. 2017, 121, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Bourgi, K.; Rebeiro, P.F.; Turner, M.; Castilho, J.L.; Hulgan, T.; Raffanti, S.P.; Koethe, J.R.; Sterling, T.R. Greater Weight Gain in Treatment-naive Persons Starting Dolutegravir-based Antiretroviral Therapy. Clin. Infect. Dis. 2020, 70, 1267–1274. [Google Scholar] [CrossRef]
- Tiendrebeogo, T.; Malateste, K.; Poda, A.; Minga, A.; Lahiri, C.D.; Ezechi, O.; Ekouevi, D.K.; Ofotokun, I.; Jaquet, A. Impact of switching to a dolutegravir-based regimen on body weight changes: Insights from West African adult HIV cohorts. J. Int. AIDS Soc. 2024, 27, e26371. [Google Scholar] [CrossRef]
- Stellbrink, H.J.; Arribas, J.R.; Stephens, J.L.; Albrecht, H.; Sax, P.E.; Maggiolo, F.; Creticos, C.; Martorell, C.T.; Wei, X.; Acosta, R.; et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: Week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV 2019, 6, e364–e372. [Google Scholar]
- Landovitz, R.J.; Donnell, D.; Clement, M.E.; Hanscom, B.; Cottle, L.; Coelho, L.; Cabello, R.; Chariyalertsak, S.; Dunne, E.F.; Frank, I.; et al. Cabotegravir for HIV Prevention in Cisgender Men and Transgender Women. N. Engl. J. Med. 2021, 385, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Ma, M.D.; Lu, N.; Yang, Y.Z.; Yang, J.X.; Li, Y.M.; Xie, C.Q.; Ma, N.Y.; Luo, R.H.; Wang, Y.P.; et al. Synthesis and anti-HIV activity of non-nucleoside reverse-transcriptase inhibitor DB02 phosphate derivatives based on water-soluble optimization. Drug Dev. Res. 2023, 84, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Tang, C.R.; Rui, R.M.; Yang, L.M.; Ding, W.; Wang, J.Y.; Li, Y.M.; Lai, C.C.; Wang, Y.P.; Luo, R.H.; et al. Synthesis and biological evaluation of a series of 2-(((5-akly/aryl-1H-pyrazol-3-yl)methyl)thio)-5-alkyl-6-(cyclohexylmethyl)-pyrimidin-4(3H)-ones as potential HIV-1 inhibitors. Acta Pharm. Sin. B 2020, 10, 512–528. [Google Scholar] [CrossRef]
- Kang, D.W.; Yang, J.X.; Kong, L.J.; Luo, R.H.; Huang, X.S.; Zhang, T.; Ma, M.D.; Feng, D.; Wang, Z.; Fang, H.; et al. Structure-Based Discovery and Characterization of a Preclinical Drug Candidate for the Treatment of HIV-1 Infection. Viruses 2022, 14, 2390. [Google Scholar] [CrossRef]
- Huang, X.S.; Luo, R.H.; Hu, X.L.; Chen, H.; Xiang, S.Y.; Tang, C.R.; Zhang, C.T.; Shen, X.N.; Zheng, Y.T. The New NNRTI ACC007 Combined with Lamivudine and Tenofovir Disoproxil Fumarate Show Synergy Anti-HIV Activity In Vitro. Curr. HIV Res. 2020, 18, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Bilello, J.A.; Bilello, P.A.; Stellrecht, K.; Leonard, J.; Norbeck, D.W.; Kempf, D.J.; Robins, T.; Drusano, G.L. Human serum alpha 1 acid glycoprotein reduces uptake, intracellular concentration, and antiviral activity of A-80987, an inhibitor of the human immunodeficiency virus type 1 protease. Antimicrob. Agents Chemother. 1996, 40, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
| Virus a | Cell | CC50 (μM) b (Mean ± SD) | Testing Method | EC50 (nM) c (Mean ± SD) | SI d | |||
|---|---|---|---|---|---|---|---|---|
| ACC017 | DTG | ACC017 | DTG | ACC017 | DTG | |||
| HIV-1IIIB | C8166 | 20.37 ± 0.10 | 20.34 ± 0.07 | Syncytia | 0.59 ± 0.09 | 0.73 ± 0.02 | 34,525.42 | 27,863.01 |
| HIV-1IIIB | C8166 | 20.37 ± 0.10 | 20.34 ± 0.07 | p24 | 0.59 ± 0.09 | 0.64 ± 0.07 | 34,525.42 | 31,781.25 |
| HIV-1IIIB (20% HS) e | C8166 | / f | / | p24 | 8.28 ± 2.02 | 33.57 ± 5.90 | / | / |
| HIV-1IIIB | MT4 | 24.80 ± 0.08 | 24.91 ± 0.01 | MTT | 51.47 ± 0.02 | 51.44 ± 0.14 | 496,000.00 | 498,200.00 |
| (pM) | (pM) | |||||||
| HIV-14755-5 | C8166 | 20.37 ± 0.10 | 20.34 ± 0.07 | p24 | 9.12 ± 2.31 | 9.96 ± 2.78 | 2233.55 | 2042.17 |
| HIV-1A17 | C8166 | 20.37 ± 0.10 | 20.34 ± 0.07 | p24 | 8.51 ± 0.25 | 4.78 ± 5.13 | 2393.65 | 4255.23 |
| pNL4-3gp41(36G) V38A, N42T | C8166 | 20.37 ± 0.10 | 20.34 ± 0.07 | p24 | 0.34 ± 0.09 | 1.95 ± 0.12 | 59,911.76 | 10,430.77 |
| HIV-1RF/V82F/184V | C8166 | 20.37 ± 0.10 | 20.34 ± 0.07 | p24 | 1.45 ± 0.48 | 1.69 ± 1.22 | 14,048.28 | 12,035.50 |
| pNL4-3G140S/Q148H | C8166 | 20.37 ± 0.10 | 20.34 ± 0.07 | p24 | 8.19 ± 4.88 | 13.46 ± 8.41 | 2487.18 | 1511.14 |
| HIV-1WAN | PBMC | 15.24 ± 1.15 | 14.56 ± 3.62 | p24 | 1.78 ± 0.29 | 8.23 ± 0.20 | 8561.80 | 1769.14 |
| HIV-1KIZ011 | PBMC | 15.24 ± 1.15 | 14.56 ± 3.62 | p24 | 0.33 ± 0.06 | 0.28 ± 0.03 | 46,181.82 | 52,000.00 |
| HIV-1TC-1 | PBMC | 15.24 ± 1.15 | 14.56 ± 3.62 | p24 | 0.11 ± 0.06 | 0.31 ± 0.18 | 138,545.45 | 46,967.74 |
| Compd. | IC50 (nM) (Mean, n = 4) a | |
|---|---|---|
| HIV-1 WT | HIV-1N155H | |
| ACC017 | 9.19 | 9.91 |
| DTG | 13.60 | 9.26 |
| Drugs | Drug Type | Single Drug | DRI Value a | CI Value f | Description | |||
|---|---|---|---|---|---|---|---|---|
| ED50 b | ED75 c | ED90 d | ED95 e | |||||
| ACC017 + ACC017 | INSTIs | ACC017 | 1.56 | 1.77 | 2.06 | 2.32 | 0.95 ± 0.33 | additive |
| INSTIs | ACC017 | 4.48 | 3.46 | 2.77 | 2.42 | |||
| ACC017 + DTG | INSTIs | ACC017 | 3.65 | 2.89 | 2.45 | 2.27 | 1.57 ± 0.87 | antagonism |
| INSTIs | DTG | 1.80 | 1.40 | 1.40 | 1.60 | |||
| ACC017 + 3TC | INSTIs | ACC017 | 3.04 | 5.25 | 9.07 | 13.15 | 0.54 ± 0.24 | synergism |
| NRTIs | 3TC | 4.89 | 6.21 | 7.90 | 9.30 | |||
| DTG + 3TC | INSTIs | DTG | 2.34 | 3.00 | 4.01 | 4.98 | 0.51 ± 0.32 | synergism |
| NRTIs | 3TC | 3.73 | 5.03 | 6.79 | 8.33 | |||
| ACC017 + ACC007 | INSTIs | ACC017 | 2.61 | 4.07 | 6.48 | 8.97 | 0.38 ± 0.12 | synergism |
| NNRTIs | ACC007 | 2.41 | 3.77 | 6.12 | 8.66 | |||
| DTG + ACC007 | INSTIs | DTG | 2.27 | 3.00 | 4.13 | 5.21 | 0.49 ± 0.22 | synergism |
| NNRTIs | ACC007 | 3.03 | 4.66 | 7.34 | 10.16 | |||
| ACC017 + FTC + TAF | INSTIs | ACC017 | 3.30 | 4.62 | 6.79 | 9.05 | 0.47 ± 0.18 | synergism |
| NRTIs | FTC | 4.90 | 7.82 | 12.57 | 17.41 | |||
| NRTIs | TAF | 3.61 | 4.78 | 6.99 | 8.96 | |||
| DTG + FTC + TAF | INSTIs | DTG | 2.46 | 3.25 | 4.37 | 5.41 | 0.61 ± 0.19 | synergism |
| NRTIs | FTC | 4.27 | 7.15 | 12.20 | 17.77 | |||
| NRTIs | TAF | 3.10 | 4.40 | 6.33 | 8.18 | |||
| Compd. | Testing Method | EC50 (nM) a | FC b | EC50 (nM) | FC | ||
|---|---|---|---|---|---|---|---|
| HIV-1IIIB | HIV-1DRACC017 | NL4-3 | NL4-3IN-R263K | ||||
| ACC017 | p24 | 1.25 ± 0.48 | >5000.00 | >4000.00 | 1.70 ± 0.47 | 28.09 ± 4.22 | 16.52 |
| DTG | p24 | 1.18 ± 0.15 | >5000.00 | >4237.29 | 2.41 ± 0.43 | 32.21 ± 3.74 | 13.37 |
| 3TC | p24 | 181.45 ± 41.48 | 322.32 ± 34.63 | 1.78 | 197.81 ± 100.51 | 180.05 ± 38.49 | 0.91 |
| EFV | p24 | 0.60 ± 0.18 | 1.24 ± 0.22 | 2.07 | 1.51 ± 0.48 | 1.62 ± 0.31 | 1.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ma, M.-D.; Luo, R.-H.; Li, C.-Y.; Huang, G.-C.; Long, X.-Y.; He, F.-Y.; Yang, L.-M.; Fu, H.-L.; Zheng, Y.-T. Anti-HIV-1 Activity of the Integrase Strand Transfer Inhibitor ACC017. Viruses 2026, 18, 33. https://doi.org/10.3390/v18010033
Ma M-D, Luo R-H, Li C-Y, Huang G-C, Long X-Y, He F-Y, Yang L-M, Fu H-L, Zheng Y-T. Anti-HIV-1 Activity of the Integrase Strand Transfer Inhibitor ACC017. Viruses. 2026; 18(1):33. https://doi.org/10.3390/v18010033
Chicago/Turabian StyleMa, Meng-Di, Rong-Hua Luo, Chun-Yan Li, Guan-Cheng Huang, Xin-Yan Long, Feng-Ying He, Liu-Meng Yang, He-Liang Fu, and Yong-Tang Zheng. 2026. "Anti-HIV-1 Activity of the Integrase Strand Transfer Inhibitor ACC017" Viruses 18, no. 1: 33. https://doi.org/10.3390/v18010033
APA StyleMa, M.-D., Luo, R.-H., Li, C.-Y., Huang, G.-C., Long, X.-Y., He, F.-Y., Yang, L.-M., Fu, H.-L., & Zheng, Y.-T. (2026). Anti-HIV-1 Activity of the Integrase Strand Transfer Inhibitor ACC017. Viruses, 18(1), 33. https://doi.org/10.3390/v18010033






