Abstract
Infectious agents account for millions of deaths every year. The Herpes Simplex Viruses (HSVs) are large double-stranded DNA viruses that infect more than 90% of the human population and can establish life-long latency in human hosts. Currently, effective FDA approved anti-herpetic drugs include acyclovir and later-generation derivatives (valacyclovir and famciclovir), which inhibit viral DNA synthesis. In previous work, we demonstrated that the small molecule Ruvidar® could inhibit numerous pathogenic human viruses when added to solutions of viruses both with and without light activation. In these experiments, we evaluated the ability of Ruvidar® to restrict HSV-1 replication in Vero cells, both by itself and in combination with acyclovir and metformin in the absence of light activation to mimic deep tissue. Ruvidar® successfully inhibited HSV-1 replication at significantly lower concentrations and more effectively than either acyclovir or metformin alone. We also discovered additive and synergistic anti-HSV-1 effects when combinational therapy was tested. Ruvidar® also restricted HSV-1 replication in human U251 glioblastoma astrocytoma cells, remained highly effective against acyclovir-resistant HSV-1 mutants, and protected infected cells from virus-induced cytopathology.
Keywords:
antivirals; lipid envelope; photodynamic therapy; Ruvidar®; Rutherrin®; TLD-1433; acyclovir; metformin 1. Introduction
Infectious agents account for millions of deaths every year (www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death, accessed on 8 January 2025). Currently, the most effective ways to protect against infection involve the use of vaccines and anti-microbials. Vaccines are useful when administered prior to infection to allow immunity to develop, whereas antibiotics and antivirals are most useful before immunity to a vaccine has had time to develop. The primary disadvantages of vaccines are that knowledge of the agent is required in advance to manufacture an effective vaccine, and substantial time is needed to produce relevant vaccines. Furthermore, the developed vaccine may not match the eventual strain that circulates [1,2]. A growing number of anti-viral agents have been developed and some are effective against numerous viruses; however, because viruses replicate and many lack genome proof-reading capabilities, resistance to the anti-viral agent may develop rapidly [3,4,5].
The herpesviruses are large double-stranded DNA viruses (reviewed in [6]). Several different types, including Herpes Simplex Virus (HSV), type 1 (HSV-1), HSV-2, Cytomegalovirus, Varicella Zoster virus (chickenpox), and Kaposi’s sarcoma human herpes virus, have been described in the literature. In addition to causing acute infections, human herpesviruses have the capacity to establish life-long latency. Since many different herpesviruses—many of which are capable of infecting more than 90% of the population—exist, they are a significant human health concern [7]. Several anti-herpetic drugs have been developed. The “gold standard” is acyclovir. Acyclovir is a nucleoside analog that inhibits viral DNA synthesis. Due to the risk of developing resistance, several derivatives, such as valacyclovir and famciclovir, have been developed. A variety of other agents, such as foscarnet and adefovir (reviewed in [8,9,10]), also are considered effective anti-herpetic compounds.
We previously demonstrated that the small molecule Ruvidar® could inhibit numerous pathogenic human viruses, when added to solutions of viruses, both with and without light activation [11]. When light-activated with green light, 2.4 nM Ruvidar® was able to inhibit 50% of HSV-1 infectivity, while 53 nM was able to inhibit 99.9% of HSV-1 infectivity. Even if not light-activated, 40 nM and 200 nM Ruvidar® were able to inhibit 50% and 99.9% HSV-1 infectivity, respectively.
Ruvidar® can be activated by several different methodologies. As a light-activated small molecule, the primary method involves activation by an appropriate wavelength and energy of light. In the case of Ruvidar®, 45 Joules per cm2 of green light (wavelength 520 nm) is sufficient to induce photoactivation and the subsequent generation of Reactive Oxygen Species (ROS), specifically singlet oxygen, which has been demonstrated to modify lipids [11]. Secondarily, Ruvidar® can be activated by various forms of ionized radiation, and thirdly by the use of ROS-producing drugs, such as the popular diabetes drug metformin (https://theralase.com/theralase-demonstrates-unique-ability-to-activate-rutherrin-with-diabetes-drug/, accessed on 21 February 2025). Metformin is an FDA drug approved for the treatment of type 2 diabetes [12,13,14] that affects major metabolic pathways, including AMPK and mTORC1 [15], and amino acid homeostasis [16]. Thus, in some clinical settings where light may not be able to penetrate, it would be advantageous to use an alternate activation method, such as metformin, which is also able to reduce HSV-1 replication on its own [17].
In our latest research, we evaluated the ability of Ruvidar® to restrict HSV-1 replication in Vero cells and in human U251 glioblastoma astrocytoma cells in the absence of light activation. Ruvidar® appeared to be more effective than acyclovir, both when added pre-infection (prophylactically) and post-infection (therapeutically). When added prophylactically, 10 µM Ruvidar® inhibited HSV-1 replication 99.993% in both Vero cells and in U251 cells, whereas 20 µM acyclovir inhibited HSV-1 replication 99.88% in Vero cells. When used therapeutically, 24 h post-infection, acyclovir had no effect on HSV-1 replication; however, 10 µM Ruvidar® was able to inhibit HSV-1 replication >99.99% in both cell types. When acyclovir and Ruvidar® were used in combination in Vero cells, there was an additive anti-HSV-1 effect. Furthermore, the combination was synergistic when used prophylactically. Combinations of Ruvidar® and metformin demonstrated additive effects in most cases.
2. Materials and Methods
2.1. Cells and Viruses
Monkey kidney Vero cells (ATCC #CCL-81) were cultured in DMEM supplemented with 1× L-glutamine, 1× non-essential amino acids, 1× sodium pyruvate, and 10% FBS (Gibco; Ottawa, ON, Canada, Cat #10437028). Human U-251 glioblastoma astrocytoma [U-251 MG (formerly known as U-373 MG; European Collection of Authenticated Cell Cultures—ECACC 09063001)] were cultured in DMEM/F12 supplemented with 1× L-glutamine, 1× non-essential amino acids, 1× sodium pyruvate, and 10% FBS. Cells were trypsinized and sub-cultured at 1:6–1:10 ratios 3 times a week. HSV-1 (strain F) was propagated and titrated in Vero cells.
2.2. Drug Treatments
Ruvidar® was freshly dissolved in sterile d.H2O and the concentration was determined by OD440 as described in [11]. Acyclovir was dissolved to 100 mM in sterile DMSO. Metformin was dissolved to 2 M in d.H2O. Working 100× sub-stocks were prepared such that the final DMSO concentration did not exceed 0.5%. Vero and U251 cells were treated with various concentrations of each of the compounds at various times pre- and post HSV-1 infection.
2.3. Cell Viability Determinations
Sets of Vero cells and U251 cells in 96-well plates (5 replicates) were treated with various concentrations of each drug for 72 h and cell viabilities determined by the WST-1 reagent according to the manufacturer’s directions. Absorbances and cell viabilities were compared to non-drug-treated and to media-only blank controls, and, because Ruvidar® is a deep red color, to Ruvidar®-in-media blank controls.
2.4. Selection of Drug-Resistant HSV-1 Mutants
HSV-1-infected Vero cells were treated through seven passages with increasing doses of drugs. For generation of acyclovir-resistant HSV (HSVAc-R), cells were treated with 1.25 μM acyclovir for two passages, in the additional presence of 1.0 μg/mL 5-bromodeoxyuridine (BUdr) during the initial passage to enhance mutational effects [18]. Resultant supernatants were then treated with 3.16 μM acyclovir for two passages, and plaque-purified in agarose containing 10 μM acyclovir. Ten individual well-separated plaques were then amplified through two final passages in the presence of 10 μM acyclovir. The sensitivities of resultant clones were then tested against 10.0 and 20.0 μM acyclovir.
To attempt to create Ruvidar®-resistant HSV clones (HSVRu-R), we followed a similar strategy, using 1.25, 3.16, and 10 μM Ruvidar®, but for two, three, and four passages, respectively, for a total of nine passages. Sensitivities of resultant clones were then tested against 10.0 μM Ruvidar®.
2.5. Infectious Virus Enumeration
Virus yields from treated cells were determined by plating 1:10 dilutions of each sample on 12-well plates of Vero cells. Cells were overlaid with 0.6% Agarose in 1× M199 supplemented with 3% FBS and 1 × L-glutamine, incubated at 37 °C for 4 days, and stained with 0.04% neutral red. Virus yields were compared to yields from non-treated controls.
3. Results
3.1. Ruvidar®, Acyclovir, and Metformin All Demonstrate Anti-HSV-1 Activity When Used Prophylactically
We initially tested each drug’s toxicity and their capacity to inhibit HSV-1 replication in Vero cells when each drug was added to cells 4 h prior to infection and the same concentrations of drug were maintained throughout infection in the absence of light activation (Figure 1).
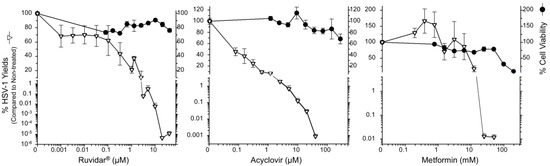
Figure 1.
The effects of Ruvidar®, acyclovir, and metformin on Vero cell viability and HSV-1 yields. Vero cells were treated with the indicated concentrations of drugs for 72 h to measure cell viability, or cells were pre-treated for 4 h with the indicated drug concentrations, infected with HSV-1 at MOI ~0.01, incubated in the presence of the same drug concentrations as pre-treatment for 68 h, and virus yields were determined. Error bars represent the standard error of the means (SEMs) from at least three replicates.
Cell viability remained above 60% at all tested Ruvidar® and acyclovir concentrations, so a Selectivity Index (SI) could not be determined for these drugs. Metformin’s SI was calculated as 8.4; 50% cell viability was observed at 76 mM and 50% virus inhibition at 9 mM. Acyclovir was highly effective as an anti-herpetic compound, as demonstrated in numerous previous studies (i.e., [19,20,21]). Additionally, 10 µM acyclovir reduced HSV-1 replication by approximately 3 Log10, to 0.12% that of the untreated sample, and 40 µM acyclovir reduced HSV-1 replication by approximately 5 Log10, to ~8.3 × 10−4% of the untreated sample.
Ruvidar® was more effective: 3 µM reduced HSV-1 replication to 0.06%; 10 µM reduced HSV-1 replication to ~6.5 × 10−3%; and 20 µM Ruvidar® reduced HSV-1 replication approximately 7.5 Log10, to <10−5% of the untreated sample. Metformin by itself also showed anti-HSV-1 activity; 12.5 mM inhibited ~80% HSV-1 replication; and 25 mM metformin reduced HSV-1 replication by almost 4 Log10 to ~0.012% of the untreated sample. These results indicate that all three drugs are effective when used prophylactically.
3.2. Ruvidar® Alone Demonstrates Anti-HSV-1 Activity When Used Therapeutically
We then examined each drug’s effectiveness, if used therapeutically (added 24 h after HSV-1 infection had been established) (Figure 2).
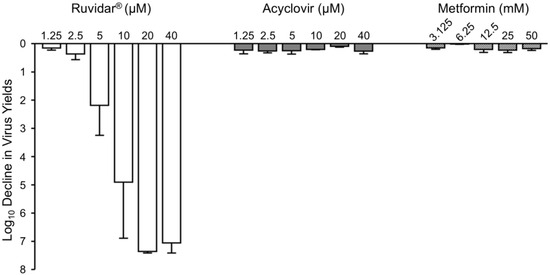
Figure 2.
Effects of Ruvidar®, acyclovir, and metformin on HSV-1 yields when added 24 hpi. Vero cells were infected with HSV-1 at MOI ~ 1.5, incubated for 24 h, then treated at 24 hpi with the indicated concentrations of drugs for an additional 44 h. Virus yields were then determined, and reductions in virus yields were compared to non-treated controls. Error bars represent SEMs from at least three replicates.
Ruvidar® continued to dramatically inhibit HSV-1 replication, even when added after HSV-1 infection had been established. It was found that 5 µM Ruvidar® inhibited HSV-1 replication more than 2 Log10, 10 µM Ruvidar® inhibited HSV-1 replication approximately 5 Log10, and 20 µM Ruvidar® inhibited HSV-1 replication approximately 7.3 Log10. Acyclovir had no major impact on HSV-1 replication at any tested concentration, if added 24 h post-infection (hpi), consistent with its known mode of action on viral DNA synthesis, which occurs relatively early in viral replication. Similarly, none of the tested metformin concentrations had a major impact on HSV-1 replication if added 24 hpi.
To determine the kinetics of each drug’s inhibitory effects, we treated Vero cells with 20 µM Ruvidar®, 20 µM acyclovir, or 25 mM metformin at various times pre- and post-infection (Figure 3). There were gradual changes in acyclovir’s anti-HSV-1 activity after 4 hpi and a dramatic change (~2 Log10) in acyclovir’s capacity to inhibit HSV-1 replication between 12 hpi and 16 hpi, consistent with its known mode of action on viral DNA synthesis. Metformin appeared to have earlier anti-HSV-1 effects; there were gradual changes in metformin’s anti-HSV-1 activity after 0 hpi and metformin had a more dramatic effect on HSV-1 replication at 12 hpi than acyclovir. Both drugs had a <1 Log10 effect on HSV-1 replication by 16 hpi. By contrast, Ruvidar® continued to inhibit HSV-1 replication by almost 99.9%, even when added 48 hpi.
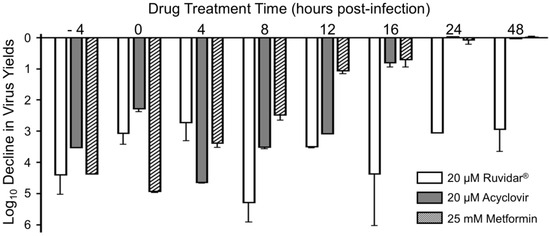
Figure 3.
Effects of Ruvidar®, acyclovir, and metformin on HSV-1 yields when added at various times pre- and post-infection. Vero cells were infected with HSV-1 at MOI ~ 1.5 and treated at indicated times with the indicated concentrations of drugs. Virus yields were determined at 68 hpi, and reductions in virus yields were compared to non-treated controls. Error bars represent SEMs from at least three replicates.
3.3. Ruvidar® and Acyclovir in Combination Had a Greater Anti-HSV-1 Effect
Many therapeutic drugs are more effective when used as a combinational therapy. For example, human immunodeficiency virus type 1 (HSV-1) is clinically inhibited by Highly Active Anti-Retroviral Therapy (HAART), which uses a combination of two reverse transcriptase inhibitors and either another non-nucleoside reverse transcriptase inhibitor or a protease inhibitor (reviewed in [22,23,24]). We tested various combinations of Ruvidar® with either various concentrations of acyclovir or metformin, both prophylactically and therapeutically. Combinations of Ruvidar® and acyclovir are shown in Figure 4.
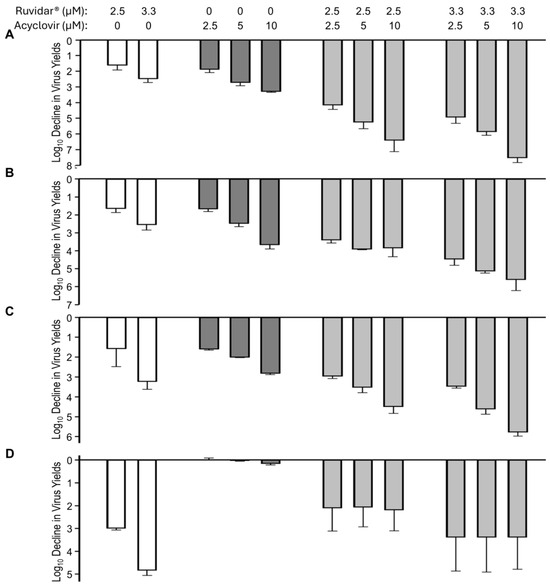
Figure 4.
The effects of Ruvidar® in combination with acyclovir on HSV-1 yields when added at various times pre- and post-infection. Vero cells were infected with HSV-1 at MOI ~ 1.5 and treated at (A) 4 h pre-infection; (B) at the same time as infection; (C) 8 hpi; or (D) 24 hpi with indicated concentrations of drugs. Virus yields were determined at 68 hpi, and reductions in virus yields were compared to non-treated controls. Error bars represent SEM from at least three replicates.
When used alone and when added 4 h pre-infection (prophylactically), 2.5 and 3.3 µM Ruvidar® inhibited HSV-1 replication by ~1.5 and ~2.5 Log10, respectively (Figure 4A, left-most white bars). Similarly, when used alone, and when added 4 h pre-infection, 2.5, 5 and 10 µM acyclovir inhibited HSV-1 replication ~1.8, ~2.6, and ~3 Log10, respectively (Figure 4A, dark gray bars). When used in combination, most doses had an additive effect (Figure 4A, right-most light gray bars); however, 3.3 µM Ruvidar® combined with 10 µM acyclovir had a synergistic effect (p = 0.015), inhibiting HSV-1 replication by approximately 7.5 Log10.
The combination of Ruvidar® with acyclovir had additive inhibitory effects on HSV-1 replication when added at the same time infection was initiated (Figure 4B) or if drugs were added 8 hpi (Figure 4C); however, if combinational drugs were added 24 hpi, HSV-1 replication inhibition appeared to be slightly less effective than if Ruvidar® was used alone at this time (Figure 4D), although there was greater experimental variability.
Combinations of Ruvidar® and metformin are shown in Figure 5. When used alone and when added 4 h pre-infection, 2.5 and 3.3 µM Ruvidar® inhibited HSV-1 replication ~1.5 and ~2.5 Log10, respectively (Figure 5A, left-most white bars). Similarly, when used alone and when added 4 h pre-infection, 3.125, 6.5 and 12.5 mM metformin inhibited HSV-1 replication ~1.1, ~1.2, and ~1.8 Log10, respectively (Figure 5A, cross-hatched bars). When used in combination, most doses had a slightly additive effect (Figure 5A, right-most light gray cross-hatched bars). The combination of Ruvidar® with metformin in most cases had additive effects on HSV-1 replication inhibition when added at the same time as the infection was initiated (Figure 5B) or if 2.5 µM Ruvidar® was added 8 hpi (Figure 5C); however, if 3.3 µM Ruvidar® was added 8 hpi, or combinational drugs were added 24 hpi, the inhibition of HSV-1 replication was less effective than if Ruvidar® was used alone (Figure 5C,D).
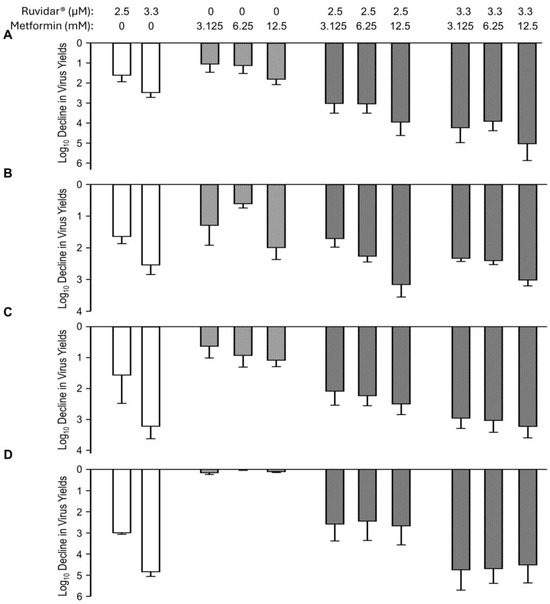
Figure 5.
Effects of Ruvidar® in combination with metformin on HSV-1 yields when added at various times pre- and post-infection. Vero cells were infected with HSV-1 at MOI ~ 1.5 and treated at 4 h pre-infection (A); at the same time as infection (B); 8 hpi (C); or 24 hpi (D) with indicated concentrations of drugs. Virus yields were determined at 68 hpi, and reductions in virus yields were compared to non-treated controls. Error bars represent SEMs from at least three replicates.
3.4. Acyclovir-Resistant HSV-1 Mutants Are Inhibited by Ruvidar®
A major problem with HSV-1 anti-viral therapy is that acyclovir-resistant mutants can commonly develop. To test the anti-viral effects of Ruvidar® on such mutants (HSVAc-R), we passaged HSV-1 in the presence of increasing concentrations of acyclovir. We initially determined the concentration of 5′-Bromo-2′-deoxyuridine (BrdU) required to reduce viral progeny production 1–2 Log10, conditions reported to favor mutant production [18,25]. Addition of 1 μg/mL of BrdU resulted in ~5% HSV-1 production compared to that in non-drug-treated samples (Figure 6A).
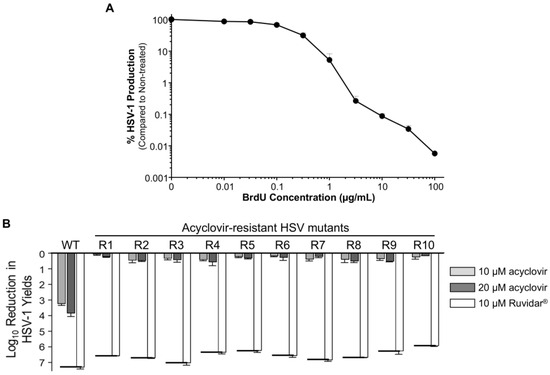
Figure 6.
Generation of acyclovir-resistant HSV-1 (HSVAc-R) mutants. (A) Effects of 5′-Bromo-2′-deoxyuridine (BrdU) on HSV-1 yields. Vero cells were infected with HSV-1 at MOI = 1 PFU/cell and overlayed with DMEM + 5% FBS that contained the indicated amounts of BrdU. Virus yields were determined at 48 hpi, and virus yields were compared to non-treated controls. Error bars represent S.E.M. from at least two replicates. Note that 1 μg/mL BrdU resulted in virus yields reduced to ~5% of the control. (B) Inhibition of wild-type (WT) HSV and HSVAc-R mutants by 10 and 20 μM acyclovir, and by 10 μM Ruvidar™, respectively. Vero cells were pre-treated with the indicated drugs for 2 h, infected at MOI ~ 1 PFU/cell, and overlaid with media containing same drug concentrations. Virus yields were determined at 68 hpi, and reductions in virus yields were compared to non-treated controls. Error bars represent SEMs from two replicates. The short thick horizontal bars represent the limit of detection of the plaque assays.
We pre-treated Vero cells for 2 h with 1.25 μM acyclovir to generate HSVAc-R mutants, or with 1.25 μM Ruvidar® to attempt to generate HSVRu-R mutants. We then infected the cells with HSV-1 at MOI = 1 PFU/cell, and overlaid them with DMEM that contained the same amounts of acyclovir or Ruvidar®, and were supplemented with 1 μg/mL of BrdU. After 48 h incubation, supernatants were used to set up 2nd passage infections with the same concentrations of acyclovir or Ruvidar®. The amounts of acyclovir or Ruvidar® were increased through five additional passages at 3.16 and 10 μM of acyclovir or Ruvidar® as detailed in the Section 2. For the 5th passage, the supernatants from the 4th passage were titrated under agarose that contained 3.16 or 10 μM acyclovir or Ruvidar®, respectively. Ten well-separated plaques were picked from the acyclovir-treated plates into media containing 10 μM acyclovir and passaged in the presence of 10 μM drug for 2 additional passages. We were unable to recover any plaques from the Ruvidar®-treated plates, so attempts to generate HSVRu-R mutants were discontinued.
These putative HSVAc-R mutants, and wild-type HSV-1, were then used to infect Vero cells that had been pre-treated for 2 h with either 0, 10, or 20 μM acyclovir or 10 μM Ruvidar®. Cells were infected with HSV-1 at MOI ~ 1.5, overlaid with 0, 10, or 20 μM acyclovir or 10 μM Ruvidar®, respectively, and virus yields were determined at 68 hpi (Figure 6B).
Wild-type (wt) HSV-1 grew to about 1 × 108 PFU/mL, and growth was inhibited 3.26 Log10 (>1850-fold) by 10 μM acyclovir, 3.86 Log10 (>8000-fold) by 20 μM acyclovir, and 7.34 Log10 (>22,000,000-fold) by 10 μM Ruvidar®, comparable to earlier results. The various HSVAc-R mutants grew to between 4.1 × 106 to 8 × 107 PFU/mL in the absence of drugs and were inhibited no more than 3.2-fold by 10 μM acyclovir and no more than 4.4-fold by 20 μM acyclovir, but were inhibited >890,000-fold by 10 μM Ruvidar™ (Figure 6B).
3.5. Ruvidar® Also Inhibits HSV-1 and Acyclovir-Resistant HSV-1 Mutants in U251 Astrocytes
All previous work had been performed in monkey kidney Vero cells. Astrocytes are another cell type that can be infected by several herpesviruses, including HSV-1 [26,27]. To test whether these drugs could also inhibit HSV-1 in this more relevant cell type, we initially pre-treated human U251 glioblastoma astrocytoma cells with different concentrations of acyclovir or Ruvidar® and determined cell viabilities and the capacity of WT HSV-1 to replicate (Figure 7). Cell viability remained >50% at all tested Ruvidar® concentrations, and wild-type HSV-1 yields were reduced >10,000-fold by 3.16 μM Ruvidar® (Figure 7A). Cell viability also was unaffected by any tested acyclovir concentration up to 100 μM and HSV-1 yields were reduced ~10-fold at 10 μM acyclovir and ~25-fold at 100 μM (Figure 7B).
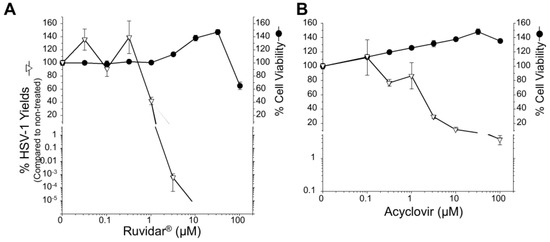
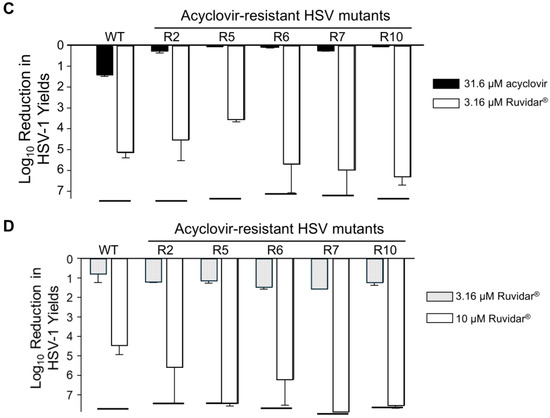
Figure 7.
Effects of Ruvidar® and acyclovir on wild-type (WT) HSV-1 and acyclovir-resistant HSV-1 (HSVAc-R) yields in human U251 glioblastoma astrocytoma cells. Effects of Ruvidar® (A) and acyclovir (B) on U251 cell viability and HSV-1 yields. U251 cells were treated with the indicated concentrations of drugs for 72 h to measure cell viability, or cells were pre-treated for 4 h with the indicated drug concentrations, infected with HSV-1 at MOI ~ 0.01, incubated in the presence of same drug concentrations as pre-treatment for 68 h, and virus yields were determined. Error bars represent SEMs from at least three replicates. The broken line at bottom of the virus yield curve in A denotes reaching the limit of detection. (C) Prophylactic effects of acyclovir and Ruvidar® on HSV-1 and HSVAc-R yields when drugs were added pre-infection. U251 cells were pre-treated for 2 h with indicated drugs, infected with HSV-1 or each of five HSVAc-R clones at MOI ~ 1.5, and incubated in the continued presence of appropriate drugs. Virus yields were determined at 68 hpi, and reductions in virus yields were compared to non-treated controls. Error bars represent SEMs from two replicates. (D) Therapeutic effects of Ruvidar® on HSV-1 and HSVAc-R yields when drugs were added 24 h post-infection. U251 cells were infected with HSV-1 or each of the five HSVAc-R clones at MOI ~ 1.5, treated at 24 hpi with the indicated drugs, and incubated in the continued presence of appropriate drugs. Virus yields were determined at 68 hpi, and reductions in virus yields were compared to non-treated controls. Error bars represent SEMs from two replicates. The short thick horizontal bars represent the limit of detection of the plaque assays.
Comparison of the dose response curves of the two drugs suggests that higher doses of acyclovir and lower doses of Ruvidar® are needed in the U251 cells compared to the Vero cells to inhibit HSV-1 to the same extent (compare Figure 7A to Figure 1, left, and Figure 7B to Figure 1, center). For example, 10 μM acyclovir induced nearly a 1000-fold reduction in HSV yields in Vero cells but only about a 10-fold reduction in U251 cells (p < 10−5 by T-test), whereas 3.16 μM Ruvidar® induced about a 1000-fold reduction in HSV yields in Vero cells but more than a 100,000-fold reduction in U251 cells (p = 0.003 by T-test).
We then pre-treated U251 cells with nothing, 31.6 μM acyclovir, or 3.16 μM Ruvidar®. Cells were infected with wt HSV-1, or with five selected HSVAc-R mutants at MOI ~ 1.5, overlaid with media lacking drugs, or media containing the same drugs, and virus yields were determined at 68 hpi (Figure 7C). WT HSV-1 grew to ~2 × 108 PFU/mL yield in the absence of drugs. Yields were inhibited 25-fold by 31.6 μM acyclovir, and >165,000-fold by 3.16 μM Ruvidar®. All of the acyclovir-resistant clones grew to between 5.95 × 107 and 2.2 × 108 in the absence of drugs, showed <2-fold reduction for 31.6 μM acyclovir, and, except in the case of HSVAc-R5, which was inhibited 3783-fold, they showed a > 170,000-fold reduction to 3.16 μM Ruvidar®, indicating they all remained resistant to acyclovir but remained sensitive to Ruvidar® in this other cell system.
To test the therapeutic efficacy of Ruvidar® on already-established infections of wt HSV-1 and of the HSVAc-R mutants, we also infected U251 cells with the same six virus clones (wt + five HSVAc-R mutants) at MOI ~ 1.5 PFU/cell, incubated the infections for 24 h, then added no drug, or 3.16 or 10 μM of Ruvidar®. Infections were harvested 44 h later and virus yields were determined to see if therapeutic application of Ruvidar® would affect HSVAc-R replication (Figure 7D).
WT HSV-1 grew to almost 3 × 108 PFU/mL, and growth was inhibited 10-fold by 3.16 μM Ruvidar® and 47,000-fold by 10 μM Ruvidar®, respectively, even when added 24 h after infection had started. The various HSVAc-R mutants grew to ~1 × 108 PFU/mL in U251 cells in the absence of drugs, were inhibited >14-fold by 3.16 μM Ruvidar®, and were inhibited >13,000,000-fold by 10 μM Ruvidar®, indicating that therapeutic application of Ruvidar® remained effective, even against acyclovir-resistant HSV-1.
3.6. Ruvidar® Protects HSV-1-Infected Cells from Cytopathology
To determine if Ruvidar® application would affect the HSV-1-induced cytopathic effect (CPE), we pre-treated both types of cells with 0, 3.16, or 10 μM Ruvidar®, infected with HSV-1 at MOI 1.5, and monitored cell morphology over time (Figure 8). HSV-1 infection induced a small amount of CPE in non-drug-treated cells by 24 hpi in both cell types, and there was substantial CPE in both non-drug-treated cells by 48 hpi. By contrast, there was little, if any, CPE in both cell types treated with either drug concentration by 48 hpi. There also was little, if any, CPE in the Vero cells treated with either drug concentration by 68 hpi. U251 cells showed stress in both drug concentrations by 68 hpi.
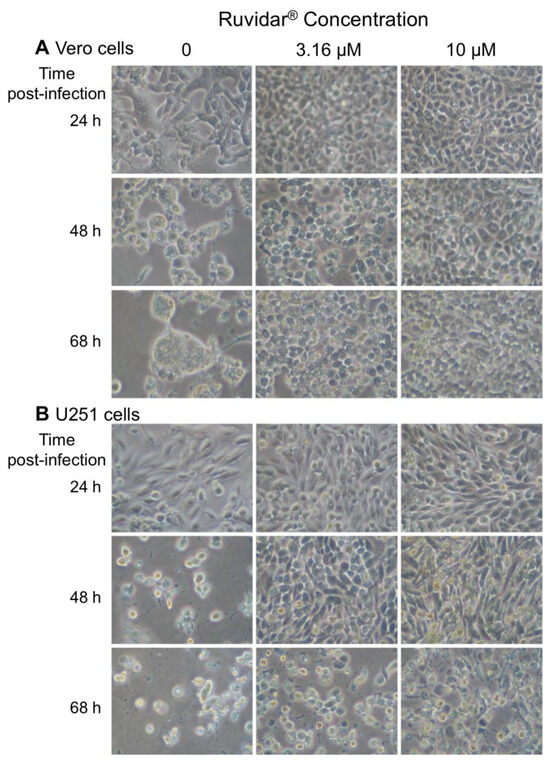
Figure 8.
Cytopathic effects induced in (A) Vero and (B) U251 cells in the presence of 0, 3.16, and 10 μM Ruvidar® at the indicated times post-infection (left). Cells were pre-treated with the indicated concentrations of compound for 2 h, infected with HSV-1 at MOI ~ 1.5, and incubated in the same concentrations of compounds.
4. Discussion
The herpesviruses are significant human pathogens. Although many, such as Herpes Simplex Virus (HSV), rarely cause mortality, they are ubiquitous and virtually the entire human population has been exposed to one or another of the eight currently recognized human herpesvirus types. These viruses are capable of entering human hosts by various methods and can damage numerous tissues, including the central nervous system (CNS), which can be fatal (reviewed in [6]).
Furthermore, in addition to causing acute infections, these agents can establish life-long latency and spontaneously reactivate. For example, the disease Shingles occurs when Varicella Zoster virus, the causative agent of Chickenpox, is reactivated.
There are several treatments for HSV. Vaccines have been developed against a few herpesviruses, such as Varicella Zoster [28], but despite decades of research, such vaccines are not yet available for HSV-1 [29]. To date, anti-HSV-1 strategies have focused on pharmacologic therapies; including acyclovir [19,20,21], its derivatives such as famciclovir and valaciclovir [9,30], and other compounds, such as foscarnet [10,31] and cidofovir [10,32]. Viruses mutate and therefore can develop resistance to anti-viral treatments [3,4,5]; thus, there is need to develop additional strategies.
Activated small molecules are a potential strategy that is effective against various cancers [33,34,35,36,37], bacterial pathogens [38,39], and viruses [40,41,42,43,44]. This methodology is based upon the use of a small molecule that is administered to the pathogen in question, then activated by exposure to a particular form of energy—in this case, wavelength(s) of light—to produce Reactive Oxygen Species (ROS), specifically singlet oxygen, which causes microbial or cancer oxidative stress and subsequent death [39,42]. Originally found to kill paramecia, a variety of different light-activated small molecules have been developed and tested over the years, with third- and fourth-generation light-activated small molecules now under investigation (reviewed in [36,45]).
Our prior work showed that Ruvidar® could effectively inactivate numerous human viruses when added to a virus at nanomolar concentrations [11], and that, while not quite as effective at equivalent doses, Ruvidar® could inactivate all tested viruses when not activated by light. Thus, Ruvidar® could potentially be used in clinical niches, where light is unable to penetrate or be activated by other methodologies, such as ionizing radiation or other drugs, dependent on the application. This latest research has determined what effects, if any, Ruvidar® could have on the replication of HSV-1 in model cell culture systems of Vero and U251 astrocytoma cells; whether Ruvidar® was as effective or more effective than acyclovir, the current “Gold Standard”; and whether combinational treatments could have additive anti-HSV-1 effects.
When used prophylactically (added prior to infection), acyclovir is an effective anti-herpetic agent (as previously demonstrated (i.e., [19,20,21]), with 10 μM inhibiting HSV-1 yields almost 99.9% in Vero cells. Acyclovir was not as effective in U251 cells, with 10 μM inhibiting HSV-1 yields only by about 10%.
Ruvidar® was significantly more effective than acyclovir, especially in the astrocytoma cells, with 3.16 μM inhibiting HSV-1 yields > 99.9% and 10 μM inhibiting HSV-1 yields almost 10-fold more in Vero cells, and 3.16 μM inhibiting HSV-1 yields > 5 Log10 in the U251 cells. Metformin also showed anti-HSV-1 activity when added prior to infection, with ~20 mM inhibiting HSV-1 approximately 99.9% and 25 mM inhibiting HSV-1 almost 10-fold more in Vero cells. Thus, of these three tested agents, Ruvidar® was by far the most effective on a molar basis. The differences in the effectiveness of acyclovir and Ruvidar® in the different cell types indicate that in addition to its virucidal activity [11], there are cell-specific factor(s) that contribute to Ruvidar®’s anti-HSV activity.
When used therapeutically, Ruvidar® was substantially more effective than the other two compounds due to the fact that no tested acyclovir or metformin concentrations were effective if added to Vero cells 24 hpi. On the contrary, Ruvidar® remained highly effective, with 5 μM inhibiting HSV-1 yield by approximately 99%, while 10 and 20 μM inhibited HSV-1 yields by approximately 5 Log10 and 7 Log10, respectively, in Vero cells. Ruvidar® also remained highly effective when used therapeutically in U251 cells, with 10 μM inhibiting yields of wt and all tested HSVAc-R mutants more than 4 Log10. This suggests that Ruvidar® could be used effectively both before and during HSV infection, making it a much more attractive and commercially viable anti-viral therapeutic against this ubiquitous virus.
Viruses are more susceptible to mutation than eukaryotic cells and therefore can escape anti-viral treatment. This has been seen in many cases, particularly with RNA viruses [3,4,5], but also with DNA viruses. For example, several clinical HSV isolates have become resistant to acyclovir [46], making it necessary to switch to derivatives such as ganciclovir [8,10] and valacyclovir [10,46], or to other compounds, such as cidofovir or foscarnet [10,32]. When Ruvidar® was evaluated against a number of acyclovir-resistant HSV-1 mutants (HSVAc-R), it confirmed that they were resistant to acyclovir in both U251 and Vero cells and that Ruvidar® remained effective against all tested HSVAc-R in both cell types.
Using combinational drug therapy leads to reduced, or slower, resistance development, particularly if the drugs target different aspects of virus replication [22,23,24,47]. To ascertain whether the same held true for our tested compounds, we tested whether Ruvidar®, in combination with either metformin, which activates Ruvidar® (https://theralase.com/theralase-demonstrates-unique-ability-to-activate-rutherrin-with-diabetes-drug/, accessed on 21 February 2025), or acyclovir, could increase the anti-HSV-1 activity compared to any drug used alone. Combinational therapy had an additive effect in the majority of cases. For example, 2.5 μM Ruvidar® alone inhibited HSV-1 yields 41-fold, and 2.5 μM acyclovir alone inhibited HSV-1 yields 75-fold, but the combination of 2.5 μM of each inhibited HSV-1 yields > 14,000-fold when used prophylactically. There also was an additive effect when these doses were added 8 hpi. The combination of 3.3 μM Ruvidar® with 10 μM acyclovir appeared to be synergistic when used prophylactically. When added prior to infection, 3.3 μM Ruvidar® inhibited HSV-1 yields 298-fold and 10 μM acyclovir inhibited HSV-1 yields 1892-fold. Synergistically, the combination inhibited HSV-1 yields > 31 million-fold (p = 0.015). Synergistic and additive effects were also seen when Ruvidar® and metformin were used in combination, although some combinations, particularly when tested therapeutically, were not always additive, and in some cases they were subtractive in nature. For example, 3.3 μM Ruvidar® alone added at the time of infection inhibited HSV-1 replication 346-fold (2.539 Log10), 3.125 mM metformin alone inhibited HSV-1 replication 19-fold (1.29Log10), but the combination, added at the time of infection, only inhibited HSV-1 replication 215-fold (2.33 Log10) instead of nearly 6734-fold (3.828Log10). The authors attribute this unexpected subtractive/nil effect to the timing of when the various agents were added to virus-infected cells versus the agent’s various mechanisms of action against the virus. In other words, the proliferation stage of the virus versus when the agent was administered plays a key role in the synergistic/additive effect of the agents.
In addition to greatly reducing HSV-1 replication, whether used prophylactically or therapeutically, Ruvidar® treatment also reduced and/or delayed cytopathology (CPE) in both tested cell types, with the CPE reduction being more apparent in Vero cells than in U251 cells. Thus, Ruvidar® appears to be a highly effective anti-HSV-1 agent, as demonstrated by the detailed experiments completed. The next steps include the completion of a Phase I/II clinical study in an attempt to replicate these results in a clinical model.
5. Conclusions
In conclusion, the above results indicate that Ruvidar® is a much more effective anti-HSV treatment than either acyclovir or metformin. These studies involved treating a population of cells either before they were exposed to HSV-1 (prophylactically), in which viral replication was dramatically reduced by all tested pharmacologic compounds, or after virtually every cell had been infected (therapeutically). While acyclovir and metformin had little, if any, beneficial effect once infection was established, Ruvidar® continued to have a dramatic inhibitory effect. In a normal human infection setting, a small proportion of cells are initially infected and subsequent progeny viruses infect a greater number of additional cells. Thus, adding acyclovir or metformin alone after symptoms present has no beneficial effect upon the already-infected cells; however, treatment with any of the compounds could help reduce viral replication once neighboring non-infected cells become infected. This inhibitory effect was significantly enhanced when Ruvidar® was used in combination with acyclovir or metformin. Ruvidar® is also highly effective at protecting cells from ongoing HSV infection and highly effective against acyclovir-resistant HSV-1 mutants.
Author Contributions
Conceptualization, K.M.C., R.D.-W. and A.M.; formal analysis, K.M.C.; investigation, K.M.C.; writing—original draft preparation, K.M.C.; writing—review and editing, K.M.C., R.D.-W. and A.M.; visualization, K.M.C.; project administration, K.M.C.; funding acquisition, K.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by unrestricted funding provided by Theralase® Technologies, Inc. to K.M.C.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are contained within the manuscript figures.
Acknowledgments
The authors thank Mark Roufaiel and Pavel Kaspler for their expert technical assistance.
Conflicts of Interest
Authors Roger DuMoulin-White and Arkady Mandel are employed by the company Theralase® Technologies Inc. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Theralase® Technologies Inc. Author KMC received this funding in the form of an unrestricted research contract. The funder had the following involvement with the study: (1) Suggestion to compare anti-herpetic effects of Ruvidar® to acyclovir and to metformin, as well as their combination. (2) Providing author KMC with aliquots of Ruvidar®. (3) In consultation with author KMC, choice of drug doses and treatment timings to investigate. (4) Periodic meetings with author KMC to discuss ongoing work and results. (5) Minor editing of the final manuscript that did not significantly alter the results or their interpretation.
Abbreviations
The following abbreviations are used in this manuscript:
| DMEM | Dulbecco’s Minimal Essential Medium |
| DMSO | Dimethyl Sulfoxide |
| HSV | Herpes Simplex Virus |
References
- de Jong, J.C.; Beyer, W.E.P.; Palache, A.M.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J. Med. Virol. 2000, 61, 94–99. [Google Scholar] [CrossRef]
- Chan, M.C.W.; Wang, M.H.; Chen, Z.G.; Hui, D.S.C.; Kwok, A.K.; Yeung, A.C.M.; Liu, K.M.; Yeoh, Y.K.; Lee, N.; Chan, P.K.S. Frequent genetic mismatch between vaccine strains and circulating seasonal Influenza viruses, Hong Kong, China, 1996–2012. Emerging Infect. Dis. 2018, 24, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Monto, A.S.; McKimm-Breschkin, J.L.; Macken, C.; Hampson, A.W.; Hay, A.; Klimov, A.; Tashiro, M.; Webster, R.G.; Aymard, M.; Hayden, F.G.; et al. Detection of influenza viruses resistant to neuraminidase inhibitors in global surveillance during the first 3 years of their use. Antimicrob. Agents Chemother. 2006, 50, 2395–2402. [Google Scholar] [CrossRef]
- Colman, P.M. New antivirals and drug resistance. Annu. Rev. Biochem. 2009, 78, 95–118. [Google Scholar] [CrossRef]
- Krol, E.; Rychowska, M.; Szewczyk, B. Antivirals—Current trends in fighting influenza. Acta Biochim. Pol. 2014, 61, 495–504. [Google Scholar] [CrossRef]
- Pellett, P.E.; Roizman, B. Herpesviridae. In Fields Virology; Knipe, D., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 1802–1821. [Google Scholar]
- Wald, A.; Corey, L. Persistence in the population: Epidemiology, transmission. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Field, H.J.; Hodge, R.A.V. Recent developments in anti-herpesvirus drugs. Br. Med. Bull. 2013, 106, 213–249. [Google Scholar] [CrossRef]
- Poole, C.L.; James, S.H. Antiviral therapies for herpesviruses: Current agents and new directions. Clin. Ther. 2018, 40, 1282–1298. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, L.A.; Upadhyay, R.; Greeley, Z.W.; Margulies, B.J. Current drugs to treat infections with Herpes Simplex viruses-1 and-2. Viruses 2021, 13, 1228. [Google Scholar] [CrossRef]
- Coombs, K.M.; Glover, K.K.M.; Russell, R.; Kaspler, P.; Roufaiel, M.; Graves, D.; Pelka, P.; Kobasa, D.; DuMoulin-White, R.; Mandel, A. Nanomolar concentrations of the photodynamic compound TLD-1433 effectively inactivate numerous human pathogenic viruses. Heliyon 2024, 10, e32140. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Pernicova, I.; Korbonits, M. Metformin-mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Nageeta, F.; Sohail, R.; Butt, T.S.; Ganesan, S.; Madhurita, F.; Ahmed, M.; Zafar, M.; Zafar, W.; Zaman, M.U.; et al. Comparative efficacy and safety profile of once-weekly Semaglutide versus once-daily Sitagliptin as an add-on to metformin in patients with type 2 diabetes: A systematic review and meta-analysis. Ann. Med. 2023, 55, 2239830. [Google Scholar] [CrossRef]
- Apostolova, N.; Iannantuoni, F.; Gruevska, A.; Muntane, J.; Rocha, M.; Victor, V.M. Mechanisms of action of metformin in type 2 diabetes: Effects on mitochondria and leukocyte-endothelium interactions. Redox Biol. 2020, 34, 101517. [Google Scholar] [CrossRef]
- Forteath, C.; Mordi, I.; Nisr, R.; Gutierrez-Lara, E.J.; Alqurashi, N.; Phair, I.R.; Cameron, A.R.; Beall, C.; Bahr, I.; Mohan, M.; et al. Amino acid homeostasis is a target of metformin therapy. Mol. Metab. 2023, 74, 101750. [Google Scholar] [CrossRef]
- Movaqar, A.; Abdoli, A.; Aryan, E.; Jazaeri, E.O.; Meshkat, Z. Metformin promotes autophagy activity and constrains HSV-1 replication in neuroblastoma cells. Gene Rep. 2021, 25, 101370. [Google Scholar] [CrossRef]
- Stark, R.M.; Littlefield, J.W. Mutagenic effect of BUdr in diploid human fibroblasts. Mutat. Res. 1974, 22, 281–286. [Google Scholar] [CrossRef]
- Larder, B.A.; Darby, G. Selection and characterization of acyclovir-resistant Herpes-Simplex virus type-1 mutants inducing altered DNA-polymerase activities. Virology 1985, 146, 262–271. [Google Scholar] [CrossRef]
- Klysik, K.; Pietraszek, A.; Karewicz, A.; Nowakowska, M. Acyclovir in the treatment of Herpes viruses—A review. Curr. Med. Chem. 2020, 27, 4118–4137. [Google Scholar] [CrossRef]
- Andrei, G.; Snoeck, R. Advances and perspectives in the management of Varicella-Zoster virus infections. Molecules 2021, 26, 1132. [Google Scholar] [CrossRef] [PubMed]
- Alam, C.; Whyte-Allman, S.K.; Omeragic, A.; Bendayan, R. Role and modulation of drug transporters in HIV-1 therapy. Adv. Drug Del. Rev. 2016, 103, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Nordin, M.A.C.; Teow, S.Y. Review of current cell-penetrating antibody developments for HIV-1 therapy. Molecules 2018, 23, 335. [Google Scholar] [CrossRef]
- Chen, V.H.; Patterson, K.M.; Montaner, J.; Wiseman, S.M. Improved outcomes following gastrointestinal surgery among people living with HIV in the HAART-era: A scoping review. Am. J. Surg. 2024, 235, 115710. [Google Scholar] [CrossRef]
- Pringle, C.R. Temperature-sensitive mutant vaccines. Meth Mol. Med. 1996, 4, 17–32. [Google Scholar]
- Feige, L.; Zaeck, L.M.; Sehl-Ewert, J.; Finke, S.; Bourhy, H. Innate immune signaling and role of glial cells in herpes simplex virus- and rabies virus-induced encephalitis. Viruses 2021, 13, 2364. [Google Scholar] [CrossRef]
- Rashidi, A.S.; Tran, D.N.; Peelen, C.R.; van Gent, M.; Ouwendijk, W.J.D.; Verjans, G.M.G.M. Herpes simplex virus infection induces necroptosis of neurons and astrocytes in human fetal organotypic brain slice cultures. J. Neuroinflamm. 2024, 21, 38. [Google Scholar] [CrossRef]
- Gershon, A.A.; Gershon, M.D. Pathogenesis and current approaches to control of Varicella-Zoster virus infections. Clin. Microbiol. Rev. 2013, 26, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Aschner, C.B.; Herold, B.C. Alphaherpesvirus vaccines. Curr. Issues Mol. Biol. 2021, 41, 469–508. [Google Scholar] [CrossRef] [PubMed]
- Faulds, D.; Heel, R.C. Ganciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs 1990, 39, 597–638. [Google Scholar] [CrossRef] [PubMed]
- Stránská, R.; van Loon, A.M.; Polman, M.; Beersma, M.F.C.; Bredius, R.G.M.; Lankester, A.C.; Meijer, E.; Schuurman, R. Genotypic and phenotypic characterization of acyclovir-resistant herpes simplex viruses isolated from haematopoietic stem cell transplant recipients. Antivir. Ther. 2004, 9, 565–575. [Google Scholar] [CrossRef]
- Saijo, M.; Suzutani, T.; De Clercq, E.; Niikura, M.; Maeda, A.; Morikawa, S.; Kurane, I. Genotypic and phenotypic characterization of the thymidine kinase of ACV-resistant HSV-1 derived from an acyclovirsensitive herpes simplex virus type 1 strain. Antiviral Res. 2002, 56, 253–262. [Google Scholar] [CrossRef]
- Fong, J.; Kasimova, K.; Arenas, Y.; Kaspler, P.; Lazic, S.; Mandel, A.; Lilge, L. A novel class of ruthenium-based photosensitizers effectively kills in vitro cancer cells and in vivo tumors. Photochem. Photobiol. Sci. 2015, 14, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Lazic, S.; Kaspler, P.; Mandel, A.; Jewett, M.A.S.; Kulkarni, G.; Lilge, L. Photodynamic therapy for non-muscle invasive bladder cancer mediated by instilled photosensitizer TLD1433 and green light activation. J. Urol. 2016, 195, E805. [Google Scholar] [CrossRef]
- Roque, J.A.; Barrett, P.C.; Cole, H.D.; Lifshits, L.M.; Shi, G.; Monro, S.; von Dohlen, D.; Kim, S.; Russo, N.; Deep, G.; et al. Breaking the barrier: An osmium photosensitizer with unprecedented hypoxic phototoxicity for real world photodynamic therapy. Chem. Sci. 2020, 11, 9784–9806. [Google Scholar] [CrossRef] [PubMed]
- McFarland, S.A.; Mandel, A.; Dumoulin-White, R.; Gasser, G. Metal-based photosensitizers for photodynamic therapy: The future of multimodal oncology? Curr. Opin. Chem. Biol. 2020, 56, 23–27. [Google Scholar] [CrossRef]
- Konda, P.; Lifshits, L.M.; Roque, J.A.; Cole, H.D.; Cameron, C.G.; McFarland, S.A.; Gujar, S. Discovery of immunogenic cell death-inducing ruthenium-based photosensitizers for anticancer photodynamic therapy. Oncoimmunology 2021, 10, 1863626. [Google Scholar] [CrossRef]
- Arenas, Y.; Monro, S.; Shi, G.; Mandel, A.; McFarland, S.; Lilge, L. Photodynamic inactivation of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus with Ru(II)-based type I/type II photosensitizers. Photodiagn. Photodyn. 2013, 10, 615–625. [Google Scholar] [CrossRef]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of photosensitizing agents for targeted antimicrobial photodynamic therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef]
- Papin, J.F.; Floyd, R.A.; Dittmer, D.P. Methylene blue photoinactivation abolishes West Nile virus infectivity in vivo. Antiviral Res. 2005, 68, 84–87. [Google Scholar] [CrossRef]
- Costa, L.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, A.; Almeida, A. Photodynamic inactivation of mammalian viruses and bacteriophages. Viruses 2012, 4, 1034–1074. [Google Scholar] [CrossRef] [PubMed]
- Monjo, A.L.A.; Pringle, E.S.; Thornbury, M.; Duguay, B.A.; Monro, S.M.A.; Hetu, M.; Knight, D.; Cameron, C.G.; McFarland, S.A.; McCormick, C. Photodynamic inactivation of Herpes simplex viruses. Viruses 2018, 10, 532. [Google Scholar] [CrossRef]
- Lebedeva, N.S.; Gubarev, Y.A.; Koifman, M.O.; Koifman, O.I. The application of porphyrins and their analogues for inactivation of viruses. Molecules 2020, 25, 4368. [Google Scholar] [CrossRef]
- Conrado, P.C.V.; Sakita, K.M.; Arita, G.S.; Galinari, C.B.; Goncalves, R.S.; Lopes, L.D.G.; Lonardoni, M.V.C.; Teixeira, J.J.V.; Bonfim-Mendonca, P.S.; Kioshima, E.S. A systematic review of photodynamic therapy as an antiviral treatment: Potential guidance for dealing with SARS-CoV-2. Photodiagn. Photodyn. 2021, 34, 102221. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Antimicrobial photodynamic therapy: New anti-infectives in the age of resistance. Compr. Ser. Photoch 2016, 15, 549–571. [Google Scholar]
- Jiang, Y.C.; Feng, H.; Lin, Y.C.; Guo, X.R. New strategies against drug resistance to herpes simplex virus. Int. J. Oral. Sci. 2016, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Maggi, F.; D’Abramo, A.; Nicastri, E.; Sullivan, D.J. Antiviral combination therapies for persistent COVID-19 in immunocompromised patients. Int. J. Infect. Dis. 2023, 137, 55–59. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).