Prevalence and Genetic Diversity of Torque teno felis virus (FcTTV) in Domestic Cats from Kazakhstan
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Data Collection
2.2. DNA Isolation
2.3. PCR Testing
2.4. Sequencing ORF1
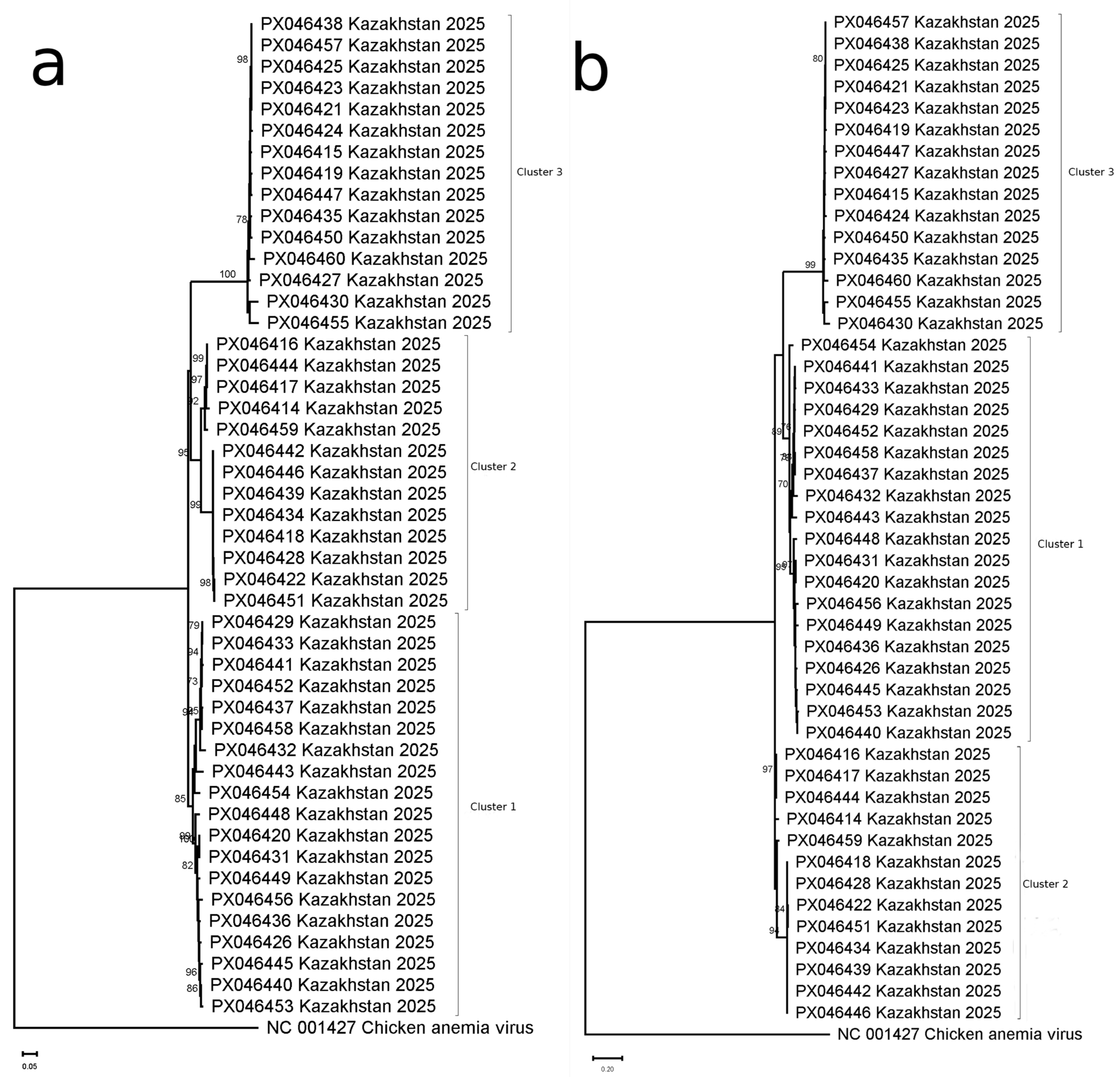
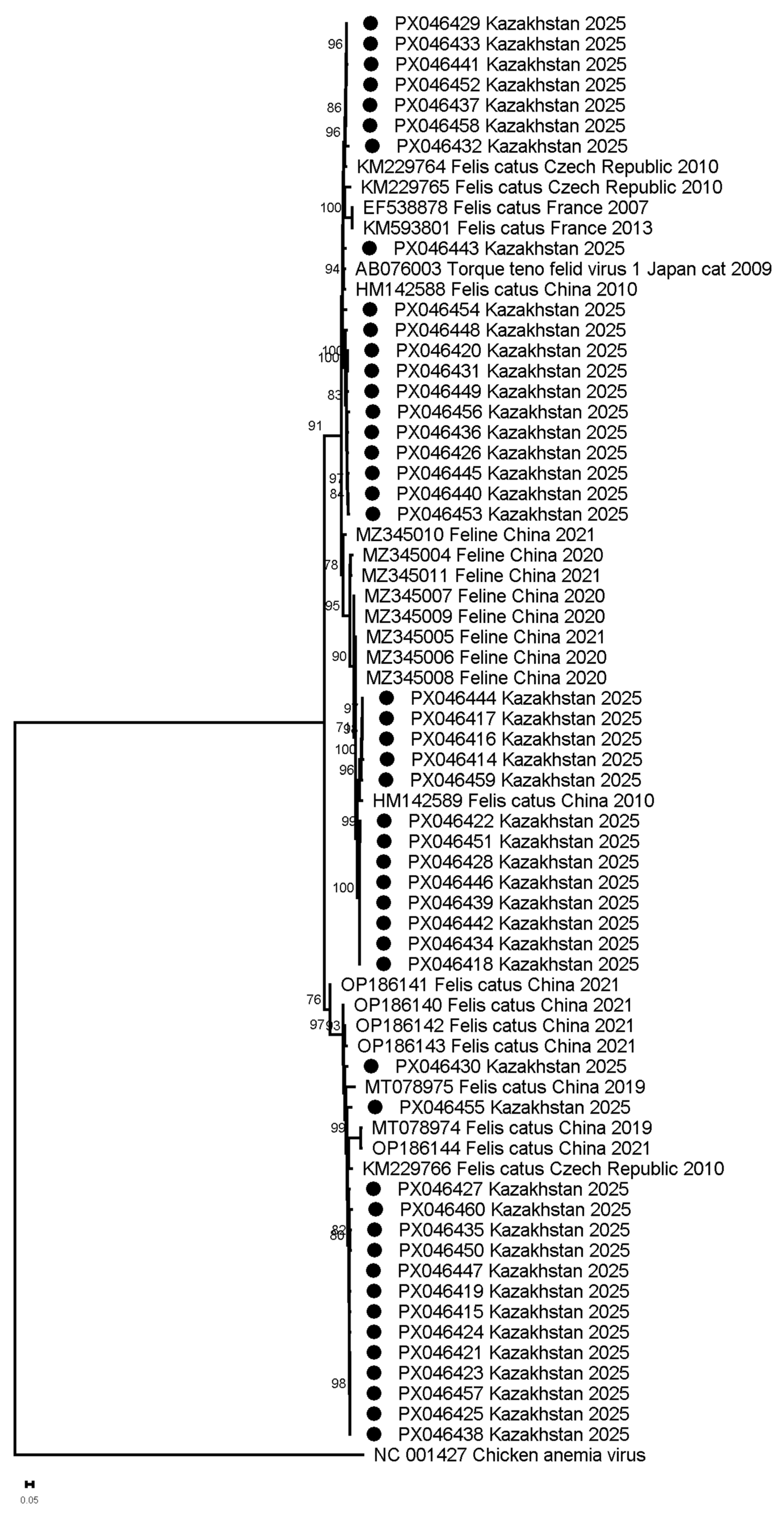
2.5. Phylogenetic Analysis
3. Results
3.1. Prevalence and Genetic Diversity of FcTTV in Domestic Cats
3.2. FcTTV Isolates in Kazakhstan as Compared to Global Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, B.; Liu, B.; Cheng, M.; Dong, J.; Hu, Y.; Jin, Q.; Yang, F. An atlas of the blood virome in healthy individuals. Virus Res. 2023, 323, 199004. [Google Scholar] [CrossRef]
- Timmerman, A.L.; Schönert, A.L.; van der Hoek, L. Anelloviruses versus human immunity: How do we control these viruses? FEMS Microbiol. Rev. 2024, 48, fuae005. [Google Scholar] [CrossRef]
- Nishizawa, T.; Okamoto, H.; Konishi, K.; Yoshizawa, H.; Miyakawa, Y.; Mayumi, M. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem. Biophys. Res. Commun. 1997, 241, 92–97. [Google Scholar] [CrossRef]
- Biagini, P. Classification of TTV and related viruses (anelloviruses). TT Viruses Still Elus. Hum. Pathog. 2009, 331, 21–33. [Google Scholar]
- Nokhova, A.R.; Dubovitskiy, N.A.; Derko, A.A.; Khozyainova, A.A.; Kurskaya, O.G.; Shestopalov, A.M.; Sharshov, K.A. Genetic characteristics of anelloviruses detected in individual viromes of children with acute respiratory symptoms using the metagenomic approach. Acta Virol. 2025, 69, 13512. [Google Scholar] [CrossRef]
- Okamoto, H. History of discoveries and pathogenicity of TT viruses. TT Viruses Still Elus. Hum. Pathog. 2009, 331, 1–20. [Google Scholar]
- Simmonds, P.; Adriaenssens, E.M.; Lefkowitz, E.J.; Oksanen, H.M.; Siddell, S.G.; Zerbini, F.M.; Alfenas-Zerbini, P.; Aylward, F.O.; Dempsey, D.M.; Dutilh, B.E. Changes to virus taxonomy and the ICTV Statutes ratified by the International Committee on Taxonomy of Viruses (2024). Arch. Virol. 2024, 169, 236. [Google Scholar] [CrossRef]
- Varsani, A.; Kraberger, S.; Opriessnig, T.; Maggi, F.; Celer, V.; Okamoto, H.; Biagini, P. Anelloviridae taxonomy update 2023. Arch. Virol. 2023, 168, 277. [Google Scholar] [CrossRef] [PubMed]
- Varsani, A.; Opriessnig, T.; Celer, V.; Maggi, F.; Okamoto, H.; Blomström, A.-L.; Cadar, D.; Harrach, B.; Biagini, P.; Kraberger, S. Taxonomic update for mammalian anelloviruses (family Anelloviridae). Arch. Virol. 2021, 166, 2943–2953. [Google Scholar] [CrossRef] [PubMed]
- Brani, P.; Manzoor, H.Z.; Spezia, P.G.; Vigezzi, A.; Ietto, G.; Dalla Gasperina, D.; Minosse, C.; Bosi, A.; Giaroni, C.; Carcano, G. Torque teno virus: Lights and shades. Viruses 2025, 17, 334. [Google Scholar] [CrossRef] [PubMed]
- Hazanudin, S.N.; Othman, Z.; Sekawi, Z.; Kqueen, C.; Rasdi, R. Torque Teno Virus and Hepatitis: A review on correlation. Life Sci. Med. Biomed. 2019, 3, 31. [Google Scholar] [CrossRef]
- Hu, Z.-J.; Lang, Z.-W.; Zhou, Y.-S.; Yan, H.-P.; Huang, D.-Z.; Chen, W.-R.; Luo, Z.-X. Clinicopathological study on TTV infection in hepatitis of unknown etiology. World J. Gastroenterol. 2002, 8, 288. [Google Scholar] [CrossRef]
- Gergely, P., Jr.; Perl, A.; Poór, G. Possible pathogenic nature of the recently discovered TT virus: Does it play a role in autoimmune rheumatic diseases? Autoimmun. Rev. 2006, 6, 5–9. [Google Scholar] [CrossRef]
- Mancuso, R.; Saresella, M.; Hernis, A.; Agostini, S.; Piancone, F.; Caputo, D.; Maggi, F.; Clerici, M. Torque teno virus (TTV) in multiple sclerosis patients with different patterns of disease. J. Med. Virol. 2013, 85, 2176–2183. [Google Scholar] [CrossRef]
- Kelly, E.; Awan, A.; Sweeney, C.; Wildes, D.; De Gascun, C.; Hassan, J.; Riordan, M. Torque Teno Virus Loads as a Marker of Immunosuppression in Pediatric Kidney Transplant Recipients. Pediatr. Transplant. 2024, 28, e14857. [Google Scholar] [CrossRef]
- Redondo, N.; Navarro, D.; Aguado, J.M.; Fernández-Ruiz, M. Viruses, friends, and foes: The case of Torque Teno Virus and the net state of immunosuppression. Transpl. Infect. Dis. 2022, 24, e13778. [Google Scholar] [CrossRef]
- Gao, J.; Liu, C.; Yi, J.; Shi, Y.; Li, H.; Liu, H. Genomic Characteristics of Feline Anelloviruses Isolated from Domestic Cats in Shanghai, China. Vet. Sci. 2023, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Jarošová, V.; Hrazdilová, K.; Filipejová, Z.; Schánilec, P.; Celer, V. Whole genome sequencing and phylogenetic analysis of feline anelloviruses. Infect. Genet. Evol. 2015, 32, 130–134. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Wang, Y.; Liu, Z.; Li, J.; Guo, L.; Yang, S.; Shen, Q.; Zhao, X.; Cui, L. Identification and genomic characterization of a novel species of feline anellovirus. Virol. J. 2016, 13, 146. [Google Scholar] [CrossRef]
- Zhu, C.; Shan, T.; Cui, L.; Luo, X.; Liu, Z.; Tang, S.; Liu, Z.; Yuan, C.; Lan, D.; Zhao, W. Molecular detection and sequence analysis of feline Torque teno virus (TTV) in China. Virus Res. 2011, 156, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Kanai, N.; Fujii, T.; Saito, K.; Tokoyama, T. Rapid and simple method for preparation of genomic DNA from easily obtainable clotted blood. J. Clin. Pathol. 1994, 47, 1043–1044. [Google Scholar] [CrossRef]
- Li, D.; Luo, R.; Liu, C.-M.; Leung, C.-M.; Ting, H.-F.; Sadakane, K.; Yamashita, H.; Lam, T.-W. MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 2016, 102, 3–11. [Google Scholar] [CrossRef]
- Molina-Mora, J.A.; Cordero-Laurent, E.; Calderón-Osorno, M.; Chacón-Ramírez, E.; Duarte-Martínez, F. Metagenomic pipeline for identifying co-infections among distinct SARS-CoV-2 variants of concern: Study cases from Alpha to Omicron. Sci. Rep. 2022, 12, 9377. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhang, L.; Zhang, X.; Yun, F.; Chang, Y.; Tuersun, A.; Aisaiti, K.; Ma, Z. Metagenome-assembled viral genomes analysis reveals diversity and infectivity of the RNA virome of gerbillinae species. Viruses 2022, 14, 356. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Kishino, H.; Yano, T.-A. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Cavalcante, L.T.; Cosentino, M.A.; D’arc, M.; Moreira, F.R.; Mouta, R.; Augusto, A.M.; Troccoli, F.; Soares, M.A.; Santos, A.F. Characterization of a new anellovirus species infecting an ocelot (Leopardus pardalis) in Brazil. Genet. Mol. Biol. 2023, 46, e20230015. [Google Scholar] [CrossRef] [PubMed]
- Di Profio, F.; Sarchese, V.; Fruci, P.; Aste, G.; Martella, V.; Palombieri, A.; Di Martino, B. Exploring the enteric virome of cats with acute gastroenteritis. Vet. Sci. 2023, 10, 362. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, L.; Tu, Z.; Sun, S.; Sun, Y.; Yi, L.; Tu, C.; He, B. Virome profiling of an amur leopard cat reveals multiple anelloviruses and a bocaparvovirus. Vet. Sci. 2022, 9, 640. [Google Scholar] [CrossRef]
- Al-Qahtani, A.A.; Alabsi, E.S.; AbuOdeh, R.; Thalib, L.; El Zowalaty, M.E.; Nasrallah, G.K. Prevalence of anelloviruses (TTV, TTMDV, and TTMV) in healthy blood donors and in patients infected with HBV or HCV in Qatar. Virol. J. 2016, 13, 208. [Google Scholar] [CrossRef]
- Sabbaghian, M.; Gheitasi, H.; Shekarchi, A.A.; Tavakoli, A.; Poortahmasebi, V. The mysterious anelloviruses: Investigating its role in human diseases. BMC Microbiol. 2024, 24, 40. [Google Scholar] [CrossRef] [PubMed]
- Rammohan, L.; Xue, L.; Wang, C.; Chittick, W.; Ganesan, S.; Ramamoorthy, S. Increased prevalence of torque teno viruses in porcine respiratory disease complex affected pigs. Vet. Microbiol. 2012, 157, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, H.; Yang, X.; Guan, Z.; Zhou, Y. Molecular detection of Torque teno virus in different breeds of swine. Virol. J. 2011, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.-T.; Giménez-Lirola, L.; Huang, Y.-W.; Meng, X.-J.; Halbur, P.G.; Opriessnig, T. The prevalence of Torque teno sus virus (TTSuV) is common and increases with the age of growing pigs in the United States. J. Virol. Methods 2012, 183, 40–44. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Ahn, H.-S.; Han, S.-H.; Go, H.-J.; Kim, D.-H.; Kim, J.-H.; Lee, J.-B.; Park, S.-Y.; Song, C.-S.; Lee, S.-W. Genetic analysis of torque teno canis virus identified in Republic of Korea. Vet. Sci. 2022, 9, 693. [Google Scholar] [CrossRef]
- Li, X.; Tavares, Y.; Carneiro, C.M.; Phillips, C.; Subramaniam, K.; Lednicky, J.; Boughton, R.K.; Pepin, K.M.; Miller, R.S.; VerCauteren, K.C. Whole genome characterization of Torque teno sus virus 1 (TTSuV1) in wild and domestic pigs: Insights into genetic classification, host differentiation, and intra-host variation. Front. Microbiol. 2025, 16, 1585558. [Google Scholar] [CrossRef]
- Nishiyama, S.; Dutia, B.M.; Stewart, J.P.; Meredith, A.L.; Shaw, D.J.; Simmonds, P.; Sharp, C.P. Identification of novel anelloviruses with broad diversity in UK rodents. J. Gen. Virol. 2014, 95, 1544–1553. [Google Scholar] [CrossRef]
- van den Brand, J.M.; van Leeuwen, M.; Schapendonk, C.M.; Simon, J.H.; Haagmans, B.L.; Osterhaus, A.D.; Smits, S.L. Metagenomic analysis of the viral flora of pine marten and European badger feces. J. Virol. 2012, 86, 2360–2365. [Google Scholar] [CrossRef]
- Xiong, Y.-Q.; Mo, Y.; Chen, M.-J.; Cai, W.; He, W.-Q.; Chen, Q. Detection and phylogenetic analysis of torque teno virus (TTV) carried by murine rodents and house shrews in China. Virology 2018, 516, 189–195. [Google Scholar] [CrossRef]
- Venkataraman, T.; Swaminathan, H.; Arze, C.A.; Jacobo, S.M.; Bhattacharyya, A.; David, T.; Nawandar, D.M.; Delagrave, S.; Mani, V.; Yozwiak, N.L. Comprehensive profiling of antibody responses to the human anellome using programmable phage display. Cell Rep. 2022, 41, 111754. [Google Scholar] [CrossRef]
- Focosi, D.; Spezia, P.G.; Macera, L.; Salvadori, S.; Navarro, D.; Lanza, M.; Antonelli, G.; Pistello, M.; Maggi, F. Assessment of prevalence and load of torquetenovirus viraemia in a large cohort of healthy blood donors. Clin. Microbiol. Infect. 2020, 26, 1406–1410. [Google Scholar] [CrossRef]
- Li, X.; Parker, B.M.; Boughton, R.K.; Beasley, J.C.; Smyser, T.J.; Austin, J.D.; Pepin, K.M.; Miller, R.S.; Vercauteren, K.C.; Wisely, S.M. Torque Teno sus virus 1: A potential surrogate pathogen to study pig-transmitted transboundary animal diseases. Viruses 2024, 16, 1397. [Google Scholar] [CrossRef] [PubMed]
- Väisänen, E.; Kuisma, I.; Mäkinen, M.; Ilonen, J.; Veijola, R.; Toppari, J.; Hedman, K.; Söderlund-Venermo, M. Torque teno virus primary infection kinetics in early childhood. Viruses 2022, 14, 1277. [Google Scholar] [CrossRef] [PubMed]
- Blois, S.; Mallus, F.; Liciardi, M.; Pilo, C.; Camboni, T.; Macera, L.; Maggi, F.; Manzin, A. High prevalence of co-infection with multiple Torque teno sus virus species in Italian pig herds. PLoS ONE 2014, 9, e113720. [Google Scholar] [CrossRef] [PubMed]
- Kraberger, S.; Serieys, L.E.; Richet, C.; Fountain-Jones, N.M.; Baele, G.; Bishop, J.M.; Nehring, M.; Ivan, J.S.; Newkirk, E.S.; Squires, J.R. Complex evolutionary history of felid anelloviruses. Virology 2021, 562, 176–189. [Google Scholar] [CrossRef]
- Maggi, F.; Andreoli, E.; Lanini, L.; Fornai, C.; Vatteroni, M.; Pistello, M.; Presciuttini, S.; Bendinelli, M. Relationships between total plasma load of torquetenovirus (TTV) and TTV genogroups carried. J. Clin. Microbiol. 2005, 43, 4807–4810. [Google Scholar] [CrossRef]
- Lipinski, M.J.; Froenicke, L.; Baysac, K.C.; Billings, N.C.; Leutenegger, C.M.; Levy, A.M.; Longeri, M.; Niini, T.; Ozpinar, H.; Slater, M.R. The ascent of cat breeds: Genetic evaluations of breeds and worldwide random-bred populations. Genomics 2008, 91, 12–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yessembekova, G.; Abdigulov, B.; Shevtsov, A.; Amirgazin, A.; Abdrakhmanov, S.; Shevtsova, E.; Bolysbekkyzy, S.; Baduanova, S.; Shustov, A. Prevalence and Genetic Diversity of Torque teno felis virus (FcTTV) in Domestic Cats from Kazakhstan. Viruses 2025, 17, 1265. https://doi.org/10.3390/v17091265
Yessembekova G, Abdigulov B, Shevtsov A, Amirgazin A, Abdrakhmanov S, Shevtsova E, Bolysbekkyzy S, Baduanova S, Shustov A. Prevalence and Genetic Diversity of Torque teno felis virus (FcTTV) in Domestic Cats from Kazakhstan. Viruses. 2025; 17(9):1265. https://doi.org/10.3390/v17091265
Chicago/Turabian StyleYessembekova, Gulzhan, Bolat Abdigulov, Alexandr Shevtsov, Asylulan Amirgazin, Sarsenbay Abdrakhmanov, Elena Shevtsova, Symbat Bolysbekkyzy, Salima Baduanova, and Alexandr Shustov. 2025. "Prevalence and Genetic Diversity of Torque teno felis virus (FcTTV) in Domestic Cats from Kazakhstan" Viruses 17, no. 9: 1265. https://doi.org/10.3390/v17091265
APA StyleYessembekova, G., Abdigulov, B., Shevtsov, A., Amirgazin, A., Abdrakhmanov, S., Shevtsova, E., Bolysbekkyzy, S., Baduanova, S., & Shustov, A. (2025). Prevalence and Genetic Diversity of Torque teno felis virus (FcTTV) in Domestic Cats from Kazakhstan. Viruses, 17(9), 1265. https://doi.org/10.3390/v17091265





