Immunogenicity and Safety Results of a Randomized, Three-Arm, Phase IV Clinical Trial of Concomitant Administration of Typhoid Vi Conjugate Vaccine with Measles and Rubella Vaccine in Healthy Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Objectives
2.3. Study Population
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
2.4. Vaccine Administration
2.5. Study Procedure
2.6. Immunogenicity Assessment
2.7. Safety Assessment
2.8. Statistical Analysis
2.9. Ethical Considerations
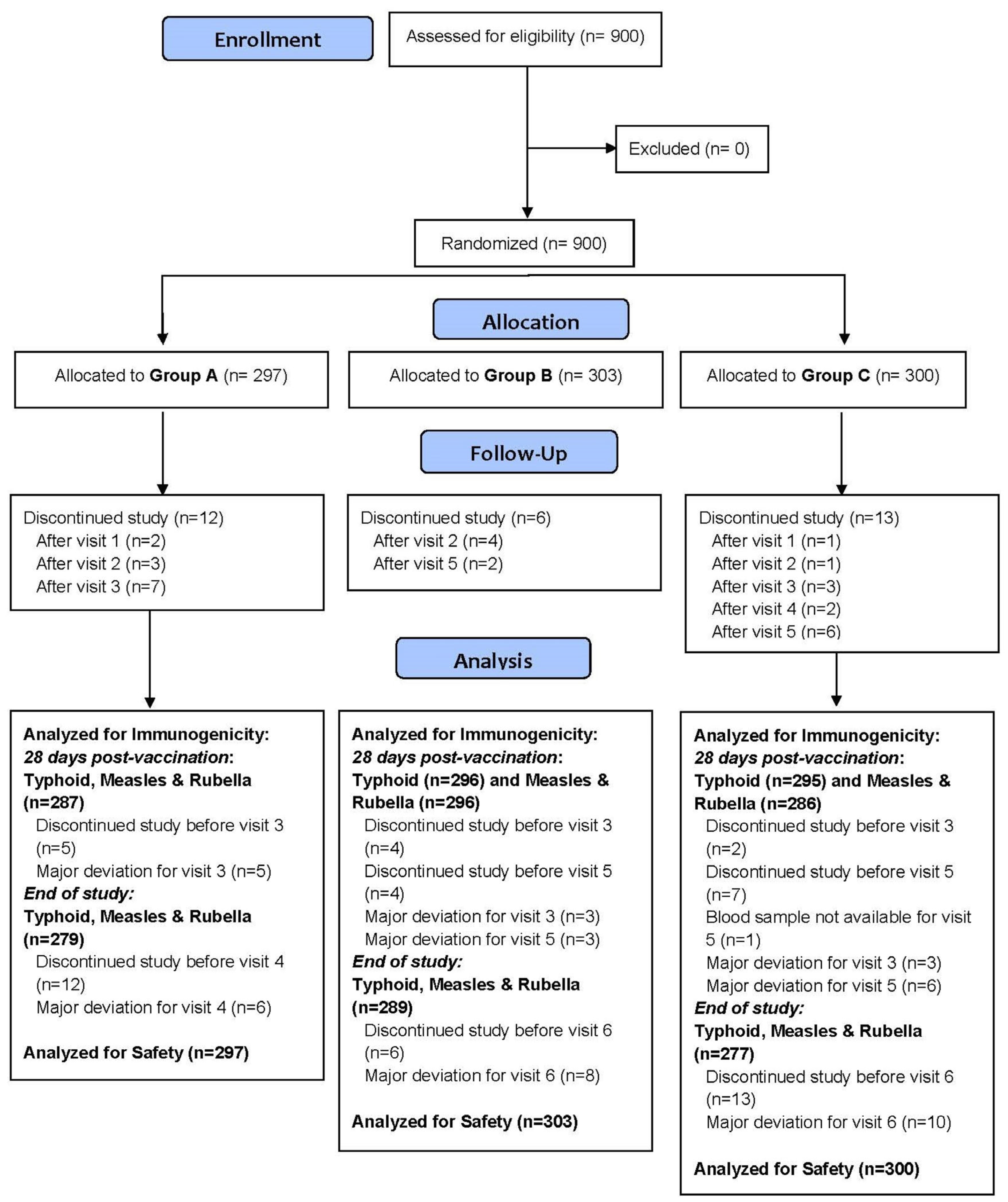
3. Results
3.1. Immunogenicity Outcomes
3.1.1. Seroconversion Rates for Anti-Vi IgG
3.1.2. Seroconversion for Anti-Measles IgG Antibodies
3.1.3. Seroconversion for Anti-Rubella IgG Antibodies
3.2. Geometric Mean Titres of Antibodies
3.3. Safety Outcomes
3.3.1. Local Adverse Events by Vaccine Type
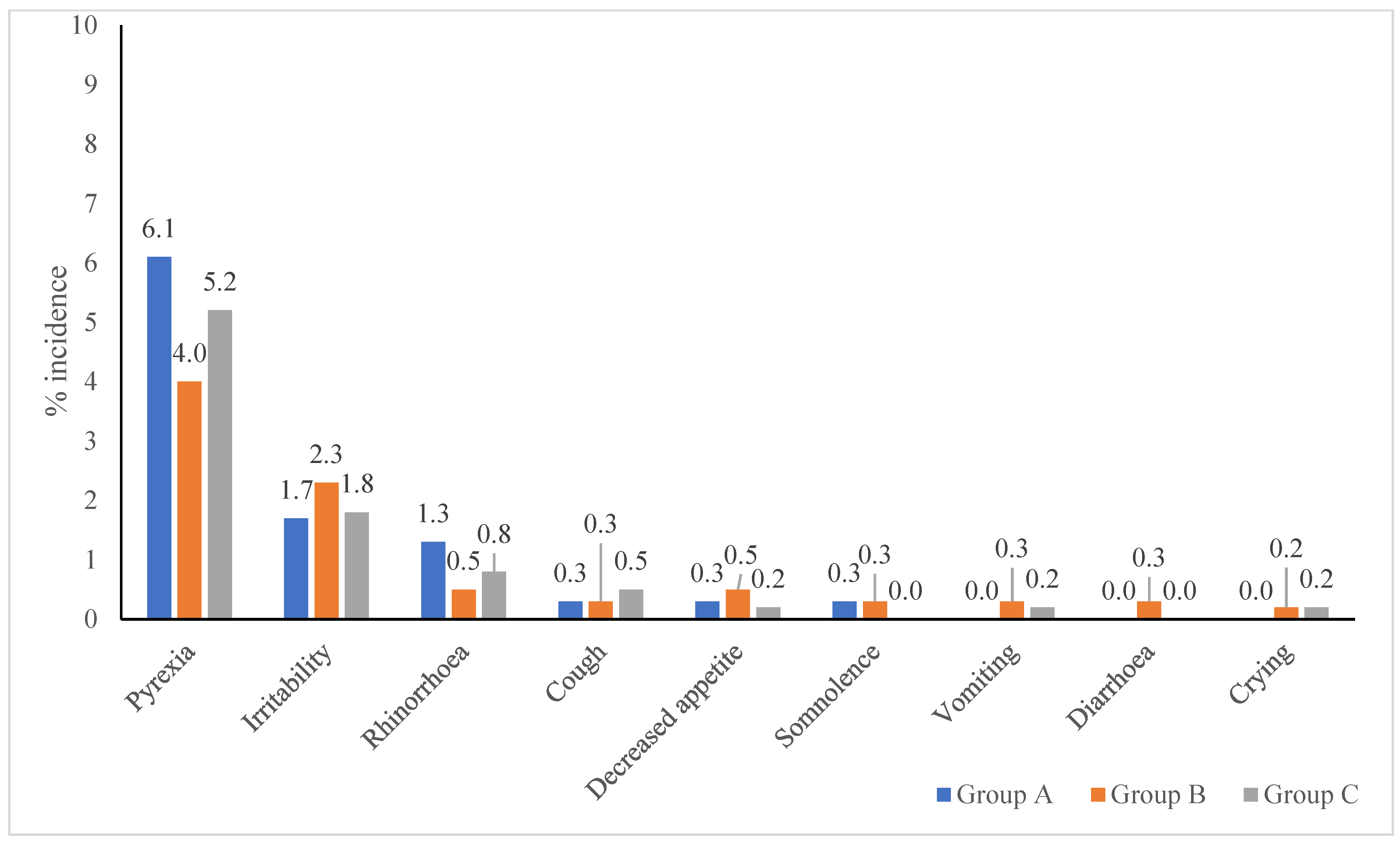
3.3.2. Systemic Adverse Events by Vaccine Type
3.4. Medically Attended Adverse Events (MAAEs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchello, C.S.; Birkhold, M.; Crump, J.A. Complications and Mortality of Typhoid Fever: A Global Systematic Review and Meta-Analysis. J. Infect. 2020, 81, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Stanaway, J.D.; Reiner, R.C.; Blacker, B.F.; Goldberg, E.M.; Khalil, I.A.; Troeger, C.E.; Andrews, J.R.; Bhutta, Z.A.; Crump, J.A.; Im, J.; et al. The Global Burden of Typhoid and Paratyphoid Fevers: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019, 19, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, B.; Sur, D.; Gupta, S.S.; Ganguly, N.K. Typhoid Fever. Indian J. Med. Res. 2019, 150, 437–447. [Google Scholar] [CrossRef] [PubMed]
- John, J.; Bavdekar, A.; Rongsen-Chandola, T.; Dutta, S.; Gupta, M.; Kanungo, S.; Sinha, B.; Srinivasan, M.; Shrivastava, A.; Bansal, A.; et al. Burden of Typhoid and Paratyphoid Fever in India. New Engl. J. Med. 2023, 388, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Piovani, D.; Figlioli, G.; Nikolopoulos, G.K.; Bonovas, S. The Global Burden of Enteric Fever, 2017–2021: A Systematic Analysis from the Global Burden of Disease Study 2021. EClinicalMedicine 2024, 77, 102883. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Measles and Rubella in India—World Health Organization (WHO). Available online: https://www.who.int/india/health-topics/measles (accessed on 7 July 2025).
- India Vaccinates 30 Million Children against Measles and Rubella in Seven Months. Available online: https://www.who.int/india/news-room/feature-stories/detail/india-vaccinates-30-million-children-against-measles-and-rubella-in-7-months (accessed on 7 July 2025).
- De Vries, R.D.; Stittelaar, K.J.; Osterhaus, A.D.M.E.; De Swart, R.L. Measles Vaccination: New Strategies and Formulations. Expert. Rev. Vaccines 2008, 7, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Sahastrabuddhe, S.; Saluja, T. Overview of the Typhoid Conjugate Vaccine Pipeline: Current Status and Future Plans. Clin. Infect. Dis. 2019, 68, S22–S26. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Typhoid Vaccines: WHO Position Paper, March 2018—Recommendations. Vaccine 2019, 37, 214–216. [Google Scholar] [CrossRef] [PubMed]
- ZyVac® TCV. World Health Organization. Available online: https://extranet.who.int/prequal/vaccines/p/zyvacr-tcv (accessed on 7 July 2025).
- WHO. Annex 3—Guidelines on the Quality, Safety and Efficacy of Typhoid Conjugate Vaccines. WHO Expert Committee on Biological Standardization. Sixty Fourth Report. WHO Technical Report Series No. 987. World Health Organization: Geneva, Switzerland, 2014. Available online: https://cdn.who.int/media/docs/default-source/biologicals/vaccine-standardization/typhoid-fever/trs_987_annex3.pdf?sfvrsn=39234f5b_3&download=true (accessed on 7 July 2025).
- Sirima, S.B.; Ouedraogo, A.; Barry, N.; Siribie, M.; Tiono, A.; Nébié, I.; Konaté, A.; Berges, G.D.; Diarra, A.; Ouedraogo, M.; et al. Safety and Immunogenicity of Vi-Typhoid Conjugate Vaccine Co-Administration with Routine 9-Month Vaccination in Burkina Faso: A Randomized Controlled Phase 2 Trial. Int. J. Infect. Dis. 2021, 108, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Sirima, S.B.; Ouedraogo, A.; Barry, N.; Siribie, M.; Tiono, A.B.; Nébié, I.; Konaté, A.T.; Berges, G.D.; Diarra, A.; Ouedraogo, M.; et al. Safety and Immunogenicity of Co-Administration of Meningococcal Type A and Measles–Rubella Vaccines with Typhoid Conjugate Vaccine in Children Aged 15–23 Months in Burkina Faso. Int. J. Infect. Dis. 2021, 102, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.R.; Corrado, M.; Perry, J.; Watts, T.H.; Bolotin, S. The Contributions of T Cell-Mediated Immunity to Protection from Vaccine-Preventable Diseases: A Primer. Hum. Vaccines Immunother. 2024, 20, 2395679. [Google Scholar] [CrossRef] [PubMed]
- Vadrevu, K.M.; Dugyala, R.; Mahantashetti, N.S.; Khalatkar, V.; Murthy, K.; Mogre, S.; Mitra, M. Safety, Immunogenicity and Non-Interference of Concomitant Typhoid Vi Capsular Polysaccharide-Tetanus Toxoid Conjugate Vaccine (Typbar-TCV®) and Measles or Measles-Mumps-Rubella Vaccines in 8–9 Months-Old Indian Children. Hum. Vaccines Immunother. 2022, 18, 2150030. [Google Scholar] [CrossRef] [PubMed]
- Findlow, H.; Borrow, R. Interactions of Conjugate Vaccines and Co-Administered Vaccines. Hum. Vaccines Immunother. 2015, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Saluja, T.; Rai, G.K.; Chaudhary, S.; Kanodia, P.; Giri, B.R.; Kim, D.R.; Yang, J.S.; Park, I.-Y.; Kyung, S.-E.; Vemula, S.; et al. Immune Non-Interference and Safety Study of Vi-DT Typhoid Conjugate Vaccine with a Measles, Mumps and Rubella Containing Vaccine in 9–15 Months Old Nepalese Infants. Vaccine 2022, 40, 5828–5834. [Google Scholar] [CrossRef] [PubMed]
- Nampota-Nkomba, N.; Nyirenda, O.M.; Khonde, L.; Mapemba, V.; Mbewe, M.; Ndaferankhande, J.M.; Msuku, H.; Masesa, C.; Misiri, T.; Mwakiseghile, F.; et al. Safety and Immunogenicity of a Typhoid Conjugate Vaccine among Children Aged 9 Months to 12 Years in Malawi: A Nested Substudy of a Double-Blind, Randomised Controlled Trial. Lancet Glob. Health 2022, 10, e1326–e1335. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.E. Measles Vaccine. Viral Immunol. 2018, 31, 86. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Taha, A.; Abouelmagd, K.; Mahmoud, A.M.; Elkasaby, M.H.; Nguyen, D.; Ahmed, R.; Patel, P.; Bonilla-Aldana, D.K.; Luna, C.; Rodriguez-Morales, A.J. Safety and Immunogenicity of Vi-Diphtheria Toxoid Typhoid Conjugate Vaccine among Children below 2 Years: A Systematic Review and Meta-Analysis. Front. Microbiol. 2024, 15, 1385834. [Google Scholar] [CrossRef] [PubMed]
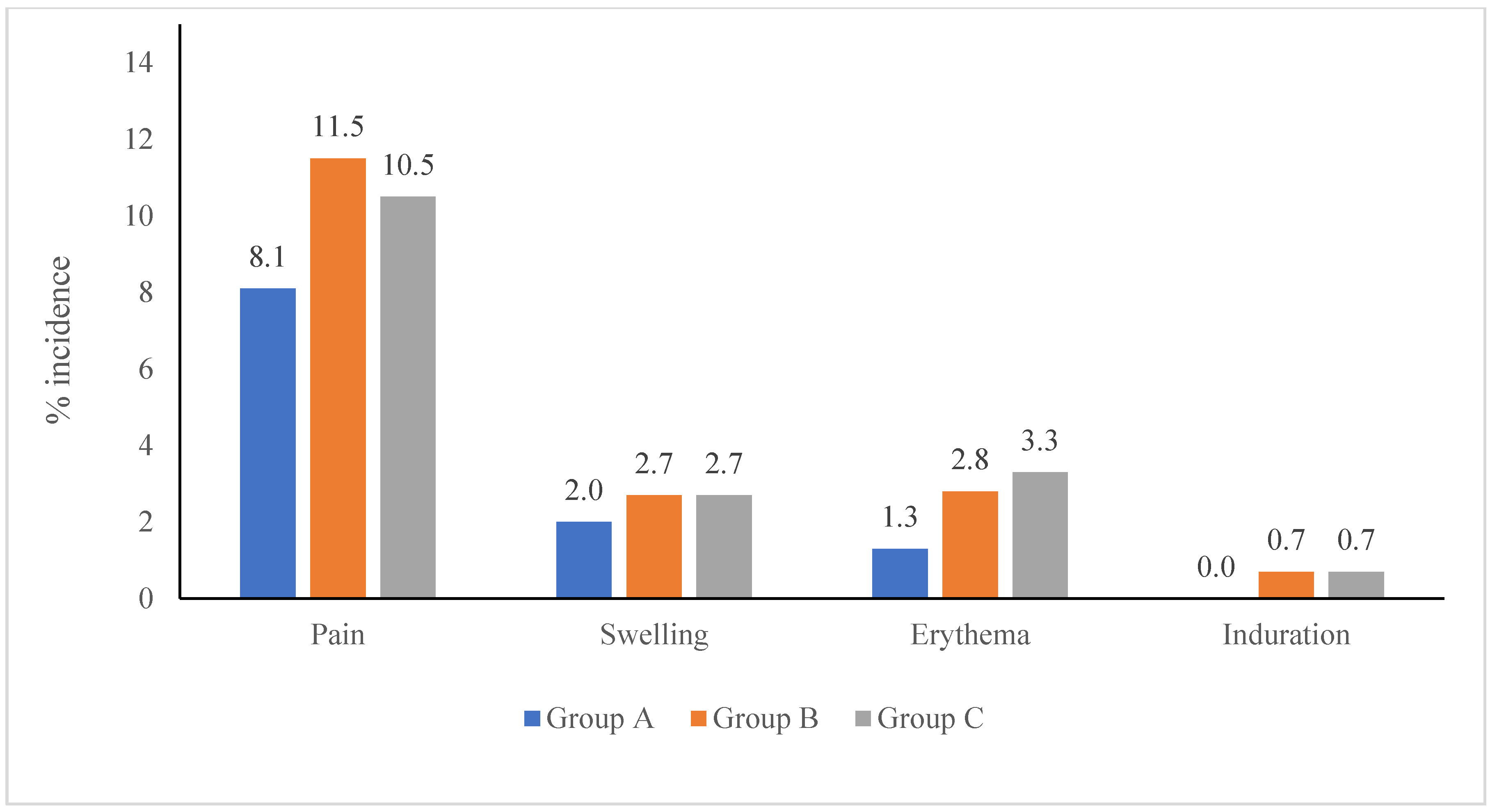
| Parameter | Group A (TCV + MR) [N = 297] | Group B (MR/TCV) [N = 303] | Group C (TCV/MR) [N = 300] | p Value (A vs. B) | p Value (A vs. C) | |
|---|---|---|---|---|---|---|
| Age (months) * | 9.2 ± 0.4 | 9.2 ± 0.4 | 9.2 ± 0.4 | 0.43 | 0.58 | |
| Gender # | Male | 161 (54.2%) | 153 (50.5%) | 161 (53.7%) | 0.37 | 0.93 |
| Female | 136 (45.8%) | 150 (49.5%) | 139 (46.3%) | |||
| Length (cm) * | 68.9 ± 6.4 | 69.3 ± 6.5 | 69.2 ± 6.2 | 0.47 | 0.51 | |
| Weight (kg) * | 8.2 ± 1.0 | 8.1 ± 1.1 | 8.2 ± 1.1 | 0.74 | 0.54 | |
| Temperature (°C) * | 36.8 ± 0.3 | 36.8 ± 0.3 | 36.8 ± 0.3 | 0.24 | 0.49 | |
| Heart Rate (/min) * | 112.2 ± 11.1 | 112.3 ± 10.1 | 113.7 ± 10.4 | 0.87 | 0.08 | |
| Respiratory Rate (/min) * | 32.7 ± 6.3 | 32.9 ± 6.2 | 33.0 ± 5.6 | 0.68 | 0.58 | |
| Study Group | Seroconversion Rate * | Group A–Group B or C # | p Value |
|---|---|---|---|
| Seroconversion Rate at Day 28 | |||
| Group A [N = 287] | 259 (90.2%) | NA | NA |
| Group B [N = 296] | 223 (75.3%) | 14.9% (8.6%, 21.1%) | <0.0001 $ |
| Group C [N = 295] | 264 (89.5%) | 0.8% (–4.5%, 6.0%) | 0.79 @ |
| Seroconversion Rate at the end of the study | |||
| Group A [N = 287] | 243 (87.1%) | NA | NA |
| Group B [N = 296] | 207 (71.6%) | 15.5% (8.6%, 22.2%) | <0.0001 $ |
| Group C [N = 295] | 230 (83.0%) | 4.1% (−2.1%, 10.3%) | 0.19 @ |
| Study Group | n ^ | Seroconversion Rate * | Group A–Group B or C # | p Value |
|---|---|---|---|---|
| Seroconversion rate on Day 28 | ||||
| Group A [N = 287] | 271 | 218 (80.4%) | NA | NA |
| Group B [N = 296] | 278 | 209 (75.2%) | 5.3% (−2.0%, 12.4%) | 0.15 $ |
| Group C [N = 286] | 238 | 185 (77.7%) | 2.7% (−4.6%, 10.1%) | 0.51 @ |
| Seroconversion rate at the end of study | ||||
| Group A [N = 287] | 263 | 237 (90.1%) | NA | NA |
| Group B [N = 296] | 271 | 244 (90.0%) | 0.1% (−5.4%, 5.5%) | 1.0 $ |
| Group C [N = 286] | 228 | 206 (90.4%) | −0.2% (−6.0%, 5.3%) | 1.0 @ |
| Study Group | n ^ | Seroconversion Rate * | Group A–Group B or C # | p Value |
|---|---|---|---|---|
| Seroconversion rate on Day 28 | ||||
| Group A [N = 287] | 261 | 229 (87.7%) | NA | NA |
| Group B [N = 296] | 263 | 221 (84.0%) | 3.7% (−2.6%, 10.0%) | 0.26 $ |
| Group C [N = 286] | 223 | 190 (85.2%) | 2.5% (−3.8%, 9.1%) | 0.43 @ |
| Seroconversion rate at the end of study | ||||
| Group A [N = 287] | 254 | 252 (99.2%) | NA | NA |
| Group B [N = 296] | 258 | 256 (99.2%) | 0.0% (−2.4%, 2.4%) | 1.0 $ |
| Group C [N = 286] | 213 | 210 (98.6%) | 0.6% (−1.9%, 3.7%) | 0.66 @ |
| Group A [N = 287] | Group B [N = 296] | Group C [N = 295] | p Value (A vs. B) | p Value (A vs. C) | |
|---|---|---|---|---|---|
| Anti-Vi IgG (U/mL) | |||||
| Pre-vaccination | 4.8 (4.2, 5.4) | 11.5 (9.0, 14.7) | 5.1 (4.5, 5.9) | <0.0001 | 0.42 |
| 28 d post-vaccination | 1338.8 (1074.1, 1668.7) | 749.6 (583.1, 963.6) | 1218.4 (971.4, 1528.1) | <0.01 | 0.56 |
| p value | <0.0001 | <0.0001 | <0.0001 | NA | NA |
| Anti-measles IgG (NTU) | |||||
| Pre-vaccination | 1.9 (1.8, 2.1) | 1.8 (1.7, 2.0) | 2.4 (2.2, 2.8) | 0.50 | <0.01 |
| 28 d post-vaccination | 12.0 (11.3, 12.8) | 11.3 (10.5, 12.2) | 11.8 (11.0, 12.6) | 0.22 | 0.69 |
| p value | <0.0001 | <0.0001 | <0.0001 | NA | NA |
| Anti-rubella IgG (IU/mL) | |||||
| Pre-vaccination | 1.3 (1.1, 1.6) | 1.5 (1.2, 1.8) | 2.2 (1.8, 2.7) | 0.31 | <0.001 |
| 28 d post-vaccination | 26.8 (23.7, 30.3) | 24.8 (22.1, 27.8) | 26.4 (23.2, 30.0) | 0.38 | 0.88 |
| p value | <0.0001 | <0.0001 | <0.0001 | NA | NA |
| Group A GMT (N = 279) | Group B GMT (N = 289) | Group C GMT (N = 277) | p Value (A vs. B) | p Value (A vs. C) | |
|---|---|---|---|---|---|
| Anti-Vi IgG (U/mL) | |||||
| Pre-vaccination | 4.8 (4.2, 5.5) | 11.8 (9.2, 15.2) | 5.1 (4.4, 5.8) | <0.0001 | 0.61 |
| End of study | 90.6 (77.9, 105.5) | 111.5 (93.6, 132.9) | 90.3 (77.0, 105.9) | 0.08 | 0.97 |
| p value | <0.0001 | <0.0001 | <0.0001 | NA | NA |
| Anti-measles IgG (NTU) | |||||
| Pre-vaccination | 1.9 (1.8, 2.1) | 1.8 (1.7, 2.0) | 2.5 (2.2, 2.9) | 0.49 | <0.01 |
| End of study | 16.7 (15.8, 17.7) | 16.7 (15.8, 17.8) | 16.9 (16.0, 17.9) | 0.97 | 0.75 |
| p value | <0.0001 | <0.0001 | <0.0001 | NA | NA |
| Anti-rubella IgG (IU/mL) | |||||
| Pre-vaccination | 1.3 (1.1, 1.6) | 1.5 (1.2, 1.8) | 2.3 (1.8, 2.8) | 0.40 | <0.001 |
| End of study | 89.2 (82.3, 96.8) | 75.3 (69.4, 81.7) | 77.9 (70.7, 85.7) | <0.01 | 0.03 |
| p value | <0.0001 | <0.0001 | <0.0001 | NA | NA |
| Parameter | Group A (TCV + MR) [N = 297] | Group B (MR/TCV) [N = 303] | Group C (TCV/MR) [N = 300] |
|---|---|---|---|
| No. (%) of subjects with AEs @ | 71 (23.9%) | 97 (32.0%) | 98 (32.7%) |
| No. of AEs reported * | 111 | 184 | 187 |
| Adverse Events by Severity Grade # | |||
| Mild (Grade 1) | 111 (100.0%) | 183 (99.5%) | 186 (99.5%) |
| Moderate (Grade 2) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) |
| Severe (Grade 3) | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) |
| Adverse Events by Association # | |||
| Related—TCV | 52 (46.8%) | 84 (45.7%) | 118 (63.1%) |
| Related—MR vaccine | 21 (18.9%) | 75 (40.8%) | 39 (20.9%) |
| Related—Both | 25 (22.5%) | 0 (0.0%) | 0 (0.0%) |
| Not related | 13 (11.7%) | 25 (13.6%) | 30 (16.0%) |
| Adverse Events by Reporting (Solicited/Unsolicited) # | |||
| Solicited | 98 (88.3%) | 159 (86.4%) | 156 (83.4%) |
| Unsolicited | 13 (11.7%) | 25 (13.6%) | 31 (16.6%) |
| Serious Adverse Events # | |||
| No. of SAEs | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) |
| No. of patients with SAEs * | 0 | 0 | 1 |
| Parameter | Group A (TCV + MR) [N = 297] | Group B (MR/TCV) [N = 303] | Group C (TCV/MR) [N = 300] | p Value (A vs. B) | p Value (A vs. C) |
|---|---|---|---|---|---|
| No. of unsolicited AEs reported * | 13 | 25 | 31 | NA | NA |
| Local AEs * | 0 | 0 | 0 | NA | NA |
| Systemic AEs * | 13 | 25 | 31 | NA | NA |
| Pyrexia | 4 (1.3%) | 6 (2.0%) | 7 (2.3%) | 0.75 | 0.55 |
| Rhinorrhoea | 3 (1.0%) | 2 (0.7%) | 5 (1.7%) | 0.68 | 0.72 |
| Cough | 2 (0.7%) | 3 (1.0%) | 7 (2.3%) | 1.0 | 0.18 |
| Diarrhea | 1 (0.3%) | 3 (1.0%) | 2 (0.7%) | 0.62 | 1.0 |
| URTI | 1 (0.3%) | 2 (0.7%) | 2 (0.7%) | 1.0 | 1.0 |
| Rhinitis | 1 (0.3%) | 1 (0.3%) | 0 (0.0%) | 1.0 | 0.50 |
| Ear pain | 1 (0.3%) | 0 (0.0%) | 1 (0.3%) | 0.50 | 1.0 |
| Nasopharyngitis | 0 (0.0%) | 2 (0.7%) | 1 (0.3%) | 0.5 | 1.0 |
| Rash | 0 (0.0%) | 2 (0.7%) | 1 (0.3%) | 0.50 | 1.0 |
| Vomiting | 0 (0.0%) | 2 (0.7%) | 0 (0.0%) | 0.50 | NA |
| Acarodermatitis | 0 (0.0%) | 1 (0.3%) | 0 (0.0%) | 1.0 | NA |
| Dermatitis atopic | 0 (0.0%) | 1 (0.3%) | 0 (0.0%) | 1.0 | NA |
| Eczema | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) | NA | 1.0 |
| Febrile convulsion | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) | NA | 1.0 |
| Gastroenteritis | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) | NA | 1.0 |
| Miliaria | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) | NA | 1.0 |
| Seborrhoeic dermatitis | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) | NA | 1.0 |
| Group A (TCV + MR) [N = 297] | Group B (MR/TCV) [N = 303] | Group C (TCV/MR) [N = 300] | |
|---|---|---|---|
| Solicited local AEs reported after TCV administration | |||
| No. of TCV administration, n | 297 | 299 | 300 |
| Local AEs, n | 52 | 63 | 84 |
| Pain, n (%) | 39 (13.1%) | 42 (14.0%) | 48 (16.0%) |
| Swelling, n (%) | 7 (2.4%) | 9 (3.0%) | 14 (4.7%) |
| Erythema, n (%) | 6 (2.0%) | 10 (3.3%) | 18 (6.0%) |
| Induration, n (%) | 0 (0.0%) | 2 (0.7%) | 4 (1.3%) |
| Solicited local AEs reported after MR administration | |||
| No. of MR vaccine administrations, n | 297 | 303 | 298 |
| Local AEs, n | 16 | 43 | 19 |
| Pain, n (%) | 9 (3.0%) | 27 (8.9%) | 15 (5.0%) |
| Swelling, n (%) | 5 (1.7%) | 7 (2.3%) | 2 (0.7%) |
| Erythema, n (%) | 2 (0.7%) | 7 (2.3%) | 2 (0.7%) |
| Induration, n (%) | 0 (0.0%) | 2 (0.7%) | 0 (0.0%) |
| Group A (TCV + MR) [N = 297] | Group B (MR/TCV) [N = 303] | Group C (TCV/MR) [N = 300] | |||
|---|---|---|---|---|---|
| Concomitant (TCV + MR) | First Vaccine (MR) | Second Vaccine (TCV) | First Vaccine (TCV) | Second Vaccine (MR) | |
| Solicited systemic AEs reported after TCV, MR vaccine and Concomitant administration | |||||
| No. of vaccinated sub., n | 297 | 303 | 299 | 300 | 298 |
| Systemic AEs, n | 30 | 32 | 21 | 33 | 20 |
| Pyrexia, n (%) | 18 (6.1%) | 12 (4.0%) | 12 (4.0%) | 20 (6.7%) | 11 (3.7%) |
| Irritability, n (%) | 5 (1.7%) | 8 (2.6%) | 6 (2.0%) | 11 (3.7%) | 0 (0.0%) |
| Rhinorrhoea, n (%) | 4 (1.3%) | 3 (1.0%) | 0 (0.0%) | 0 (0.0%) | 5 (1.7%) |
| Cough, n (%) | 1 (0.3%) | 2 (0.7%) | 0 (0.0%) | 0 (0.0%) | 3 (1.0%) |
| Decreased appetite, n (%) | 1 (0.3%) | 2 (0.7%) | 1 (0.3%) | 0 (0.0%) | 1 (0.3%) |
| Somnolence, n (%) | 1 (0.3%) | 2 (0.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Vomiting, n (%) | 0 (0.0%) | 1 (0.3%) | 1 (0.3%) | 1 (0.3%) | 0 (0.0%) |
| Diarrhea, n (%) | 0 (0.0%) | 2 (0.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Crying, n (%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) | 1 (0.3%) | 0 (0.0%) |
| Unsolicited systemic AEs reported after TCV, MR vaccine and Concomitant administration | |||||
| Systemic AEs, n | 13 | 3 | 22 | 10 | 21 |
| Pyrexia, n (%) | 4 (1.3%) | 1 (0.3%) | 5 (1.7%) | 2 (0.7%) | 5 (1.7%) |
| Rhinorrhoea, n (%) | 3 (1.0%) | 0 (0.0%) | 2 (0.7%) | 2 (0.7%) | 3 (1.0%) |
| Cough, n (%) | 2 (0.7%) | 1 (0.3%) | 2 (0.7%) | 3 (1.0%) | 4 (1.3%) |
| Diarrhea, n (%) | 1 (0.3%) | 0 (0.0%) | 3 (1.0%) | 1 (0.3%) | 1 (0.3%) |
| URTI, n (%) | 1 (0.3%) | 0 (0.0%) | 2 (0.7%) | 1 (0.3%) | 1 (0.3%) |
| Rhinitis, n (%) | 1 (0.3%) | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Ear pain, n (%) | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) |
| Nasopharyngitis, n (%) | 0 (0.0%) | 0 (0.0%) | 2 (0.7%) | 0 (0.0%) | 1 (0.3%) |
| Rash, n (%) | 0 (0.0%) | 0 (0.0%) | 2 (0.7%) | 0 (0.0%) | 1 (0.3%) |
| Vomiting, n (%) | 0 (0.0%) | 0 (0.0%) | 2 (0.7%) | 0 (0.0%) | 0 (0.0%) |
| Acarodermatitis, n (%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) |
| Dermatitis atopic, n (%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) |
| Eczema, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) |
| Febrile convulsion, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) | 0 (0.0%) |
| Gastroenteritis, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) |
| Miliaria, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) |
| Seborrhoeic dermatitis, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) |
| Group A (TCV + MR) [N = 297] | Group B (MR/TCV) [N = 303] | Group C (TCV/MR) [N = 300] | |
|---|---|---|---|
| No. of vaccinated sub. | 297 | 299 | 298 |
| Local AEs, n | 0 | 0 | 0 |
| Systemic AEs, n | 10 | 20 | 17 |
| Pyrexia, n (%) | 3 (1.0%) | 5 (1.7%) | 4 (1.3%) |
| Rhinorrhoea, n (%) | 2 (0.7%) | 1 (0.3%) | 3 (1.0%) |
| Cough, n (%) | 1 (0.3%) | 2 (0.7%) | 4 (1.3%) |
| Diarrhea, n (%) | 1 (0.3%) | 3 (1.0%) | 1 (0.3%) |
| URTI, n (%) | 1 (0.3%) | 1 (0.3%) | 1 (0.3%) |
| Rhinitis, n (%) | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) |
| Ear pain, n (%) | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) |
| Nasopharyngitis, n (%) | 0 (0.0%) | 2 (0.7%) | 1 (0.3%) |
| Rash, n (%) | 0 (0.0%) | 2 (0.7%) | 0 (0.0%) |
| Vomiting, n (%) | 0 (0.0%) | 2 (0.7%) | 0 (0.0%) |
| Acarodermatitis, n (%) | 0 (0.0%) | 1 (0.3%) | 0 (0.0%) |
| Dermatitis atopic, n (%) | 0 (0.0%) | 1 (0.3%) | 0 (0.0%) |
| Eczema, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Febrile convulsion, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Gastroenteritis, n (%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) |
| Miliaria, n (%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) |
| Seborrhoeic dermatitis, n (%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, S.N.; Ambike, D.; Patil, M.; Mankar, S.V.; Verma, N.; Hanumante, N.; Sarangi, L.; Mitra, M.; Preeti, G.; Deshmukh, B.J.; et al. Immunogenicity and Safety Results of a Randomized, Three-Arm, Phase IV Clinical Trial of Concomitant Administration of Typhoid Vi Conjugate Vaccine with Measles and Rubella Vaccine in Healthy Infants. Viruses 2025, 17, 1237. https://doi.org/10.3390/v17091237
Rao SN, Ambike D, Patil M, Mankar SV, Verma N, Hanumante N, Sarangi L, Mitra M, Preeti G, Deshmukh BJ, et al. Immunogenicity and Safety Results of a Randomized, Three-Arm, Phase IV Clinical Trial of Concomitant Administration of Typhoid Vi Conjugate Vaccine with Measles and Rubella Vaccine in Healthy Infants. Viruses. 2025; 17(9):1237. https://doi.org/10.3390/v17091237
Chicago/Turabian StyleRao, Songa Narayana, Deepali Ambike, Mahantesh Patil, Sanjay Vasant Mankar, Nishant Verma, Neeta Hanumante, Lisa Sarangi, Monjori Mitra, Godatwar Preeti, Bhaskar Jedhe Deshmukh, and et al. 2025. "Immunogenicity and Safety Results of a Randomized, Three-Arm, Phase IV Clinical Trial of Concomitant Administration of Typhoid Vi Conjugate Vaccine with Measles and Rubella Vaccine in Healthy Infants" Viruses 17, no. 9: 1237. https://doi.org/10.3390/v17091237
APA StyleRao, S. N., Ambike, D., Patil, M., Mankar, S. V., Verma, N., Hanumante, N., Sarangi, L., Mitra, M., Preeti, G., Deshmukh, B. J., Nanoti, G., Faridi, M. M. A., Daultani, P., Mittal, R., Maithal, K., Kansagra, K., Parmar, D. V., Kunnathamman, R., Elaiyaraja, M., ... Desai, S. (2025). Immunogenicity and Safety Results of a Randomized, Three-Arm, Phase IV Clinical Trial of Concomitant Administration of Typhoid Vi Conjugate Vaccine with Measles and Rubella Vaccine in Healthy Infants. Viruses, 17(9), 1237. https://doi.org/10.3390/v17091237






