Insights into Novel Viral Threats in Sweetpotato from Burkina Faso: Characterisation of Unexplored Pathogens
Abstract
1. Introduction
2. Materials and Methods
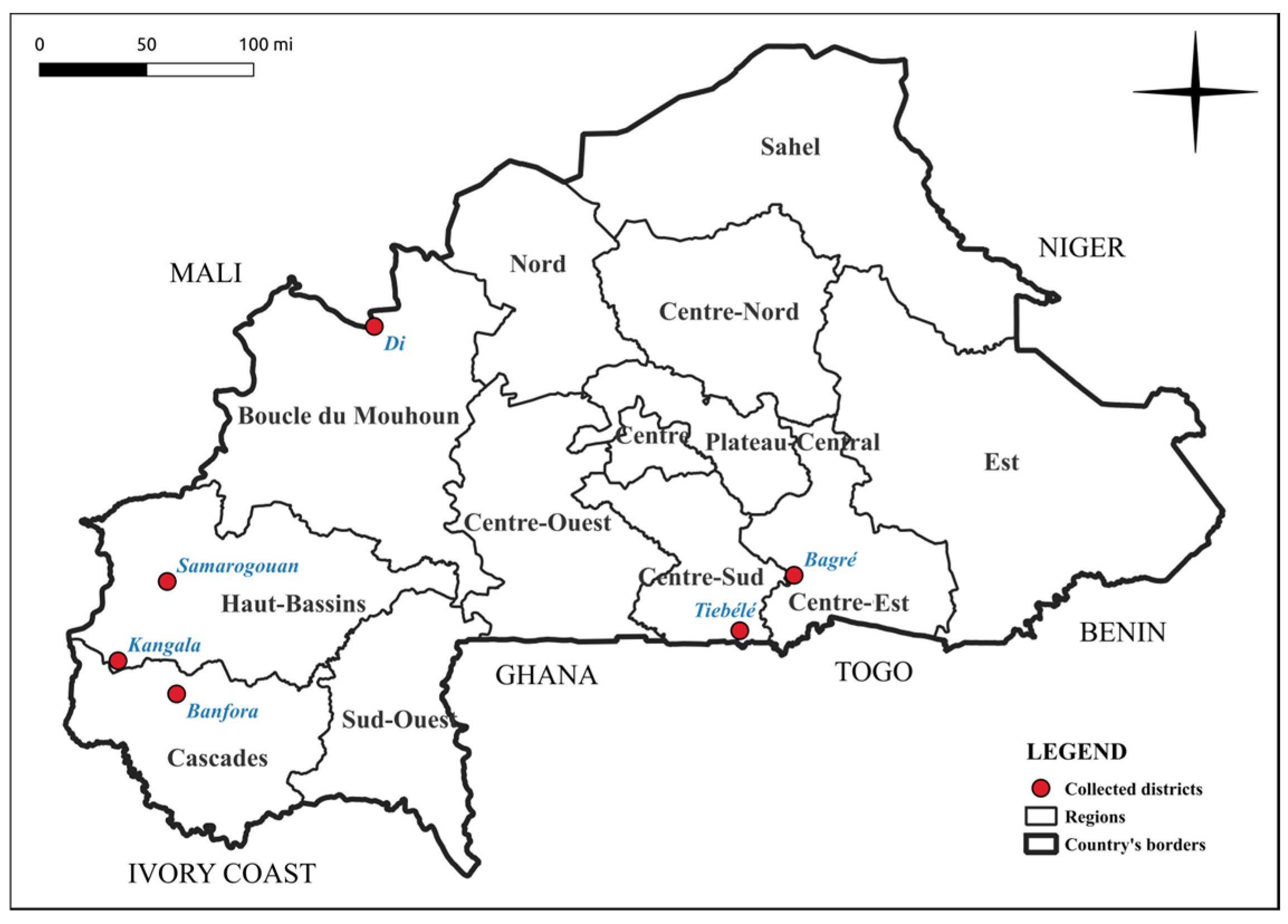
2.1. Screening of Study Samples
2.2. Extraction of Nucleic Acids and Enrichment
2.3. Library Preparation and Nanopore Sequencing
2.4. Bioinformatics Analysis
2.5. PCR Screening and Sanger Sequencing
2.6. Phylogenetic Analysis
3. Results
3.1. Viral Genome Assembly
3.2. Sequence Analysis of Assembled Viral Genomes
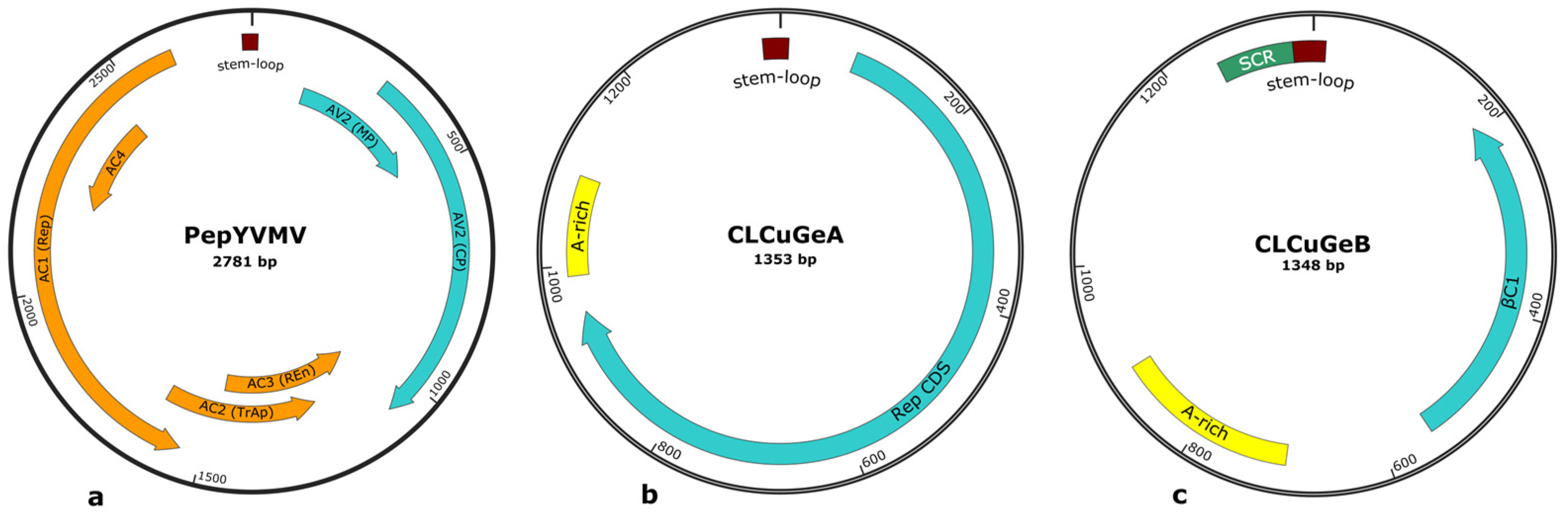
3.3. Genomic Organization and Phylogenetic Relationships of PepYVMV
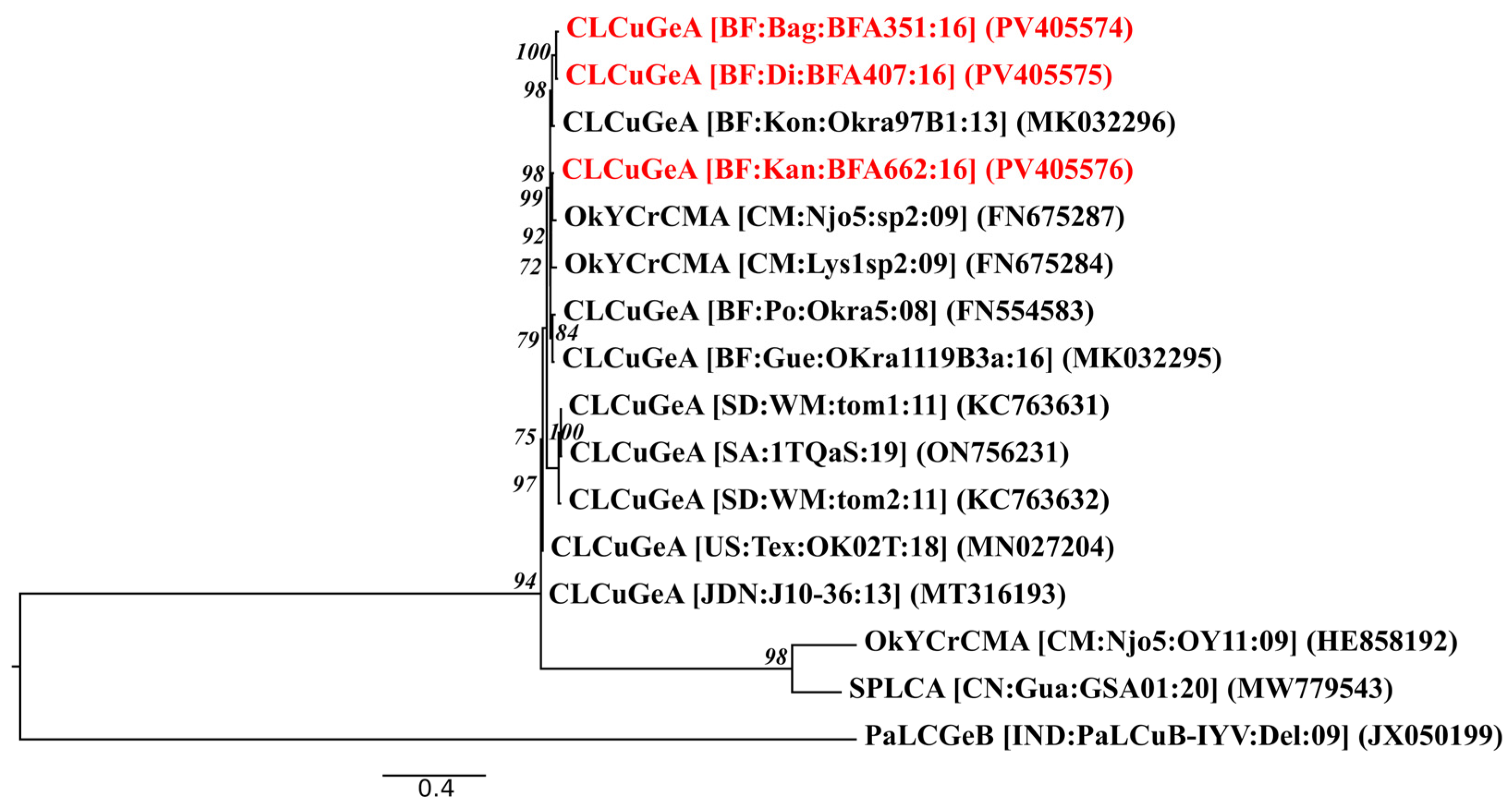
3.4. Genomic Organization and Phylogenetic Relationships of CLCuGeA
3.5. Genomic Organization and Phylogenetic Relationships of CLCuGeB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CLCuGeA | cotton leaf curl Gezira alphasatellite |
| CLCuGeB | cotton leaf curl Gezira betasatellite |
| CP | coat protein |
| HTS | high-throughput sequencing |
| MeCSV | melon chlorotic spot virus |
| MP | movement protein |
| OkLCMB | okra leaf curl Mali virus betasatellite |
| ONT | Oxford Nanopore Technologies |
| ORF | Open Reading Frame |
| OYCrCMA | okra yellow crinkle Cameroon alphastellite |
| PaLCGeB | papaya leaf curl Gezira betasatellite |
| PepYVMV | pepper yellow vein Mali virus |
| RCA | Rolling Circle Amplification |
| REn | Replication enhancer |
| Rep | Replication protein |
| SCR | satellite conserved region |
| SPCSV | sweet potato chlorotic stunt virus |
| SPFMV | sweet potato feathery mottle virus |
| SPLCD3 | sweet potato leaf curl deltasatellite 3 |
| SPLCA | sweet potato leaf curl alphasatellite |
| SPLCV | sweet potato leaf curl virus |
| ToCV | tomato chlorosis virus |
| ToLCV | tomato leaf curl virus |
| ToLCMV | tomato leaf curl Mali virus |
| ToYLCMV | tomato yellow leaf curl Mali virus |
| TrAp | Transcriptional activator protein |
References
- Somé, K.; Ouedraogo, T.J.; Belem, J.; Asante, K.I.; Vernon, G.; Danquah, Y.E. Breeding sweetpotato for yield and beta-carotene content in Burkina Faso. In Potato and Sweetpotato in Africa: Transforming the Value Chains for Food and Nutrition Security; CABI: Wallingford, UK, 2015; pp. 69–78. [Google Scholar]
- Kim, H.-J.; Woo, K.S.; Lee, H.-U.; Nam, S.S.; Lee, B.W.; Kim, M.Y.; Lee, Y.-Y.; Lee, J.Y.; Kim, M.H.; Lee, B. Physicochemical Characteristics of Starch in Sweet Potato Cultivars Grown in Korea. Prev. Nutr. Food Sci. 2020, 25, 212–218. [Google Scholar] [CrossRef]
- Qin, Y.; Naumovski, N.; Ranadheera, C.S.; D’Cunha, N.M. Nutrition-related health outcomes of sweet potato (Ipomoea batatas) consumption: A systematic review. Food Biosci. 2022, 50, 102208. [Google Scholar] [CrossRef]
- Abrham, T.; Beshir, H.M.; Haile, A. Sweetpotato production practices, constraints, and variety evaluation under different storage types. Food Energy Secur. 2020, 10, e263. [Google Scholar] [CrossRef]
- Chabi, N.K.A.; Name, P.E.; Tibiri, E.B.; Moumouni-Moussa, I.; Sikirou, R.; Desoignies, N.; Zinsou, V.A.; Tiendrebeogo, F.; Afouda, C.L.A. Identification of viruses infecting sweetpotato (Ipomoea batatas Lam.) in Benin. Open Agric. 2024, 9, 20220403. [Google Scholar] [CrossRef]
- Kreuze, J.F.; Ramírez, D.A.; Fuentes, S.; Loayza, H.; Ninanya, J.; Rinza, J.; David, M.; Gamboa, S.; De Boeck, B.; Diaz, F.; et al. High-throughput characterization and phenotyping of resistance and tolerance to virus infection in sweetpotato. Virus Res. 2023, 339, 199276. [Google Scholar] [CrossRef] [PubMed]
- Adikini, S.; Mukasa, S.B.; Mwanga, R.O.; Gibson, R.W. Virus Movement from Infected Sweetpotato Vines to Roots and Reversion on Root Sprouts. HortScience 2019, 54, 117–124. [Google Scholar] [CrossRef]
- Clark, C.A.; Davis, J.A.; Abad, J.A.; Cuellar, W.J.; Fuentes, S.; Kreuze, J.F.; Valkonen, J.P. Sweetpotato Viruses: 15 Years of Progress on Understanding and Managing Complex Diseases. Plant Dis. 2012, 96, 168–185. [Google Scholar] [CrossRef]
- Tibiri, E.B.; Pita, J.S.; Tiendrébéogo, F.; Bangratz, M.; Néya, J.B.; Brugidou, C.; Somé, K.; Barro, N. Characterization of virus species associated with sweetpotato virus diseases in Burkina Faso. Plant Pathol. 2020, 69, 1003–1017. [Google Scholar] [CrossRef]
- Tibiri, E.B.; Somé, K.; Pita, J.S.; Tiendrébéogo, F.; Bangratz, M.; Néya, J.B.; Brugidou, C.; Barro, N. Effects of sweet potato feathery mottle virus, sweet potato chlorotic stunt virus and their co-infection on sweet potato yield in Western Burkina Faso. Open Agric. 2019, 4, 758–766. [Google Scholar] [CrossRef]
- Zhang, K.; Lu, H.; Wan, C.; Tang, D.; Zhao, Y.; Luo, K.; Li, S.; Wang, J. The Spread and Transmission of Sweet Potato Virus Disease (SPVD) and Its Effect on the Gene Expression Profile in Sweet Potato. Plants 2020, 9, 492. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, L.; Wang, B.; Yin, Z.; Feng, C.; Wang, Q. Sweetpotato viruses in China. Crop Prot. 2010, 29, 110–114. [Google Scholar] [CrossRef]
- Kim, J.; Kil, E.; Kim, S.; Seo, H.; Byun, H.; Park, J.; Chung, M.; Kwak, H.; Kim, M.; Kim, C.; et al. Seed transmission of Sweet potato leaf curl virus in sweet potato (Ipomoea batatas). Plant Pathol. 2015, 64, 1284–1291. [Google Scholar] [CrossRef]
- Tibiri, E.B.; Tiendrébéogo, F.; Pita, J.S.; Somé, K.; Bangratz, M.; Néya, J.B.; Brugidou, C.; Barro, N. Molecular and Biological Features of Sweet Potato Leaf Curl Virus in Burkina Faso. Acta Sci. Microbiol. 2019, 2, 170–177. [Google Scholar]
- Rivarez, M.P.S.; Pecman, A.; Bačnik, K.; Maksimović, O.; Vučurović, A.; Seljak, G.; Mehle, N.; Gutiérrez-Aguirre, I.; Ravnikar, M.; Kutnjak, D. In-depth study of tomato and weed viromes reveals undiscovered plant virus diversity in an agroecosystem. Microbiome 2023, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, A.; Nana, T.A.; Sogoba, K.H.; Koïta, K.; Lefeuvre, P.; Lett, J.M. First report of a naturally occurring isolate of Pepper yellow vein Mali virus causing tobacco yellow leaf curl disease in Burkina Faso. New Dis. Rep. 2023, 48, e12217. [Google Scholar] [CrossRef]
- Temple, C.; Blouin, A.G.; Fontdevila, N.; Steyer, S.; Massart, S. First Report of Melon Chlorotic Spot Virus in Cultivated Sorrel (Rumex acetosa) in Belgium. Plant Dis. 2023, 108, 824. [Google Scholar] [CrossRef]
- Fiallo-Olivé, E.; Navas-Castillo, J. Tomato chlorosis virus, an emergent plant virus still expanding its geographical and host ranges. Mol. Plant Pathol. 2019, 20, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Inoue-Nagata, A.K.; Albuquerque, L.C.; Rocha, W.B.; Nagata, T. A simple method for cloning the complete begomovirus genome using the bacteriophage φ29 DNA polymerase. J. Virol. Methods 2004, 116, 209–211. [Google Scholar] [CrossRef]
- De Coster, W.; D’Hert, S.; Schultz, D.T.; Cruts, M.; Van Broeckhoven, C. NanoPack: Visualizing and processing long-read sequencing data. Bioinformatics 2018, 34, 2666–2669. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of long, error-prone reads using repeat graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Delatte, H.; Martin, D.P.; Naze, F.; Goldbach, R.; Reynaud, B.; Peterschmitt, M.; Lett, J.-M. South West Indian Ocean islands tomato begomovirus populations represent a new major monopartite begomovirus group. J. Gen. Virol. 2005, 86, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S.; Battistuzzi, F.U. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Ouattara, A.; Tiendrebeogo, F.; Lefeuvre, P.; Hoareau, M.; Claverie, S.; Allibert, A.; Lett, J.M. Diversity, distribution and prevalence of vegetable-infecting geminiviruses in Burkina Faso. Plant Pathol. 2020, 69, 379–392. [Google Scholar] [CrossRef]
- Séka, K.; Ouattara, A.; Assiri, K.; Kra, K.; Hoareau, M.; Lefeuvre, P.; Diallo, H.A.; Lett, J. First report of Pepper yellow vein Mali virus associated with pepper yellow vein disease in Cote d’Ivoire. New Dis. Rep. 2017, 35, 11. [Google Scholar] [CrossRef]
- Tiendrebeo, F.; Traore, V.E.; Barro, N.; Traore, A.S.; Konate, G.; Traore, O. Characterization of Pepper yellow vein mali virus in Capsicum sp. in Burkina Faso. Plant Pathol. J. 2008, 7, 155–161. [Google Scholar] [CrossRef][Green Version]
- Tiendrebeogo, F.; Akakpo, A.; BTibiri, E.; Soro, M.; Zongo, C.; Savadogo, A.; Bouma Neya, J.; Djodji Adjata, K.; Barro, N. Diversity of Begomoviruses Infecting Okra [Abelmoschus esculentus (L.) Moench] in Togo. Acta Sci. Microbiol. 2020, 3, 29–33. [Google Scholar] [CrossRef]
- Tiendrébéogo, F.; Lefeuvre, P.; Hoareau, M.; Villemot, J.; Konaté, G.; Traoré, A.S.; Barro, N.; Traoré, V.S.; Reynaud, B.; Traoré, O.; et al. Molecular diversity of Cotton leaf curl Gezira virus isolates and their satellite DNAs associated with okra leaf curl disease in Burkina Faso. Virol. J. 2010, 7, 48–57. [Google Scholar] [CrossRef]
- Tiendrébéogo, F.; Lefeuvre, P.; Hoareau, M.; Traoré, V.S.E.; Barro, N.; Péréfarres, F.; Reynaud, B.; Traoré, A.S.; Konaté, G.; Lett, J.-M.; et al. Molecular and biological characterization of Pepper yellow vein Mali virus (PepYVMV) isolates associated with pepper yellow vein disease in Burkina Faso. Arch. Virol. 2010, 156, 483–487. [Google Scholar] [CrossRef]
- Badial, A.B.; Sherman, D.; Stone, A.; Gopakumar, A.; Wilson, V.; Schneider, W.; King, J. Nanopore Sequencing as a Surveillance Tool for Plant Pathogens in Plant and Insect Tissues. Plant Dis. 2018, 102, 1648–1652. [Google Scholar] [CrossRef]
- Boykin, L.M.; Sseruwagi, P.; Alicai, T.; Ateka, E.; Mohammed, I.U.; Stanton, J.-A.L.; Kayuki, C.; Mark, D.; Fute, T.; Erasto, J.; et al. Tree Lab: Portable Genomics for Early Detection of Plant Viruses and Pests in Sub-Saharan Africa. Genes 2019, 10, 632. [Google Scholar] [CrossRef]
- Chalupowicz, L.; Dombrovsky, A.; Gaba, V.; Luria, N.; Reuven, M.; Beerman, A.; Lachman, O.; Dror, O.; Nissan, G.; Manulis-Sasson, S. Diagnosis of plant diseases using the Nanopore sequencing platform. Plant Pathol. 2019, 68, 229–238. [Google Scholar] [CrossRef]
- Kon, T.; Rojas, M.R.; Abdourhamane, I.K.; Gilbertson, R.L. Roles and interactions of begomoviruses and satellite DNAs associated with okra leaf curl disease in Mali, West Africa. J. Gen. Virol. 2009, 90, 1001–1013. [Google Scholar] [CrossRef]
- Zhou, X. Advances in Understanding Begomovirus Satellites. Annu. Rev. Phytopathol. 2013, 51, 357–381. [Google Scholar] [CrossRef]
- Cui, X.; Li, G.; Wang, D.; Hu, D.; Zhou, X. A Begomovirus DNAβ-Encoded Protein Binds DNA, Functions as a Suppressor of RNA Silencing, and Targets the Cell Nucleus. J. Virol. 2005, 79, 10764–10775. [Google Scholar] [CrossRef]
- Yang, X.; Xie, Y.; Raja, P.; Li, S.; Wolf, J.N.; Shen, Q.; Bisaro, D.M.; Zhou, X.; Vance, V. Suppression of Methylation-Mediated Transcriptional Gene Silencing by βC1-SAHH Protein Interaction during Geminivirus-Betasatellite Infection. PLOS Pathog. 2011, 7, e1002329. [Google Scholar] [CrossRef] [PubMed]
- Nawaz-ul-Rehman, M.S.; Nahid, N.; Mansoor, S.; Briddon, R.W.; Fauquet, C.M. Post-transcriptional gene silencing suppressor activity of two non-pathogenic alphasatellites associated with a begomovirus. Virology 2010, 405, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Patil, B.L.; Fauquet, C.M. Differential interaction between cassava mosaic geminiviruses and geminivirus satellites. J. Gen. Virol. 2010, 91, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Yadava, P.; Suyal, G.; Mukherjee, S.K. Begomovirus DNA replication and pathogenicity. Curr. Sci. 2010, 98, 360–368. [Google Scholar]
- Leke, W.N.; Mignouna, D.B.; Brown, J.K.; Kvarnheden, A. Begomovirus disease complex: Emerging threat to vegetable production systems of West and Central Africa. Agric. Food Secur. 2015, 4, 14. [Google Scholar] [CrossRef]
- Fiallo-Olivé, E.; Navas-Castillo, J. Begomoviruses: What is the secret(s) of their success? Trends Plant Sci. 2023, 28, 715–727. [Google Scholar] [CrossRef]
- Moreno, A.B.; Lopez-Moya, J.J. When viruses play team sports: Mixed infections in plants. Phytopathology 2020, 110, 29–48. [Google Scholar] [CrossRef]
- Allen-Perkins, A.; Estrada, E. Mathematical modelling for sustainable aphid control in agriculture via intercropping. Proc. R. Soc. A: Math. Phys. Eng. Sci. 2019, 475, 20190136. [Google Scholar] [CrossRef]
- Kluger, D.M.; Owen, A.B.; Lobell, D.B. Combining randomized field experiments with observational satellite data to assess the benefits of crop rotations on yields. Environ. Res. Lett. 2022, 17, 044066. [Google Scholar] [CrossRef]
- Valverde, R.A.; Sim, J.; Lotrakul, P. Whitefly transmission of sweet potato viruses. Virus Res. 2004, 100, 123–128. [Google Scholar] [CrossRef]
- Simmons, A.; Harrison, H.; Ling, K. Forty-nine new host plant species for Bemisia tabaci (Hemiptera: Aleyrodidae). Entomol. Sci. 2008, 11, 385–390. [Google Scholar] [CrossRef]
- Tiwari, S.P.; Nema, S.; Khare, M.N. Whitefly- A Strong Transmitter of Plant Viruses. Int. J. Phytopathol. 2013, 2, 102–120. [Google Scholar] [CrossRef]
- Romba, R.; Gnankine, O.; Drabo, S.F.; Tiendrebeogo, F.; Henri, H.; Mouton, L.; Vavre, F. Abundance of Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) and its parasitoids on vegetables and cassava plants in Burkina Faso (West Africa). Ecol. Evol. 2018, 8, 6091–6103. [Google Scholar] [CrossRef] [PubMed]
- Delêtre, M.; Lett, J.-M.; Sulpice, R.; Spillane, C. Kinship networks of seed exchange shape spatial patterns of plant virus diversity. Nat. Commun. 2021, 12, 4505. [Google Scholar] [CrossRef] [PubMed]
- Soro, M.; Tiendrébéogo, F.; Pita, J.S.; Traoré, E.T.; Somé, K.; Tibiri, E.B.; Néya, J.B.; Mutuku, J.M.; Simporé, J.; Koné, D. Epidemiological assessment of cassava mosaic disease in Burkina Faso. Plant Pathol. 2021, 70, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Whattam, M.; Dinsdale, A.; Elliott, C.E. Evolution of Plant Virus Diagnostics Used in Australian Post Entry Quarantine. Plants 2021, 10, 1430. [Google Scholar] [CrossRef] [PubMed]



| Virus Isolate | Strain | Host | Geographical Origin | Accession No. |
|---|---|---|---|---|
| [BF:Sam:BFA942:16] | Pepper yellow vein Mali virus | Ipomoea batatas | Burkina Faso | PV405580 |
| [BF:Tou:796BE:15] | Pepper yellow vein Mali virus | Solanum lycopersicum | Burkina Faso | MH778658 |
| [BF:Po:Hpe:08] | Pepper yellow vein Mali virus | Capsicum frutescens | Burkina Faso | FN555171 |
| [BF:Tie:1108B5g:16] | Pepper yellow vein Mali virus | Sida acuta | Burkina Faso | MH778662 |
| [BF:Oua:Sp2:08] | Pepper yellow vein Mali virus | Capsicum annuum | Burkina Faso | FM876851 |
| [BF:Mog:12PA:14] | Pepper yellow vein Mali virus | Capsicum frutescens | Burkina Faso | MH778651 |
| [CI:Fer:CI11-1:17] | Pepper yellow vein Mali virus | Solanum melongena | Ivory Coast | MH460532 |
| [BF:Tab:3BF:22] | Pepper yellow vein Mali virus | Tobacco | Burkina Faso | OR483374 |
| [CI:Tou:CI29:12] | Pepper yellow vein Mali virus | Capsicum sp | Ivory Coast | KY271077 |
| [BF:Bou:39BE:13] | Pepper yellow vein Mali virus | Solanum lycopersicum | Burkina Faso | MH778685 |
| [CI:Kor:CI241:19] | Pepper yellow vein Mali virus | Solanum lycopersicum | Ivory Coast | ON367443 |
| [CI:Fer:CI251:19] | Pepper yellow vein Mali virus | Capsicum frutescens | Ivory Coast | ON367442 |
| [BF:Yam:15B1:13] | Pepper yellow vein Mali virus | Capsicum annuum | Burkina Faso | MH778673 |
| [BF:Ban:Hpe1:09] | Pepper yellow vein Mali virus | Capsicum frutescens | Burkina Faso | FN555173 |
| [MLI:03] | Pepper yellow vein Mali virus | - | Mali | NC_005347 |
| [CN:Fuz:LC1-3:06] | Pepper yellow vein Mali virus | Eclipta prostrata | China | AM691547 |
| [CN:Fuz:FY1:06] | Pepper yellow vein Mali virus | Bougainvillea | China | AM691549 |
| [CM:09] | Pepper yellow vein Mali virus | Malvastrum | Cameroon | MN372224 |
| [IR:Ker:08] | Tomato yellow leaf curl virus | Lycopersicon esculentum | Iran | EU635776 |
| [BF:Tom-141:14] | Tomato yellow leaf curl Mali virus | Solanum lycopersicum | Burkina Faso | LM651400 |
| [MLI:03] | Tomato leaf curl Mali virus | - | Mali | AY502936 |
| [BF:Sam:BFA942:16] | Sweet potato leaf curl virus | Ipomoea batatas | Burkina Faso | This study |
| [BF:Bag:BFA351:16] | Cotton leaf curl Gezira Alphasatellite | Ipomoea batatas | Burkina Faso | PV405574 |
| [BF:Di:BFA407:16] | Cotton leaf curl Gezira Alphasatellite | Ipomoea batatas | Burkina Faso | PV405575 |
| [BF:Kan:BFA662:16] | Cotton leaf curl Gezira Alphasatellite | Ipomoea batatas | Burkina Faso | PV405576 |
| [SD:WM:tom1:11] | Cotton leaf curl Gezira alphasatellite | Solanum lycopersicum | Sudan | KC763631 |
| [SA:1TQaS:19] | Cotton leaf curl Gezira alphasatellite | Solanum lycopersicum | Saudi Arabia | ON756231 |
| [SD:WM:tom2:11] | Cotton leaf curl Gezira alphasatellite | Solanum lycopersicum | Sudan | KC763632 |
| [JDN:J10-36:13] | Cotton leaf curl Gezira alphasatellite | Okara | Jordan | MT316193 |
| [US:Tex:OK02T:18] | Cotton leaf curl Gezira alphasatellite | Abelmoschus esculentus | USA | MN027204 |
| [BF:Kon:Okra97B1:13] | Cotton leaf curl Gezira alphasatellite | Abelmoschus esculentus | Burkina Faso | MK032296 |
| [BF:Gue:OKra1119B3a:16] | Cotton leaf curl Gezira alphasatellite | Abelmoschus esculentus | Burkina Faso | MK032295 |
| [BF:Po:Okra5:08] | Cotton leaf curl Gezira alphasatellite | Abelmoschus esculentus | Burkina Faso | FN554583 |
| [CM:Njo5:sp2:09] | Okra yellow crinkle Cameroon alphasatellite | Abelmoschus esculentus | Cameroon | FN554587 |
| [CM:Lys1sp2:09] | Okra yellow crinkle Cameroon alphasatellite | Abelmoschus esculentus | Cameroon | FN675284 |
| [CM:Njo5:OY11:09] | Okra yellow crinkle Cameroon alphasatellite | Abelmoschus esculentus | Cameroon | HE858192 |
| [CN:Gua:GSA01:20] | Sweet potato leaf curl alphasatellite | Ipomoea batatas | China | MW779543 |
| [IND:PaLCuB-IYV:Del:09] | Papaya leaf curl Gezira betasatellite | Ipomoea purpurea | India | JX050199 |
| [BF:Bag:BFA362:16] | Cotton leaf curl Gezira betasatellite | Ipomoea batatas | Burkina Faso | PV405578 |
| [BF:Sam:BFA942:16] | Cotton leaf curl Gezira betasatellite | Ipomoea batatas | Burkina Faso | PV405579 |
| [BF:Ban:BFA1094:16] | Cotton leaf curl Gezira betasatellite | Ipomoea batatas | Burkina Faso | PV405577 |
| [CM:Dou:ODLAR11:08] | Okra leaf curl Mali virus betasatellite | Abelmoschus esculentus | Cameroon | FM164728 |
| [BF:Kam:Okra3:08] | Cotton leaf curl Gezira betasatellite | Abelmoschus esculentus | Burkina Faso | FN554576 |
| [BF:Kap:Okra1-1:08] | Cotton leaf curl Gezira betasatellite | Abelmoschus esculentus | Burkina Faso | FN554574 |
| [BF:So:Okra87PA:14] | Cotton leaf curl Gezira betasatellite | Abelmoschus esculentus | Burkina Faso | MK032305 |
| [BF:Tie:Okra2:08] | Cotton leaf curl Gezira betasatellite | Abelmoschus esculentus | Burkina Faso | FN554573 |
| [NG:Sad:NG1:07] | Cotton leaf curl Gezira betasatellite | Abelmoschus esculentus | Niger | FJ469628 |
| [BF:Lou:Okra97PF:14] | Cotton leaf curl Gezira betasatellite | Abelmoschus esculentus | Burkina Faso | MK032303 |
| [SDN:Okra44-2:96] | Cotton leaf curl Gezira betasatellite | Abelmoschus esculentus | Sudan | AY044142 |
| [IS:Rev:16] | Cotton leaf curl Gezira betasatellite | Solanum lycopersicum | Israel | MK456609 |
| [JDN:J10-47:13] | Cotton leaf curl Gezira betasatellite | Okara | Jordan | MT316190 |
| [IQ:Bag:s88:23] | Cotton leaf curl Gezira betasatellite | Abelmoschus esculentus | Iraq | PP093771 |
| [SA:1TQb_S:19] | Cotton leaf curl Gezira betasatellite | Solanum lycopersicum | Saudi Arabia | ON756227 |
| [IR:Anb:Okra2:Okra:19] | Cotton leaf curl Gezira betasatellite | Abelmoschus esculentus | Iran | MZ911859 |
| [SA:AZB24-3:15] | Cotton leaf curl Gezira betasatellite | Solanum lycopersicum | Saudi Arabia | MN508949 |
| [CN:Gua:GSA01:20] | Sweet potato leaf curl alphasatellite | Ipomoea batatas | China | MW779543 |
| Samples | Regions | District | Virus Identified | ||||
|---|---|---|---|---|---|---|---|
| SPLCV | SPLCD | PepYVMV | CLCuGeA | CLCuGeB | |||
| BFA310 | Centre-Sud | Tiébélé | + | + | + | - | - |
| BFA313 | Centre-Sud | Tiébélé | + | + | + | - | - |
| BFA351 | Centre-Est | Bagré | + | + | - | + | - |
| BFA362 | Centre-Est | Bagré | + | + | - | - | + |
| BFA942 | Haut-Bassins | Samorogouan | + | + | + | - | + |
| BFA1094 | Cascades | Banfora | + | + | + | ||
| BFA407 | Boucle du Mouhoun | Di | + | + | + | ||
| BFA662 | Haut-Bassins | Kangala | + | + | + | ||
| Isolates of This Study | Closest Relatives Retrieved from GenBank Organisms | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolates | District | Virus Identified | N° Accession | Length (bp) | % GC | Number of Reads | Coverage (%) | Mean Depth | Strains | Query Cover (%) | % of Identity | N° Accession |
| [BF:Bag:BFA351:16] | Bagré | CLCuGeA | PV405574 | 1353 | 38.9 | 1511 | 100 | 382.622 | CLCuGeA Okra97B1 | 100 | 96.61 | MK032296 |
| [BF:Bag:BFA362:16] | Bagré | CLCuGeB | PV405578 | 1348 | 37.7 | 6 | 99.77 | 1.52819 | CLCuGeB Okra1-1 | 100 | 97.78 | FN554574 |
| [BF:Di:BFA407:16] | Di | CLCuGeA | PV405575 | 1353 | 38.7 | 789 | 100 | 342.786 | CLCuGeA Okra97B1 | 100 | 96.90 | MK032296 |
| [BF:Kan:BFA662:16] | Kangala | CLCuGeA | PV405576 | 1353 | 38.6 | 70 | 100 | 25.2 | OkYCrCMA Njo5sp2 | 100 | 97.71 | FN675287 |
| [BF:Sam:BFA942:16] | Samorogouan | CLCuGeB | PV405579 | 1333 | 38.2 | 2 | 100 | 1.02326 | CLCuGeB Okra3 | 100 | 96.52 | FN554576 |
| PepYVMV | PV405580 | 2781 | 43.9 | 62039 | 100 | 5578.1 | PepYVMV 796BE | 100 | 99.17 | MH778658 | ||
| [BF:Ban:BFA1094:16] | Banfora | CLCuGeB | PV405577 | 1348 | 38.2 | 423 | 100 | 188.497 | CLCuGeB Okra3 | 100 | 97.78 | FN554576 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Name, P.E.; Tibiri, E.B.; Tiendrébéogo, F.; Sawadogo, S.; Djigma, F.; Traoré, L.; Eni, A.O.; Pita, J.S. Insights into Novel Viral Threats in Sweetpotato from Burkina Faso: Characterisation of Unexplored Pathogens. Viruses 2025, 17, 1222. https://doi.org/10.3390/v17091222
Name PE, Tibiri EB, Tiendrébéogo F, Sawadogo S, Djigma F, Traoré L, Eni AO, Pita JS. Insights into Novel Viral Threats in Sweetpotato from Burkina Faso: Characterisation of Unexplored Pathogens. Viruses. 2025; 17(9):1222. https://doi.org/10.3390/v17091222
Chicago/Turabian StyleName, Pakyendou E., Ezechiel B. Tibiri, Fidèle Tiendrébéogo, Seydou Sawadogo, Florencia Djigma, Lassina Traoré, Angela O. Eni, and Justin S. Pita. 2025. "Insights into Novel Viral Threats in Sweetpotato from Burkina Faso: Characterisation of Unexplored Pathogens" Viruses 17, no. 9: 1222. https://doi.org/10.3390/v17091222
APA StyleName, P. E., Tibiri, E. B., Tiendrébéogo, F., Sawadogo, S., Djigma, F., Traoré, L., Eni, A. O., & Pita, J. S. (2025). Insights into Novel Viral Threats in Sweetpotato from Burkina Faso: Characterisation of Unexplored Pathogens. Viruses, 17(9), 1222. https://doi.org/10.3390/v17091222









