Development of a Safe and Effective mRNA Candidate Vaccine Against PEDV G2c Genotype Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, and Antibodies
2.2. Construction of Recombinant Plasmids
2.3. mRNA Vaccine Preparation
2.4. Indirect Immunofluorescence Assay
2.5. Western Blotting
2.6. Animal Experiments
2.6.1. Safety Experiment
2.6.2. Analysis of Immunogenicity
2.6.3. Immune Challenge Test
2.7. Neutralization Test
2.8. Viral Shedding
2.9. Histopathology and Immunohistochemistry
2.10. Statistical Analysis
3. Results
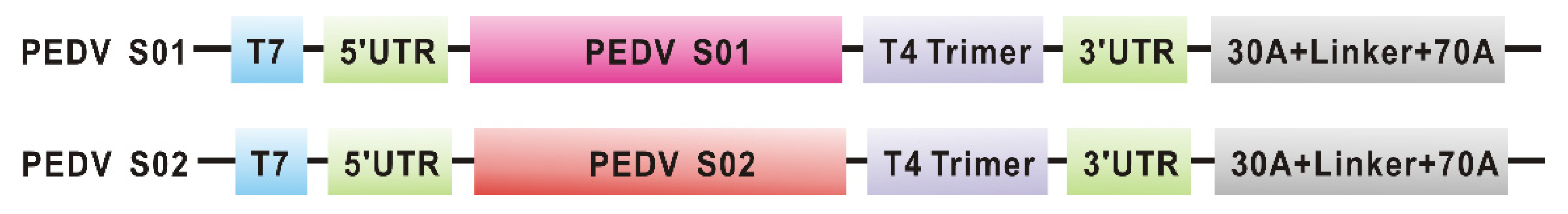
3.1. Construction of mRNA Vaccine
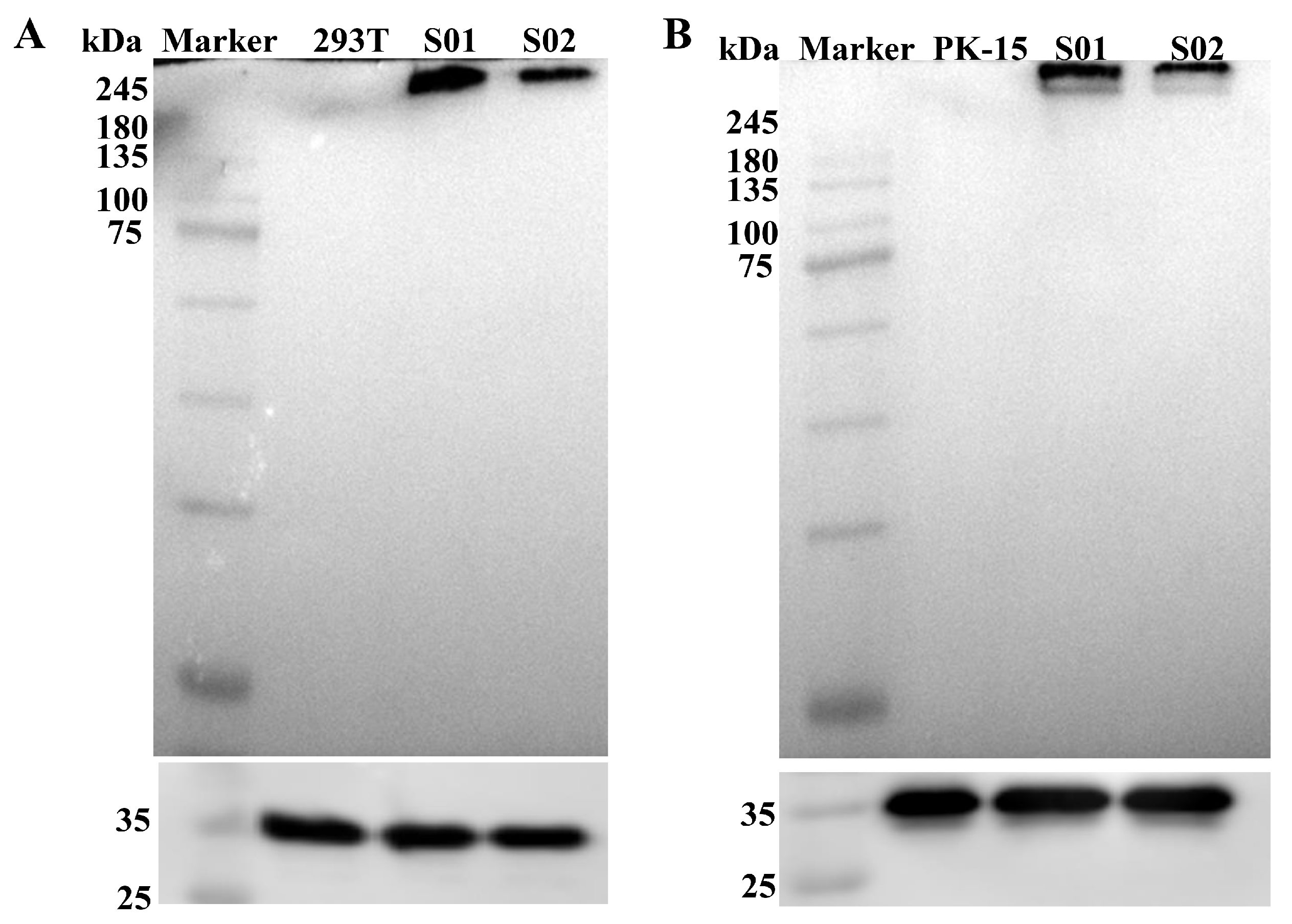
3.2. Expression of mRNA Vaccine
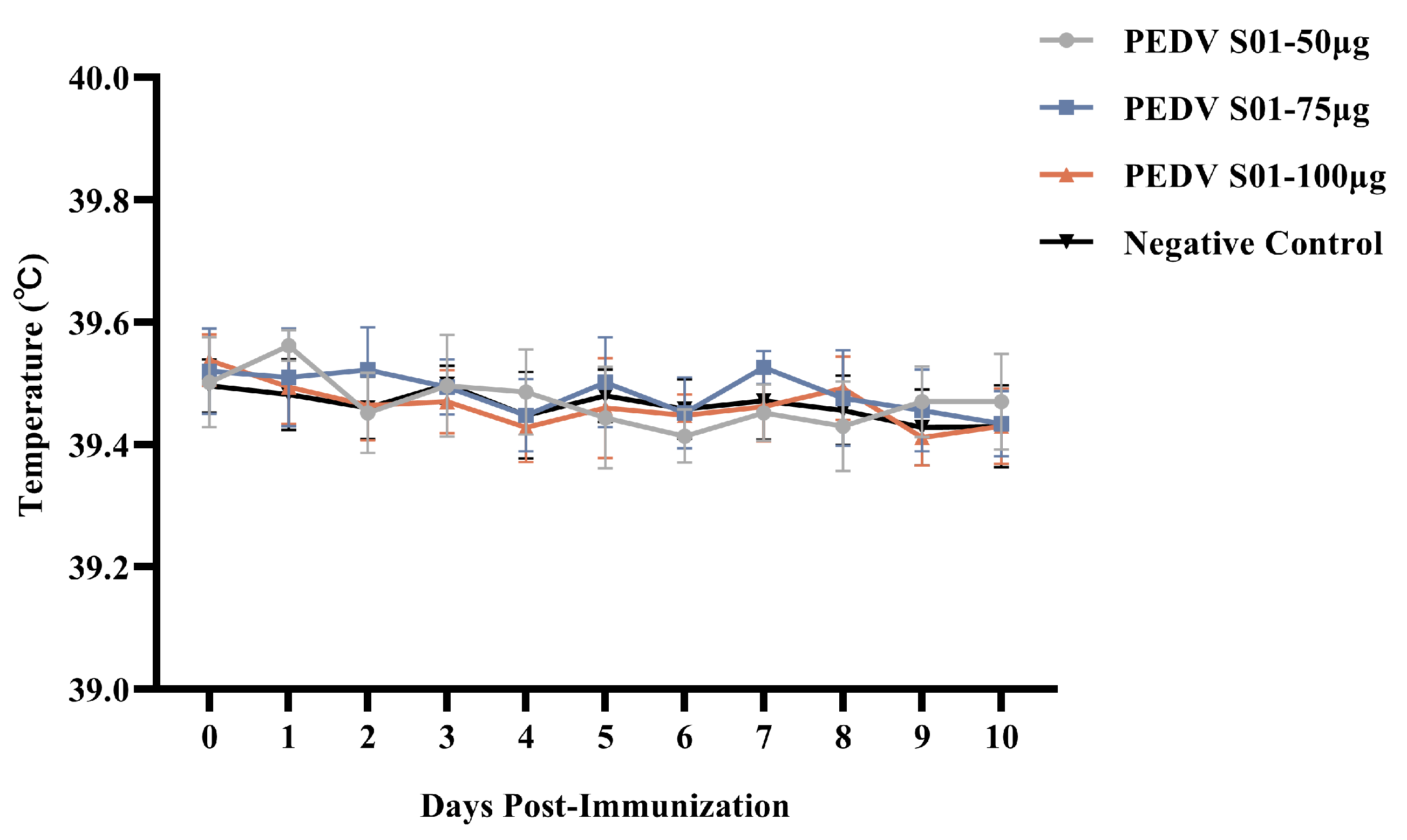
3.3. Safety of the mRNA S01
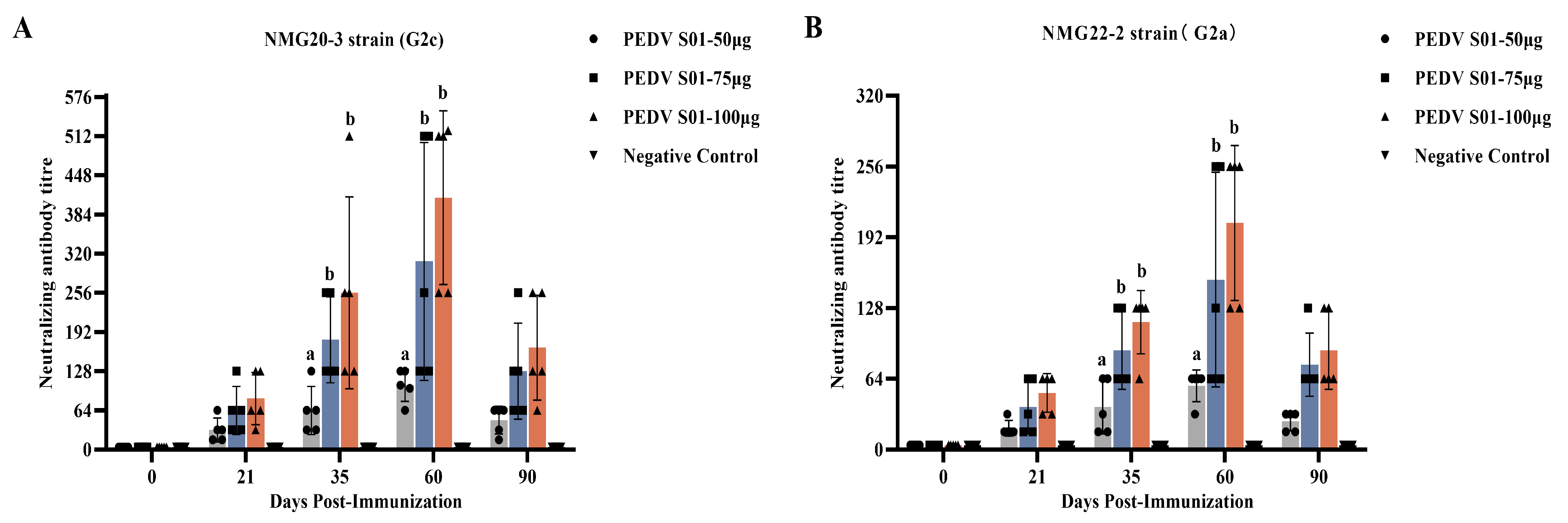
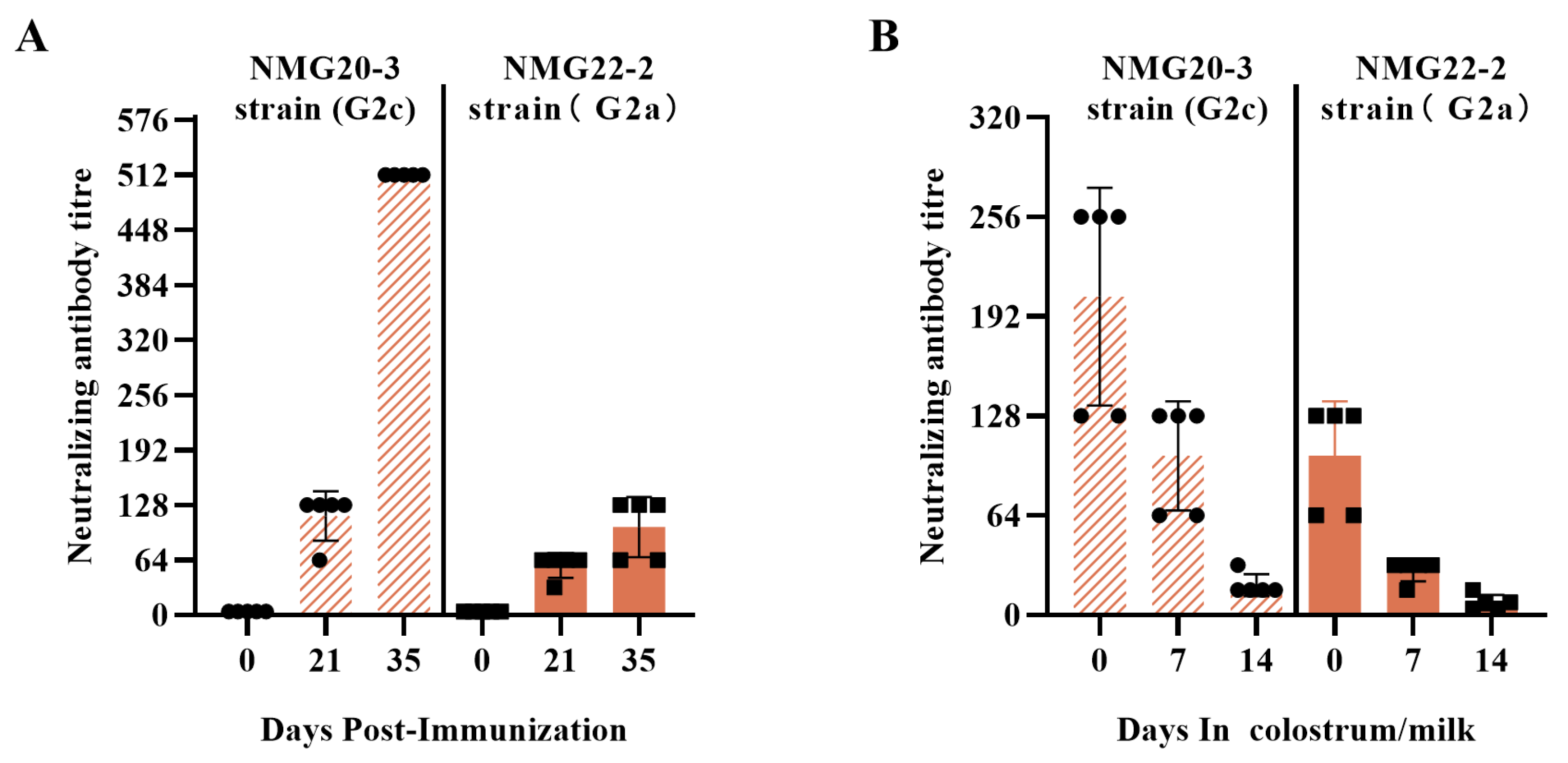
3.4. Immunogenicity of the mRNA S01
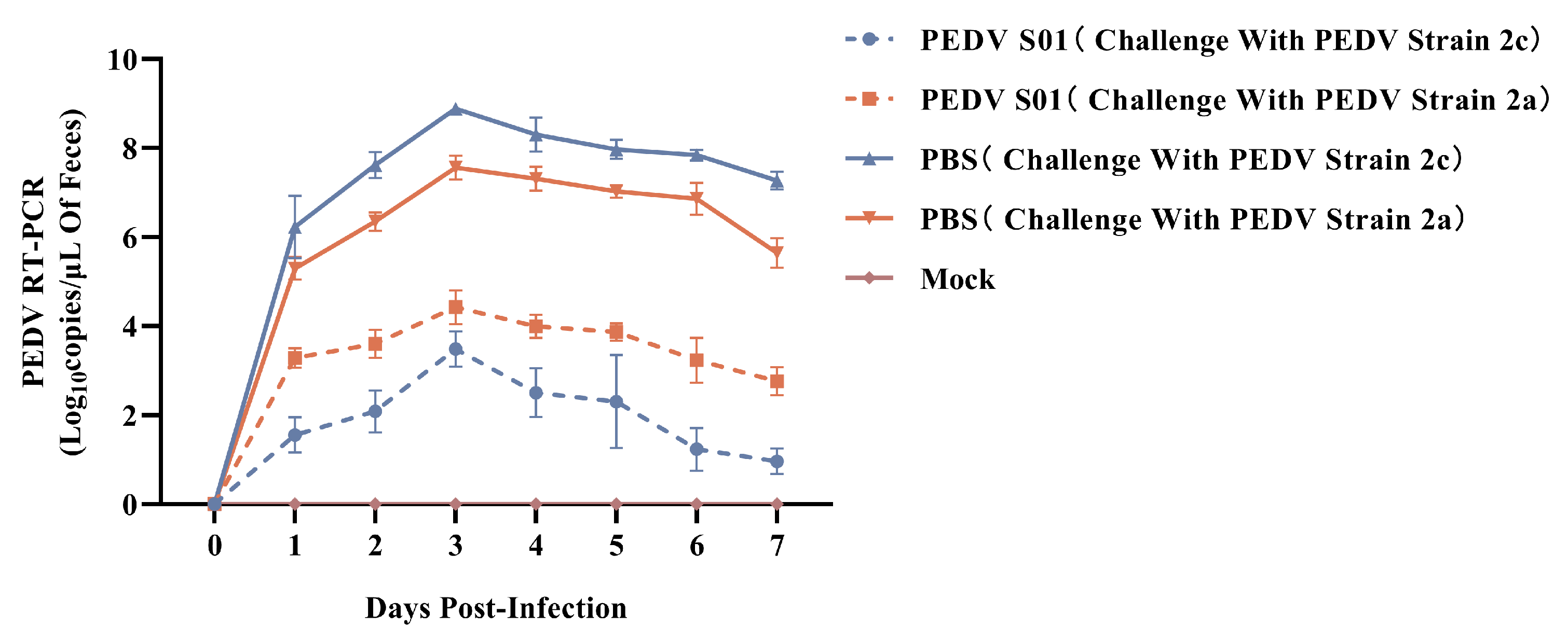
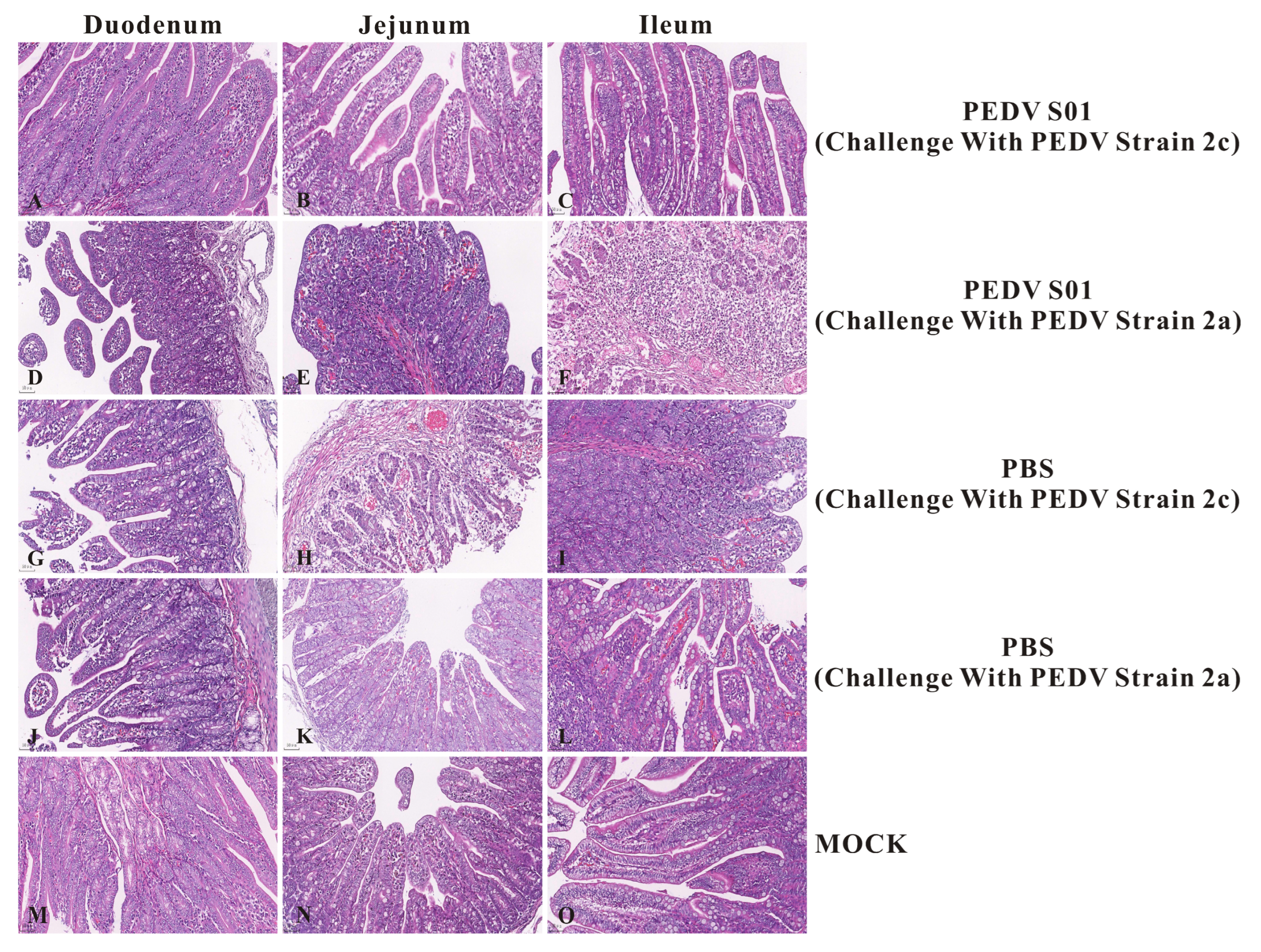
3.5. Protective Efficacy of the mRNA S01 Against PEDV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vlasova, A.N.; Amimo, J.O.; Saif, L.J. Porcine rotaviruses: Epidemiology, immune responses and control strategies. Viruses 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Cui, E.; Song, Y.; Yan, R.; Wang, J. Porcine deltacoronavirus and its prevalence in China: A review of epidemiology, evolution, and vaccine development. Arch. Virol. 2021, 166, 2975–2988. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.; Liang, G. Getah Virus (Alphavirus): An Emerging, Spreading Zoonotic Virus. Pathogens 2022, 11, 945. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, C.; Peng, O.; Ashraf, U.; Xu, Q.; Gong, L.; Fan, B.; Zhang, Y.; Xu, Z.; Xue, C.; et al. Global Dynamics of Porcine Enteric Coronavirus PEDV Epidemiology, Evolution, and Transmission. Mol. Biol. Evol. 2023, 40, msad052. [Google Scholar] [CrossRef]
- Kong, F.; Jia, H.; Xiao, Q.; Fang, L.; Wang, Q. Prevention and Control of Swine Enteric Coronaviruses in China: A Review of Vaccine Development and Application. Vaccines 2024, 12, 11. [Google Scholar] [CrossRef]
- Sun, J.; Cheng, J.; Shi, D.; Xu, X.; Liu, Y.; Ying, J.; Zhao, Y.; Zheng, H.; Yan, J.; Sun, D.; et al. Genetic Epidemiology of Porcine Epidemic Diarrhea Virus Circulating in China From 2010 to 2024: Characterization of Phylogenetic and Genetic Diversity of S1-Based Genes. J. Med. Virol. 2025, 97, e70198. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Yan, G.; Li, H.; Zhou, J.; Yang, A. Epidemiological investigation, isolation, and pathogenicity of porcine epidemic diarrhea virus subtype G2c in Sichuan province. Arch. Virol. 2025, 170, 129. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tian, L.; Liu, Y.; Sun, Y.; Li, Z.; Cai, X.; Meng, Q.; Qiao, J. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea virus in Xinjiang, China, from 2020 to 2022. Arch. Virol. 2024, 169, 96. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Fu, P.; Zhou, Y.; Lang, Y.; Zhao, S.; Wen, Y.; Wang, Y.; Wu, R.; Zhao, Q.; Du, S.; et al. Phylogenetic Analysis of Porcine Epidemic Diarrhea Virus (PEDV) during 2020–2022 and Isolation of a Variant Recombinant PEDV Strain. Int. J. Mol. Sci. 2024, 25, 10878. [Google Scholar] [CrossRef]
- Guo, W.; Wang, C.; Song, X.; Xu, H.; Zhao, S.; Gu, J.; Zou, Z.; Li, J.; Qian, J.; Zhang, X.; et al. Immunogenicity and protective efficacy of a trimeric full-length S protein subunit vaccine for porcine epidemic diarrhea virus. Vaccine 2024, 42, 828–839. [Google Scholar] [CrossRef]
- Li, L.; Yin, S.; Zhou, J.; Zhang, L.; Teng, Z.; Qiao, L.; Wang, Y.; Yu, J.; Zang, H.; Ding, Y.; et al. Spike 1 trimer, a nanoparticle vaccine against porcine epidemic diarrhea virus induces protective immunity challenge in piglets. Front. Microbiol. 2024, 15, 1386136. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, Z.; Wu, T.; Peng, O.; Huang, L.; Zhang, Y.; Xue, C.; Wen, Z.; Zhou, Q.; Cao, Y. A flagellin-adjuvanted PED subunit vaccine improved protective efficiency against PEDV variant challenge in pigs. Vaccine 2018, 36, 4228–4235. [Google Scholar] [CrossRef]
- Thavorasak, T.; Chulanetra, M.; Glab-Ampai, K.; Teeranitayatarn, K.; Songserm, T.; Yodsheewan, R.; Sae-Lim, N.; Lekcharoensuk, P.; Sookrung, N.; Chaicumpa, W. Novel Neutralizing Epitope of PEDV S1 Protein Identified by IgM Monoclonal Antibody. Viruses 2022, 14, 125. [Google Scholar] [CrossRef]
- Kirby, T. mRNA vaccine technology for a multivalent flu vaccine. Lancet Infect. Dis. 2023, 23, 157. [Google Scholar] [CrossRef]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Szabó, G.T.; Mahiny, A.J.; Vlatkovic, I. COVID-19 mRNA vaccines: Platforms and current developments. Mol. Ther. 2022, 30, 1850–1868. [Google Scholar] [CrossRef]
- Mochida, Y.; Uchida, S. mRNA vaccine designs for optimal adjuvanticity and delivery. RNA Biol. 2024, 21, 422–448. [Google Scholar] [CrossRef]
- Le, T.; Sun, C.; Chang, J.; Zhang, G.; Yin, X. mRNA Vaccine Development for Emerging Animal and Zoonotic Diseases. Viruses 2022, 14, 401. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, Y.; Zhang, S.; Chen, S.J. mRNA vaccine sequence and structure design and optimization: Advances and challenges. J. Biol. Chem. 2025, 301, 108015. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, J.; Li, Y.; Wang, D.; Song, X.; Tian, S.; Zhou, J.; Wang, W.; Guo, R.; Li, J.; et al. Genetic characterization and phylogenetic analysis of the S genes of porcine epidemic diarrhea virus isolates from China from 2020 to 2023. Arch. Virol. 2024, 169, 180. [Google Scholar] [CrossRef]
- Kim, D.M.; Moon, S.H.; Kim, S.C.; Cho, H.S.; Tark, D. Genetic and Pathogenic Analysis of a Novel Porcine Epidemic Diarrhea Virus Strain Isolated in the Republic of Korea. Viruses 2024, 16, 1108. [Google Scholar] [CrossRef]
- Li, J.; Ulitzky, L.; Silberstein, E.; Taylor, D.R.; Viscidi, R. Immunogenicity and protection efficacy of monomeric and trimeric recombinant SARS coronavirus spike protein subunit vaccine candidates. Viral Immunol. 2013, 26, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Sreenivasan, C.; Uprety, T.; Gao, R.; Huang, C.; Lee, E.J.; Lawson, S.; Nelson, J.; Christopher-Hennings, J.; Kaushik, R.S.; et al. Piglet immunization with a spike subunit vaccine enhances disease by porcine epidemic diarrhea virus. npj Vaccines 2021, 6, 22. [Google Scholar] [CrossRef]
- Miller, L.C.; Crawford, K.K.; Lager, K.M.; Kellner, S.G.; Brockmeier, S.L. Evaluation of two real-time polymerase chain reaction assays for Porcine epidemic diarrhea virus (PEDV) to assess PEDV transmission in growing pigs. J. Vet. Diagn. Investig. 2016, 28, 20–29. [Google Scholar] [CrossRef]
- Jin, Y.; Hou, C.; Li, Y.; Zheng, K.; Wang, C. mRNA Vaccine: How to Meet the Challenge of SARS-CoV-2. Front. Immunol. 2022, 12, 821538. [Google Scholar] [CrossRef]
- Yu, R.; Dong, S.; Chen, B.; Liu, Y.; Li, F.; Si, F.; Xie, C.; Li, Z. Antigenicity Alternations of Variant PEDV S Protein Disclosed by Linear B Cell Epitope Mapping. Viruses 2022, 14, 1371. [Google Scholar] [CrossRef]
- Li, W.; Hangalapura, B.N.; Elzen, P.v.D.; Born, E.v.D.; van Kuppeveld, F.J.M.; Rottier, P.J.M.; Bosch, B.-J.; Gallagher, T. Spike gene variability in porcine epidemic diarrhea virus as a determinant for virulence. J. Virol. 2025, 99, e0216524. [Google Scholar] [CrossRef]
- Masuda, A.; Lee, J.M.; Miyata, T.; Ebihara, T.; Kakino, K.; Hino, M.; Fujita, R.; Mon, H.; Kusakabe, T. Stable trimer formation of spike protein from porcine epidemic diarrhea virus improves the efficiency of secretory production in silkworms and induces neutralizing antibodies in mice. Vet. Res. 2021, 52, 102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, B.; Song, X.; Gao, J.; Guo, R.; Yi, C.; He, Z.; Hu, H.; Jiang, J.; Zhong, T.; et al. PEDV-spike-protein-expressing mRNA vaccine protects piglets against PEDV challenge. mBio 2024, 15, e0295823. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Cao, N.; Zhang, H.; Sun, L. Development of a Safe and Effective mRNA Candidate Vaccine Against PEDV G2c Genotype Infection. Viruses 2025, 17, 1210. https://doi.org/10.3390/v17091210
Zhu S, Cao N, Zhang H, Sun L. Development of a Safe and Effective mRNA Candidate Vaccine Against PEDV G2c Genotype Infection. Viruses. 2025; 17(9):1210. https://doi.org/10.3390/v17091210
Chicago/Turabian StyleZhu, Shixuan, Nan Cao, Huawei Zhang, and Leqiang Sun. 2025. "Development of a Safe and Effective mRNA Candidate Vaccine Against PEDV G2c Genotype Infection" Viruses 17, no. 9: 1210. https://doi.org/10.3390/v17091210
APA StyleZhu, S., Cao, N., Zhang, H., & Sun, L. (2025). Development of a Safe and Effective mRNA Candidate Vaccine Against PEDV G2c Genotype Infection. Viruses, 17(9), 1210. https://doi.org/10.3390/v17091210



