Peste des Petits Ruminants Vaccine: Criteria for Assessing Its Thermotolerance
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccine Receipt, Handling, and Registration
2.2. Incubation of Vaccines at High Temperature
2.3. Residual Moisture Testing
2.4. Titration of PPR Vaccine Virus
2.5. Vaccination of Goats with Vaccines Incubated at 40 °C and 45 °C
3. Results
3.1. Residual Moisture in Vaccine Vials
3.2. Accuracy of PPR Vaccine Titration
3.3. Titres of PPR Vaccines Before and After the Incubation Period
- -
- On Day 3, data showed that 6/8 (75%) vaccines submitted as ThT PPR maintain titre above the minimum required vaccine dose of 102.5 TCID50/mL, and one (37-LSG ThT) with a titre slightly below the standard. For the conventional PPR freeze-dried vaccines, 15/29 (51.72%) presented titres above the threshold of 102.5 TCID50/mL.
- -
- On Day 5, a total of 17 batches maintained a titre above the reference titre of 102.5 TCID50/mL. Among these vaccines, 4/8 (50%) are vaccines submitted as ThT PPR vaccine, while 13/29 (44.82%) are conventional freeze-dried PPR vaccines. All these vaccines (ThT and conventional vaccines) are formulated with stabilisers such as skimmed milk, Stabiliser30, lactalbumin–sucrose, Lactose–NZ Amine, Weybridge, or Trehalose.
3.4. Assessment of the Seroconversion Using the PPR Vaccine Without Maintaining the Cold Chain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pruvot, M.; Fine, A.E.; Hollinger, C.; Strindberg, S.; Damdinjav, B.; Buuveibaatar, B.; Chimeddorj, B.; Bayandonoi, G.; Khishgee, B.; Sandag, B.; et al. Outbreak of Peste des Petits Ruminants among Critically Endangered Mongolian Saiga and Other Wild Ungulates, Mongolia, 2016–2017. Emerg. Infect. Dis. 2020, 26, 51–62. [Google Scholar] [CrossRef]
- Aguilar, X.F.; Fine, A.E.; Pruvot, M.; Njeumi, F.; Walzer, C.; Kock, R.; Shiilegdamba, E. PPR virus threatens wildlife conservation. Science 2018, 362, 165–166. [Google Scholar] [CrossRef]
- Baron, M.D.; Diallo, A.; Lancelot, R.; Libeau, G. Peste des Petits Ruminants Virus. Adv. Virus Res. 2016, 95, 1–42. [Google Scholar] [CrossRef]
- Marashi, M.; Masoudi, S.; Moghadam, M.K.; Modirrousta, H.; Marashi, M.; Parvizifar, M.; Dargi, M.; Saljooghian, M.; Homan, F.; Hoffmann, B.; et al. Peste des Petits Ruminants Virus in Vulnerable Wild Small Ruminants, Iran, 2014–2016. Emerg. Infect. Dis. 2017, 23, 704–706. [Google Scholar] [CrossRef]
- Hoffmann, B.; Wiesner, H.; Maltzan, J.; Mustefa, R.; Eschbaumer, M.; Arif, F.A.; Beer, M. Fatalities in wild goats in Kurdistan associated with Peste des Petits Ruminants virus. Transbound. Emerg. Dis. 2012, 59, 173–176. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Amarasinghe, G.K.; Ayllon, M.A.; Bao, Y.; Basler, C.F.; Bavari, S.; Blasdell, K.R.; Briese, T.; Brown, P.A.; Bukreyev, A.; Balkema-Buschmann, A.; et al. Taxonomy of the order Mononegavirales: Update 2019. Arch. Virol. 2019, 164, 1967–1980. [Google Scholar] [CrossRef] [PubMed]
- Dundon, W.G.; Diallo, A.; Cattoli, G. Peste des petits ruminants in Africa: A review of currently available molecular epidemiological data. Arch. Virol. 2020, 165, 2147–2163. [Google Scholar] [CrossRef] [PubMed]
- Banyard, A.C.; Parida, S.; Batten, C.; Oura, C.; Kwiatek, O.; Libeau, G. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J. Gen. Virol. 2010, 91, 2885–2897. [Google Scholar] [CrossRef] [PubMed]
- Kwiatek, O.; Ali, Y.H.; Saeed, I.K.; Khalafalla, A.I.; Mohamed, O.I.; Obeida, A.A.; Abdelrahman, M.B.; Osman, H.M.; Taha, K.M.; Abbas, Z.; et al. Asian lineage of peste des petits ruminants virus, Africa. Emerg. Infect. Dis. 2011, 17, 1223–1231. [Google Scholar] [CrossRef]
- Gargadennec, L.; Lalanne, A. La Peste des Petits Ruminants. Bull. Serv. Zoo. A. O. F. 1942, 5, 15–21. [Google Scholar]
- First Detection of Peste Des Petits Ruminants (PPR) in Greece and Romania. Available online: https://www.woah.org/en/first-detection-of-peste-des-petits-ruminants-ppr-in-greece-and-romania/ (accessed on 13 February 2025).
- Geographical Distribution of Peste des Petitis Ruminants. Available online: https://www.woah.org/en/disease/peste-des-petits-ruminants/#ui-id-2 (accessed on 11 May 2024).
- Hungary-Peste des Petits Ruminants Virus (Inf. with)-Follow Up Report 11. Available online: https://wahis.woah.org/#/in-review/6216?fromPage=event-dashboard-url (accessed on 2 June 2025).
- Idoga, E.S.; Armson, B.; Alafiatayo, R.; Ogwuche, A.; Mijten, E.; Ekiri, A.B.; Varga, G.; Cook, A.J.C. A Review of the Current Status of Peste des Petits Ruminants Epidemiology in Small Ruminants in Tanzania. Front. Vet. Sci. 2020, 25, 592662. [Google Scholar] [CrossRef]
- Balamurugan, V.; Saravanan, P.; Sen, A.; Rajak, K.K.; Venkatesan, G.; Krishnamoorthy, P.; Bhanuprakash, V.; Singh, R.K. Prevalence of peste des petits ruminants among sheep and goats in India. J. Vet. Sci. 2012, 13, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, M.; Sayalel, K.; Muniraju, M.; Eblate, E.; Fyumagwa, R.; Shilinde, L.; Mdaki, M.; Keyyu, J.; Parida, S.; Kock, R. Spillover of peste des petits ruminants virus from domestic to wild ruminants in the serengeti ecosystem, Tanzania. Emerg. Infect. Dis. 2015, 21, 2230–2234. [Google Scholar] [CrossRef]
- Kumar, N.; Maherchandani, S.; Kashyap, S.K.; Singh, S.V.; Sharma, S.; Chaubey, K.K.; Ly, H. Peste des petits ruminants virus infection of small ruminants: A comprehensive review. Viruses 2014, 6, 2287–2327. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (WOAH) & Food and Agriculture Organization of the United Nations (FAO). Global Strategy for the Control and Eradication of PPR; WOAH & FAO: Rome, Italy, 2015; Available online: https://www.woah.org/app/uploads/2021/03/ppr-global-strategy-avecannexes-2015-03-28.pdf (accessed on 10 September 2023).
- Diallo, A.; Taylor, W.P.; Lefèvre, P.C.; Provost, A. Atténuation d’une souche du virus de la PPR. Candidat pour un vaccin homologue vivant. Rev. Elev. Med. Vet. Pays Trop. 1989, 42, 311–319. [Google Scholar] [CrossRef]
- Sen, A.; Saravanan, P.; Balamurugan, V.; Rajak, K.K.; Sudhakar, S.B.; Bhanuprakash, V.; Parida, S.; Singh, R.K. Vaccines against peste des petits ruminants virus. Expert. Rev. Vaccines 2010, 9, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Balamurugan, V.; Bhanuprakash, V.; Sen, A.; Saravanan, P.; Yadav, M.P. Possible control and eradication of peste des petits ruminants from India: Technical aspects. Vet. Ital. 2009, 45, 449–462. [Google Scholar]
- WOAH Glossery of Terms. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/0.04_GLOSSARY.pdf (accessed on 18 March 2025).
- Mariner, J.C.; Gachanja, J.; Tindih, S.H.; Toye, P. A thermostable presentation of the live, attenuated peste des petits ruminants vaccine in use in Africa and Asia. Vaccine 2017, 35, 3773–3779. [Google Scholar] [CrossRef]
- Silva, A.C.; Carrondo, M.J.; Alves, P.M. Strategies for improved stability of Peste des Petits Ruminants Vaccine. Vaccine 2011, 29, 4983–4991. [Google Scholar] [CrossRef]
- Sarkar, J.; Sreenivasa, B.P.; Singh, R.P.; Dhar, P.; Bandyopadhyay, S.K. Comparative efficacy of various chemical stabilizers on the thermostability of a live-attenuated peste des petits ruminants (PPR) vaccine. Vaccine 2003, 21, 4728–4735. [Google Scholar] [CrossRef]
- Worrall, E.E.; Litamoi, J.K.; Seck, B.M.; Ayelet, G. Xerovac: An ultra-rapid method for the dehydration and preservation of live attenuated Rinderpest and Peste des Petits ruminants vaccines. Vaccine 2000, 19, 834–839. [Google Scholar] [CrossRef]
- Rweyemamu, M.M.; Sylla, D.; Palya, V.; Prandota, J.; Mark, M. Quality Control Testing of Rinderpest Cell Culture Vaccine: Standard Operating Procedures; Food and Agriculture Organization of the United Nations (FAO) Animal Production and Health: Rome, Italy, 1994; 118p. [Google Scholar]
- World Health Organization. Temperature Sensitivity of Vaccines; World Health Organization: Geneva, Switzerland, 2006; Available online: https://iris.who.int/handle/10665/69387 (accessed on 16 July 2025).
- May, J.C.; Wheeler, R.M.; Etz, N.; Del Grosso, A. Measurement of final container residual moisture in freeze-dried biological products. Dev. Biol. Stand. 1992, 74, 153–164. [Google Scholar] [PubMed]
- Kärber, G. Beitrag zur kollektiven behandlung pharmakologiseher reihenversuche. Arch. Exp. Path. Pharmacol. 1931, 162, 480–484. [Google Scholar] [CrossRef]
- Peste des Petits Ruminants (Infection with Small Ruminant Morbillivirus): Chapter 3.8.9. in WOAH Terrestrial Manual. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.08.09_PPR.pdf (accessed on 11 May 2025).
- Bodjo, S.C.; Baziki, J.-D.; Nwankpa, N.; Chitsungo, E.; Koffi, Y.M.; Couacy-Hymann, E.; Tounkara, K.; Diop, M.; Gizaw, D.; Tajelser, I.B.A.; et al. Development and validation of an epitope-blocking ELISA using an anti-haemagglutinin monoclonal antibody for specific detection of antibodies in sheep and goat sera directed against peste des petits ruminants virus. Arch. Virol. 2018, 163, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Libeau, G.; Prehaud, C.; Lancelot, R.; Colas, F.; Guerre, L.; Bishop, D.H.; Diallo, A. Development of a competitive ELISA for detecting antibodies to the peste des petits ruminants virus using a recombinant nucleoprotein. Res. Vet. Sci. 1995, 58, 50–55. [Google Scholar] [CrossRef]
- Diallo, A.; Motsoane, M.C.; Gelaw, H.B.; Baziki, J.D.; Boukary, C.R.M.; Melesse, G.A.; Chitsungo, E.; Gebresillassie, M.; Tessema, Y.D.; Olugasa, B.O.; et al. An Evaluation of the Thermotolerance of Various Formulations of Freeze-Dried and Reconstituted Peste des Petits Ruminant Vaccines. Vet. Sci. 2024, 11, 525. [Google Scholar] [CrossRef]
- Bora, M.; Sunil, S.; Suni, S.; Reddy, G.S. Effect of chemical stabilizers on pellet profile and stability of lyophilized Peste-Des-Petits ruminants, sheep pox and goat pox vaccines at different temperatures. Pharma Innov. J. 2019, 8, 1182–1187. [Google Scholar]

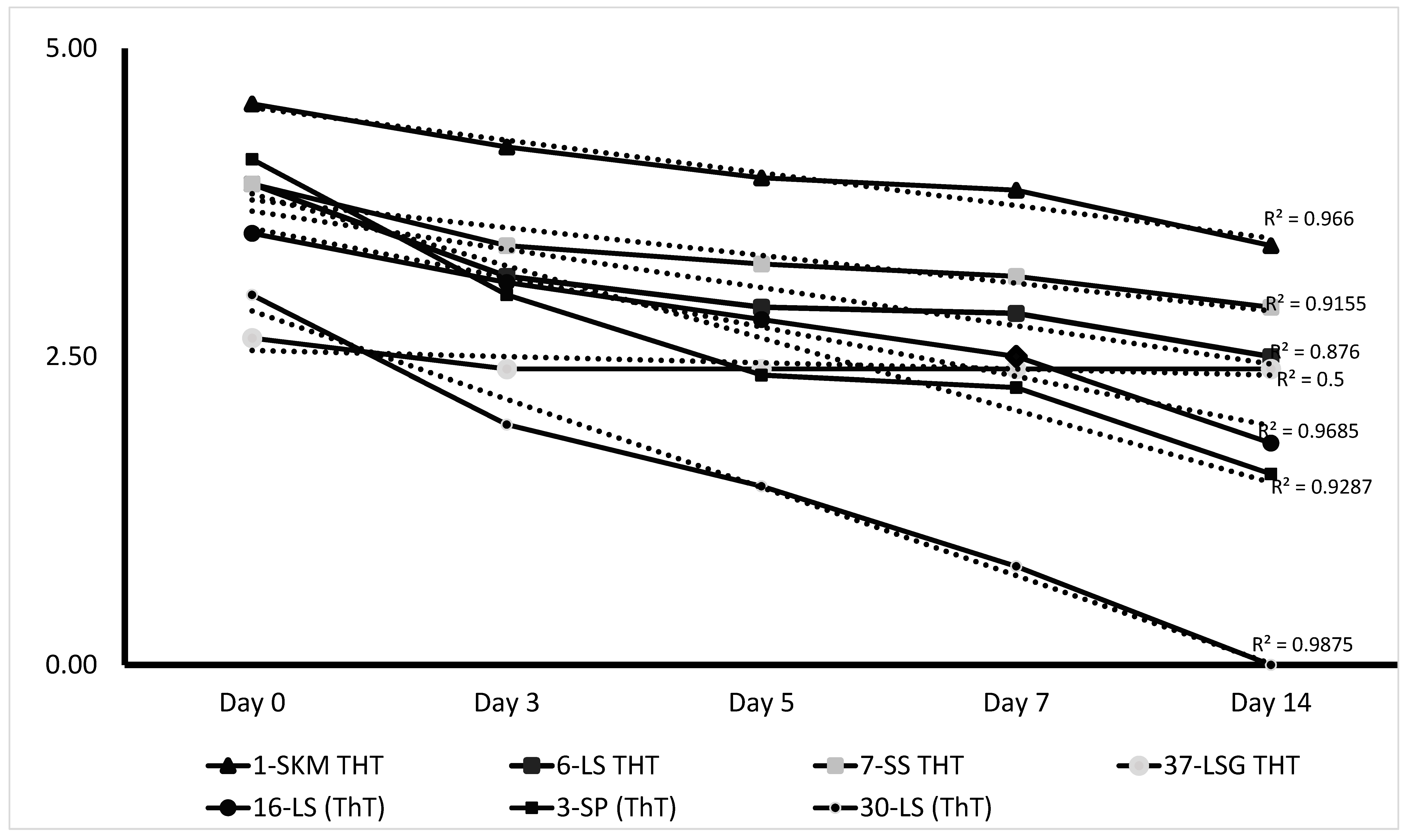
| Vaccine Titre (log10 TCID50/mL) | Titre Loss (log10 TCID50/mL) | |||||
|---|---|---|---|---|---|---|
| Residual moisture [≤3%] | Day 0 | Day 3 | Day 5 | Day 0–Day 3 | Day 0–Day 5 | |
| 1-SKM (ThT) | 1.72% | 4.55 | 4.2 | 3.95 | 0.35 | 0.6 |
| 2-SP | 2.0% | 4.2 | 3 | 2.4 | 1.2 | 1.8 |
| 3-SP (ThT) | 1.52% | 4.1 | 3 | 2.25 | 1.1 | 1.85 |
| 4-SKM | 1.7% | 4 | 3.2 | 3.1 | 0.8 | 0.9 |
| 5-SKM | 1.1% | 4 | 3.3 | 3.2 | 0.7 | 0.8 |
| 6-LS (ThT) | 1.17% | 3.9 | 3.15 | 2.90 | 0.75 | 1 |
| 7-SS (ThT) | 0.76% | 3.9 | 3.4 | 3.25 | 0.5 | 0.65 |
| 8-SP | 2.1% | 3.9 | 2.5 | 1.9 | 1.4 | 2 |
| 9-SKM | 1.26% | 3.8 | 2.8 | 2.75 | 1 | 1.05 |
| 10-WB | 2.3% | 3.8 | 3.2 | 2.6 | 0.6 | 1.2 |
| 11-WB | 3.0% | 3.8 | 3.4 | 2.4 | 0.4 | 1.4 |
| 12-LS | 2.51% | 3.7 | 2.7 | 2.55 | 1 | 1.15 |
| 13-SP | 1.9% | 3.65 | 2.2 | 2 | 1.45 | 1.65 |
| 14-LS | 1.1% | 3.6 | 3 | 3 | 0.6 | 0.6 |
| 15-LS | 1.1% | 3.5 | 3.15 | 3.05 | 0.35 | 0.45 |
| 16-LS (ThT) | 0.88% | 3.5 | 3.1 | 2.8 | 0.4 | 0.7 |
| 17-LA | 1.6% | 3.5 | 2.5 | 2.1 | 1 | 1.4 |
| 18-LS (ThT) | 0.84% | 3.45 | 2.85 | 2.35 | 0.6 | 1.1 |
| 19-LS | 1.6% | 3.4 | 3.1 | 2.6 | 0.3 | 0.8 |
| 20-LS | 2.9% | 3.4 | 1.9 | 1.6 | 1.5 | 1.8 |
| 21-LS | 2.2% | 3.4 | 1.6 | 1.8 | 1.8 | 1.6 |
| 22-LA | 1.7% | 3.3 | 2.85 | 2.55 | 0.45 | 0.75 |
| 23-LA | 1.9% | 3.3 | 2.3 | 1.9 | 1 | 1.4 |
| 24-LA | 1.8% | 3.2 | 3 | 2.65 | 0.2 | 0.55 |
| 25-WB | 2.6% | 3.1 | 0.4 | 0.1 | 2.7 | 3 |
| 26-TT | 2.2% | 3.05 | 2.8 | 2.55 | 0.25 | 0.5 |
| 27-LS | 2.5% | 3.05 | 2.5 | 2 | 0.55 | 1.05 |
| 28-SP | 2.28% | 3.05 | 1.65 | 1.3 | 1.4 | 1.75 |
| 29-LS | 2.3% | 3 | 2.9 | 2.6 | 0.1 | 0.4 |
| 30-LS (ThT) | 2.17% | 3 | 1.95 | 1.45 | 1.05 | 1.55 |
| 31-WB | 3.8% | 3 | 1.35 | 1 | 1.65 | 2 |
| 32-SKM | 2.2% | 2.95 | 2.5 | 2.25 | 0.45 | 0.7 |
| 33-LS | 2.8% | 2.95 | 1.35 | 1 | 1.6 | 1.95 |
| 34-LA | 2.7% | 2.9 | 2.45 | 1.55 | 0.45 | 1.35 |
| 35-WB | 2.8% | 2.9 | 0.5 | 0.1 | 2.4 | 2.8 |
| 36-WB | 2.4% | 2.7 | 2.05 | 1.9 | 0.65 | 0.8 |
| 37-LSG (ThT) | n,d | 2.65 | 2.4 | 2.4 | 0.25 | 0.25 |
| Day 0–Day 3 | Day 0–Day 5 | Day 3–Day 5 | |
|---|---|---|---|
| Average Titre Loss | 0.52 log10 | 0.72 log10 | 0.20 log10 |
| SD | 0.26 | 0.27 | 0.2 |
| Average Titre Loss + SD | 0.78 log10 | 0.99 log10 | 0.40 log10 |
| 1-SKM THT | 3-SP (ThT) | 6-LS THT | 7-SS THT | 16-LS (ThT) | 30-LS (ThT) | 37-LSG THT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incubation Time | 40 °C | 45 °C | 40 °C | 45 °C | 40 °C | 45 °C | 40 °C | 45 °C | 40 °C | 45 °C | 40 °C | 45 °C | 40 °C | 45 °C |
| Day 0 | 4.55 | 4.55 | 4.10 | 4.10 | 3.90 | 3.9 | 3.90 | 3.9 | 3.50 | 3.50 | 3.00 | 3.00 | 2.65 | 2.65 |
| Day 3 | 4.20 | 3.95 | 3.00 | 2.00 | 3.15 | 3 | 3.40 | 3.25 | 3.10 | 2.80 | 1.95 | 0 | 2.40 | 2.4 |
| Day 5 | 3.95 | 3.65 | 2.35 | 1.95 | 2.90 | 2 | 3.25 | 3 | 2.80 | 2.45 | 1.45 | 0 | 2.40 | 2.4 |
| Day 7 | 3.85 | 3.1 | 2.25 | 1.80 | 2.85 | 1.85 | 3.15 | 2.85 | 2.50 | 2.05 | 0.80 | 0 | 2.40 | 2.4 |
| Day 14 | 3.40 | 2.7 | 1.55 | 1.50 | 2.50 | 1.5 | 2.90 | 2.55 | 1.80 | 1.15 | 0.00 | 0 | 2.40 | 1.9 |
| 1-SKM THT | 3-SP (ThT) | 6-LS THT | 7-SS THT | 16-LS (ThT) | 30-LS (ThT) | 37-LSG THT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40 °C | 45 °C | 40 °C | 45 °C | 40 °C | 45 °C | 40 °C | 45 °C | 40 °C | 45 °C | 40 °C | 45 °C | 40 °C | 45 °C | |
| Days 0–3 | 0.35 | 0.6 | 1.1 | 2.1 | 0.75 | 0.9 | 0.5 | 0.65 | 0.4 | 0.7 | 1.05 | 3 | 0.25 | 0.25 |
| Days 0–5 | 0.6 | 0.9 | 1.75 | 2.15 | 1 | 1.9 | 0.65 | 0.9 | 0.7 | 1.05 | 1.55 | 3 | 0.25 | 0.25 |
| Days 0–7 | 0.7 | 1.45 | 1.85 | 2.3 | 1.05 | 2.05 | 0.75 | 1.05 | 1 | 1.45 | 2.2 | 3 | 0.25 | 0.25 |
| Days 0–14 | 1.15 | 1.85 | 2.55 | 2.6 | 1.4 | 2.4 | 1 | 1.35 | 1.7 | 2.35 | 3 | 3 | 0.25 | 0.75 |
| Incubation of 1-SKM PPR ThT Vaccine | Number of Vaccinated Goats | ELISAs Results: (%) POSITIVE | ||||
|---|---|---|---|---|---|---|
| Temperature | Incubation Time | IDVET PPR cELISA | HPPR bELISA | |||
| Sera Collected at Day 0 | Sera Collected at Day 28 | Sera Collected at Day 0 | Sera Collected at Day 28 | |||
| +4 °C | Day 0 | 3 | 0 | 3/3 (100%) | 0 | 3/3 (100%) |
| 40 °C | Day 7 (titre of 3.70 log10 TCID50/mL) | 3 | 0 | 3/3 (100%) | 0 | 3/3 (100%) |
| Day 21 (titre 2.55 log10 TCID50/mL) | 3 | 0 | 3/3 (100%) | 0 | 3/3 (100%) | |
| Day 28 (titre 2.30 log10 TCID50/mL) | 3 | 0 | 3/3 (100%) | 0 | 3/3 (100%) | |
| 45 °C | Day 7 (titre 2.95 log10 TCID50/mL) | 3 | 0 | 3/3(100%) | 0 | 3/3 (100%) |
| Day 21 (titre 2.05 log10 TCID50/mL) | 3 | 0 | 3/3 (100%) | 0 | 3/3 (100%) | |
| Day 28 (titre 1.35 log10 TCID50/mL) | 3 | 0 | 2 (66.66%) | 0 | 1/3 (33%) | |
| Unvaccinated | 2 | 0 | 0 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodjo, C.S.; Gelaw, H.B.; Luhanga, Z.D.; Tessema, Y.D.; Baziki, J.-D.-D.; Boukary, C.R.M.; Melesse, G.A.; Chitsungo, E.; Nwankpa, N.; Kihu, S.; et al. Peste des Petits Ruminants Vaccine: Criteria for Assessing Its Thermotolerance. Viruses 2025, 17, 1151. https://doi.org/10.3390/v17091151
Bodjo CS, Gelaw HB, Luhanga ZD, Tessema YD, Baziki J-D-D, Boukary CRM, Melesse GA, Chitsungo E, Nwankpa N, Kihu S, et al. Peste des Petits Ruminants Vaccine: Criteria for Assessing Its Thermotolerance. Viruses. 2025; 17(9):1151. https://doi.org/10.3390/v17091151
Chicago/Turabian StyleBodjo, Charles S., Hassen Belay Gelaw, Zione D. Luhanga, Yebechaye Degefa Tessema, Jean-De-Dieu Baziki, Cisse R. Moustapha Boukary, Gelagay Ayelet Melesse, Ethel Chitsungo, Nick Nwankpa, Simon Kihu, and et al. 2025. "Peste des Petits Ruminants Vaccine: Criteria for Assessing Its Thermotolerance" Viruses 17, no. 9: 1151. https://doi.org/10.3390/v17091151
APA StyleBodjo, C. S., Gelaw, H. B., Luhanga, Z. D., Tessema, Y. D., Baziki, J.-D.-D., Boukary, C. R. M., Melesse, G. A., Chitsungo, E., Nwankpa, N., Kihu, S., Njeumi, F., Parida, S., & Diallo, A. (2025). Peste des Petits Ruminants Vaccine: Criteria for Assessing Its Thermotolerance. Viruses, 17(9), 1151. https://doi.org/10.3390/v17091151







