Serologic Evidence of Human Exposure to Bat-Borne Zoonotic Paramyxoviruses, Cambodia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sera Collection
2.2. Multiplex Microsphere-Based Immunoassay
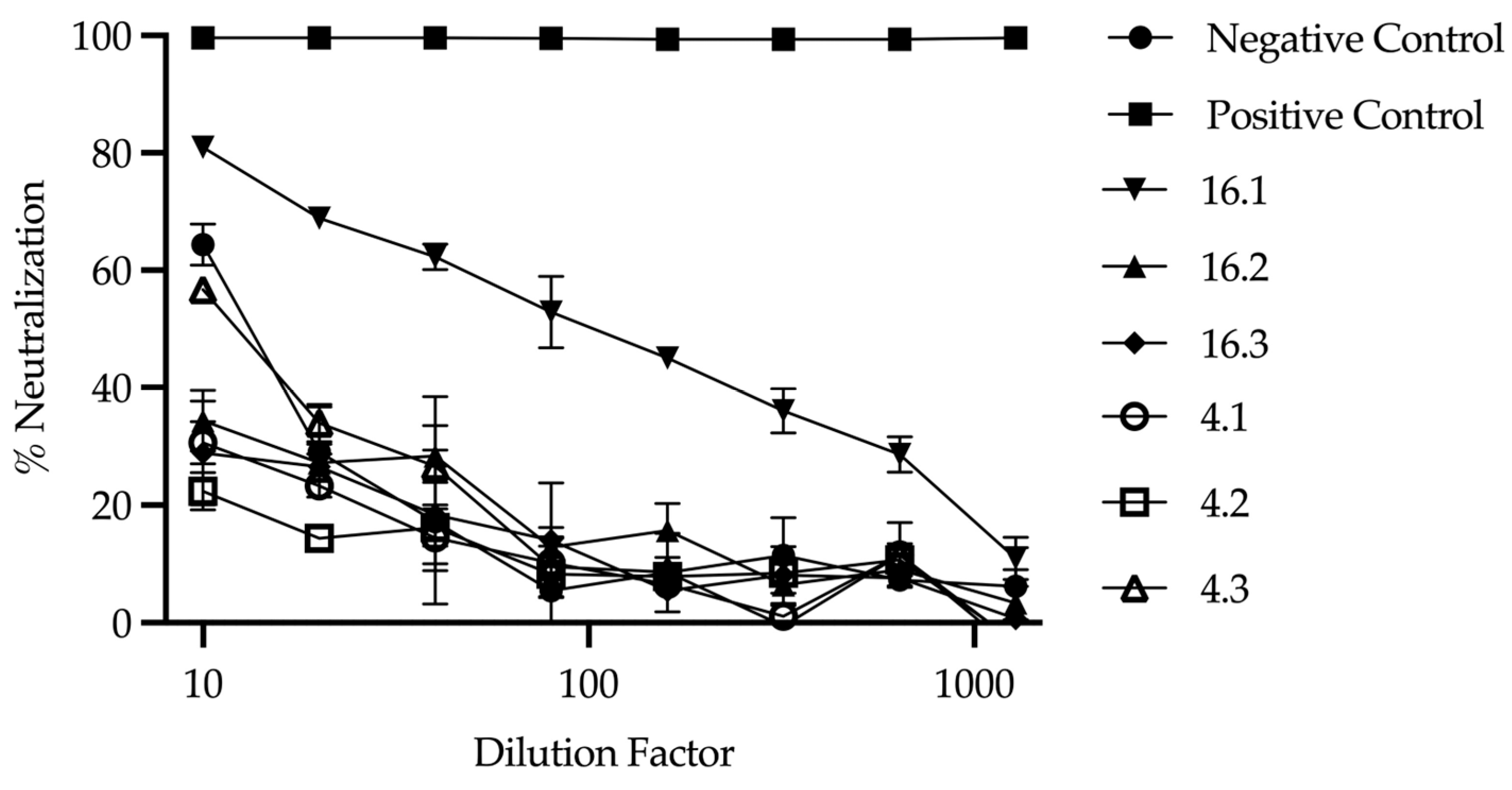
2.3. Cedar Virus Neutralization Test
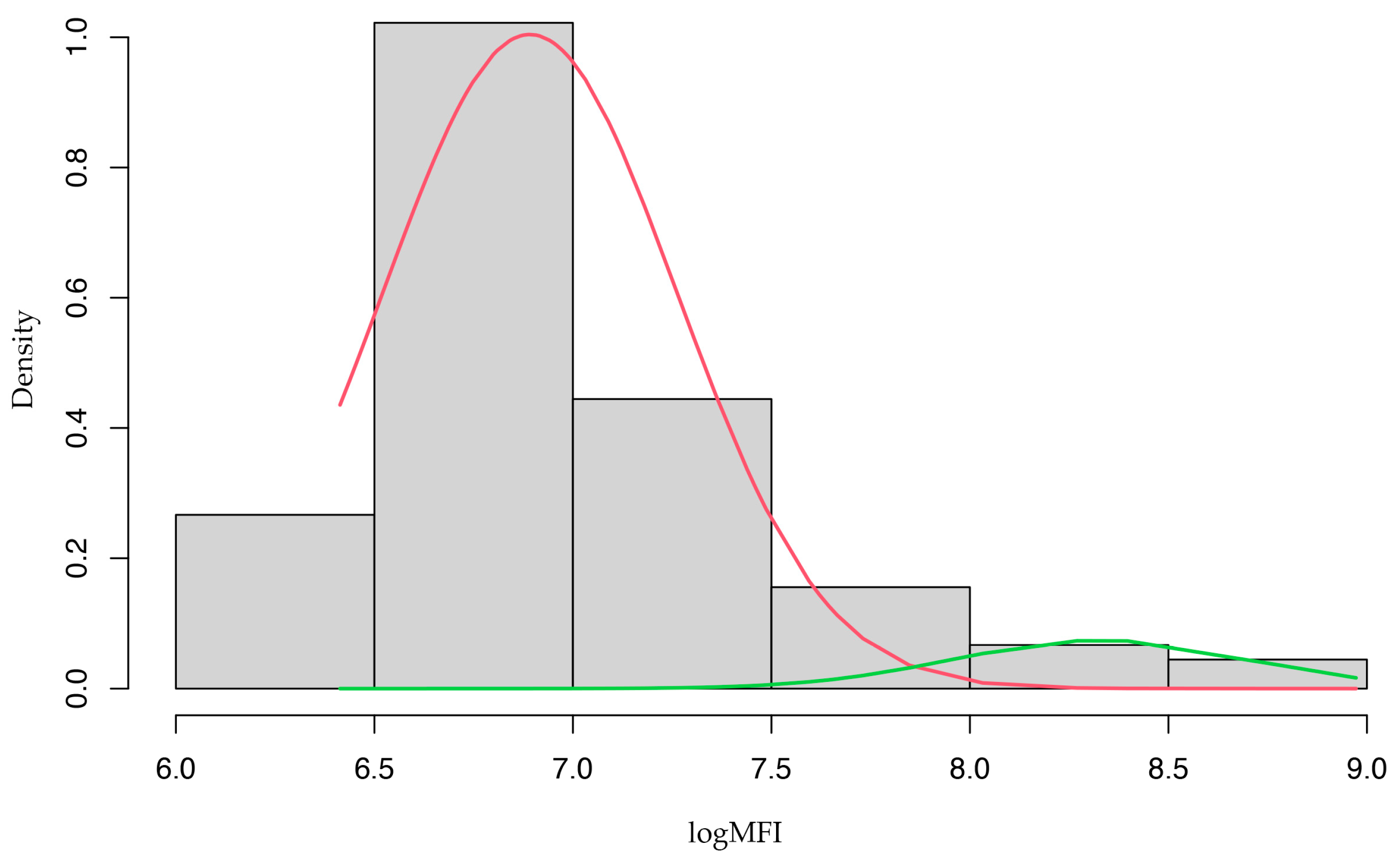
2.4. Statistical Analysis
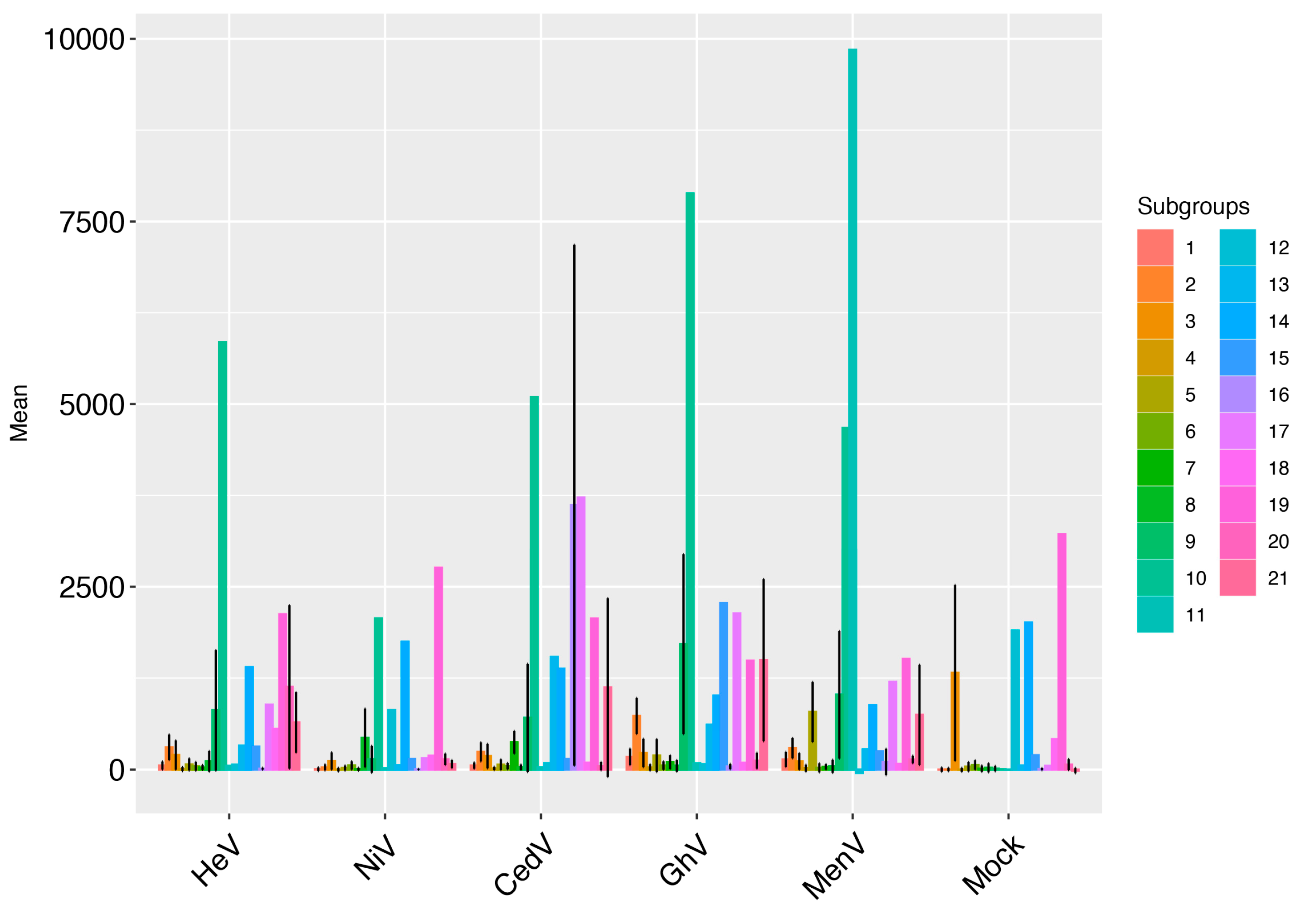
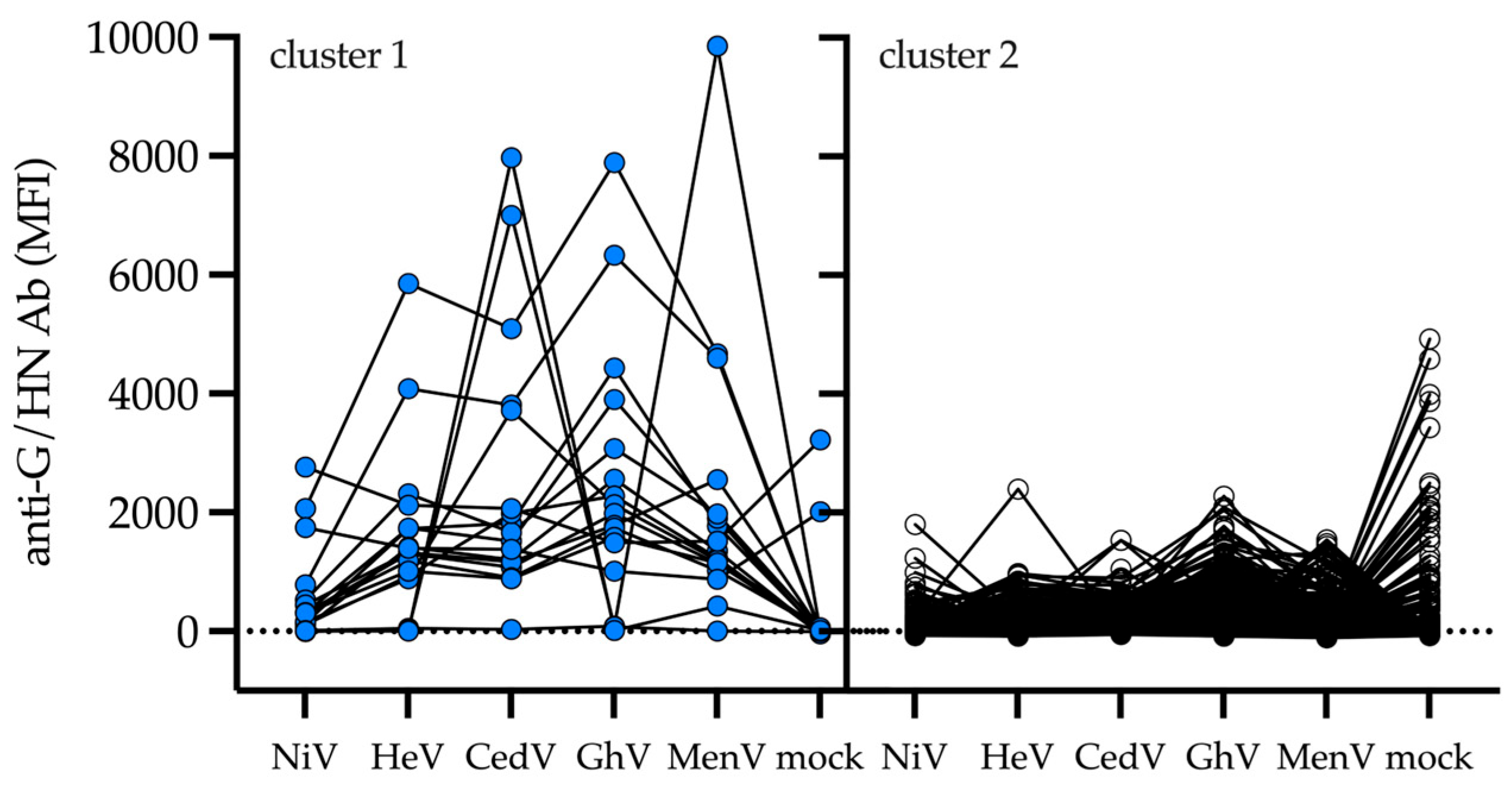
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
Abbreviations
| NiV | Nipah virus |
| HeV | Hendra virus |
| CedV | Cedar virus |
| Ghana virus | GhV |
| Menangle virus | MenV |
| MMIA | Multiplex microsphere immunoassay |
| G | Henipavirus attachment glycoprotein |
| HN | Pararubulavirus hemagglutinin-neuraminidase protein |
| xMAP | Multi-analyte profiling |
| PBS | Phosphate-buffered saline |
| PBST | Phosphate-buffered saline, Tween-20 (0.05%) |
| rCedV | Recombinant Cedar virus |
| DPMM | Dirichlet process mixture model |
| PCA | Principal component analysis |
Appendix A
| Cluster.ID | GhV | HeV | CedV | NiV | MenV | Mock |
|---|---|---|---|---|---|---|
| 9.1 | 6331 | 4084.25 | 3808.75 | 779 | 4600 | −22.5 |
| 9.2 | 4431 | 1730 | 1820.5 | 145.5 | 1776 | −3.75 |
| 9.3 | 3077.5 | 2317.75 | 1662.25 | 526.5 | 1975.5 | 46.25 |
| 9.4 | 3903.5 | 1740 | 1519.75 | 305.5 | 1900 | −38 |
| 9.5 | 2559.5 | 1412 | 1208.5 | 54.5 | 1349.5 | −25.25 |
| 9.6 | 1785.75 | 1322.75 | 1166.5 | 448 | 2553.75 | 20.25 |
| 9.7 | 1987.25 | 1307.5 | 1079.5 | 309.25 | 1140.5 | 10.5 |
| 9.8 | 2041.5 | 930.75 | 901 | 57.5 | 782 | 13.5 |
| 9.9 | 1735.25 | 1187.5 | 901 | 314.75 | 1025 | 46.75 |
| 9.10 | 1577 | 1014.5 | 886.75 | 306.25 | 1154 | 56.5 |
| 9.11 | 1564.5 | 975 | 822 | 203.5 | 980.25 | 81.25 |
| 9.12 | 1700.75 | 959.75 | 648.5 | 58 | 690.75 | 23.75 |
| 9.13 | 2089.75 | 657.25 | 598.5 | 62 | 1154 | 13.5 |
| 9.14 | 1438.75 | 400.75 | 582.5 | 26 | 951 | 34.75 |
| 9.15 | 1311.25 | 652.75 | 567 | 183.5 | 697.5 | 11.5 |
| 9.16 | 1474.75 | 719.5 | 531 | 146.25 | 1205.25 | 100.75 |
| 9.17 | 961.25 | 659.875 | 516.875 | 187.5 | 529.625 | 64.75 |
| 9.18 | 658.75 | 336.25 | 480.5 | 43.5 | 586.25 | −14 |
| 9.19 | 1742 | 354.75 | 325 | 17 | 597.5 | 28.75 |
| 9.20 | 1130.75 | 359 | 323.75 | 67 | 683.75 | −32.75 |
| 9.21 | 1199.5 | 354.25 | 293.5 | 85.5 | 1342.75 | 14 |
| 9.22 | 934.75 | 330.25 | 289.5 | 36.25 | 702 | 8 |
| 9.23 | 1068.25 | 256.75 | 246 | 59.5 | 982 | −33.25 |
| 9.24 | 1772.25 | 481.25 | 240.25 | −11.5 | 143 | −19.5 |
| 9.25 | 808.5 | 423.25 | 229 | −21.5 | -29 | −26.25 |
| 9.26 | 999.25 | 115.75 | 228.5 | 7.5 | 379 | 14 |
| 9.27 | 999.25 | 115.75 | 228.5 | 7.5 | 379 | 14 |
| 9.28 | 999.25 | 115.75 | 228.5 | 7.5 | 379 | 14 |
| 9.29 | 663.25 | 115.5 | 100.5 | 15.5 | 7 | −41.5 |
| 9.30 | 559.5 | 64 | 72 | 7 | 984 | 74.75 |
| 9.31 | 757.75 | −14.25 | −2.5 | −18.5 | 255.5 | 7 |
| 10.1 | 7883.25 | 5847.75 | 5095 | 2065.5 | 4673 | 4 |
| 11.1 | 84 | 50 | 30 | 17 | 9850 | −4 |
| 15.1 | 2275 | 311.75 | 144.25 | 144.75 | 249 | 196 |

References
- Centers for Disease Control and Prevention (CDC). Update: Outbreak of Nipah virus--Malaysia and Singapore, 1999. MMWR Morb. Mortal. Wkly. Rep. 1999, 48, 335–337. [Google Scholar]
- Miranda, M.E.; Miranda, N.L. Reston ebolavirus in humans and animals in the Philippines: A review. J. Infect. Dis. 2011, 204 (Suppl. S3), S757–S760. [Google Scholar] [CrossRef]
- Chua, K.B.; Goh, K.J.; Wong, K.T.; Kamarulzaman, A.; Tan, P.S.; Ksiazek, T.G.; Zaki, S.R.; Paul, G.; Lam, S.K.; Tan, C.T. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet 1999, 354, 1257–1259. [Google Scholar] [CrossRef]
- Mohd Nor, M.N.; Gan, C.H.; Ong, B.L. Nipah virus infection of pigs in peninsular Malaysia. Rev. Sci. Tech. 2000, 19, 160–165. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Nipah virus outbreak(s) in Bangladesh, January-April 2004. Wkly. Epidemiol. Rec. 2004, 79, 168–171. [Google Scholar]
- Chadha, M.S.; Comer, J.A.; Lowe, L.; Rota, P.A.; Rollin, P.E.; Bellini, W.J.; Ksiazek, T.G.; Mishra, A. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg. Infect. Dis. 2006, 12, 235–240. [Google Scholar] [CrossRef]
- Bellini, W.J.; Harcourt, B.H.; Bowden, N.; Rota, P.A. Nipah virus: An emergent paramyxovirus causing severe encephalitis in humans. J. Neurovirol. 2005, 11, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Clayton, B.A. Nipah virus: Transmission of a zoonotic paramyxovirus. Curr. Opin. Virol. 2017, 22, 97–104. [Google Scholar] [CrossRef]
- Luby, S.P.; Rahman, M.; Hossain, M.J.; Blum, L.S.; Husain, M.M.; Gurley, E.; Khan, R.; Ahmed, B.N.; Rahman, S.; Nahar, N.; et al. Foodborne transmission of Nipah virus, Bangladesh. Emerg. Infect. Dis. 2006, 12, 1888–1894. [Google Scholar] [CrossRef] [PubMed]
- Ching, P.K.; de los Reyes, V.C.; Sucaldito, M.N.; Tayag, E.; Columna-Vingno, A.B.; Malbas, F.F., Jr.; Bolo, G.C., Jr.; Sejvar, J.J.; Eagles, D.; Playford, G.; et al. Outbreak of henipavirus infection, Philippines, 2014. Emerg. Infect. Dis. 2015, 21, 328–331. [Google Scholar] [CrossRef]
- Yadav, P.D.; Shete, A.M.; Kumar, G.A.; Sarkale, P.; Sahay, R.R.; Radhakrishnan, C.; Lakra, R.; Pardeshi, P.; Gupta, N.; Gangakhedkar, R.R.; et al. Nipah Virus Sequences from Humans and Bats during Nipah Outbreak, Kerala, India, 2018. Emerg. Infect. Dis. 2019, 25, 1003–1006. [Google Scholar] [CrossRef]
- Arunkumar, G.; Chandni, R.; Mourya, D.T.; Singh, S.K.; Sadanandan, R.; Sudan, P.; Bhargava, B. Outbreak Investigation of Nipah Virus Disease in Kerala, India, 2018. J. Infect. Dis. 2019, 219, 1867–1878. [Google Scholar] [CrossRef]
- Thiagarajan, K. Nipah virus: Kerala reports second death in four months. Bmj 2024, 386, q2058. [Google Scholar] [CrossRef]
- As, A.K.; Sahay, R.R.; Radhakrishnan, C.; P, S.; Kandath, S.; Patil, D.Y.; Shete, A.M.; M, S.; Ramakrishnan, G.; Moorkoth, A.P.; et al. Clinico-epidemiological presentations and management of Nipah virus infection during the outbreak in Kozhikode district, Kerala state, India 2023. J. Med. Virol. 2024, 96, e29559. [Google Scholar] [CrossRef] [PubMed]
- Sudeep, A.B.; Yadav, P.D.; Gokhale, M.D.; Balasubramanian, R.; Gupta, N.; Shete, A.; Jain, R.; Patil, S.; Sahay, R.R.; Nyayanit, D.A.; et al. Detection of Nipah virus in Pteropus medius in 2019 outbreak from Ernakulam district, Kerala, India. BMC Infect. Dis. 2021, 21, 162. [Google Scholar] [CrossRef]
- Cappelle, J.; Hoem, T.; Hul, V.; Furey, N.; Nguon, K.; Prigent, S.; Dupon, L.; Ken, S.; Neung, C.; Hok, V.; et al. Nipah virus circulation at human-bat interfaces, Cambodia. Bull. World Health Organ. 2020, 98, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Wacharapluesadee, S.; Boongird, K.; Wanghongsa, S.; Ratanasetyuth, N.; Supavonwong, P.; Saengsen, D.; Gongal, G.N.; Hemachudha, T. A longitudinal study of the prevalence of Nipah virus in Pteropus lylei bats in Thailand: Evidence for seasonal preference in disease transmission. Vector-Borne Zoonotic Dis. 2010, 10, 183–190. [Google Scholar] [CrossRef]
- Wacharapluesadee, S.; Ghai, S.; Duengkae, P.; Manee-Orn, P.; Thanapongtharm, W.; Saraya, A.W.; Yingsakmongkon, S.; Joyjinda, Y.; Suradhat, S.; Ampoot, W.; et al. Two decades of one health surveillance of Nipah virus in Thailand. One Health Outlook 2021, 3, 12. [Google Scholar] [CrossRef]
- Reynes, J.-M.; Counor, D.; Ong, S.; Faure, C.; Seng, V.; Molia, S.; Walston, J.; Georges-Courbot, M.C.; Deubel, V.; Sarthou, J.-L. Nipah Virus in Lyle’s Flying Foxes, Cambodia. Emerg. Infect. Dis. 2005, 11, 1042–1047. [Google Scholar] [CrossRef]
- Halpin, K.; Hyatt, A.D.; Fogarty, R.; Middleton, D.; Bingham, J.; Epstein, J.H.; Rahman, S.A.; Hughes, T.; Smith, C.; Field, H.E.; et al. Pteropid bats are confirmed as the reservoir hosts of henipaviruses: A comprehensive experimental study of virus transmission. Am. J. Trop. Med. Hyg. 2011, 85, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Marsh, G.A.; de Jong, C.; Barr, J.A.; Tachedjian, M.; Smith, C.; Middleton, D.; Yu, M.; Todd, S.; Foord, A.J.; Haring, V.; et al. Cedar virus: A novel Henipavirus isolated from Australian bats. PLoS Pathog. 2012, 8, e1002836. [Google Scholar] [CrossRef]
- Eaton, B.T.; Broder, C.C.; Middleton, D.; Wang, L.F. Hendra and Nipah viruses: Different and dangerous. Nat. Rev. Microbiol. 2006, 4, 23–35. [Google Scholar] [CrossRef]
- Drexler, J.F.; Corman, V.M.; Gloza-Rausch, F.; Seebens, A.; Annan, A.; Ipsen, A.; Kruppa, T.; Müller, M.A.; Kalko, E.K.; Adu-Sarkodie, Y.; et al. Henipavirus RNA in African bats. PLoS ONE 2009, 4, e6367. [Google Scholar] [CrossRef]
- Mortlock, M.; Geldenhuys, M.; Dietrich, M.; Epstein, J.H.; Weyer, J.; Pawęska, J.T.; Markotter, W. Seasonal shedding patterns of diverse henipavirus-related paramyxoviruses in Egyptian rousette bats. Sci. Rep. 2021, 11, 24262. [Google Scholar] [CrossRef]
- Madera, S.; Kistler, A.; Ranaivoson, H.C.; Ahyong, V.; Andrianiaina, A.; Andry, S.; Raharinosy, V.; Randriambolamanantsoa, T.H.; Ravelomanantsoa, N.A.F.; Tato, C.M.; et al. Discovery and Genomic Characterization of a Novel Henipavirus, Angavokely Virus, from Fruit Bats in Madagascar. J. Virol. 2022, 96, e0092122. [Google Scholar] [CrossRef]
- Peel, A.J.; Wells, K.; Giles, J.; Boyd, V.; Burroughs, A.; Edson, D.; Crameri, G.; Baker, M.L.; Field, H.; Wang, L.-F.; et al. Synchronous shedding of multiple bat paramyxoviruses coincides with peak periods of Hendra virus spillover. Emerg. Microbes Infect. 2019, 8, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.S.; Todd, S.; Marsh, G.; Fernandez-Loras, A.; Suu-Ire, R.; Wood, J.L.N.; Wang, L.F.; Murcia, P.R.; Cunningham, A.A. Co-circulation of diverse paramyxoviruses in an urban African fruit bat population. J. Gen. Virol. 2012, 93, 850–856. [Google Scholar] [CrossRef]
- Kohl, C.; Tachedjian, M.; Todd, S.; Monaghan, P.; Boyd, V.; Marsh, G.A.; Crameri, G.; Field, H.; Kurth, A.; Smith, I.; et al. Hervey virus: Study on co-circulation with Henipaviruses in Pteropid bats within their distribution range from Australia to Africa. PLoS ONE 2018, 13, e0191933. [Google Scholar] [CrossRef]
- Philbey, A.W.; Kirkland, P.D.; Ross, A.D.; Davis, R.J.; Gleeson, A.B.; Love, R.J.; Daniels, P.W.; Gould, A.R.; Hyatt, A.D. An apparently new virus (family Paramyxoviridae) infectious for pigs, humans, and fruit bats. Emerg. Infect. Dis. 1998, 4, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.A.; Smith, C.; Marsh, G.A.; Field, H.; Wang, L.F. Evidence of bat origin for Menangle virus, a zoonotic paramyxovirus first isolated from diseased pigs. J. Gen. Virol. 2012, 93, 2590–2594. [Google Scholar] [CrossRef] [PubMed]
- Bowden, T.R.; Westenberg, M.; Wang, L.F.; Eaton, B.T.; Boyle, D.B. Molecular characterization of Menangle virus, a novel paramyxovirus which infects pigs, fruit bats, and humans. Virology 2001, 283, 358–373. [Google Scholar] [CrossRef]
- Kirkland, P.D.; Love, R.J.; Philbey, A.W.; Ross, A.D.; Davis, R.J.; Hart, K.G. Epidemiology and control of Menangle virus in pigs. Aust. Vet. J. 2001, 79, 199–206. [Google Scholar] [CrossRef]
- Chant, K.; Chan, R.; Smith, M.; Dwyer, D.E.; Kirkland, P. Probable human infection with a newly described virus in the family Paramyxoviridae. The NSW Expert Group. Emerg. Infect. Dis. 1998, 4, 273–275. [Google Scholar] [CrossRef]
- Yaiw, K.C.; Bingham, J.; Crameri, G.; Mungall, B.; Hyatt, A.; Yu, M.; Eaton, B.; Shamala, D.; Wang, L.F.; Thong Wong, K. Tioman virus, a paramyxovirus of bat origin, causes mild disease in pigs and has a predilection for lymphoid tissues. J. Virol. 2008, 82, 565–568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mackenzie, J.S.; Chua, K.B.; Daniels, P.W.; Eaton, B.T.; Field, H.E.; Hall, R.A.; Halpin, K.; Johansen, C.A.; Kirkland, P.D.; Lam, S.K.; et al. Emerging viral diseases of Southeast Asia and the Western Pacific. Emerg. Infect. Dis. 2001, 7 (3 Suppl.), 497–504. [Google Scholar] [CrossRef]
- Rachmat, A.; Kelly, G.C.; Tran, L.K.; Christy, N.; Supaprom, C.; Heang, V.; Dul, S.; Garcia-Rivera, J.A.; Prom, S.; Sopheab, H.; et al. Clinical Presentation, Risk Factors, and Comparison of Laboratory Diagnostics for Seasonal Influenza Virus Among Cambodians From 2007 to 2020. Open Forum Infect. Dis. 2024, 11, ofae062. [Google Scholar] [CrossRef]
- Rachmat, A.; Kelly, G.C.; Hontz, R.D.; Supaprom, C.; Heang, V.; Hip, P.; Garcia-Rivera, J.A.; Prom, S.; Chhea, C.; Sutherland, I.W.; et al. Clinical and epidemiologic evaluation of a 2020 chikungunya outbreak in Cambodia. BMC Infect. Dis. 2022, 22, 949. [Google Scholar] [CrossRef]
- Kelly, G.C.; Rachmat, A.; Hontz, R.D.; Sklar, M.J.; Tran, L.K.; Supaprom, C.; Luy, M.; Lina, S.; Gregory, M.J.; Sopheab, H.; et al. Etiology and risk factors for diarrheal disease amongst rural and peri-urban populations in Cambodia, 2012-2018. PLoS ONE 2023, 18, e0283871. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Sterling, S.L.; Fusco, D.L.; Chan, Y.P.; Xu, K.; Laing, E.D.; Broder, C.C. Recombinant Soluble Henipavirus Glycoprotein Preparation. Methods Mol. Biol. 2023, 2682, 33–58. [Google Scholar] [CrossRef] [PubMed]
- Pulscher, L.A.; Peel, A.J.; Rose, K.; Welbergen, J.A.; Baker, M.L.; Boyd, V.; Low-Choy, S.; Edson, D.; Todd, C.; Dorrestein, A.; et al. Serological evidence of a pararubulavirus and a betacoronavirus in the geographically isolated Christmas Island flying-fox (Pteropus natalis). Transbound. Emerg. Dis. 2022, 69, e2366–e2377. [Google Scholar] [CrossRef]
- Laing, E.D.; Amaya, M.; Navaratnarajah, C.K.; Feng, Y.R.; Cattaneo, R.; Wang, L.F.; Broder, C.C. Rescue and characterization of recombinant cedar virus, a non-pathogenic Henipavirus species. Virol. J. 2018, 15, 56. [Google Scholar] [CrossRef]
- Amaya, M.; Cheng, H.; Borisevich, V.; Navaratnarajah, C.K.; Cattaneo, R.; Cooper, L.; Moore, T.W.; Gaisina, I.N.; Geisbert, T.W.; Rong, L.; et al. A recombinant Cedar virus based high-throughput screening assay for henipavirus antiviral discovery. Antivir. Res. 2021, 193, 105084. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing version 4.5.1; R Core Team: Vienna, Austria, 2025.
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses, R package version 1.0.7; R Core Team: Vienna, Austria, 2020.
- Ross, G.J.; Markwick, D. Dirichletprocess: Build Dirichlet Process Objects for Bayesian Modelling, R package version 0.4.2; R Core Team: Vienna, Austria, 2023.
- Benaglia, T.; Chauveau, D.; Hunter, D.R.; Young, D.S. mixtools: An R Package for Analyzing Mixture Models. J. Stat. Softw. 2009, 32, 1–29. [Google Scholar] [CrossRef]
- Tong, D.D.M.; Buxser, S.; Vidmar, T.J. Application of a mixture model for determining the cutoff threshold for activity in high-throughput screening. Comput. Stat. Data Anal. 2007, 51, 4002–4012. [Google Scholar] [CrossRef]
- Kafatos, G.; Andrews, N.J.; McConway, K.J.; Maple, P.A.; Brown, K.; Farrington, C.P. Is it appropriate to use fixed assay cut-offs for estimating seroprevalence? Epidemiol. Infect. 2016, 144, 887–895. [Google Scholar] [CrossRef]
- Lee, B.; Pernet, O.; Ahmed, A.A.; Zeltina, A.; Beaty, S.M.; Bowden, T.A. Molecular recognition of human ephrinB2 cell surface receptor by an emergent African henipavirus. Proc. Natl. Acad. Sci. USA 2015, 112, E2156–E2165. [Google Scholar] [CrossRef] [PubMed]
- Zeltina, A.; Bowden, T.A.; Lee, B. Emerging Paramyxoviruses: Receptor Tropism and Zoonotic Potential. PLoS Pathog. 2016, 12, e1005390. [Google Scholar] [CrossRef] [PubMed]
- Laing, E.D.; Navaratnarajah, C.K.; Cheliout Da Silva, S.; Petzing, S.R.; Xu, Y.; Sterling, S.L.; Marsh, G.A.; Wang, L.F.; Amaya, M.; Nikolov, D.B.; et al. Structural and functional analyses reveal promiscuous and species specific use of ephrin receptors by Cedar virus. Proc. Natl. Acad. Sci. USA 2019, 116, 20707–20715. [Google Scholar] [CrossRef] [PubMed]
- Satterfield, B.A.; Cross, R.W.; Fenton, K.A.; Agans, K.N.; Basler, C.F.; Geisbert, T.W.; Mire, C.E. The immunomodulating V and W proteins of Nipah virus determine disease course. Nat. Commun. 2015, 6, 7483. [Google Scholar] [CrossRef]
- Lieu, K.G.; Marsh, G.A.; Wang, L.-F.; Netter, H.J. The non-pathogenic Henipavirus Cedar paramyxovirus phosphoprotein has a compromised ability to target STAT1 and STAT2. Antivir. Res. 2015, 124, 69–76. [Google Scholar] [CrossRef]
- Schountz, T.; Campbell, C.; Wagner, K.; Rovnak, J.; Martellaro, C.; DeBuysscher, B.L.; Feldmann, H.; Prescott, J. Differential Innate Immune Responses Elicited by Nipah Virus and Cedar Virus Correlate with Disparate In Vivo Pathogenesis in Hamsters. Viruses 2019, 11, 291. [Google Scholar] [CrossRef]
- Natasha, A.; Pye, S.E.; Park, K.; Rajoriya, S.; Yang, I.; Park, J.; Pangestu, H.S.; Kim, J.; Oh, Y.; López, C.B.; et al. Detection and characterization of Langya virus in Crocidura lasiura (the Ussuri white-toothed shrew), Republic of Korea. One Health 2025, 20, 101017. [Google Scholar] [CrossRef]
- Anthony, S.J.; Epstein, J.H.; Murray, K.A.; Navarrete-Macias, I.; Zambrana-Torrelio, C.M.; Solovyov, A.; Ojeda-Flores, R.; Arrigo, N.C.; Islam, A.; Ali Khan, S.; et al. A strategy to estimate unknown viral diversity in mammals. mBio 2013, 4, e00598-13. [Google Scholar] [CrossRef]
- Pernet, O.; Schneider, B.S.; Beaty, S.M.; LeBreton, M.; Yun, T.E.; Park, A.; Zachariah, T.T.; Bowden, T.A.; Hitchens, P.; Ramirez, C.M.; et al. Evidence for henipavirus spillover into human populations in Africa. Nat. Commun. 2014, 5, 5342. [Google Scholar] [CrossRef]
- Weatherman, S.; Feldmann, H.; de Wit, E. Transmission of henipaviruses. Curr. Opin. Virol. 2017, 28, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Halpin, K.; Young, P.L.; Field, H.E.; Mackenzie, J.S. Isolation of Hendra virus from pteropid bats: A natural reservoir of Hendra virus. J. Gen. Virol. 2000, 81 Pt 8, 1927–1932. [Google Scholar] [CrossRef]
- Pulliam, J.R.; Epstein, J.H.; Dushoff, J.; Rahman, S.A.; Bunning, M.; Jamaluddin, A.A.; Hyatt, A.D.; Field, H.E.; Dobson, A.P.; Daszak, P. Agricultural intensification, priming for persistence and the emergence of Nipah virus: A lethal bat-borne zoonosis. J. R. Soc. Interface 2012, 9, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.H.; Field, H.E.; Luby, S.; Pulliam, J.R.; Daszak, P. Nipah virus: Impact, origins, and causes of emergence. Curr. Infect. Dis. Rep. 2006, 8, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Wilson, M.E.; Luby, S.P.; Gurley, E.S.; Hossain, M.J. Transmission of Human Infection with Nipah Virus. Clin. Infect. Dis. 2009, 49, 1743–1748. [Google Scholar] [CrossRef]

| Cluster.ID | GhV | HeV | CedV | NiV | MenV | mock | VNT 2 |
|---|---|---|---|---|---|---|---|
| 16.1 1 | 11 | 7 | 7968 | −0.5 | 422.25 | 9.5 | + |
| 16.2 | 77.5 | 35 | 6997.5 | −2 | 5 | −9.5 | − |
| 16.3 | 85 | −0.25 | 1533 | −1 | 6 | −5.5 | − |
| 16.4 | 16.5 | 14.75 | 1051.25 | 13 | 15.5 | 10.25 | |
| 16.5 | 18.75 | 3.75 | 532.5 | 7.5 | 67.25 | 28 | |
| 17.1 | 2134.25 | 886.25 | 3720.75 | 155.25 | 1197.75 | 51 | |
| 4.1 3 | 12.5 | 2 | 13.25 | 24.75 | 9 | 1.75 | − |
| 4.2 | −0.5 | −7 | 0.5 | −4.5 | −23.25 | −21 | − |
| 4.3 | 10 | 6.75 | 10 | 7 | 37 | 9.75 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mittal, N.; Sterling, S.L.; Hip, P.; Low, D.H.W.; Ly, P.; Mao, M.; Ouch, P.; Paskey, A.C.; Yan, L.; Hitch, A.; et al. Serologic Evidence of Human Exposure to Bat-Borne Zoonotic Paramyxoviruses, Cambodia. Viruses 2025, 17, 1146. https://doi.org/10.3390/v17081146
Mittal N, Sterling SL, Hip P, Low DHW, Ly P, Mao M, Ouch P, Paskey AC, Yan L, Hitch A, et al. Serologic Evidence of Human Exposure to Bat-Borne Zoonotic Paramyxoviruses, Cambodia. Viruses. 2025; 17(8):1146. https://doi.org/10.3390/v17081146
Chicago/Turabian StyleMittal, Neil, Spencer L. Sterling, Phireak Hip, Dolyce H. W. Low, Piseth Ly, Menghou Mao, Pidor Ouch, Adrian C. Paskey, Lianying Yan, Alan Hitch, and et al. 2025. "Serologic Evidence of Human Exposure to Bat-Borne Zoonotic Paramyxoviruses, Cambodia" Viruses 17, no. 8: 1146. https://doi.org/10.3390/v17081146
APA StyleMittal, N., Sterling, S. L., Hip, P., Low, D. H. W., Ly, P., Mao, M., Ouch, P., Paskey, A. C., Yan, L., Hitch, A., Smith, G. J. D., Hertz, J., Letizia, A. G., Mendenhall, I. H., & Laing, E. D. (2025). Serologic Evidence of Human Exposure to Bat-Borne Zoonotic Paramyxoviruses, Cambodia. Viruses, 17(8), 1146. https://doi.org/10.3390/v17081146







