Novel Role of the Epstein-Barr Virus Encoded Deubiquitinating Enzyme (BPLF1) in mTOR-Mediated Cell Growth and Proliferation Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines, Growth, and Transfection
2.2. BPLF1 Full Length (FL) and BPLF1 FL C61S Construction
2.3. Construction of Untagged BPLF1 1-246 and BPLF1 1-246 C61S Plasmids
2.4. Mass Spectrometry Screen
2.5. In-Vitro DUB Assay
2.6. Western Blots
2.7. Immunoprecipitations
2.8. Infection and Flow Cytometry
2.9. Real-Time PCR
2.10. Lysosomal Fractionation
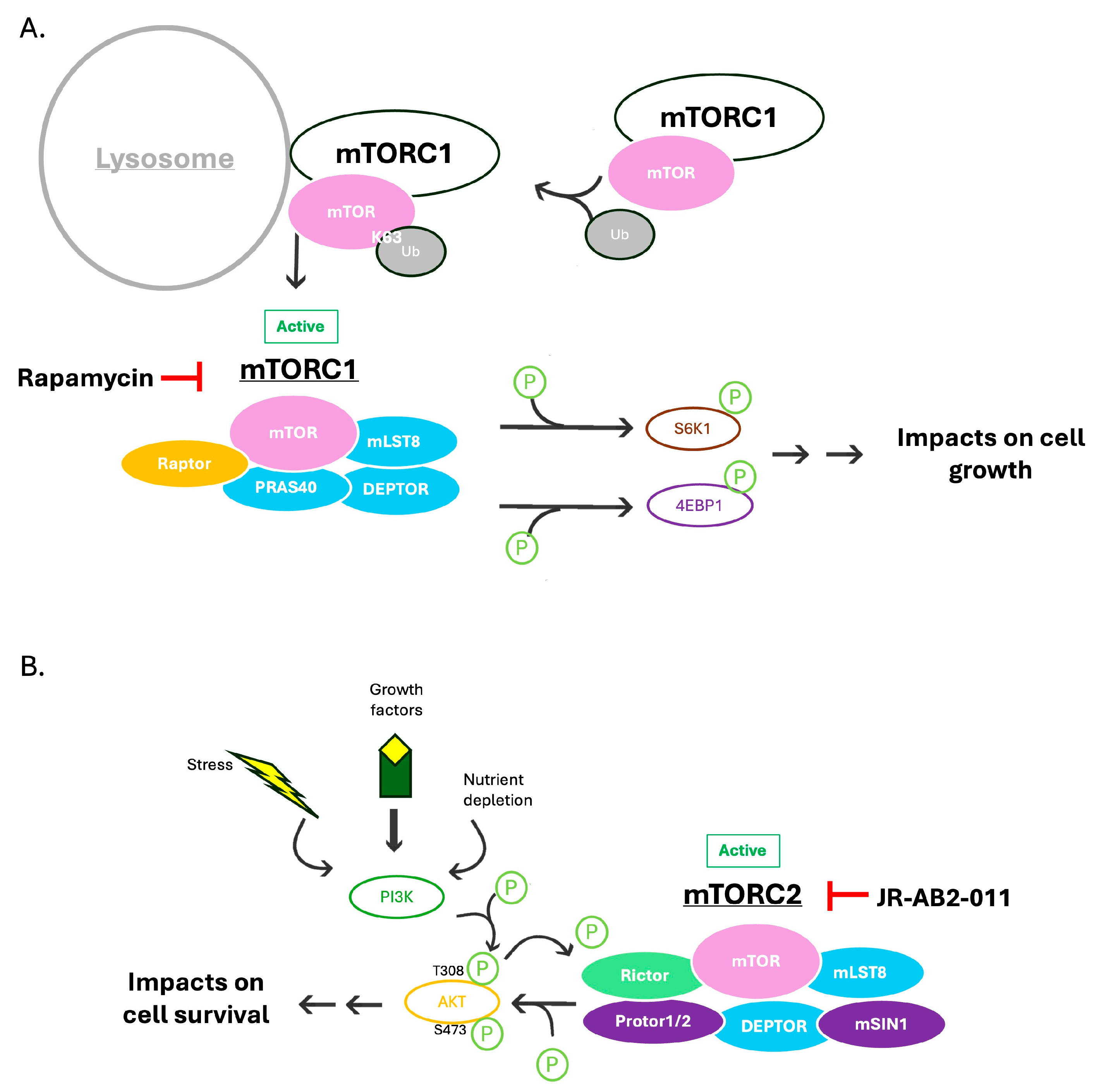
3. Results
3.1. BPLF1 Interactome
3.2. BPLF1 Deubiquitinates mTOR
3.3. BPLF1 Decreases Activation of mTORC1
3.4. BPLF1 Interacts with Raptor
3.5. BPLF1 Disrupts mTORC1 but Not mTORC2 Complex Formation
3.6. Inhibition of mTORC1 Increases Infectious Virus Production of EBV
3.7. mTORC2 Inhibition with JR-AB2-011 Decreases EBV Infectious Virus Production
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Epstein, M.A.; Achong, B.G.; Barr, Y.M. Virus Particles in Cultured Lymphoblasts from Burkitt’s Lymphoma. Lancet 1964, 1, 702–703. [Google Scholar] [CrossRef]
- Weiss, L.M.; Movahed, L.A.; Warnke, R.A.; Sklar, J. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin’s disease. N. Engl. J. Med. 1989, 320, 502–506. [Google Scholar] [CrossRef]
- Raab-Traub, N. Epstein-Barr virus and nasopharyngeal carcinoma. Semin. Cancer Biol. 1992, 3, 297–307. [Google Scholar]
- zur Hausen, H.; Schulte-Holthausen, H.; Klein, G.; Henle, W.; Henle, G.; Clifford, P.; Santesson, L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature 1970, 228, 1056–1058. [Google Scholar] [CrossRef]
- Langer-Gould, A.; Wu, J.; Lucas, R.; Smith, J.; Gonzales, E.; Amezcua, L.; Haraszti, S.; Chen, L.H.; Quach, H.; James, J.A.; et al. Epstein-Barr virus, cytomegalovirus, and multiple sclerosis susceptibility: A multiethnic study. Neurology 2017, 89, 1330–1337. [Google Scholar] [CrossRef]
- Laurence, M.; Benito-Leon, J. Epstein-Barr virus and multiple sclerosis: Updating Pender’s hypothesis. Mult. Scler. Relat. Disord. 2017, 16, 8–14. [Google Scholar] [CrossRef]
- Damania, B.; Kenney, S.C.; Raab-Traub, N. Epstein-Barr virus: Biology and clinical disease. Cell 2022, 185, 3652–3670. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, P.K.; Damania, B. The regulation of KSHV lytic reactivation by viral and cellular factors. Curr. Opin. Virol. 2022, 52, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Swatek, K.N.; Komander, D. Ubiquitin modifications. Cell Res. 2016, 26, 399–422. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Schulman, B.A. An expanded lexicon for the ubiquitin code. Nat. Rev. Mol. Cell Biol. 2023, 24, 273–287. [Google Scholar] [CrossRef]
- Reyes-Turcu, F.E.; Ventii, K.H.; Wilkinson, K.D. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu. Rev. Biochem. 2009, 78, 363–397. [Google Scholar] [CrossRef]
- Kattenhorn, L.M.; Korbel, G.A.; Kessler, B.M.; Spooner, E.; Ploegh, H.L. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol. Cell 2005, 19, 547–557. [Google Scholar] [CrossRef]
- Schlieker, C.; Korbel, G.A.; Kattenhorn, L.M.; Ploegh, H.L. A deubiquitinating activity is conserved in the large tegument protein of the herpesviridae. J. Virol. 2005, 79, 15582–15585. [Google Scholar] [CrossRef]
- Whitehurst, C.B.; Ning, S.; Bentz, G.L.; Dufour, F.; Gershburg, E.; Shackelford, J.; Langelier, Y.; Pagano, J.S. The Epstein-Barr virus (EBV) deubiquitinating enzyme BPLF1 reduces EBV ribonucleotide reductase activity. J. Virol. 2009, 83, 4345–4353. [Google Scholar] [CrossRef]
- Whitehurst, C.B.; Vaziri, C.; Shackelford, J.; Pagano, J.S. Epstein-Barr virus BPLF1 deubiquitinates PCNA and attenuates polymerase eta recruitment to DNA damage sites. J. Virol. 2012, 86, 8097–8106. [Google Scholar] [CrossRef]
- Dyson, O.F.; Pagano, J.S.; Whitehurst, C.B. The Translesion Polymerase Pol eta Is Required for Efficient Epstein-Barr Virus Infectivity and Is Regulated by the Viral Deubiquitinating Enzyme BPLF1. J. Virol. 2017, 91, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- van Gent, M.; Braem, S.G.; de Jong, A.; Delagic, N.; Peeters, J.G.; Boer, I.G.; Moynagh, P.N.; Kremmer, E.; Wiertz, E.J.; Ovaa, H.; et al. Epstein-Barr virus large tegument protein BPLF1 contributes to innate immune evasion through interference with toll-like receptor signaling. PLoS Pathog. 2014, 10, e1003960. [Google Scholar] [CrossRef] [PubMed]
- Yla-Anttila, P.; Gupta, S.; Masucci, M.G. The Epstein-Barr virus deubiquitinase BPLF1 targets SQSTM1/p62 to inhibit selective autophagy. Autophagy 2021, 17, 3461–3474. [Google Scholar] [CrossRef] [PubMed]
- Lui, W.Y.; Bharti, A.; Wong, N.M.; Jangra, S.; Botelho, M.G.; Yuen, K.S.; Jin, D.Y. Suppression of cGAS- and RIG-I-mediated innate immune signaling by Epstein-Barr virus deubiquitinase BPLF1. PLoS Pathog. 2023, 19, e1011186. [Google Scholar] [CrossRef]
- Gupta, S.; Yla-Anttila, P.; Callegari, S.; Tsai, M.H.; Delecluse, H.J.; Masucci, M.G. Herpesvirus deconjugases inhibit the IFN response by promoting TRIM25 autoubiquitination and functional inactivation of the RIG-I signalosome. PLoS Pathog. 2018, 14, e1006852. [Google Scholar] [CrossRef] [PubMed]
- Whitehurst, C.B.; Li, G.; Montgomery, S.A.; Montgomery, N.D.; Su, L.; Pagano, J.S. Knockout of Epstein-Barr virus BPLF1 retards B-cell transformation and lymphoma formation in humanized mice. mBio 2015, 6, e01574-01515. [Google Scholar] [CrossRef]
- Saito, S.; Murata, T.; Kanda, T.; Isomura, H.; Narita, Y.; Sugimoto, A.; Kawashima, D.; Tsurumi, T. Epstein-Barr virus deubiquitinase downregulates TRAF6-mediated NF-kappaB signaling during productive replication. J. Virol. 2013, 87, 4060–4070. [Google Scholar] [CrossRef]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Maruki, Y.; Long, X.; Yoshino, K.; Oshiro, N.; Hidayat, S.; Tokunaga, C.; Avruch, J.; Yonezawa, K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 2002, 110, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Sarbassov, D.D.; Ali, S.M.; King, J.E.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 2002, 110, 163–175. [Google Scholar] [CrossRef]
- Kim, D.H.; Sarbassov, D.D.; Ali, S.M.; Latek, R.R.; Guntur, K.V.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol. Cell 2003, 11, 895–904. [Google Scholar] [CrossRef]
- Nojima, H.; Tokunaga, C.; Eguchi, S.; Oshiro, N.; Hidayat, S.; Yoshino, K.; Hara, K.; Tanaka, N.; Avruch, J.; Yonezawa, K. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J. Biol. Chem. 2003, 278, 15461–15464. [Google Scholar] [CrossRef]
- Guertin, D.A.; Stevens, D.M.; Thoreen, C.C.; Burds, A.A.; Kalaany, N.Y.; Moffat, J.; Brown, M.; Fitzgerald, K.J.; Sabatini, D.M. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev. Cell 2006, 11, 859–871. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Peterson, T.R.; Laplante, M.; Thoreen, C.C.; Sancak, Y.; Kang, S.A.; Kuehl, W.M.; Gray, N.S.; Sabatini, D.M. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 2009, 137, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Thoreen, C.C.; Peterson, T.R.; Lindquist, R.A.; Kang, S.A.; Spooner, E.; Carr, S.A.; Sabatini, D.M. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol. Cell 2007, 25, 903–915. [Google Scholar] [CrossRef]
- Holz, M.K.; Ballif, B.A.; Gygi, S.P.; Blenis, J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 2005, 123, 569–580. [Google Scholar] [CrossRef]
- Gingras, A.C.; Gygi, S.P.; Raught, B.; Polakiewicz, R.D.; Abraham, R.T.; Hoekstra, M.F.; Aebersold, R.; Sonenberg, N. Regulation of 4E-BP1 phosphorylation: A novel two-step mechanism. Genes Dev. 1999, 13, 1422–1437. [Google Scholar] [CrossRef]
- Zhao, J.; Zhai, B.; Gygi, S.P.; Goldberg, A.L. mTOR inhibition activates overall protein degradation by the ubiquitin proteasome system as well as by autophagy. Proc. Natl. Acad. Sci. USA 2015, 112, 15790–15797. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef]
- Linares, J.F.; Duran, A.; Yajima, T.; Pasparakis, M.; Moscat, J.; Diaz-Meco, M.T. K63 polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Mol. Cell 2013, 51, 283–296. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Kim, D.H.; Guertin, D.A.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004, 14, 1296–1302. [Google Scholar] [CrossRef]
- Frias, M.A.; Thoreen, C.C.; Jaffe, J.D.; Schroder, W.; Sculley, T.; Carr, S.A.; Sabatini, D.M. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr. Biol. 2006, 16, 1865–1870. [Google Scholar] [CrossRef]
- Thedieck, K.; Polak, P.; Kim, M.L.; Molle, K.D.; Cohen, A.; Jeno, P.; Arrieumerlou, C.; Hall, M.N. PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS ONE 2007, 2, e1217. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef]
- Jacinto, E.; Loewith, R.; Schmidt, A.; Lin, S.; Ruegg, M.A.; Hall, A.; Hall, M.N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004, 6, 1122–1128. [Google Scholar] [CrossRef]
- Chen, J.; Hu, C.F.; Hou, J.H.; Shao, Q.; Yan, L.X.; Zhu, X.F.; Zeng, Y.X.; Shao, J.Y. Epstein-Barr virus encoded latent membrane protein 1 regulates mTOR signaling pathway genes which predict poor prognosis of nasopharyngeal carcinoma. J. Transl. Med. 2010, 8, 30. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, L.; Lin, W.; Yip, Y.L.; Lo, K.W.; Lau, V.M.Y.; Zhu, D.; Tsang, C.M.; Zhou, Y.; Deng, W.; et al. Epstein-Barr Virus-Encoded Latent Membrane Protein 1 Upregulates Glucose Transporter 1 Transcription via the mTORC1/NF-kappaB Signaling Pathways. J. Virol. 2017, 91, e02168-16. [Google Scholar] [CrossRef]
- Zhu, N.; Wang, Q.; Wu, Z.; Wang, Y.; Zeng, M.S.; Yuan, Y. Epstein-Barr Virus LMP1-Activated mTORC1 and mTORC2 Coordinately Promote Nasopharyngeal Cancer Stem Cell Properties. J. Virol. 2022, 96, e0194121. [Google Scholar] [CrossRef]
- Moody, C.A.; Scott, R.S.; Amirghahari, N.; Nathan, C.O.; Young, L.S.; Dawson, C.W.; Sixbey, J.W. Modulation of the cell growth regulator mTOR by Epstein-Barr virus-encoded LMP2A. J. Virol. 2005, 79, 5499–5506. [Google Scholar] [CrossRef]
- Xiang, T.; Lin, Y.X.; Ma, W.; Zhang, H.J.; Chen, K.M.; He, G.P.; Zhang, X.; Xu, M.; Feng, Q.S.; Chen, M.Y.; et al. Vasculogenic mimicry formation in EBV-associated epithelial malignancies. Nat. Commun. 2018, 9, 5009. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Adamson, A.L.; Le, B.T.; Siedenburg, B.D. Inhibition of mTORC1 inhibits lytic replication of Epstein-Barr virus in a cell-type specific manner. Virol. J. 2014, 11, 110. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, N.; Hu, J.; Wang, Y.; Xu, J.; Gu, Q.; Lieberman, P.M.; Yuan, Y. The mTOR inhibitor manassantin B reveals a crucial role of mTORC2 signaling in Epstein-Barr virus reactivation. J. Biol. Chem. 2020, 295, 7431–7441. [Google Scholar] [CrossRef]
- Furukawa, S.; Wei, L.; Krams, S.M.; Esquivel, C.O.; Martinez, O.M. PI3Kdelta inhibition augments the efficacy of rapamycin in suppressing proliferation of Epstein-Barr virus (EBV)+ B cell lymphomas. Am. J. Transplant. 2013, 13, 2035–2043. [Google Scholar] [CrossRef]
- Delecluse, H.J.; Hilsendegen, T.; Pich, D.; Zeidler, R.; Hammerschmidt, W. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc. Natl. Acad. Sci. USA 1998, 95, 8245–8250. [Google Scholar] [CrossRef]
- Ma, S.D.; Hegde, S.; Young, K.H.; Sullivan, R.; Rajesh, D.; Zhou, Y.; Jankowska-Gan, E.; Burlingham, W.J.; Sun, X.; Gulley, M.L.; et al. A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. J. Virol. 2011, 85, 165–177. [Google Scholar] [CrossRef]
- Pal, M.; Munoz-Hernandez, H.; Bjorklund, D.; Zhou, L.; Degliesposti, G.; Skehel, J.M.; Hesketh, E.L.; Thompson, R.F.; Pearl, L.H.; Llorca, O.; et al. Structure of the TELO2-TTI1-TTI2 complex and its function in TOR recruitment to the R2TP chaperone. Cell Rep. 2021, 36, 109317. [Google Scholar] [CrossRef]
- Zhang, L.X.; Yang, X.; Wu, Z.B.; Liao, Z.M.; Wang, D.G.; Chen, S.W.; Lu, F.; Wu, Y.B.; Zhu, S.Q. TTI1 promotes non-small-cell lung cancer progression by regulating the mTOR signaling pathway. Cancer Sci. 2023, 114, 855–869. [Google Scholar] [CrossRef]
- Gonzalez-Estevez, C.; Felix, D.A.; Smith, M.D.; Paps, J.; Morley, S.J.; James, V.; Sharp, T.V.; Aboobaker, A.A. SMG-1 and mTORC1 act antagonistically to regulate response to injury and growth in planarians. PLoS Genet. 2012, 8, e1002619. [Google Scholar] [CrossRef]
- Huang, J.; Manning, B.D. The TSC1-TSC2 complex: A molecular switchboard controlling cell growth. Biochem. J. 2008, 412, 179–190. [Google Scholar] [CrossRef]
- Josse, L.; Xie, J.; Proud, C.G.; Smales, C.M. mTORC1 signalling and eIF4E/4E-BP1 translation initiation factor stoichiometry influence recombinant protein productivity from GS-CHOK1 cells. Biochem. J. 2016, 473, 4651–4664. [Google Scholar] [CrossRef]
- Ross, F.A.; MacKintosh, C.; Hardie, D.G. AMP-activated protein kinase: A cellular energy sensor that comes in 12 flavours. FEBS J. 2016, 283, 2987–3001. [Google Scholar] [CrossRef]
- Fonseca, B.D.; Zakaria, C.; Jia, J.J.; Graber, T.E.; Svitkin, Y.; Tahmasebi, S.; Healy, D.; Hoang, H.D.; Jensen, J.M.; Diao, I.T.; et al. La-related Protein 1 (LARP1) Represses Terminal Oligopyrimidine (TOP) mRNA Translation Downstream of mTOR Complex 1 (mTORC1). J. Biol. Chem. 2015, 290, 15996–16020. [Google Scholar] [CrossRef]
- Wrobel, L.; Siddiqi, F.H.; Hill, S.M.; Son, S.M.; Karabiyik, C.; Kim, H.; Rubinsztein, D.C. mTORC2 Assembly Is Regulated by USP9X-Mediated Deubiquitination of RICTOR. Cell Rep. 2020, 33, 108564. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Chen, Y.T.; Schilling, B.; Gibson, B.W.; Hughes, R.E. Ubiquitin-specific peptidase 9, X-linked (USP9X) modulates activity of mammalian target of rapamycin (mTOR). J. Biol. Chem. 2012, 287, 21164–21175. [Google Scholar] [CrossRef]
- Bridges, C.R.; Tan, M.C.; Premarathne, S.; Nanayakkara, D.; Bellette, B.; Zencak, D.; Domingo, D.; Gecz, J.; Murtaza, M.; Jolly, L.A.; et al. USP9X deubiquitylating enzyme maintains RAPTOR protein levels, mTORC1 signalling and proliferation in neural progenitors. Sci. Rep. 2017, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Feldman, A.L.; Das, C.; Ziesmer, S.C.; Ansell, S.M.; Galardy, P.J. Ubiquitin hydrolase UCH-L1 destabilizes mTOR complex 1 by antagonizing DDB1-CUL4-mediated ubiquitination of raptor. Mol. Cell. Biol. 2013, 33, 1188–1197. [Google Scholar] [CrossRef]
- Ghosh, P.; Wu, M.; Zhang, H.; Sun, H. mTORC1 signaling requires proteasomal function and the involvement of CUL4-DDB1 ubiquitin E3 ligase. Cell Cycle 2008, 7, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.A.; Gaumann, A.; Koehl, G.E.; Seidel, U.; Bataille, F.; Klein, D.; Ellis, L.M.; Bolder, U.; Hofstaedter, F.; Schlitt, H.J.; et al. Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model. Int. J. Cancer 2007, 120, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Mund, R.; Whitehurst, C.B. Ubiquitin-Mediated Effects on Oncogenesis during EBV and KSHV Infection. Viruses 2024, 16, 1523. [Google Scholar] [CrossRef]
- Chouhan, S.; Kumar, A.; Piprode, V.; Dasgupta, A.; Singh, S.; Khalique, A. Regulatory-Associated Protein of mTOR-Mediated Signaling: A Nexus Between Tumorigenesis and Disease. Targets 2024, 2, 341–371. [Google Scholar] [CrossRef]
- Gastaldello, S.; Hildebrand, S.; Faridani, O.; Callegari, S.; Palmkvist, M.; Di Guglielmo, C.; Masucci, M.G. A deneddylase encoded by Epstein-Barr virus promotes viral DNA replication by regulating the activity of cullin-RING ligases. Nat. Cell Biol. 2010, 12, 351–361. [Google Scholar] [CrossRef]
- Jackson, S.; Xiong, Y. CRL4s: The CUL4-RING E3 ubiquitin ligases. Trends Biochem. Sci. 2009, 34, 562–570. [Google Scholar] [CrossRef]
- Wang, F.F.; Zhang, X.J.; Yan, Y.R.; Zhu, X.H.; Yu, J.; Ding, Y.; Hu, J.L.; Zhou, W.J.; Zeng, Z.C.; Liao, W.T.; et al. FBX8 is a metastasis suppressor downstream of miR-223 and targeting mTOR for degradation in colorectal carcinoma. Cancer Lett. 2017, 388, 85–95. [Google Scholar] [CrossRef]








| Protein (Interacting with BPLF1) | Protein Function in mTOR Pathway | No Virus Induction with FLAG-BPLF1 FL Expression (Log2(FC)) | No Virus Induction with FLAG-BPLF1_1-246 Expression (Log2(FC)) | Virus Induction with FLAG-BPLF1 FL Expression (Log2(FC)) | Virus Induction with FLAG-BPLF1_1-246 Expression (Log2(FC)) |
|---|---|---|---|---|---|
| mTOR | Protein kinase responsible for controlling cellular metabolism, catabolism, immune responses, autophagy, survival, proliferation, and migration, to maintain cellular homeostasis. | 2.17 | 4.56 | 2.33 | 4.69 |
| Raptor | Regulates mTORC1 formation and signaling. | 1.52 | 3.03 | NA | NA |
| TELO2 | Stabilize mTOR complexes and regulate mTOR signaling. | 1.60 | 4.33 | 2.28 | 4.63 |
| TELO2-IP 1 | Stabilize mTOR complexes and regulate mTOR signaling. | 1.22 | 5.60 | 1.90 | 4.03 |
| SMG1 | Functions related to the mTOR signaling pathway | −0.89 | 3.13 | NA | 3.36 |
| TSC2 | Regulation of the common mTOR pathway of protein synthesis, cell growth, and viability in response to cellular energy levels | 2.97 | 5.50 | 3.43 | 5.24 |
| EIF4G1 | Has mTOR signaling mediated tumorigenesis-promoting functions. | 0.93 | 3.65 | 1.41 | 3.35 |
| PRKAG1 | Subunit of the AMPK protein kinase, which is involved in regulating cell growth and energy metabolism. | 0.74 | 2.46 | −0.05 | 1.69 |
| LARP1 | RNA-binding protein that functions as a molecular switch for mTORC1-mediated translation of mRNAs. | 0.12 | −0.36 | 1.19 | 0.15 |
| USP9X | Negatively regulates mTOR activity. | 0.98 | 3.52 | 1.35 | 3.50 |
| BPLF1 | EBV encoded DUB enzyme | 13.32 | 8.76 | 12.83 | 7.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mund, R.; Atkins, S.L.; Cao, A.; Diallo, A.; Whitehurst, C.B. Novel Role of the Epstein-Barr Virus Encoded Deubiquitinating Enzyme (BPLF1) in mTOR-Mediated Cell Growth and Proliferation Pathways. Viruses 2025, 17, 1139. https://doi.org/10.3390/v17081139
Mund R, Atkins SL, Cao A, Diallo A, Whitehurst CB. Novel Role of the Epstein-Barr Virus Encoded Deubiquitinating Enzyme (BPLF1) in mTOR-Mediated Cell Growth and Proliferation Pathways. Viruses. 2025; 17(8):1139. https://doi.org/10.3390/v17081139
Chicago/Turabian StyleMund, Rachel, Sage L. Atkins, Anwen Cao, Aminatou Diallo, and Christopher B. Whitehurst. 2025. "Novel Role of the Epstein-Barr Virus Encoded Deubiquitinating Enzyme (BPLF1) in mTOR-Mediated Cell Growth and Proliferation Pathways" Viruses 17, no. 8: 1139. https://doi.org/10.3390/v17081139
APA StyleMund, R., Atkins, S. L., Cao, A., Diallo, A., & Whitehurst, C. B. (2025). Novel Role of the Epstein-Barr Virus Encoded Deubiquitinating Enzyme (BPLF1) in mTOR-Mediated Cell Growth and Proliferation Pathways. Viruses, 17(8), 1139. https://doi.org/10.3390/v17081139





