First Complete Genome Sequence of Palo Verde Broom Emaravirus, Virus-Derived siRNA Signatures, and Phytohormone-Metabolite Profiling of Witches’ Broom-Affected Palo Verde Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collections
2.2. Total RNA Isolation and Discovery Illumina Sequencing
2.3. Virus Genome Sequence Analyses
2.4. Emaravirus Contigs Confirmation by Reverse Transcription-Polymerase Chain Amplification
2.5. High-Performance Liquid Chromatography-Mass Spectrometry of Plant Sample Extracts
3. Results
3.1. Genome Sequencing of Blue Palo Verde Witches Broom Disease-Associated Emaravirus
3.2. RT-PCR Verification of Emaravirus Presence in Blue Palo Verde Tree Plant Tissue
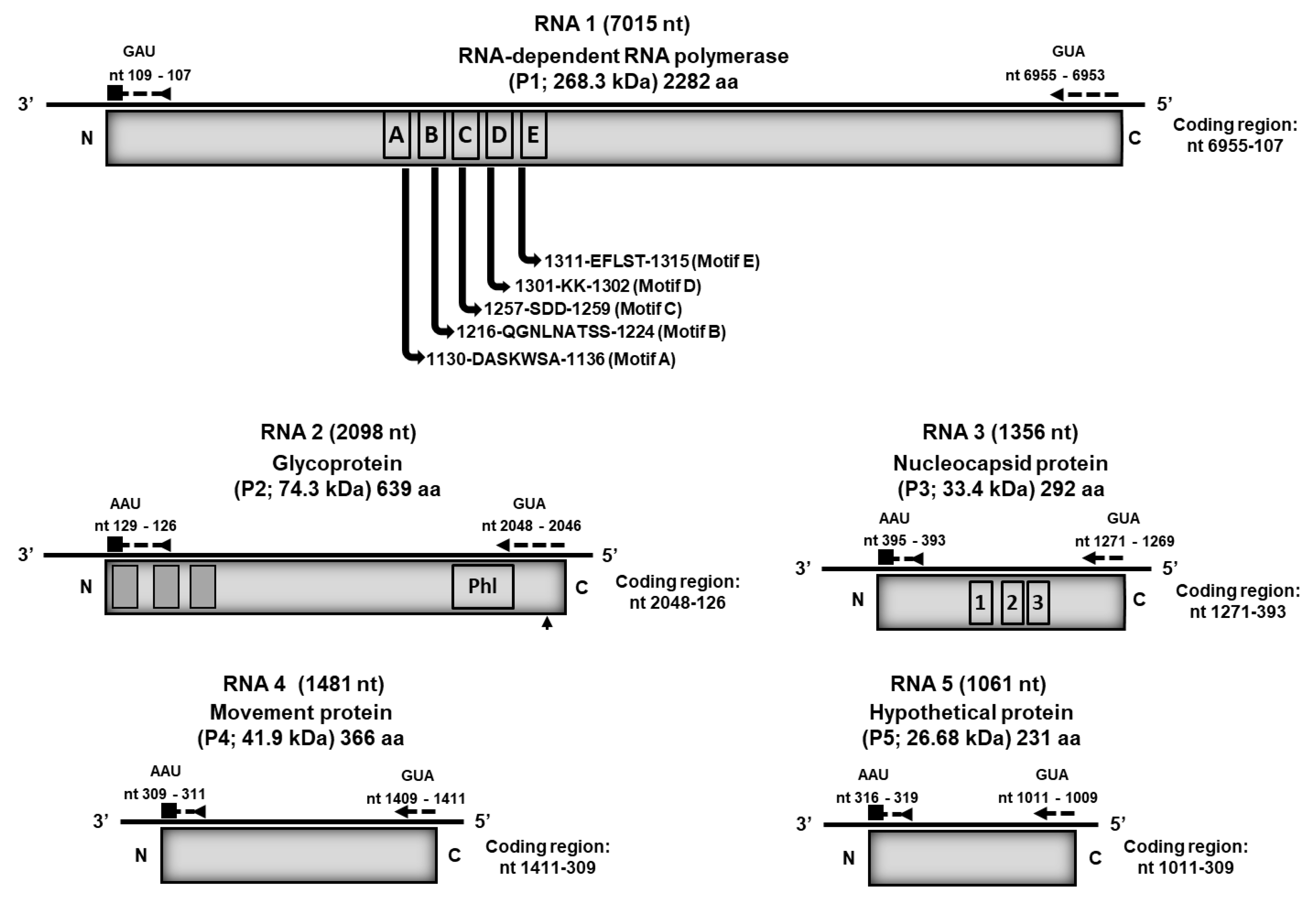
3.3. Genome Organization of Palo Verde Broom Virus and Sequence Analyses
3.4. Phylogenetic Relationships of Palo Verde Broom Virus with Known Emaravirus Species
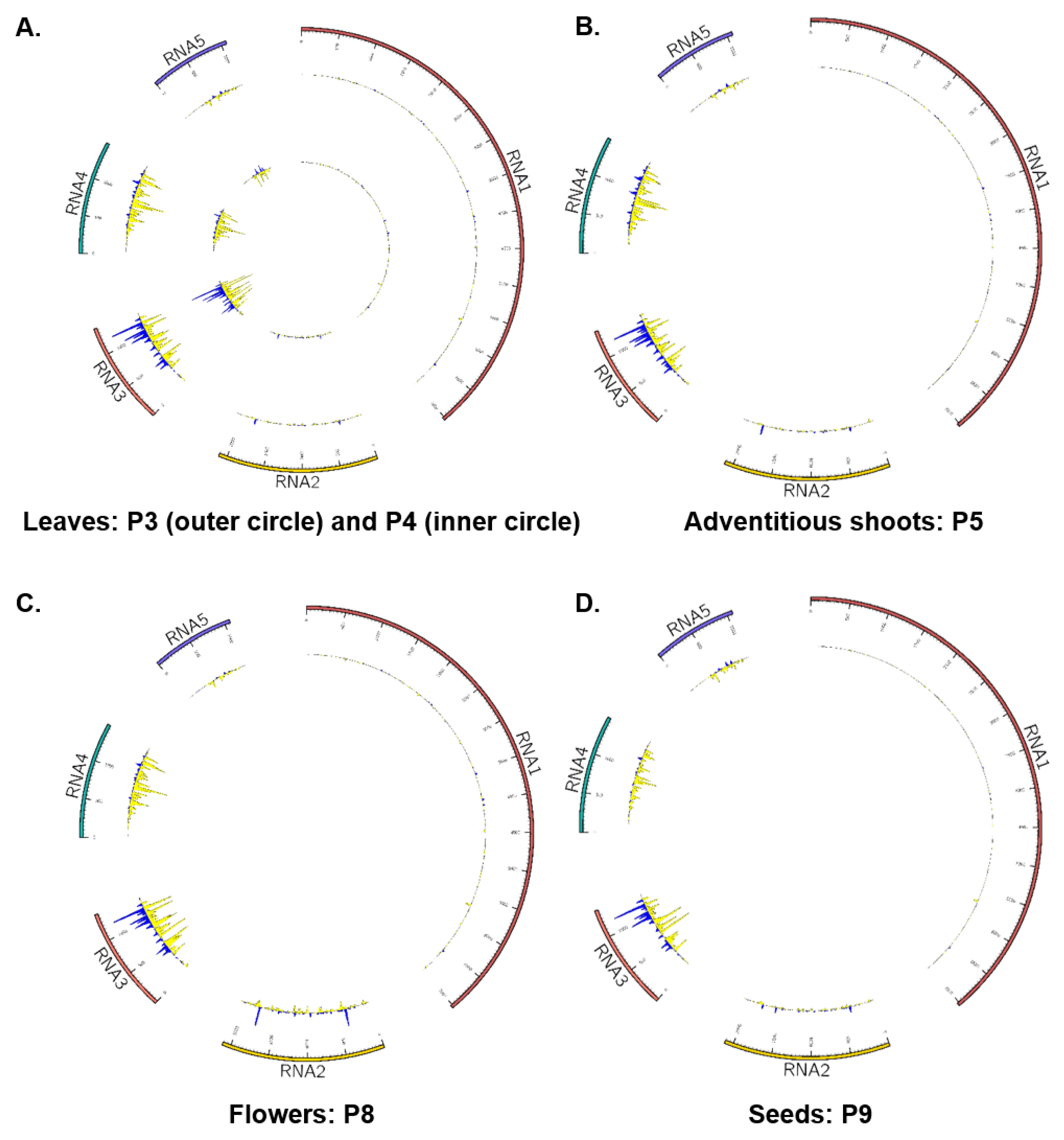
3.5. Palo Verde Broom Virus-Derived Small RNA Profiles in Blue Palo Verde Plant Tissues and/or Organs
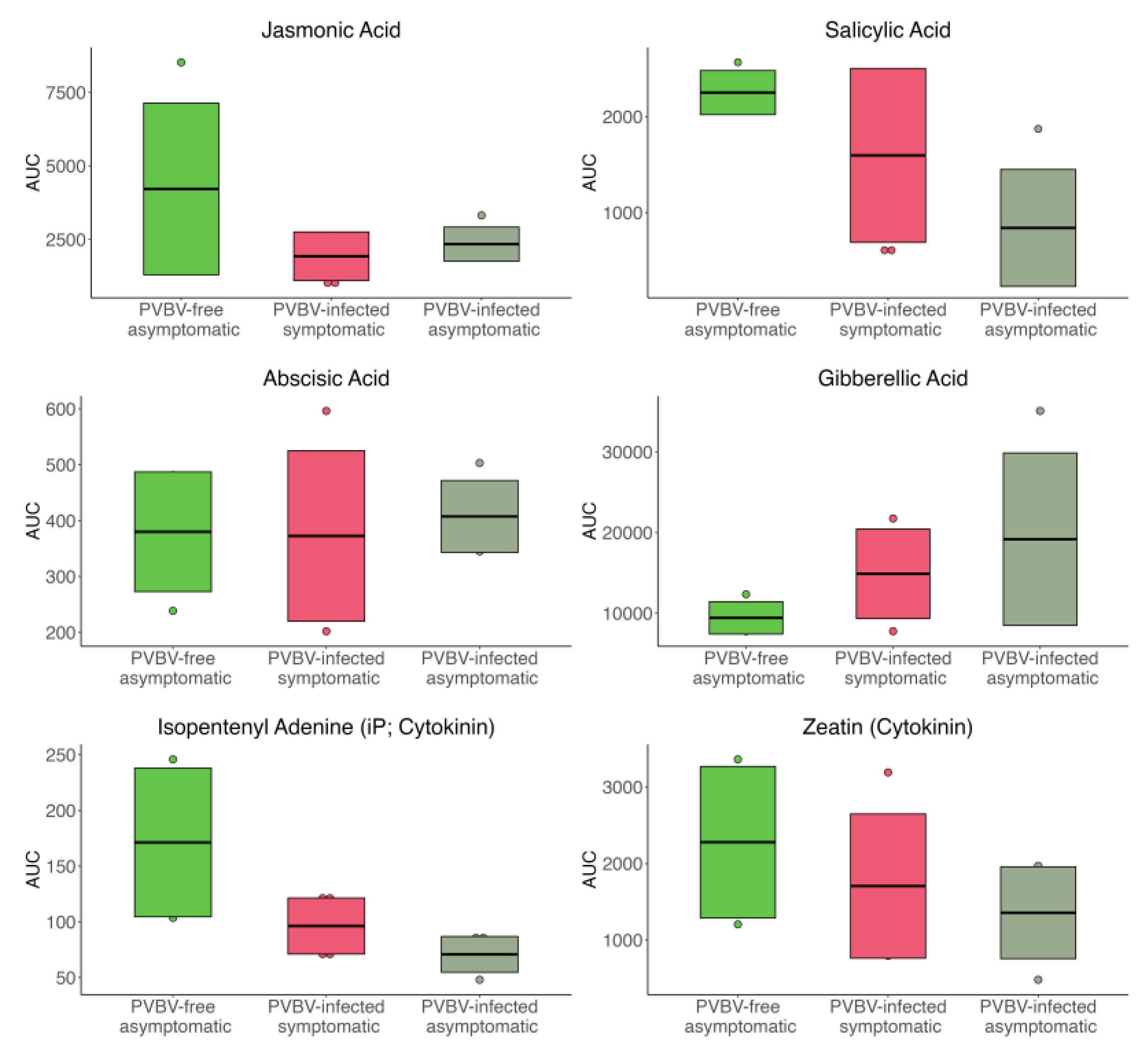
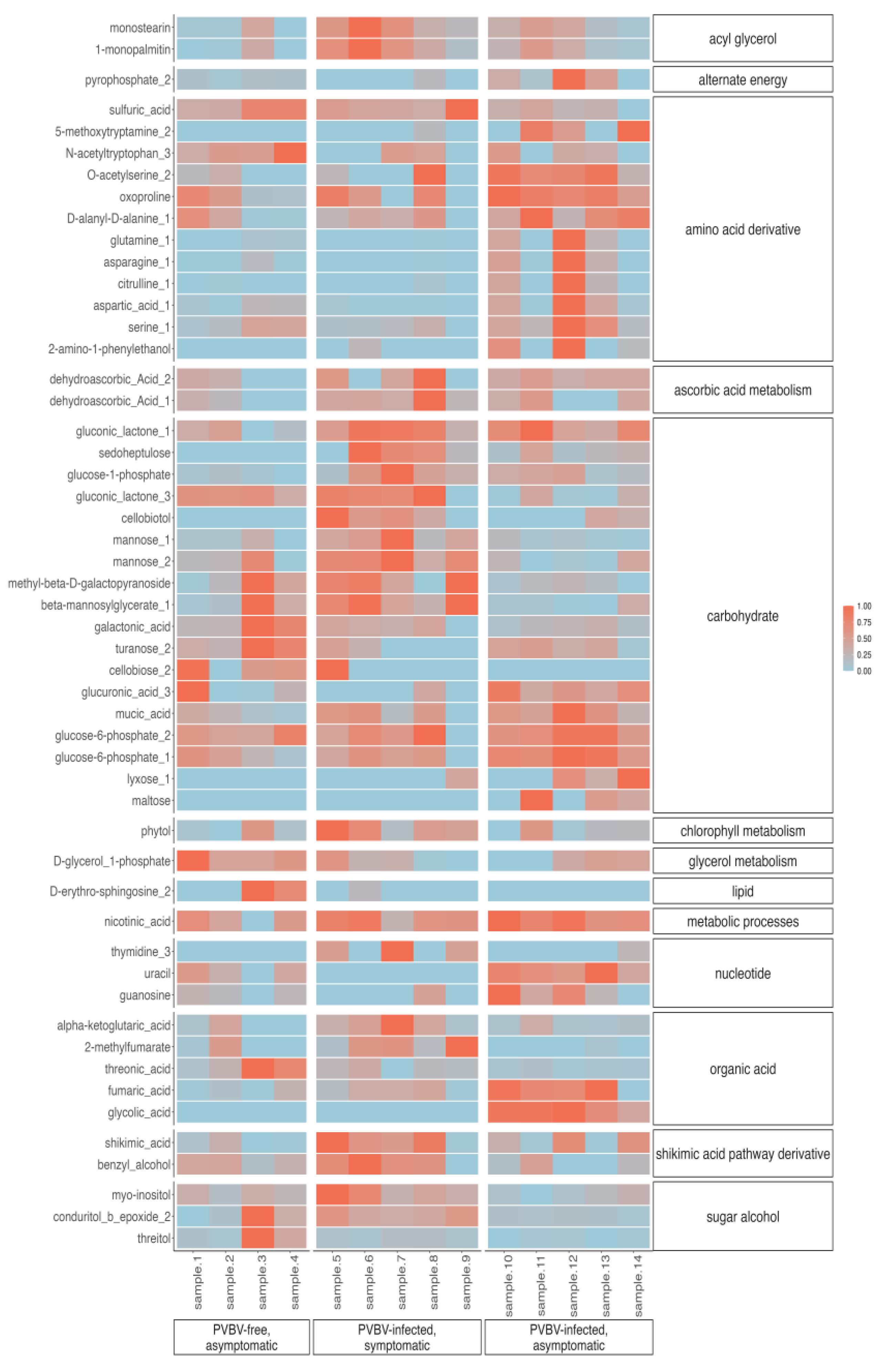
3.6. Variation in Phytohormones and Metabolites in Leaves of Asymptomatic, Apparantly Virus-Free Trees, Broom-Symptomatic Trees, and Adventitous Shoots from Broom-Symptomatic Trees
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowers, J.E.; Turner, R.M. Dieback and Episodic Mortality of Cercidium Microphyllum (Foothill Paloverde), a Dominant Sonoran Desert Tree. J. Torrey Bot. Soc. 2001, 128, 128–140. [Google Scholar] [CrossRef]
- Little, E.L. Cercídium. Checklist of United States Trees (Native and Naturalized); USDA, Forest Service: Washington, DC, USA, 1979. [Google Scholar]
- Schuch, U.K.; Kelly, J.J. Mesquite and Palo Verde Trees for the Urban Landscape. 2012; Volume AZ1429; pp. 1–10. Available online: https://extension.arizona.edu/sites/default/files/2024-08/az1429.pdf (accessed on 21 March 2025).
- Wiersema, J.H.; GRIN Taxonomy. US National Plant Germplasm System. 2019. Available online: https://doi.org/10.15468/ao14pp (accessed on 13 November 2021).
- Carter, A. The Genus Cercidium (Leguminosae: Caesalpinioideae) in the Sonoran Desert of Mexico and the United States. Proc. Calif. Acad. Sci. 1974, 40, 17–57. [Google Scholar]
- Shreve, F.; Wiggins, I.L. Vegetation and Flora of the Sonoran Desert; Stanford University Press: Redwood City, CA, USA, 1964; ISBN 978-0-8047-0163-1. [Google Scholar]
- The Arizona Native Plant Society (AZNPS) Arizona State Tree. 2021. Available online: https://aznps.com/the-plant-list/?species=parkinsonia+florida (accessed on 4 October 2021).
- Werner, F.G.; Olson, C.E. Learning About and Living with Insects of the Southwest: How to Identify Helpful, Harmful, and Venomous Insects; Grand Central Publishing: New York, NY, USA, 1994; ISBN 978-1-55561-060-9. [Google Scholar]
- Ilyas, M.; Avelar, S.; Schuch, U.K.; Brown, J.K. First Report of an Emaravirus Associated with Witches’ Broom Disease and Eriophyid Mite Infestations of the Blue Palo Verde Tree in Arizona. Plant Dis. 2018, 102, 1863. [Google Scholar] [CrossRef]
- Avelar, A.S.; Ilyas, M.; Schuch, U.; Brown, J.K. A Previously Undiscovered Emaravirus Associated with Witches Broom Symptoms in Blue Palo Verde (Parkinsonia florida) Trees in Arizona. In Proceedings of the International Congress of Plant Pathology, Boston, MA, USA, 29 July–3 August 2018. [Google Scholar]
- Elbeaino, T.; Digiaro, M.; Mielke-Ehret, N.; Muehlbach, H.-P.; Martelli, G.P. ICTV Report Consortium ICTV Virus Taxonomy Profile: Fimoviridae. J. General. Virol. 2018, 99, 1478–1479. [Google Scholar] [CrossRef]
- Mielke-Ehret, N.; Mühlbach, H.-P. Emaravirus: A Novel Genus of Multipartite, Negative Strand RNA Plant Viruses. Viruses 2012, 4, 1515–1536. [Google Scholar] [CrossRef]
- Kuhn, J.H.; Adkins, S.; Alkhovsky, S.V.; Avšič-Županc, T.; Ayllón, M.A.; Bahl, J.; Balkema-Buschmann, A.; Ballinger, M.J.; Bandte, M.; Beer, M.; et al. 2022 Taxonomic Update of Phylum Negarnaviricota (Riboviria: Orthornavirae), Including the Large Orders Bunyavirales and Mononegavirales. Arch. Virol. 2022, 167, 2857–2906. [Google Scholar] [CrossRef]
- Lu, Y.; McGavin, W.; Cock, P.J.A.; Schnettler, E.; Yan, F.; Chen, J.; MacFarlane, S. Newly Identified RNAs of Raspberry Leaf Blotch Virus Encoding a Related Group of Proteins. J. General. Virol. 2015, 96, 3432–3439. [Google Scholar] [CrossRef]
- Tatineni, S.; McMechan, A.J.; Wosula, E.N.; Wegulo, S.N.; Graybosch, R.A.; French, R.; Hein, G.L. An Eriophyid Mite-Transmitted Plant Virus Contains Eight Genomic RNA Segments with Unusual Heterogeneity in the Nucleocapsid Protein. J. Virol. 2014, 88, 11834–11845. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.N.; Elliott, R.M.; Dunn, E.F.; Wertz, G.W. Segment-Specific Terminal Sequences of Bunyamwera Bunyavirus Regulate Genome Replication. Virology 2003, 311, 326–338. [Google Scholar] [CrossRef]
- Kormelink, R.; Garcia, M.L.; Goodin, M.; Sasaya, T.; Haenni, A.-L. Negative-Strand RNA Viruses: The Plant-Infecting Counterparts. Virus Res. 2011, 162, 184–202. [Google Scholar] [CrossRef]
- Di Bello, P.L.; Laney, A.G.; Druciarek, T.; Ho, T.; Gergerich, R.C.; Keller, K.E.; Martin, R.R.; Tzanetakis, I.E. A Novel Emaravirus Is Associated with Redbud Yellow Ringspot Disease. Virus Res. 2016, 222, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, C.; Zhang, Z.; Ren, F.; Hu, G.; Shen, H.; Zhang, B.; Dong, Y. Identification and Characterization of a Novel Emaravirus From Grapevine Showing Chlorotic Mottling Symptoms. Front. Microbiol. 2021, 12, 694601. [Google Scholar] [CrossRef]
- Gaskin, T.R.; Tischendorf, M.; Günther, I.; Rehanek, M.; Büttner, C.; von Bargen, S. Characterization of a Novel Emaravirus Affecting Ash Species (Fraxinus spp.) in Europe. Forests 2021, 12, 1574. [Google Scholar] [CrossRef]
- Kubota, K.; Usugi, T.; Tomitaka, Y.; Shimomoto, Y.; Takeuchi, S.; Kadono, F.; Yanagisawa, H.; Chiaki, Y.; Tsuda, S. Perilla Mosaic Virus Is a Highly Divergent Emaravirus Transmitted by Shevtchenkella Sp. (Acari: Eriophyidae). Phytopathology 2020, 110, 1352–1361. [Google Scholar] [CrossRef]
- Rabbidge, L.O.; Blouin, A.G.; Chooi, K.M.; Higgins, C.M.; MacDiarmid, R.M. Characterisation and Distribution of Karaka Ōkahu Purepure Virus—A Novel Emaravirus Likely to Be Endemic to New Zealand. Viruses 2021, 13, 1611. [Google Scholar] [CrossRef]
- von Bargen, S.; Al Kubrusli, R.; Gaskin, T.; Fürl, S.; Hüttner, F.; Blystad, D.-R.; Karlin, D.G.; Jalkanen, R.; Büttner, C. Characterisation of a Novel Emaravirus Identified in Mosaic-Diseased Eurasian Aspen (Populus tremula). Ann. Appl. Biol. 2020, 176, 210–222. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Cao, M.; Cheng, Q.; Wu, J.; Hu, T. Identification of a Novel Emaravirus Infecting Lilac through Next-Generation Sequencing. J. Integr. Agric. 2020, 19, 2064–2071. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, L.; Ma, L.; Tian, X.; Li, R.; Zhou, C.; Cao, M. Virome of Camellia Japonica: Discovery of and Molecular Characterization of New Viruses of Different Taxa in Camellias. Front. Microbiol. 2020, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Navarro, B.; Wang, G.; Wang, Y.; Yang, Z.; Xu, W.; Zhu, C.; Wang, L.; Serio, F.D.; Hong, N. Actinidia Chlorotic Ringspot-Associated Virus: A Novel Emaravirus Infecting Kiwifruit Plants. Mol. Plant Pathol. 2017, 18, 569–581. [Google Scholar] [CrossRef]
- Jensen, S.G. A New Disease of Maize and Wheat in the High Plains. Plant Dis. 1996, 80, 1387–1390. [Google Scholar] [CrossRef]
- Preising, S.; Borges, D.F.; De Queiroz Ambrósio, M.M.; Da Silva, W.L. A Fig Deal: A Global Look at Fig Mosaic Disease and Its Putative Associates. Plant Dis. 2021, 105, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Laney, A.G.; Keller, K.E.; Martin, R.R.; Tzanetakis, I.E. A Discovery 70 Years in the Making: Characterization of the Rose Rosette Virus. J. General. Virol. 2011, 92, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Di Bello, P.L.; Keller, K.E.; Martin, R.R.; Sabanadzovic, S.; Tzanetakis, I.E. A New, Widespread Emaravirus Discovered in Blackberry. Virus Res. 2017, 235, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Coutts, B.A.; Cox, B.A.; Thomas, G.J.; Jones, R.A.C. First Report of Wheat Mosaic Virus Infecting Wheat in Western Australia. Plant Dis. 2014, 98, 285. [Google Scholar] [CrossRef]
- Chirkov, S.; Tsygankova, S.; Rastorguev, S.; Mitrofanova, I.; Chelombit, S.; Boulygina, E.; Slobodova, N.; Sharko, F. First Report of Fig Mosaic Virus on Fig in Russia. Plant Dis. 2021, 105, 2260. [Google Scholar] [CrossRef]
- Casteel, C.L.; Hansen, A.K. Evaluating Insect-Microbiomes at the Plant-Insect Interface. J. Chem. Ecol. 2014, 40, 836–847. [Google Scholar] [CrossRef]
- Walling, L.L. Chapter 13 Adaptive Defense Responses to Pathogens and Insects. Adv. Bot. Res. 2009, 51, 551–612. [Google Scholar]
- Ding, L.-N.; Li, Y.-T.; Wu, Y.-Z.; Li, T.; Geng, R.; Cao, J.; Zhang, W.; Tan, X.-L. Plant Disease Resistance-Related Signaling Pathways: Recent Progress and Future Prospects. Int. J. Mol. Sci. 2022, 23, 16200. [Google Scholar] [CrossRef]
- de Lillo, E.; Pozzebon, A.; Valenzano, D.; Duso, C. An Intimate Relationship Between Eriophyoid Mites and Their Host Plants—A Review. Front. Plant Sci. 2018, 9, 1786. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Dicke, M. Plant Interactions with Microbes and Insects: From Molecular Mechanisms to Ecology. Trends Plant Sci. 2007, 12, 564–569. [Google Scholar] [CrossRef]
- Adie, B.; Chico, J.M.; Rubio-Somoza, I.; Solano, R. Modulation of Plant Defenses by Ethylene. J. Plant Growth Regul. 2007, 26, 160–177. [Google Scholar] [CrossRef]
- Wu, X.; Ye, J. Manipulation of Jasmonate Signaling by Plant Viruses and Their Insect Vectors. Viruses 2020, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Islam, W.; Naveed, H.; Zaynab, M.; Huang, Z.; Chen, H.Y.H. Plant Defense against Virus Diseases; Growth Hormones in Highlights. Plant Signal. Behav. 2019, 14, 1596719. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.-W.; Ma, W. Phytohormone Pathways as Targets of Pathogens to Facilitate Infection. Plant Mol. Biol. 2016, 91, 713–725. [Google Scholar] [CrossRef]
- Mauck, K.E.; Chesnais, Q.; Shapiro, L.R. Chapter Seven—Evolutionary Determinants of Host and Vector Manipulation by Plant Viruses. Adv. Virus Res. 2018, 101, 189–250. [Google Scholar]
- Wu, D.; Qi, T.; Li, W.-X.; Tian, H.; Gao, H.; Wang, J.; Ge, J.; Yao, R.; Ren, C.; Wang, X.-B.; et al. Viral Effector Protein Manipulates Host Hormone Signaling to Attract Insect Vectors. Cell Res. 2017, 27, 402–415. [Google Scholar] [CrossRef]
- Sugio, A.; Kingdom, H.N.; MacLean, A.M.; Grieve, V.M.; Hogenhout, S.A. Phytoplasma Protein Effector SAP11 Enhances Insect Vector Reproduction by Manipulating Plant Development and Defense Hormone Biosynthesis. Proc. Natl. Acad. Sci. USA 2011, 108, E1254–E1263. [Google Scholar] [CrossRef]
- Bosque-Pérez, N.A.; Eigenbrode, S.D. The Influence of Virus-Induced Changes in Plants on Aphid Vectors: Insights from Luteovirus Pathosystems. Virus Res. 2011, 159, 201–205. [Google Scholar] [CrossRef]
- Shalileh, S.; Ogada, P.A.; Moualeu, D.P.; Poehling, H.-M. Manipulation of Frankliniella Occidentalis (Thysanoptera: Thripidae) by Tomato Spotted Wilt Virus (Tospovirus) Via the Host Plant Nutrients to Enhance Its Transmission and Spread. Environ. Entomol. 2016, 45, 1235–1242. [Google Scholar] [CrossRef]
- Baulcombe, D. RNA Silencing in Plants. Nature 2004, 431, 356–363. [Google Scholar] [CrossRef]
- Hamilton, A.J.; Baulcombe, D.C. A Species of Small Antisense RNA in Posttranscriptional Gene Silencing in Plants. Science 1999, 286, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Wang, Y.; Jin, Z.; Hong, Y.; Liu, Y. Transcriptional and Post-Transcriptional Regulation of RNAi-Related Gene Expression during Plant-Virus Interactions. Stress. Biol. 2022, 2, 33. [Google Scholar] [CrossRef]
- Bushnell, B. BBDuk. Available online: https://jgi.doe.gov/data-and-tools/software-tools/bbtools/bb-tools-user-guide/bbduk-guide/ (accessed on 28 July 2022).
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 28 July 2022).
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and Memory-Efficient Alignment of Short DNA Sequences to the Human Genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Watson, M.; Schnettler, E.; Kohl, A. viRome: An R Package for the Visualization and Analysis of Viral Small RNA Sequence Datasets. Bioinformatics 2013, 29, 1902–1903. [Google Scholar] [CrossRef]
- R Core Team. R A Language and Environment for Statistical Computing, R Foundation for Statistical Computing. Available online: https://cir.nii.ac.jp/crid/1370298755636824325 (accessed on 11 January 2025).
- Quinlan, A.R.; Hall, I.M. BEDTools: A Flexible Suite of Utilities for Comparing Genomic Features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, İ.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An Information Aesthetic for Comparative Genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Phanstiel, D.H.; Boyle, A.P.; Araya, C.L.; Snyder, M.P. Sushi.R: Flexible, Quantitative and Integrative Genomic Visualizations for Publication-Quality Multi-Panel Figures. Bioinformatics 2014, 30, 2808–2810. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2. WIREs Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Church, D.M.; Federhen, S.; Lash, A.E.; Madden, T.L.; Pontius, J.U.; Schuler, G.D.; Schriml, L.M.; Sequeira, E.; Tatusova, T.A.; et al. Database Resources of the National Center for Biotechnology. Nucleic Acids Res. 2003, 31, 28–33. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. ISBN 978-1-59259-890-8. [Google Scholar]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L.L. Predicting Transmembrane Protein Topology with a Hidden Markov Model: Application to Complete Genomes1. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Gupta, R.; Brunak, S. Prediction of Glycosylation across the Human Proteome and the Correlation to Protein Function. In Biocomputing 2002; World Scientific: Singapore, 2001; pp. 310–322. ISBN 978-981-02-4777-5. [Google Scholar]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 Improves Signal Peptide Predictions Using Deep Neural Networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Grishin, N.V. PROMALS3D: Multiple Protein Sequence Alignment Enhanced with Evolutionary and Three-Dimensional Structural Information. In Multiple Sequence Alignment Methods; Russell, D.J., Ed.; Humana Press: Totowa, NJ, USA, 2014; pp. 263–271. ISBN 978-1-62703-646-7. [Google Scholar]
- Pei, J.; Grishin, N.V. PROMALS: Towards Accurate Multiple Sequence Alignments of Distantly Related Proteins. Bioinformatics 2007, 23, 802–808. [Google Scholar] [CrossRef]
- Buchan, D.W.A.; Jones, D.T. The PSIPRED Protein Analysis Workbench: 20 Years On. Nucleic Acids Res. 2019, 47, W402–W407. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: A Virus Classification Tool Based on Pairwise Sequence Alignment and Identity Calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Di Bello, P.L.; Ho, T.; Tzanetakis, I.E. The Evolution of Emaraviruses Is Becoming More Complex: Seven Segments Identified in the Causal Agent of Rose Rosette Disease. Virus Res. 2015, 210, 241–244. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a Unified Platform for Metabolomics Data Processing, Analysis and Interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef] [PubMed]
- Buzkan, N.; Chiumenti, M.; Massart, S.; Sarpkaya, K.; Karadağ, S.; Minafra, A. A New Emaravirus Discovered in Pistacia from Turkey. Virus Res. 2019, 263, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, G.; Yang, Z.; Wang, Y.; Zhang, Z.; Li, L.; Waqas, M.; Hong, N.; Liu, H.; Wang, G.; et al. Identification and Characterization of a Pear Chlorotic Leaf Spot-Associated Virus, a Novel Emaravirus Associated with a Severe Disease of Pear Trees in China. Plant Dis. 2020, 104, 2786–2798. [Google Scholar] [CrossRef] [PubMed]
- Elbeaino, T.; Digiaro, M.; Uppala, M.; Sudini, H. Deep Sequencing of dsRNAs Recovered from Mosaic-Diseased Pigeonpea Reveals the Presence of a Novel Emaravirus: Pigeonpea Sterility Mosaic Virus 2. Arch. Virol. 2015, 160, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
- Elbeaino, T.; Digiaro, M.; Martelli, G.P. Complete Nucleotide Sequence of Four RNA Segments of Fig Mosaic Virus. Arch. Virol. 2009, 154, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Druffel, K.L.; Pappu, H.R. Structure and Genome Organization of the Large RNA of Iris Yellow Spot Virus (Genus Tospovirus, Family Bunyaviridae). Arch. Virol. 2010, 155, 275–279. [Google Scholar] [CrossRef]
- Duijsings, D.; Kormelink, R.; Goldbach, R. In Vivo Analysis of the TSWV Cap-snatching Mechanism: Single Base Complementarity and Primer Length Requirements. EMBO J. 2001, 20, 2545–2552. [Google Scholar] [CrossRef]
- Reguera, J.; Weber, F.; Cusack, S. Bunyaviridae RNA Polymerases (L-Protein) Have an N-Terminal, Influenza-Like Endonuclease Domain, Essential for Viral Cap-Dependent Transcription. PLOS Pathog. 2010, 6, e1001101. [Google Scholar] [CrossRef]
- Walia, J.J.; Falk, B.W. Fig Mosaic Virus mRNAs Show Generation by Cap-Snatching. Virology 2012, 426, 162–166. [Google Scholar] [CrossRef]
- Yu, C.; Karlin, D.G.; Lu, Y.; Wright, K.; Chen, J.; MacFarlane, S. Experimental and Bioinformatic Evidence That Raspberry Leaf Blotch Emaravirus P4 Is a Movement Protein of the 30K Superfamily. J. General. Virol. 2013, 94, 2117–2128. [Google Scholar] [CrossRef]
- Melcher, U. The ‘30K’ Superfamily of Viral Movement Proteins. J. General. Virol. 2000, 81, 257–266. [Google Scholar] [CrossRef]
- Rehanek, M.; von Bargen, S.; Bandte, M.; Karlin, D.G.; Büttner, C. A Novel Emaravirus Comprising Five RNA Segments Is Associated with Ringspot Disease in Oak. Arch. Virol. 2021, 166, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Hein, G.L.; Graybosch, R.A.; Tatineni, S. Octapartite Negative-Sense RNA Genome of High. Plains Wheat Mosaic Virus Encodes Two Suppressors of RNA Silencing. Virology 2018, 518, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Keifer, H.H. An Illustrated Guide to Plant Abnormalities Caused by Eriophyid Mites in North America; U.S. Department of Agriculture, Agricultural Research Service: MD, USA, 1982. [Google Scholar]
- Walia, J.J.; Willemsen, A.; Elci, E.; Caglayan, K.; Falk, B.W.; Rubio, L. Genetic Variation and Possible Mechanisms Driving the Evolution of Worldwide Fig Mosaic Virus Isolates. Phytopathology 2014, 104, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Elbeaino, T.; Digiaro, M.; Martelli, G.P. Rna-5 and -6, Two Additional Negative-Sense Rna Segments Associated with Fig Mosaic Virus. J. Plant Pathol. 2012, 94, 421–425. [Google Scholar]
- Rehanek, M.; Karlin, D.G.; Bandte, M.; Al Kubrusli, R.; Nourinejhad Zarghani, S.; Candresse, T.; Büttner, C.; von Bargen, S. The Complex World of Emaraviruses—Challenges, Insights, and Prospects. Forests 2022, 13, 1868. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, X.; Zhang, F.; Xu, M.; Ye, Z.; Wang, K.; Liu, S.; Han, X.; Cheng, Y.; Zhong, K.; et al. A Virus-Derived siRNA Activates Plant Immunity by Interfering with ROS Scavenging. Mol. Plant 2021, 14, 1088–1103. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, T.; Li, J.; Wu, N.; Wu, G.; Yang, J.; Chen, X.; He, L.; Chen, J. Chinese Wheat Mosaic Virus-Derived vsiRNA-20 Can Regulate Virus Infection in Wheat through Inhibition of Vacuolar- (H+)-PPase Induced Cell Death. New Phytol. 2020, 226, 205–220. [Google Scholar] [CrossRef]
- Pooggin, M.M. Small RNA-Omics for Plant Virus Identification, Virome Reconstruction, and Antiviral Defense Characterization. Front. Microbiol. 2018, 9, 2779. [Google Scholar] [CrossRef]
- Schuck, J.; Gursinsky, T.; Pantaleo, V.; Burgyán, J.; Behrens, S.-E. AGO/RISC-Mediated Antiviral RNA Silencing in a Plant in Vitro System. Nucleic Acids Res. 2013, 41, 5090–5103. [Google Scholar] [CrossRef] [PubMed]
- Mallory, A.; Vaucheret, H. Form, Function, and Regulation of ARGONAUTE Proteins. Plant Cell 2010, 22, 3879–3889. [Google Scholar] [CrossRef]
- Morel, J.B.; Godon, C.; Mourrain, P.; Beclin, C.; Boutet, S.; Feuerbach, F.; Proux, F.; Vaucheret, H. Fertile Hypomorphic ARGONAUTE (Ago1) Mutants Impaired in Post-Transcriptional Gene Silencing and Virus Resistance. Plant Cell 2002, 14, 629–639. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, S.; Navarro, B.; Gisel, A.; Peña, L.; Navarro, L.; Moreno, P.; Serio, F.D.; Flores, R. Citrus Tristeza Virus Infection Induces the Accumulation of Viral Small RNAs (21–24-Nt) Mapping Preferentially at the 3′-Terminal Region of the Genomic RNA and Affects the Host Small RNA Profile. Plant Mol. Biol. 2011, 75, 607–619. [Google Scholar] [CrossRef]
- Turco, S.; Golyaev, V.; Seguin, J.; Gilli, C.; Farinelli, L.; Boller, T.; Schumpp, O.; Pooggin, M.M. Small RNA-Omics for Virome Reconstruction and Antiviral Defense Characterization in Mixed Infections of Cultivated Solanum Plants. MPMI 2018, 31, 707–723. [Google Scholar] [CrossRef]
- Lisowiec-Wąchnicka, J.; Bartyś, N.; Pasternak, A. A Systematic Study on the Influence of Thermodynamic Asymmetry of 5′-Ends of siRNA Duplexes in Relation to Their Silencing Potency. Sci. Rep. 2019, 9, 2477. [Google Scholar] [CrossRef]
- Sharma, N.; Sahu, P.P.; Puranik, S.; Prasad, M. Recent Advances in Plant–Virus Interaction with Emphasis on Small Interfering RNAs (siRNAs). Mol. Biotechnol. 2013, 55, 63–77. [Google Scholar] [CrossRef] [PubMed]
- McGavin, W.J.; Mitchell, C.; Cock, P.J.A.; Wright, K.M.; MacFarlane, S.A. Raspberry Leaf Blotch Virus, a Putative New Member of the Genus Emaravirus, Encodes a Novel Genomic RNA. J. General. Virol. 2012, 93, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Rumbou, A.; Candresse, T.; von Bargen, S.; Büttner, C. Next-Generation Sequencing Reveals a Novel Emaravirus in Diseased Maple Trees From a German Urban Forest. Front. Microbiol. 2021, 11, 621179. [Google Scholar] [CrossRef]
- Adegbola, R.O.; Maheepala, D.C.; Schuch, U.K.; Brown, J.K. Prevalence, host range, and characterization of multiple palo verde broom emaravirus (PVBV) genomes and eriophyid mites from Parkinsonia spp. in Arizona. University of Arizona, Tucson, AZ. 202x, to be submitted.
- Guo, L.; Su, Q.; Yin, J.; Yang, Z.; Xie, W.; Wang, S.; Wu, Q.; Cui, H.; Zhang, Y. Amino Acid Utilization May Explain Why Bemisia Tabaci Q and B Differ in Their Performance on Plants Infected by the Tomato Yellow Leaf Curl Virus. Front. Physiol. 2019, 10, 489. [Google Scholar] [CrossRef]
- López-Gresa, M.P.; Lisón, P.; Yenush, L.; Conejero, V.; Rodrigo, I.; Bellés, J.M. Salicylic Acid Is Involved in the Basal Resistance of Tomato Plants to Citrus Exocortis Viroid and Tomato Spotted Wilt Virus. PLoS ONE 2016, 11, e0166938. [Google Scholar] [CrossRef]
- Mound, L.A. The Feeding Apparatus of Thrips. Bull. Entomol. Res. 1971, 60, 547–548. [Google Scholar] [CrossRef]
- Song, L.; Wang, Y.; Zhao, L.; Zhao, T. Transcriptome Profiling Unravels the Involvement of Phytohormones in Tomato Resistance to the Tomato Yellow Leaf Curl Virus (TYLCV). Horticulturae 2022, 8, 143. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, Y.; Zhao, L.; Chen, Y.; Zheng, L.; Zheng, K.; Mu, Y.; Zhao, X.; Gao, Y.; Zhang, J. Tripartite Interactions between Jasmonic/Salicylic Acid Pathways, Western Flower Thrips, and Thrips-Transmitted Tomato Zonate Spot Virus Infection in Capsicuum Annuum. Arthropod-Plant Interact. 2019, 13, 289–297. [Google Scholar] [CrossRef]
- Ziebell, H.; Murphy, A.M.; Groen, S.C.; Tungadi, T.; Westwood, J.H.; Lewsey, M.G.; Moulin, M.; Kleczkowski, A.; Smith, A.G.; Stevens, M.; et al. Cucumber Mosaic Virus and Its 2b RNA Silencing Suppressor Modify Plant-Aphid Interactions in Tobacco. Sci. Rep. 2011, 1, 187. [Google Scholar] [CrossRef] [PubMed]
- Lewsey, M.G.; Murphy, A.M.; Maclean, D.; Dalchau, N.; Westwood, J.H.; Macaulay, K.; Bennett, M.H.; Moulin, M.; Hanke, D.E.; Powell, G.; et al. Disruption of Two Defensive Signaling Pathways by a Viral RNA Silencing Suppressor. Mol. Plant Microbe Interact. 2010, 23, 835–845. [Google Scholar] [CrossRef]
- He, Y.; Zhang, H.; Sun, Z.; Li, J.; Hong, G.; Zhu, Q.; Zhou, X.; MacFarlane, S.; Yan, F.; Chen, J. Jasmonic Acid-Mediated Defense Suppresses Brassinosteroid-Mediated Susceptibility to Rice Black Streaked Dwarf Virus Infection in Rice. New Phytol. 2017, 214, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhang, H.; Yang, Z.; Wei, Z.; Li, Y.; Chen, J.; Sun, Z. NF-YA Transcription Factors Suppress Jasmonic Acid-Mediated Antiviral Defense and Facilitate Viral Infection in Rice. PLOS Pathog. 2022, 18, e1010548. [Google Scholar] [CrossRef] [PubMed]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silverleaf Whitefly Induces Salicylic Acid Defenses and Suppresses Effectual Jasmonic Acid Defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Yang, Z.; Wang, C.; Li, S.; Cao, C.; Yao, T.; Wei, Z.; Li, Y.; Chen, J.; et al. Independently Evolved Viral Effectors Convergently Suppress DELLA Protein SLR1-Mediated Broad-Spectrum Antiviral Immunity in Rice. Nat. Commun. 2022, 13, 6920. [Google Scholar] [CrossRef]
- Tao, T.; Zhou, C.-J.; Wang, Q.; Chen, X.-R.; Sun, Q.; Zhao, T.-Y.; Ye, J.-C.; Wang, Y.; Zhang, Z.-Y.; Zhang, Y.-L.; et al. Rice Black Streaked Dwarf Virus P7-2 Forms a SCF Complex through Binding to Oryza Sativa SKP1-like Proteins, and Interacts with GID2 Involved in the Gibberellin Pathway. PLoS ONE 2017, 12, e0177518. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, H.; Xu, Y.; Zhang, X.; Liang, X.; Xue, M. Phytohormone Content Variation Manipulated by Bemisia Tabaci Participated in Inhibiting Tobacco Growth: Gibberellin May Play a Crucial Role. Chil. J. Agric. Res. 2020, 80, 90–99. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Cui, K.; Chen, H.; Yang, Z.; Wu, H.; Shao, S.; King-Jones, K.; Chen, X. Gibberellic Acid Is Selectively Downregulated in Response to Aphid-Induced Gall Formation. Acta Physiol. Plant. 2016, 38, 214. [Google Scholar] [CrossRef]
- Clarke, S.F.; Mckenzie, M.J.; Burritt, D.J.; Guy, P.L.; Jameson, P.E. Influence of White Clover Mosaic Potexvirus Infection on the Endogenous Cytokinin Content of Bean. Plant Physiol. 1999, 120, 547–552. [Google Scholar] [CrossRef]
- Fleishon, S.; Shani, E.; Ori, N.; Weiss, D. Negative Reciprocal Interactions between Gibberellin and Cytokinin in Tomato. New Phytol. 2011, 190, 609–617. [Google Scholar] [CrossRef]
- Müllender, M.; Varrelmann, M.; Savenkov, E.I.; Liebe, S. Manipulation of Auxin Signalling by Plant Viruses. Mol. Plant Pathol. 2021, 22, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Verchot, J.; Herath, V.; Urrutia, C.D.; Gayral, M.; Lyle, K.; Shires, M.K.; Ong, K.; Byrne, D. Infectious DNA Clone Technology and Inoculation Strategy for Rose Rosette Virus That Includes All Seven Segments of the Negative-Strand RNA Genome. bioRxiv 2019. [Google Scholar] [CrossRef]
- Al-Subhi, A.M.; Al-Sadi, A.M.; Al-Yahyai, R.A.; Chen, Y.; Mathers, T.; Orlovskis, Z.; Moro, G.; Mugford, S.; Al-Hashmi, K.S.; Hogenhout, S.A. Witches’ Broom Disease of Lime Contributes to Phytoplasma Epidemics and Attracts Insect Vectors. Plant Dis. 2021, 105, 2637–2648. [Google Scholar] [CrossRef]
- Montano, H.G.; Davis, R.E.; Dally, E.L.; Hogenhout, S.; Pimentel, J.P.; Brioso, P.S. “Candidatus Phytoplasma Brasiliense”, a New Phytoplasma Taxon Associated with Hibiscus Witches’ Broom Disease. Int. J. Syst. Evol. Microbiol. 2001, 51, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.; Péron, T.; Lecerf, M.; Perez-Garcia, M.-D.; Barrière, Q.; Rolčík, J.; Boutet-Mercey, S.; Citerne, S.; Lemoine, R.; Porcheron, B.; et al. Sucrose Is an Early Modulator of the Key Hormonal Mechanisms Controlling Bud Outgrowth in Rosa Hybrida. J. Exp. Bot. 2015, 66, 2569–2582. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, C.A.; Rameau, C.; Wijerathna-Yapa, A. Lessons from a Century of Apical Dominance Research. J. Exp. Bot. 2023, 74, 3903–3922. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Inaba, J.; Zhao, Y.; Mowery, J.D.; Hammond, R. Phytoplasma Infection Blocks Starch Breakdown and Triggers Chloroplast Degradation, Leading to Premature Leaf Senescence, Sucrose Reallocation, and Spatiotemporal Redistribution of Phytohormones. Int. J. Mol. Sci. 2022, 23, 1810. [Google Scholar] [CrossRef] [PubMed]




| Isolate * | Total No. of Reads | No. of PVBV-Specific Reads (%) | No. of Reads Specific to Each RNA Segment (Percent Proportion of PVBV Specific Sequence Reads) | ||||
|---|---|---|---|---|---|---|---|
| RNA1 | RNA2 | RNA3 | RNA4 | RNA5 | |||
| P2 | 29,215,291 | 1431 (0.00%) | 135 (9%) | 146 (10%) | 700 (49%) | 355 (25%) | 95 (7%) |
| P3 | 79,809,277 | 585,276 (0.73%) | 88,963 (15%) | 62,888 (11%) | 259,148 (44%) | 140,214 (24%) | 34,063 (6%) |
| P4 | 50,523,789 | 1,286,718 (2.55%) | 84,487 (7%) | 56,810 (4%) | 737,004 (57%) | 321,399 (25%) | 87,018 (7%) |
| P5 | 54,202,178 | 16,677 (0.03%) | 1122 (7%) | 1112 (7%) | 9128 (55%) | 4061 (24%) | 1254 (4%) |
| P8 | 39,762,182 | 456,385 (1.15%) | 69,973 (15%) | 79,776 (17%) | 205,677 (45%) | 81,051 (18%) | 19,908 (4%) |
| P9 | 59,815,606 | 464,972 (0.78%) | 111,943 (24%) | 77,841 (17%) | 154,015 (33%) | 70,033 (15%) | 51,140 (11%) |
| Emaravirus * | Palo Verde Broom Virus Genome Segment and Coding Region ** | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RNA1 | RdRp | RNA2 | GP | RNA3 | NP | RNA4 | MP | RNA5 | HP | |
| HPWMoV | 62.5 | 50.8 | 60.0 | 40.6 | 64.3 | 35.2 | 63.7 | 51.7 | 64.5 | 27.2 |
| CORSaV | 60.6 | 43.8 | 59.2 | 32.6 | 59.3 | 32.8 | 58.6 | 45.7 | 57.2 | 26.9 |
| TiRSaV | 62.3 | 43.5 | 61.0 | 32.7 | 65.0 | 32.8 | 63.4 | 50.5 | 65.3 | 24.4 |
| JYMaV | 59.2 | 44.0 | 58.1 | 35.3 | 61.5 | 33.6 | 58.0 | 45.5 | 62.0 | 19.5 |
| RLBV | 61.4 | 43.1 | 59.9 | 34.0 | 62.1 | 28.5 | 62.9 | 44.4 | 64.1 | 29.3 |
| AcCRaV | 60.7 | 33.8 | 58.7 | 28.4 | 60.1 | 22.9 | 61.0 | 24.7 | 62.8 | 15.2 |
| AcEV-2 | 60.3 | 36.9 | 60.0 | 25.2 | 61.3 | 23.6 | 59.6 | 21.2 | 62.9 | 22.6 |
| AsMaV | 59.1 | 35.4 | 60.2 | 25.4 | 56.0 | 23.3 | 58.6 | 22.4 | 58.5 | 18.6 |
| BLMaV | 61.1 | 34.1 | 57.6 | 24.2 | 60.9 | 21.3 | 57.0 | 20.9 | 63.1 | 20.1 |
| ChMaV | 59.3 | 33.5 | 59.3 | 24.1 | 56.1 | 28.7 | 58.5 | 20.7 | 58.4 | 15.2 |
| CjaV-1 | 61.2 | 31.6 | 59.1 | 24.7 | 61.5 | 24.9 | 58.5 | 28.8 | 61.6 | 20.9 |
| CjaV-2 | 61.5 | 31.8 | 57.5 | 26.3 | 60.7 | 23.5 | 60.2 | 28.0 | - | - |
| EMARaV | 58.7 | 35.9 | 59.3 | 26.0 | 59.0 | 21.2 | 57.0 | 21.8 | - | - |
| FMV | 58.8 | 35.8 | 59.6 | 24.4 | 56.2 | 24.1 | 58.7 | 21.7 | 60.4 | 22.8 |
| KOPV | 60.1 | 33.8 | 61.3 | 21.4 | 61.9 | 24.4 | 59.0 | 26.2 | 63.1 | 19.5 |
| LiCRaV | 59.5 | 35.0 | 59.7 | 24.8 | 58.6 | 22.5 | 58.3 | 23.3 | 59.2 | 17.1 |
| MaMaV | 60.6 | 36.0 | 60.2 | 27.0 | 58.8 | 23.6 | 57.3 | 24.5 | 62.3 | 24.1 |
| PCLSaV | 60.3 | 32.7 | 59.1 | 22.8 | 59.0 | 24.4 | 58.8 | 24.4 | 57.4 | 15.5 |
| PerMV | 59.1 | 30.1 | 59.3 | 23.1 | 60.8 | 21.0 | 57.9 | 26.8 | 61.0 | 17.9 |
| PiVB | 60.4 | 35.7 | 58.9 | 25.1 | 59.0 | 24.3 | 56.8 | 22.6 | 60.1 | 27.8 |
| PPSMV-1 | 60.7 | 36.3 | 59.4 | 25.2 | 54.4 | 26.0 | 58.4 | 20.8 | 62.4 | 24.9 |
| PPSMV-2 | 59.0 | 35.8 | 60.1 | 24.5 | 56.1 | 21.8 | 57.5 | 22.5 | 63.0 | 27.6 |
| RRV | 61.0 | 35.8 | 59.6 | 24.6 | 63.8 | 23.3 | 60.7 | 23.0 | 62.6 | 22.8 |
| RYRSaV | 60.7 | 36.7 | 58.6 | 26.7 | 59.7 | 26.5 | 59.1 | 19.3 | 61.7 | 18.4 |
| VEV | 58.8 | 34.4 | 60.2 | 27.1 | 57.9 | 23.2 | 56.3 | 19.0 | 57.5 | 18.1 |
| Isolate * | Total No. Reads | No. PVBV-Specific Reads (cpm) * | No. of Reads Specific to Each PVBV RNA Genome Segment in Counts Per Million | ||||
|---|---|---|---|---|---|---|---|
| RNA1 | RNA2 | RNA3 | RNA4 | RNA5 | |||
| P2 | 29,624,358 | 382.00 (0.00%) | 24.98 (6%) | 25.49 (7%) | 268.26 (70%) | 17.27 (5%) | 46.01 (12.0%) |
| P3 | 18,673,896 | 197,065.36 (1.05%) | 12,163.66 (6%) | 9185.44 (5%) | 99,072.58 (50%) | 66,541.34 (34%) | 10,102.34 (5%) |
| P4 | 16,049,539 | 216,875.39 (1.35%) | 13,375.59 (6%) | 10,324.16 (5%) | 114,104.52 (53%) | 57,637.17 (26%) | 21,433.95 (10%) |
| P5 | 11,889,372 | 70,122.80 (0.59%) | 3349.97 (5%) | 4373.23 (6%) | 32,991.90 (47%) | 25,880.42 (37%) | 3527.27 (5%) |
| P8 | 9,194,688 | 110,337.29 (1.20%) | 7457.89 (7%) | 20,365.67 (18%) | 51,950.76 (47%) | 26,989.93 (25%) | 3573.04 (3%) |
| P9 | 13,380,230 | 77,302.41 (0.58%) | 3166.46 (4%) | 8220.34 (11%) | 41,403.25 (53%) | 17,826.82 (23%) | 6685.54 (9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adegbola, R.O.; Ilyas, M.; Maheepala, D.C.; Schuch, U.K.; Brown, J.K. First Complete Genome Sequence of Palo Verde Broom Emaravirus, Virus-Derived siRNA Signatures, and Phytohormone-Metabolite Profiling of Witches’ Broom-Affected Palo Verde Trees. Viruses 2025, 17, 1122. https://doi.org/10.3390/v17081122
Adegbola RO, Ilyas M, Maheepala DC, Schuch UK, Brown JK. First Complete Genome Sequence of Palo Verde Broom Emaravirus, Virus-Derived siRNA Signatures, and Phytohormone-Metabolite Profiling of Witches’ Broom-Affected Palo Verde Trees. Viruses. 2025; 17(8):1122. https://doi.org/10.3390/v17081122
Chicago/Turabian StyleAdegbola, Raphael O., Muhammad Ilyas, Dinusha C. Maheepala, Ursula K. Schuch, and Judith K. Brown. 2025. "First Complete Genome Sequence of Palo Verde Broom Emaravirus, Virus-Derived siRNA Signatures, and Phytohormone-Metabolite Profiling of Witches’ Broom-Affected Palo Verde Trees" Viruses 17, no. 8: 1122. https://doi.org/10.3390/v17081122
APA StyleAdegbola, R. O., Ilyas, M., Maheepala, D. C., Schuch, U. K., & Brown, J. K. (2025). First Complete Genome Sequence of Palo Verde Broom Emaravirus, Virus-Derived siRNA Signatures, and Phytohormone-Metabolite Profiling of Witches’ Broom-Affected Palo Verde Trees. Viruses, 17(8), 1122. https://doi.org/10.3390/v17081122






