Genetic and Antigenic Diversity of Bubaline alphaherpesvirus 1
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Genome Sequencing
2.2. Phylogenetic Analysis, Genomic Distance, and Recombination Detection
2.3. Preparation of Hyperimmune Sera
2.4. Cross-Neutralization Assay
3. Results
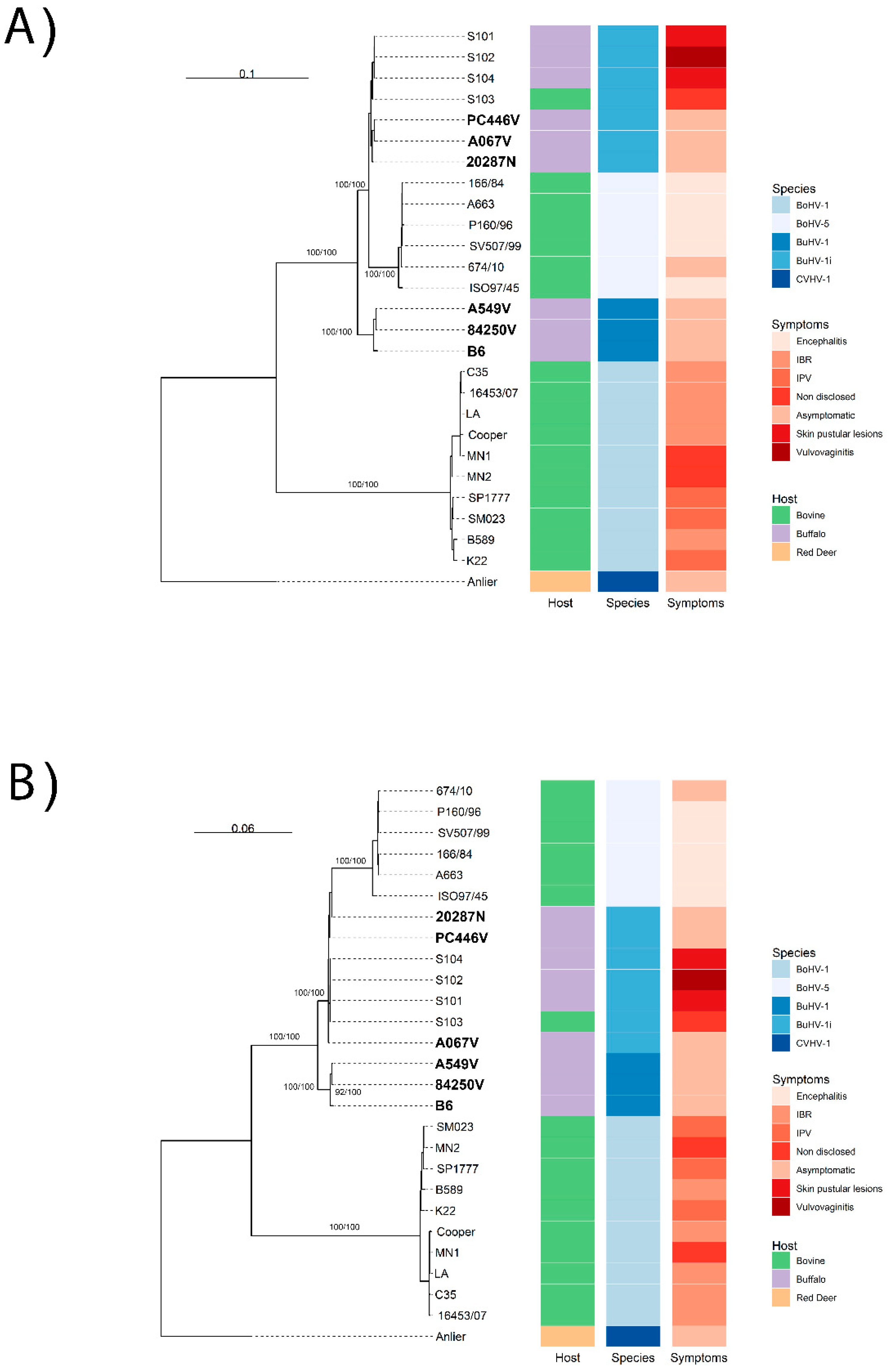
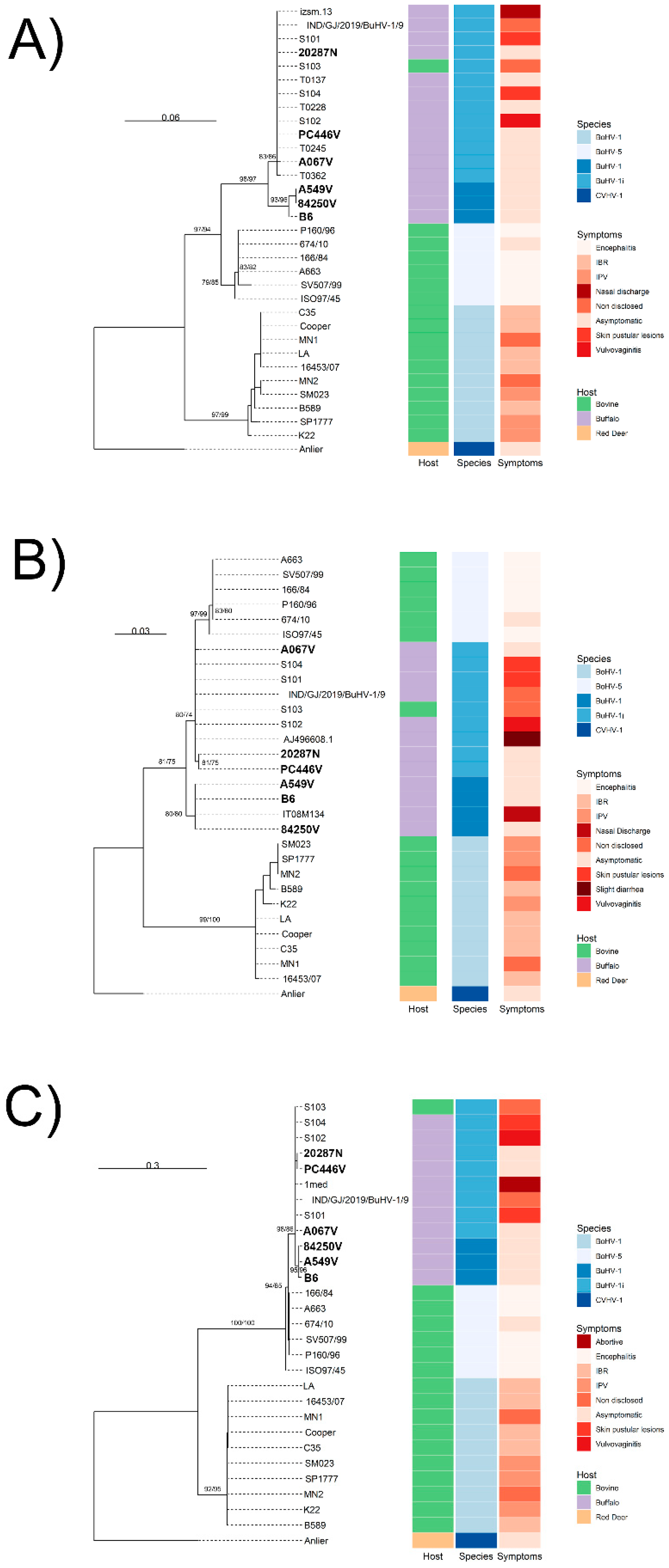
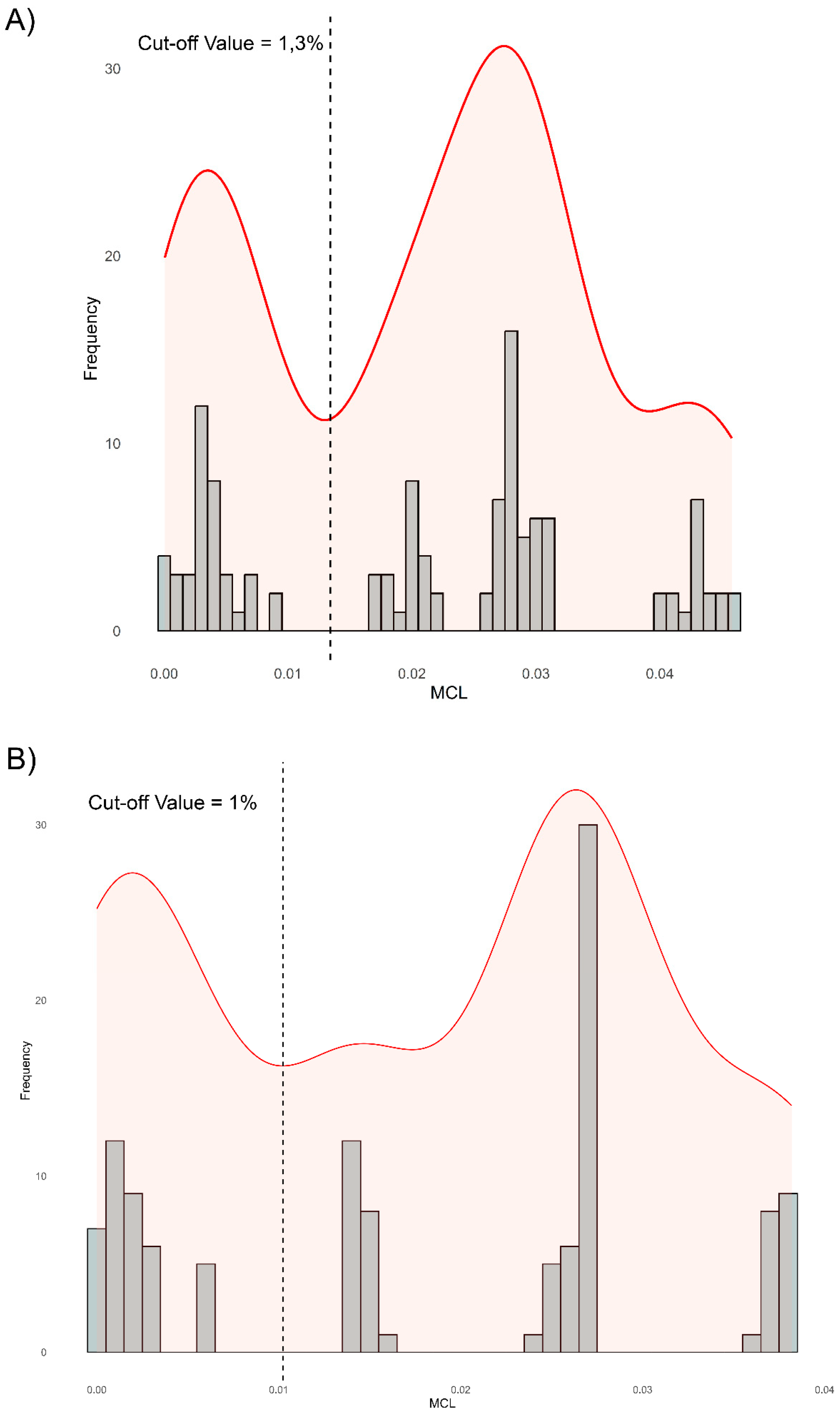
3.1. Genomic Diversity and Phylogenetic Analysis
3.2. Antigenic Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pineda, P.S.; Flores, E.B.; Villamor, L.P.; Parac, C.J.M.; Khatkar, M.S.; Thu, H.T.; Smith, T.P.L.; Rosen, B.D.; Ajmone-Marsan, P.; Colli, L.; et al. Disentangling river and swamp buffalo genetic diversity: Initial insights from the 1000 Buffalo Genomes Project. Gigascience 2024, 13, giae053. [Google Scholar] [CrossRef]
- Zhang, Y.; Colli, L.; Barker, J.S.F. Asian water buffalo: Domestication, history and genetics. Anim. Genet. 2020, 51, 177–187. [Google Scholar] [CrossRef]
- Pitt, S.J.; Gunn, A. The One Health Concept. Br. J. Biomed. Sci. 2024, 81, 12366. [Google Scholar] [CrossRef]
- Šudomová, M.; Hassan, S.T.S. Herpesvirus Diseases in Humans and Animals: Recent Developments, Challenges, and Charting Future Paths. Pathogens 2023, 12, 1422. [Google Scholar] [CrossRef]
- De Carlo, E.; Re, G.N.; Letteriello, R.; Del Vecchio, V.; Giordanelli, M.R.; Magnino, S.; Fabbi, M.; Bazzocchi, C.; Bandi, C.; Galiero, G.; et al. Molecular characterisation of a field strain of bubaline herpesvirus isolated from buffaloes (Bubalus bubalis) after pharmacological reactivation. Vet. Rec. 2004, 154, 171–174. [Google Scholar] [CrossRef]
- Scheffer, C.M.; Varela, A.P.M.; Cibulski, S.P.; Schmidt, C.; Campos, F.S.; Paim, W.P.; dos Santos, R.N.; Teixeira, T.F.; Loiko, M.R.; Tochetto, C.; et al. Genome sequence of Bubaline alphaherpesvirus 1 (BuHV1) isolated in Australia in 1972. Arch. Virol. 2017, 162, 1169–1176. [Google Scholar] [CrossRef]
- Amoroso, M.G.; Corrado, F.; De Carlo, E.; Lucibelli, M.G.; Martucciello, A.; Guarino, A.; Galiero, G. Bubaline herpesvirus 1 associated with abortion in a Mediterranean water buffalo. Res. Vet. Sci. 2013, 94, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Fiorito, F.; Miletti, G.; Serra, F.; Balestrieri, A.; Cioffi, B.; Cerracchio, C.; Galiero, G.; De Carlo, E.; Amoroso, M.G.; et al. Involvement of herpesviruses in cases of abortion among water buffaloes in southern Italy. Vet. Res. Commun. 2022, 46, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Preziuso, S.; Marenzoni, M.L.; Thiry, J.; Thiry, E.; Cuteri, V. Molecular characterization and virulence of an alphaherpesvirus isolated from a BoHV1 gB-seropositive and gE-seronegative Italian buffalo. Vet. Microbiol. 2018, 221, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Boora, A.; Thomas, P.; Kaliappan, A.; Verma, N.; Devi, P.; Dhaka, N.; Khurana, S.; Kumar, A.; Balhara, S.; et al. Genome sequence of bubaline herpesvirus-1 associated with pustular vulvovaginitis in Indian water buffalo. Microbiol. Resour. Announc. 2024, 13, e0088923. [Google Scholar] [CrossRef]
- Hedayat, N.; Haji Hajikolaei, M.R.; Seyfi Abad Shapouri, M.R.; Ghadrdan Mashhadi, A.R.; Izadnia, H.; Daghari, M. Isolation and identification of bubaline herpesvirus 1 (BuHV-1) from latently infected water buffalo (Bubalus bubalis) from Iran. Trop. Anim. Health Prod. 2020, 52, 217–226. [Google Scholar] [CrossRef]
- Maidana, S.S.; Konrad, J.L.; Craig, M.I.; Zabal, O.; Mauroy, A.; Thiry, E.; Crudeli, G.; Romera, S.A. First report of isolation and molecular characterization of bubaline herpesvirus 1 (BuHV1) from Argentinean water buffaloes. Arch. Virol. 2014, 159, 2917–2923. [Google Scholar] [CrossRef]
- Paredes-Galarza, B.; Oliveira, M.T.; Timm, F.B.; Stone, N.V.; Violet-Lozano, L.; Salvato, R.S.; Müller, N.D.; Prandi, B.A.; Gasparetto, R.; Gonçalves, M.; et al. Bovine alphaherpesvirus 1, bovine alphaherpesvirus 5, and Bubaline alphaherpesvirus 1 in palatine tonsils from water buffaloes in northern Brazil and possible links with the origin of bovine alphaherpesvirus type 5. Viruses 2024, 16, 1024. [Google Scholar] [CrossRef]
- Ferrara, G.; Iovane, V.; Moje, N.; Improda, E.; Iovane, G.; Pagnini, U.; Montagnaro, S. Cattle exposure to bubaline herpesvirus (BuHV-1) in Southern Italy: A hidden threat for IBR eradication? Prev. Vet. Med. 2024, 224, 106116. [Google Scholar] [CrossRef]
- Maidana, S.S.; Delgado, F.; Vagnoni, L.; Mauroy, A.; Thiry, E.; Romera, S. Cattle are a potential reservoir of bubaline herpesvirus 1 (BuHV1). Vet. Rec. Open 2016, 3, e000162. [Google Scholar] [CrossRef]
- Crudeli, G.A.; Patiño, J.; Maldonado, J.; Konrad, J. Los búfalos en Argentina. Rev. Vet. 2021, 32, 169–173. [Google Scholar] [CrossRef]
- Romera, S.A.; Perez, R.; Marandino, A.; Tau, L.; Campos, F.; Roehe, P.M.; Thiry, E.; Maidana, S.S. Whole-genome analysis of natural interspecific recombinant between bovine alphaherpesviruses 1 and 5. Virus Res. 2022, 309, 198656. [Google Scholar] [CrossRef]
- Paredes-Galarza, B.S.; Campos, F.S.; Oliveira, M.T.; Prandi, B.A.; de Souza, U.J.B.; Junqueira, D.M.; Martin, D.P.; Spilki, F.R.; Franco, A.C.; Roehe, P.M. Recombination between Bubaline alphaherpesvirus 1 and bovine alphaherpesvirus 1 as a possible origin of bovine alphaherpesvirus 5. Viruses 2025, 17, 198. [Google Scholar] [CrossRef] [PubMed]
- Maidana, S.S.; Destefano, G.; Romera, S.A.; Marin, M.; Combessies, G. Bovine herpesvirus 1 (BoHV-1): Update of circulating strains in Argentina. Rev. Vet. 2018, 29, 52–56. [Google Scholar] [CrossRef]
- Maidana, S.S.; Ladelfa, M.F.; Pérez, S.E.; Lomónaco, P.M.; Del Médico Zajac, M.P.; Odeón, A.; Blanco Viera, J.; Combessies, G.; Fondevila, N.; Palacios, M.; et al. Characterization of BoHV-5 field strains circulation and report of transient specific subtype of bovine herpesvirus 5 in Argentina. BMC Vet. Res. 2011, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Camero, M.; Larocca, V.; Losurdo, M.; Lorusso, E.; Patruno, G.; Staffa, V.N.; Martella, V.; Buonavoglia, C.; Tempesta, M. Goats are susceptible to Bubaline alphaherpesvirus 1 infection: Results of an experimental study. Comp. Immunol. Microbiol. Infect. Dis. 2017, 50, 97–100. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinform 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinform 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol 2015, 1, vev003. [Google Scholar] [CrossRef] [PubMed]
- Lole, K.; Bollinger, R.; Paranjape, R.; Gadkari, D.; Kulkarni, S.; Novak, N.; Ingersoll, R.; Sheppard, H.; Ray, S. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 1999, 73, 152–160. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Kolb, A.W.; Brandt, C.R. Genomic nucleotide-based distance analysis for delimiting Old World monkey-derived herpes simplex virus species. BMC Genom. 2020, 21, 436. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 36, 1188–1195. [Google Scholar] [CrossRef]
- Yu, G. Using ggtree to visualize data on tree-like structures. Curr. Protoc. Bioinform. 2020, 69, e96. [Google Scholar] [CrossRef] [PubMed]
- Romera, S.A.; Puntel, M.; Quattrocchi, V.; Del Médico Zajac, P.; Zamorano, P.; Blanco Viera, J.; Carrillo, C.; Chowdhury, S.; Borca, M.V.; Sadir, A.M. Protection induced by a glycoprotein E-deleted bovine herpesvirus type 1 marker strain used either as an inactivated or live attenuated vaccine in cattle. BMC Vet. Res. 2014, 10, 8. [Google Scholar] [CrossRef]
- Parreño, V.; Romera, S.A.; Makek, L.; Rodriguez, D.; Malacari, D.; Maidana, S.; Compaired, D.; Combessies, G.; Vena, M.M.; Garaicoechea, L.; et al. Validation of an indirect ELISA to detect antibodies against BoHV-1 in bovine and guinea-pig serum samples using ISO/IEC 17025 standards. J. Virol. Methods 2010, 169, 143–153. [Google Scholar] [CrossRef]
- Romera, S.A.; Hilgers, L.A.T.; Puntel, M.; Zamorano, P.I.; Alcon, V.L.; Santos, M.J.D.; Viera, J.B.; Borca, M.V.; Sadir, A.M. Adjuvant effects of sulfolipo-cyclodextrin in a squalane-in-water and water-in-mineral oil emulsions for BHV-1 vaccines in cattle. Vaccine 2000, 19, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Choi, K.S.; Lee, E.K.; Jeon, W.J.; Park, M.J.; Kim, J.W.; Kwon, J.H. Pathogenicity and antigenicity of a new variant of Korean nephropathogenic infectious bronchitis virus. J. Vet. Sci. 2009, 10, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Prato, R.; Ingravalle, F.; Vecchio, D.; Sciarra, A.; Ternavasio, M.; Ceccarelli, L.; Martucciello, A.; Galiero, G.; De Carlo, E.; et al. Prevalence of antibodies against bubaline herpesvirus (BuHV-1) among Mediterranean water buffalo (Bubalus bubalis) with implications in buffalo trade. Vet. Q. 2016, 36, 184–188. [Google Scholar] [CrossRef]
- Martucciello, A.; Balestrieri, A.; Righi, C.; Cappelli, G.; Scoccia, E.; Grassi, C.; Brandi, S.; Rossi, E.; Galiero, G.; Gioia, D.; et al. Evaluation of an immunization protocol using bovine alphaherpesvirus 1 gE-deleted marker vaccines against Bubaline alphaherpesvirus 1 in water buffaloes. Vaccines 2023, 11, 891. [Google Scholar] [CrossRef]
- Lecchi, C.; Ceciliani, F.; Petrini, S.; Cappelli, G.; Grassi, C.; Balestrieri, A.; Galiero, G.; De Carlo, E.; Salvi, G.; Panzeri, F.; et al. Endogenous and viral microRNAs in nasal secretions of water buffaloes (Bubalus bubalis) after Bubaline alphaherpesvirus 1 (BuHV-1) challenge infection. Vet. Res. 2023, 54, 44. [Google Scholar] [CrossRef]
- Montagnaro, S.; De Martinis, C.; Iovane, V.; Ciarcia, R.; Damiano, S.; Nizza, S.; De Martino, L.; Iovane, G.; Pagnini, U. Bovine herpesvirus type 1 marker vaccine induces cross-protection against bubaline herpesvirus type 1 in water buffalo. Prev. Vet. Med. 2014, 116, 56–62. [Google Scholar] [CrossRef]
- Petrini, S.; Martucciello, A.; Grandoni, F.; De Matteis, G.; Cappelli, G.; Giammarioli, M.; Scoccia, E.; Grassi, C.; Righi, C.; Fusco, G.; et al. Evaluation of safety and efficacy of an inactivated marker vaccine against bovine alphaherpesvirus 1 (BoHV-1) in water buffalo (Bubalus bubalis). Vaccines 2021, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Gatherer, D.; Depledge, D.P.; Hartley, C.A.; Szpara, M.L.; Vaz, P.K.; Benkő, M.; Brandt, C.R.; Bryant, N.A.; Dastjerdi, A.; Doszpoly, A.; et al. ICTV virus taxonomy profile: Herpesviridae 2021. J. Gen. Virol. 2021, 102, 001673. [Google Scholar] [CrossRef] [PubMed]
| Complete genomes MLC Distances | |||
|---|---|---|---|
| BoHV-5 | BuHV-1 | BuHV-1i | |
| BoHV-5 | 0.0428 | 0.0253 | |
| BuHV-1 | 0.0367 | 0.0223 | |
| BuHV-1i | 0.0262 | 0.0210 | |
| CDS MLC distances | |||
| Strain Comparison | R Value (%) | Antigenic Differences |
|---|---|---|
| BuHV-1 strains | 33–82 | Minor subtype differences |
| BuHV-1i vs. BoHV-5 | 70–99 | Minor differences |
| BuHV-1i vs. BoHV-1 | 25–26 | Major subtype differences |
| BuHV-1 vs. BoHV-1 | 39–66 | Minor subtype differences |
| A067V vs. BoHV-5/1 | 0 | Distinct serotype |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tau, R.L.; Marandino, A.E.; Torales, F.; Campos, F.S.; Roehe, P.M.; Konrad, J.L.; Romera, S.A.; Pérez, R.; Maidana, S.S. Genetic and Antigenic Diversity of Bubaline alphaherpesvirus 1. Viruses 2025, 17, 1110. https://doi.org/10.3390/v17081110
Tau RL, Marandino AE, Torales F, Campos FS, Roehe PM, Konrad JL, Romera SA, Pérez R, Maidana SS. Genetic and Antigenic Diversity of Bubaline alphaherpesvirus 1. Viruses. 2025; 17(8):1110. https://doi.org/10.3390/v17081110
Chicago/Turabian StyleTau, Rocío Lucía, Ana Eugenia Marandino, Fátima Torales, Fabrício Souza Campos, Paulo Michel Roehe, José Luis Konrad, Sonia Alejandra Romera, Ruben Pérez, and Silvina Soledad Maidana. 2025. "Genetic and Antigenic Diversity of Bubaline alphaherpesvirus 1" Viruses 17, no. 8: 1110. https://doi.org/10.3390/v17081110
APA StyleTau, R. L., Marandino, A. E., Torales, F., Campos, F. S., Roehe, P. M., Konrad, J. L., Romera, S. A., Pérez, R., & Maidana, S. S. (2025). Genetic and Antigenic Diversity of Bubaline alphaherpesvirus 1. Viruses, 17(8), 1110. https://doi.org/10.3390/v17081110







