Unveiling the Genomic Landscape of G2P[6] Rotavirus a Strains in Brazil: Evolutionary and Epidemiological Perspectives
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Sample Selection
2.2. Electropherotyping Analysis
2.3. Whole-Genome Constellation Profiling
2.4. Phylogenetic Inference
3. Results
3.1. Genome Constellations
3.2. Analysis of the Outer Capsid Glycoprotein VP7
3.3. Analysis of the Spike Protein VP4
3.4. Phylogenetic Analysis of VP1–VP3 and VP6
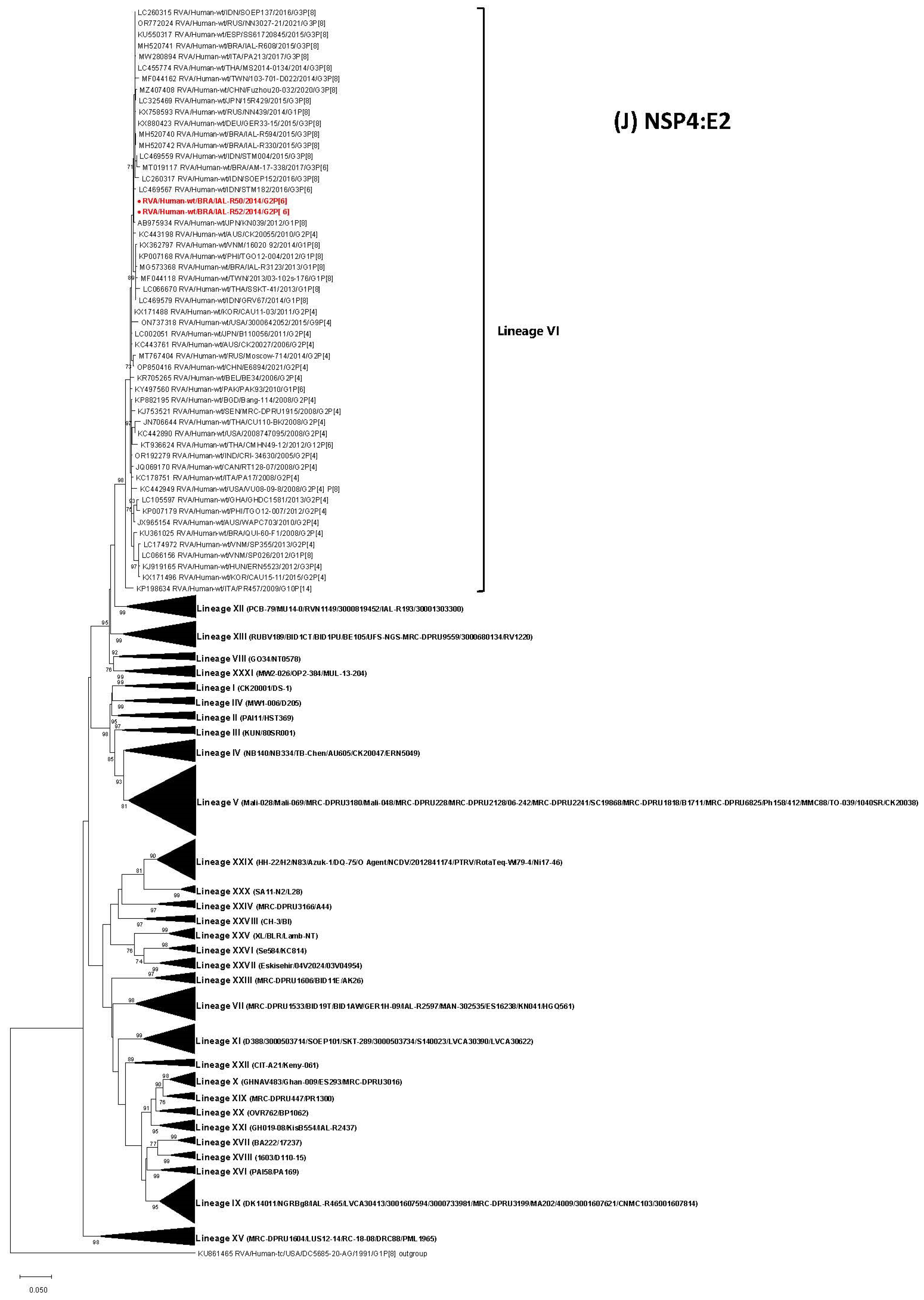
3.5. Phylogenetic Analysis of NSP1-NSP5 Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Šenica, P.; Žele Vengušt, D.; Vengušt, G.; Kuhar, U. Genomic revelations: Investigating rotavirus a presence in wild ruminants and its zoonotic potential. Front. Vet. Sci. 2024, 11, 1429654. [Google Scholar] [CrossRef] [PubMed]
- Prunas, O.; Asare, E.O.; Sajewski, E.; Li, Y.; Pithawala, Z.; Weinberger, D.M.; Warren, J.L.; Armah, G.E.; Cunliffe, N.A.; Iturriza-Gómara, M.; et al. Global estimates of rotavirus vaccine efficacy and effectiveness: A rapid review and meta-regression analysis. EClinicalMedicine 2025, 81, 103122. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Attoui, H.; Bányai, K.; Brussaard, C.P.D.; Danthi, P.; Del Vas, M.; Dermody, T.S.; Duncan, R.; Fang, Q.; Johne, R.; et al. ICTV Virus Taxonomy Profile: Sedoreoviridae 2022. J. Gen. Virol. 2022, 103, e001782. [Google Scholar] [CrossRef] [PubMed]
- Esona, M.D.; Steele, D.; Kerin, T.; Armah, G.; Peenze, I.; Geyer, A.; Page, N.; Nyangao, J.; Agbaya, V.A.; Trabelsi, A.; et al. Determination of the G and P types of previously nontypeable rotavirus strains from the African Rotavirus Network, 1996–2004: Identification of unusual G types. J. Infect. Dis. 2010, 202 (Suppl. S1), S49–S54. [Google Scholar] [CrossRef]
- Amin, A.B.; Cates, J.E.; Liu, Z.; Wu, J.; Ali, I.; Rodriguez, A.; Panjwani, J.; Tate, J.E.; Lopman, B.A.; Parashar, U.D. Rotavirus Genotypes in the Postvaccine Era: A Systematic Review and Meta-analysis of Global, Regional, and Temporal Trends by Rotavirus Vaccine Introduction. J. Infect. Dis. 2024, 229, 1460–1469. [Google Scholar] [CrossRef]
- Luchs, A.; Cilli, A.; Morillo, S.G.; Carmona, R.d.C.; Timenetsky, M.d.C.S.T. Rotavirus genotypes circulating in Brazil, 2007–2012: Implications for the vaccine program. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 305–313. [Google Scholar] [CrossRef]
- Díaz Alarcón, R.G.; Salvatierra, K.; Gómez Quintero, E.; Liotta, D.J.; Parreño, V.; Miño, S.O. Complete Genome Classification System of Rotavirus alphagastroenteritidis: An Updated Analysis. Viruses 2025, 17, 211. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Ciarlet, M.; McDonald, S.M.; Attoui, H.; Bányai, K.; Brister, J.R.; Buesa, J.; Esona, M.D.; Estes, M.K.; Gentsch, J.R.; et al. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch. Virol. 2011, 156, 1397–1413. [Google Scholar] [CrossRef]
- Hoxie, I.; Dennehy, J.J. Intragenic recombination influences rotavirus diversity and evolution. Virus Evol. 2020, 6, vez059. [Google Scholar] [CrossRef]
- Santos, N.; Soares, C.C.; Volotão, E.M.; Albuquerque, M.C.M.; Hoshino, Y. Surveillance of rotavirus strains in Rio de Janeiro, Brazil, from 1997 to 1999. J. Clin. Microbiol. 2003, 41, 3399–3402. [Google Scholar] [CrossRef]
- Leite, J.P.G.; Carvalho-Costa, F.A.; Linhares, A.C. Group A rotavirus genotypes and the ongoing Brazilian experience: A review. Mem. Inst. Oswaldo Cruz 2008, 103, 745–753. [Google Scholar] [CrossRef] [PubMed]
- da Silva Soares, L.; de Fátima Dos Santos Guerra, S.; do Socorro Lima de Oliveira, A.; da Silva Dos Santos, F.; de Fátima Costa de Menezes, E.M.; Mascarenhas, J.d.P.; Linhares, A.C. Diversity of rotavirus strains circulating in Northern Brazil after introduction of a rotavirus vaccine: High prevalence of G3P[6] genotype. J. Med. Virol. 2014, 86, 1065–1072. [Google Scholar] [CrossRef]
- Santos, V.S.; Nóbrega, F.A.; Soares, M.W.S.; Moreira, R.D.; Cuevas, L.E.; Gurgel, R.Q. Rotavirus genotypes circulating in Brazil before and after the national rotavirus vaccine program: A review. Pediatr. Infect. Dis. J. 2018, 37, e63–e65. [Google Scholar] [CrossRef]
- Gutierrez, M.B.; Fialho, A.M.; Maranhão, A.G.; Malta, F.C.; Andrade, J.d.S.R.d.; de Assis, R.M.S.; Mouta, S.D.S.E.; Miagostovich, M.P.; Leite, J.P.G.; Machado Fumian, T. Rotavirus A in Brazil: Molecular epidemiology and surveillance during 2018–2019. Pathogens 2020, 9, 515. [Google Scholar] [CrossRef]
- Gurgel, R.Q.; Cuevas, L.E.; Vieira, S.C.; Barros, V.C.; Fontes, P.B.; Salustino, E.F.; Nakagomi, O.; Nakagomi, T.; Dove, W.; Cunliffe, N.; et al. Predominance of rotavirus P[4]G2 in a vaccinated population, Brazil. Emerg. Infect. Dis. 2007, 13, 1571–1573. [Google Scholar] [CrossRef]
- Nakagomi, T.; Cuevas, L.E.; Gurgel, R.G.; Elrokhsi, S.H.; Belkhir, Y.A.; Abugalia, M.; Dove, W.; Montenegro, F.M.U.; Correia, J.B.; Nakagomi, O.; et al. Apparent extinction of non-G2 rotavirus strains from circulation in Recife, Brazil, after the introduction of rotavirus vaccine. Arch. Virol. 2008, 153, 591–593. [Google Scholar] [CrossRef]
- Montenegro, F.M.U.; Falbo, A.R.; Germano, E.M.; Correia, N.B.; Souza, E.d.S.; Nakagomi, O.; Nakagomi, T.; Cuevas, L.E.; Cunliffe, N.A.; Correia, J.B. Reduction in rotavirus disease and sustained predominance of G2P[4] rotavirus strain following introduction of rotavirus vaccine in Recife, Brazil. J. Trop. Pediatr. 2015, 61, 206–209. [Google Scholar] [CrossRef]
- Kirkwood, C.D.; Boniface, K.; Bishop, R.F.; Barnes, G.L.; Australian Rotavirus Surveillance Group. Australian rotavirus surveillance program annual report, 2008/2009. Commun. Dis. Intell. Q. Rep. 2009, 33, 382–388. [Google Scholar] [CrossRef]
- Patel, M.; Pedreira, C.; De Oliveira, L.H.; Tate, J.; Orozco, M.; Mercado, J.; Gonzalez, A.; Malespin, O.; Amador, J.J.; Umaña, J.; et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA 2009, 301, 2243–2251. [Google Scholar] [CrossRef] [PubMed]
- Dóró, R.; László, B.; Martella, V.; Leshem, E.; Gentsch, J.; Parashar, U.; Bányai, K. Review of global rotavirus strain prevalence data from six years post vaccine licensure surveillance: Is there evidence of strain selection from vaccine pressure? Infect. Genet. Evol. 2014, 28, 446–461. [Google Scholar] [CrossRef] [PubMed]
- Donato, C.M.; Zhang, Z.A.; Donker, N.C.; Kirkwood, C.D. Characterization of G2P[4] rotavirus strains associated with increased detection in Australian states using the RotaTeq® vaccine during the 2010–2011 surveillance period. Infect. Genet. Evol. 2014, 28, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Thanh, H.D.; Tran, V.T.; Lim, I.; Kim, W. Emergence of human G2P[4] rotaviruses in the post-vaccination era in South Korea: Footprints of multiple interspecies re-assortment events. Sci. Rep. 2018, 8, 6011. [Google Scholar] [CrossRef] [PubMed]
- Antunes, H.; Afonso, A.; Iturriza, M.; Martinho, I.; Ribeiro, C.; Rocha, S.; Magalhães, C.; Carvalho, L.; Branca, F.; Gray, J. G2P[4] the most prevalent rotavirus genotype in 2007 winter season in an European non-vaccinated population. J. Clin. Virol. 2009, 45, 76–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martínez, M.; Amarilla, A.A.; Galeano, M.E.; Aquino, V.H.; Fariña, N.; Russomando, G.; Parra, G.I. Predominance of rotavirus G2P[4] and emergence of G12P[9] strains in Asunción, Paraguay, 2006–2007. Arch. Virol. 2010, 155, 525–533. [Google Scholar] [CrossRef]
- Gurgel, R.Q.; Alvarez, A.D.J.; Rodrigues, A.; Ribeiro, R.R.; Dolabella, S.S.; Da Mota, N.L.; Santos, V.S.; Iturriza-Gomara, M.; Cunliffe, N.A.; Cuevas, L.E. Incidence of rotavirus and circulating genotypes in Northeast Brazil during 7 years of national rotavirus vaccination. PLoS ONE 2014, 9, e110217. [Google Scholar] [CrossRef][Green Version]
- Luchs, A.; Cilli, A.; Morillo, S.G.; de Cassia Compagnoli Carmona, R.; do Carmo Sampaio Tavares Timenetsky, M. Rotavirus in adults, Brazil, 2004–2011: G2P[4] dominance and potential impact on vaccination. Braz. J. Infect. Dis. 2014, 18, 53–59. [Google Scholar] [CrossRef]
- Kirkwood, C.D.; Roczo-Farkas, S.; Bishop, R.F.; Barnes, G.L.; Australian Rotavirus Surveillance Group. Australian Rotavirus Surveillance Program annual report, 2013. Commun. Dis. Intell. Q. Rep. 2014, 38, E334–E342. [Google Scholar] [CrossRef]
- Linhares, A.C.; Stupka, J.A.; Ciapponi, A.; Bardach, A.E.; Glujovsky, D.; Aruj, P.K.; Mazzoni, A.; Rodriguez, J.A.B.; Rearte, A.; Lanzieri, T.M.; et al. Burden and typing of rotavirus group A in Latin America and the Caribbean: Systematic review and meta-analysis. Rev. Med. Virol. 2011, 21, 89–109. [Google Scholar] [CrossRef]
- Adah, M.I.; Wade, A.; Taniguchi, K. Molecular epidemiology of rotaviruses in Nigeria: Detection of unusual strains with G2P[6] and G8P[1] specificities. J. Clin. Microbiol. 2001, 39, 3969–3975. [Google Scholar] [CrossRef]
- Clark, H.F.; Lawley, D.; DiStefano, D.; Maliga, M.; Kilby, B.; Kulnis, G.; Mallette, L.; DiNubile, M.J. An unusual outbreak of rotavirus genotype G2P[6] during the 2005–2006 epidemic season in Philadelphia. Diagn. Microbiol. Infect. Dis. 2011, 70, 218–222. [Google Scholar] [CrossRef]
- Enweronu-Laryea, C.C.; Sagoe, K.W.; Damanka, S.; Lartey, B.; Armah, G.E. Rotavirus genotypes associated with childhood severe acute diarrhoea in southern Ghana: A cross-sectional study. Virol. J. 2013, 10, 287. [Google Scholar] [CrossRef]
- Ghosh, S.; Urushibara, N.; Chawla-Sarkar, M.; Krishnan, T.; Kobayashi, N. Whole genomic analyses of asymptomatic human G1P[6], G2P[6] and G3P[6] rotavirus strains reveal intergenogroup reassortment events and genome segments of artiodactyl origin. Infect. Genet. Evol. 2013, 16, 165–173. [Google Scholar] [CrossRef]
- Heylen, E.; Zeller, M.; Ciarlet, M.; De Coster, S.; Van Ranst, M.; Matthijnssens, J. Complete genetic characterization of human G2P[6] and G3P[6] rotavirus strains. Infect. Genet. Evol. 2013, 13, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Luchs, A.; Cilli, A.; Morillo, S.G.; Gregório, D.d.S.; de Souza, K.A.F.; Vieira, H.R.; Fernandes, A.d.M.; Carmona, R.d.C.C.; Timenetsky, M.d.C.S.T. Detection of the emerging rotavirus G12P[8] genotype at high frequency in Brazil in 2014: Successive replacement of predominant strains after vaccine introduction. Acta Trop. 2016, 156, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Agbla, J.M.; Esona, M.D.; Jaimes, J.; Gautam, R.; Agbankpé, A.J.; Katz, E.; Dougnon, T.V.; Capo-Chichi, A.; Ouedraogo, N.; Razack, O.; et al. Whole genome analysis of rotavirus strains circulating in Benin before vaccine introduction, 2016–2018. Virus Res. 2022, 313, 198715. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, P.N.; Potgieter, R.-L.; Simwaka, J.; Mpabalwani, E.M.; Mwenda, J.M.; Mogotsi, M.T.; Magagula, N.; Esona, M.D.; Steele, A.D.; Seheri, M.L.; et al. Genomic analysis of G2P[4] group A rotaviruses in Zambia reveals positive selection in amino acid site 7 of viral protein 3. Viruses 2023, 15, 501. [Google Scholar] [CrossRef]
- Lestari, F.B.; Vongpunsawad, S.; Wanlapakorn, N.; Poovorawan, Y. Rotavirus infection in children in Southeast Asia 2008–2018: Disease burden, genotype distribution, seasonality, and vaccination. J. Biomed. Sci. 2020, 27, 66. [Google Scholar] [CrossRef]
- Bányai, K.; Martella, V.; Jakab, F.; Melegh, B.; Szücs, G. Sequencing and phylogenetic analysis of human genotype P[6] rotavirus strains detected in Hungary provides evidence for genetic heterogeneity within the P[6] VP4 gene. J. Clin. Microbiol. 2004, 42, 4338–4343. [Google Scholar] [CrossRef] [PubMed]
- Martella, V.; Bányai, K.; Ciarlet, M.; Iturriza-Gómara, M.; Lorusso, E.; De Grazia, S.; Arista, S.; Decaro, N.; Elia, G.; Cavalli, A.; et al. Relationships among porcine and human P[6] rotaviruses: Evidence that the different human P[6] lineages have originated from multiple interspecies transmission events. Virology 2006, 344, 509–519. [Google Scholar] [CrossRef]
- Nyaga, M.M.; Tan, Y.; Seheri, M.L.; Halpin, R.A.; Akopov, A.; Stucker, K.M.; Fedorova, N.B.; Shrivastava, S.; Duncan Steele, A.; Mwenda, J.M.; et al. Whole-genome sequencing and analyses identify high genetic heterogeneity, diversity and endemicity of rotavirus genotype P[6] strains circulating in Africa. Infect. Genet. Evol. 2018, 63, 79–88. [Google Scholar] [CrossRef]
- Linhares, A.C.; Mascarenhas, J.D.P.; Gusmão, R.H.P.; Gabbay, Y.B.; Fialho, A.M.; Leite, J.P.G. Neonatal rotavirus infection in Belém, northern Brazil: Nosocomial transmission of a P[6] G2 strain. J. Med. Virol. 2002, 67, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, J.D.P.; Linhares, A.C.; Bayma, A.P.G.; Lima, J.C.; Sousa, M.S.; Araújo, I.T.; Heinemann, M.B.; Gusmão, R.H.P.; Gabbay, Y.B.; Leite, J.P.G. Molecular analysis of VP4, VP7, and NSP4 genes of P[6]G2 rotavirus genotype strains recovered from neonates admitted to hospital in Belém, Brazil. J. Med. Virol. 2006, 78, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Papp, H.; Borzák, R.; Farkas, S.; Kisfali, P.; Lengyel, G.; Molnár, P.; Melegh, B.; Matthijnssens, J.; Jakab, F.; Martella, V.; et al. Zoonotic transmission of reassortant porcine G4P[6] rotaviruses in Hungarian pediatric patients identified sporadically over a 15 year period. Infect. Genet. Evol. 2013, 19, 71–80. [Google Scholar] [CrossRef]
- Luchs, A.; Timenetsky, M.D.C.S.T. G8P[6] rotaviruses isolated from Amerindian children in Mato Grosso do Sul, Brazil, during 2009: Close relationship of the G and P genes with those of bovine and bat strains. J. Gen. Virol. 2014, 95, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-K.; Oh, S.J.; Choi, S.; Choi, S.H.; Shin, S.-H.; Lee, E.J.; Cho, E.-J.; Hyun, J.; Kim, H.S. Relationship Between Rotavirus P[6] Infection in Korean Neonates and Histo-Blood Group Antigen: A Single-Center Study. Ann. Lab. Med. 2021, 41, 181–189. [Google Scholar] [CrossRef]
- Herring, A.J.; Inglis, N.F.; Ojeh, C.K.; Snodgrass, D.R.; Menzies, J.D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J. Clin. Microbiol. 1982, 16, 473–477. [Google Scholar] [CrossRef]
- Mijatovic-Rustempasic, S.; Bányai, K.; Esona, M.D.; Foytich, K.; Bowen, M.D.; Gentsch, J.R. Genome sequence based molecular epidemiology of unusual US Rotavirus A G9 strains isolated from Omaha, USA between 1997 and 2000. Infect. Genet. Evol. 2011, 11, 522–527. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Pang, B.-B.; Ghosh, S.; Zhou, X.; Shintani, T.; Urushibara, N.; Song, Y.-W.; He, M.-Y.; Liu, M.-Q.; Tang, W.-F.; et al. Molecular epidemiology and genetic evolution of the whole genome of G3P[8] human rotavirus in Wuhan, China, from 2000 through 2013. PLoS ONE 2014, 9, e88850. [Google Scholar] [CrossRef]
- Magagula, N.B.; Esona, M.D.; Nyaga, M.M.; Stucker, K.M.; Halpin, R.A.; Stockwell, T.B.; Seheri, M.L.; Steele, A.D.; Wentworth, D.E.; Mphahlele, M.J. Whole genome analyses of G1P[8] rotavirus strains from vaccinated and non-vaccinated South African children presenting with diarrhea. J. Med. Virol. 2015, 87, 79–101. [Google Scholar] [CrossRef]
- Varghese, V.; Ghosh, S.; Das, S.; Bhattacharya, S.K.; Krishnan, T.; Karmakar, P.; Kobayashi, N.; Naik, T.N. Characterization of VP1, VP2 and VP3 gene segments of a human rotavirus closely related to porcine strains. Virus Genes 2006, 32, 241–247. [Google Scholar] [CrossRef]
- Gentsch, J.R.; Glass, R.I.; Woods, P.; Gouvea, V.; Gorziglia, M.; Flores, J.; Das, B.K.; Bhan, M.K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 1365–1373. [Google Scholar] [CrossRef]
- Gouvea, V.; Glass, R.I.; Woods, P.; Taniguchi, K.; Clark, H.F.; Forrester, B.; Fang, Z.Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 1990, 28, 276–282. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Agbemabiese, C.A.; Nakagomi, T.; Damanka, S.A.; Dennis, F.E.; Lartey, B.L.; Armah, G.E.; Nakagomi, O. Sub-genotype phylogeny of the non-G, non-P genes of genotype 2 Rotavirus A strains. PLoS ONE 2019, 14, e0217422. [Google Scholar] [CrossRef]
- Azevedo, L.S.; Costa, F.F.; Ghani, M.B.A.; Viana, E.; França, Y.; Medeiros, R.S.; Guiducci, R.; Morillo, S.G.; Primo, D.; Lopes, R.D.; et al. Full genotype characterization of Brazilian canine G3P[3] strains during a 10-year survey (2012–2021) of rotavirus infection in domestic dogs and cats. Arch. Virol. 2023, 168, 176. [Google Scholar] [CrossRef]
- Gupta, S.; Gauhar, M.; Bubber, P.; Ray, P. Phylogenetic analysis of VP7 and VP4 genes of the most predominant human group A rotavirus G12 identified in children with acute gastroenteritis in Himachal Pradesh, India during 2013–2016. J. Med. Virol. 2021, 93, 6200–6209. [Google Scholar] [CrossRef]
- Doan, Y.H.; Nakagomi, T.; Agbemabiese, C.A.; Nakagomi, O. Changes in the distribution of lineage constellations of G2P[4] Rotavirus A strains detected in Japan over 32 years (1980–2011). Infect. Genet. Evol. 2015, 34, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Heylen, E.; Zeller, M.; Ciarlet, M.; Lawrence, J.; Steele, D.; Van Ranst, M.; Matthijnssens, J. Comparative analysis of pentavalent rotavirus vaccine strains and G8 rotaviruses identified during vaccine trial in Africa. Sci. Rep. 2015, 5, 14658. [Google Scholar] [CrossRef]
- Jere, K.C.; Chaguza, C.; Bar-Zeev, N.; Lowe, J.; Peno, C.; Kumwenda, B.; Nakagomi, O.; Tate, J.E.; Parashar, U.D.; Heyderman, R.S.; et al. Emergence of Double- and Triple-Gene Reassortant G1P[8] Rotaviruses Possessing a DS-1-Like Backbone after Rotavirus Vaccine Introduction in Malawi. J. Virol. 2018, 92, e01246-17. [Google Scholar] [CrossRef] [PubMed]
- Munlela, B.; João, E.D.; Strydom, A.; Bauhofer, A.F.L.; Chissaque, A.; Chilaúle, J.J.; Maurício, I.L.; Donato, C.M.; O’Neill, H.G.; de Deus, N. Whole-Genome Characterization of Rotavirus G9P[6] and G9P[4] Strains That Emerged after Rotavirus Vaccine Introduction in Mozambique. Viruses 2024, 16, 1140. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Matthijnssens, J.; Yang, X.; Delbeke, T.; Arijs, I.; Taniguchi, K.; Iturriza-Gómara, M.; Iftekharuddin, N.; Azim, T.; Van Ranst, M. Evolutionary history and global spread of the emerging g12 human rotaviruses. J. Virol. 2007, 81, 2382–2390. [Google Scholar] [CrossRef]
- Sharma, S.; Paul, V.K.; Bhan, M.K.; Ray, P. Genomic characterization of nontypeable rotaviruses and detection of a rare G8 strain in Delhi, India. J. Clin. Microbiol. 2009, 47, 3998–4005. [Google Scholar] [CrossRef]
- De Grazia, S.; Giammanco, G.M.; Dóró, R.; Bonura, F.; Marton, S.; Cascio, A.; Martella, V.; Bányai, K. Identification of a multi-reassortant G12P[9] rotavirus with novel VP1, VP2, VP3 and NSP2 genotypes in a child with acute gastroenteritis. Infect. Genet. Evol. 2015, 35, 34–37. [Google Scholar] [CrossRef]
- Doan, Y.H.; Dennis, F.E.; Takemae, N.; Haga, K.; Shimizu, H.; Appiah, M.G.; Lartey, B.L.; Damanka, S.A.; Hayashi, T.; Suzuki, T.; et al. Emergence of Intergenogroup Reassortant G9P[4] Strains Following Rotavirus Vaccine Introduction in Ghana. Viruses 2023, 15, 2453. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.; Roy, S.; Esona, M.D.; Mijatovic-Rustempasic, S.; Hardy, C.; Wang, Y.; Cortese, M.; Bowen, M.D. Full Genome Sequence of a Reassortant Human G9P[4] Rotavirus Strain. Genome Announc. 2014, 2, e01284-14. [Google Scholar] [CrossRef] [PubMed]
- Doan, Y.H.; Suzuki, Y.; Fujii, Y.; Haga, K.; Fujimoto, A.; Takai-Todaka, R.; Someya, Y.; Nayak, M.K.; Mukherjee, A.; Imamura, D.; et al. Complex reassortment events of unusual G9P[4] rotavirus strains in India between 2011 and 2013. Infect. Genet. Evol. 2017, 54, 417–428. [Google Scholar] [CrossRef]
- Quaye, O.; McDonald, S.; Esona, M.D.; Lyde, F.C.; Mijatovic-Rustempasic, S.; Roy, S.; Banegas, D.J.C.; Quiñonez, Y.M.; Chinchilla, B.L.; Santiago, F.G.; et al. Rotavirus G9P[4] in 3 countries in Latin America, 2009–2010. Emerg. Infect. Dis. 2013, 19, 1332–1333. [Google Scholar] [CrossRef]
- Gutierrez, M.B.; de Assis, R.M.S.; Andrade, J.d.S.R.d.; Fialho, A.M.; Fumian, T.M. Rotavirus A during the COVID-19 Pandemic in Brazil, 2020–2022: Emergence of G6P[8] Genotype. Viruses 2023, 15, 1619. [Google Scholar] [CrossRef]
- Medeiros, R.S.; França, Y.; Viana, E.; de Azevedo, L.S.; Guiducci, R.; de Lima Neto, D.F.; da Costa, A.C.; Luchs, A. Genomic Constellation of Human Rotavirus G8 Strains in Brazil over a 13-Year Period: Detection of the Novel Bovine-like G8P[8] Strains with the DS-1-like Backbone. Viruses 2023, 15, 664. [Google Scholar] [CrossRef]
- França, Y.; Medeiros, R.S.; Viana, E.; de Azevedo, L.S.; Guiducci, R.; da Costa, A.C.; Luchs, A. Genetic diversity and evolution of G12P[6] DS-1-like and G12P[9] AU-1-like Rotavirus strains in Brazil. Funct. Integr. Genom. 2024, 24, 92. [Google Scholar] [CrossRef] [PubMed]
- Moutelíková, R.; Sauer, P.; Prodělalová, J. Whole-genome sequence of a reassortant G9P[4] rotavirus A strain from two children in the Czech Republic. Arch. Virol. 2020, 165, 1703–1706. [Google Scholar] [CrossRef] [PubMed]
- Kachooei, A.; Tava Koli, A.; Minaeian, S.; Hosseini, M.; Jalilvand, S.; Latifi, T.; Arashkia, A.; Ataei-Pirkooh, A.; Shoja, Z. Molecular characterization of rotavirus infections in children less than 5 years of age with acute gastroenteritis in Tehran, Iran, 2021–2022: Emergence of uncommon G9P[4] and G9P[8] rotavirus strains. J. Med. Virol. 2023, 95, e28529. [Google Scholar] [CrossRef]
- Doan, Y.H.; Nakagomi, T.; Cunliffe, N.A.; Pandey, B.D.; Sherchand, J.B.; Nakagomi, O. The occurrence of amino acid substitutions D96N and S242N in VP7 of emergent G2P[4] rotaviruses in Nepal in 2004–2005: A global and evolutionary perspective. Arch. Virol. 2011, 156, 1969–1978. [Google Scholar] [CrossRef]
- Dennis, A.F.; McDonald, S.M.; Payne, D.C.; Mijatovic-Rustempasic, S.; Esona, M.D.; Edwards, K.M.; Chappell, J.D.; Patton, J.T. Molecular epidemiology of contemporary G2P[4] human rotaviruses cocirculating in a single U.S. community: Footprints of a globally transitioning genotype. J. Virol. 2014, 88, 3789–3801. [Google Scholar] [CrossRef]
- Pietsch, C.; Schuster, V.; Liebert, U.G. A hospital based study on inter- and intragenotypic diversity of human rotavirus A VP4 and VP7 gene segments, Germany. J. Clin. Virol. 2011, 50, 136–141. [Google Scholar] [CrossRef]
- Do, L.P.; Nakagomi, T.; Doan, Y.H.; Kitahori, Y.; Nakagomi, O. Molecular evolution of the VP7 gene of Japanese G2 rotaviruses before vaccine introduction. Arch. Virol. 2014, 159, 315–319. [Google Scholar] [CrossRef]
- Wu, F.-T.; Bányai, K.; Jiang, B.; Wu, C.-Y.; Chen, H.-C.; Fehér, E.; Huang, Y.-C.; Lin, J.-S.; Huang, F.-C.; Hsiung, C.A.; et al. Molecular epidemiology of human G2P[4] rotaviruses in Taiwan, 2004–2011. Infect. Genet. Evol. 2014, 28, 530–536. [Google Scholar] [CrossRef]
- Paul, S.K.; Kobayashi, N.; Nagashima, S.; Ishino, M.; Watanabe, S.; Alam, M.M.; Ahmed, M.U.; Hossain, M.A.; Naik, T.N. Phylogenetic analysis of rotaviruses with genotypes G1, G2, G9 and G12 in Bangladesh: Evidence for a close relationship between rotaviruses from children and adults. Arch. Virol. 2008, 153, 1999–2012. [Google Scholar] [CrossRef]
- Nakagomi, T.; Doan, Y.H.; Dove, W.; Ngwira, B.; Iturriza-Gómara, M.; Nakagomi, O.; Cunliffe, N.A. G8 rotaviruses with conserved genotype constellations detected in Malawi over 10 years (1997–2007) display frequent gene reassortment among strains co-circulating in humans. J. Gen. Virol. 2013, 94, 1273–1295. [Google Scholar] [CrossRef] [PubMed]
- Ndze, V.N.; Esona, M.D.; Achidi, E.A.; Gonsu, K.H.; Dóró, R.; Marton, S.; Farkas, S.; Ngeng, M.B.; Ngu, A.F.; Obama-Abena, M.T.; et al. Full genome characterization of human Rotavirus A strains isolated in Cameroon, 2010–2011: Diverse combinations of the G and P genes and lack of reassortment of the backbone genes. Infect. Genet. Evol. 2014, 28, 537–560. [Google Scholar] [CrossRef] [PubMed]
- Langa, J.S.; Thompson, R.; Arnaldo, P.; Resque, H.R.; Rose, T.; Enosse, S.M.; Fialho, A.; de Assis, R.M.S.; da Silva, M.F.M.; Leite, J.P.G. Epidemiology of rotavirus A diarrhea in Chókwè, Southern Mozambique, from February to September, 2011. J. Med. Virol. 2016, 88, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Galeano, M.E.; Akopov, A.; Palacios, R.; Russomando, G.; Kirkness, E.F.; Parra, G.I. Whole-genome analyses reveals the animal origin of a rotavirus G4P[6] detected in a child with severe diarrhea. Infect. Genet. Evol. 2014, 27, 156–162. [Google Scholar] [CrossRef]
- Malasao, R.; Khamrin, P.; Kumthip, K.; Ushijima, H.; Maneekarn, N. Complete genome sequence analysis of rare G4P[6] rotavirus strains from human and pig reveals the evidence for interspecies transmission. Infect. Genet. Evol. 2018, 65, 357–368. [Google Scholar] [CrossRef]
- Gutierrez, M.B.; de Assis, R.M.S.; Arantes, I.; Fumian, T.M. Full genotype constellations analysis of unusual DS-1-like G12P[6] and G6P[8] rotavirus strains detected in Brazil, 2019. Virology 2022, 577, 74–83. [Google Scholar] [CrossRef]
- Silva Serra, A.C.; Júnior, E.C.; Cruz, J.F.; Lobo, P.S.; Júnior, E.T.; Bandeira, R.S.; Bezerra, D.A.; Mascarenhas, J.D.; Santos Guerra, S.F.; Soares, L.S. Molecular analysis of G3P[6] rotavirus in the Amazon region of Brazil: Evidence of reassortment with equine-like strains. Future Microbiol. 2021, 16, 847–862. [Google Scholar] [CrossRef]
- Giammanco, G.M.; Bonura, F.; Zeller, M.; Heylen, E.; Van Ranst, M.; Martella, V.; Bányai, K.; Matthijnssens, J.; De Grazia, S. Evolution of DS-1-like human G2P[4] rotaviruses assessed by complete genome analyses. J. Gen. Virol. 2014, 95, 91–109. [Google Scholar] [CrossRef]
- Aida, S.; Nahar, S.; Paul, S.K.; Hossain, M.A.; Kabir, M.R.; Sarkar, S.R.; Ahmed, S.; Ghosh, S.; Urushibara, N.; Kawaguchiya, M.; et al. Whole genomic analysis of G2P[4] human Rotaviruses in Mymensingh, north-central Bangladesh. Heliyon 2016, 2, e00168. [Google Scholar] [CrossRef]
- Gómez, M.M.; Carvalho-Costa, F.A.; Volotão, E.d.M.; Rose, T.L.; da Silva, M.F.M.; Fialho, A.M.; de Assis, R.M.S.; Andrade, J.d.S.R.d.; Sá, A.C.C.; Zeller, M.; et al. Prevalence and genomic characterization of G2P[4] group A rotavirus strains during monovalent vaccine introduction in Brazil. Infect. Genet. Evol. 2014, 28, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Silva-Sales, M.; Leal, E.; Milagres, F.A.d.P.; Brustulin, R.; Morais, V.D.S.; Marcatti, R.; Araújo, E.L.L.; Witkin, S.S.; Deng, X.; Sabino, E.C.; et al. Genomic constellation of human Rotavirus A strains identified in Northern Brazil: A 6-year follow-up (2010–2016). Rev. Inst. Med. Trop. Sao Paulo 2020, 62, e98. [Google Scholar] [CrossRef]
- Dóró, R.; Mihalov-Kovács, E.; Marton, S.; László, B.; Deák, J.; Jakab, F.; Juhász, Á.; Kisfali, P.; Martella, V.; Melegh, B.; et al. Large-scale whole genome sequencing identifies country-wide spread of an emerging G9P[8] rotavirus strain in Hungary, 2012. Infect. Genet. Evol. 2014, 28, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Tacharoenmuang, R.; Komoto, S.; Guntapong, R.; Ide, T.; Sinchai, P.; Upachai, S.; Yoshikawa, T.; Tharmaphornpilas, P.; Sangkitporn, S.; Taniguchi, K. Full Genome Characterization of Novel DS-1-Like G8P[8] Rotavirus Strains that Have Emerged in Thailand: Reassortment of Bovine and Human Rotavirus Gene Segments in Emerging DS-1-Like Intergenogroup Reassortant Strains. PLoS ONE 2016, 11, e0165826. [Google Scholar] [CrossRef] [PubMed]
- Morozova, O.V.; Sashina, T.A.; Epifanova, N.V.; Velikzhanina, E.I.; Novikova, N.A. Phylodynamic characteristics of reassortant DS-1-like G3P[8]-strains of rotavirus type A isolated in Nizhny Novgorod (Russia). Braz. J. Microbiol. 2023, 54, 2867–2877. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, G.N.; Walimbe, A.M.; Chitambar, S.D. Molecular characterization of emerging G9P[4] rotavirus strains possessing a rare E6 NSP4 or T1 NSP3 genotype on a genogroup-2 backbone using a refined classification framework. J. Gen. Virol. 2016, 97, 3139–3153. [Google Scholar] [CrossRef]
- Komoto, S.; Tacharoenmuang, R.; Guntapong, R.; Ide, T.; Haga, K.; Katayama, K.; Kato, T.; Ouchi, Y.; Kurahashi, H.; Tsuji, T.; et al. Emergence and Characterization of Unusual DS-1-Like G1P[8] Rotavirus Strains in Children with Diarrhea in Thailand. PLoS ONE 2015, 10, e0141739. [Google Scholar] [CrossRef]
- Pietsch, C.; Liebert, U.G. Molecular characterization of different equine-like G3 rotavirus strains from Germany. Infect. Genet. Evol. 2018, 57, 46–50. [Google Scholar] [CrossRef]
- Luchs, A.; da Costa, A.C.; Cilli, A.; Komninakis, S.C.V.; Carmona, R.d.C.C.; Boen, L.; Morillo, S.G.; Sabino, E.C.; Timenetsky, M.D.C.S.T. Spread of the emerging equine-like G3P[8] DS-1-like genetic backbone rotavirus strain in Brazil and identification of potential genetic variants. J. Gen. Virol. 2019, 100, 7–25. [Google Scholar] [CrossRef]
- Komoto, S.; Tacharoenmuang, R.; Guntapong, R.; Ide, T.; Tsuji, T.; Yoshikawa, T.; Tharmaphornpilas, P.; Sangkitporn, S.; Taniguchi, K. Reassortment of Human and Animal Rotavirus Gene Segments in Emerging DS-1-Like G1P[8] Rotavirus Strains. PLoS ONE 2016, 11, e0148416. [Google Scholar] [CrossRef]
- Fujii, Y.; Doan, Y.H.; Suzuki, Y.; Nakagomi, T.; Nakagomi, O.; Katayama, K. Study of Complete Genome Sequences of Rotavirus A Epidemics and Evolution in Japan in 2012–2014. Front. Microbiol. 2019, 10, 38. [Google Scholar] [CrossRef]
- Luchs, A.; da Costa, A.C.; Cilli, A.; Komninakis, S.C.V.; Carmona, R.d.C.C.; Morillo, S.G.; Sabino, E.C.; Timenetsky, M.d.C.S.T. First Detection of DS-1-like G1P[8] Double-gene Reassortant Rotavirus Strains on The American Continent, Brazil, 2013. Sci. Rep. 2019, 9, 2210. [Google Scholar] [CrossRef] [PubMed]











| Strain | Age | Gender | City | State | Profile | VP7 | VP4 | VP6 | VP1 | VP2 | VP3 | NSP1 | NSP2 | NSP3 | NSP4 | NSP5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G2 | P[6] | I2 | R2 | C2 | M2 | A2 | N2 | T2 | E6 | H2 | ||||||
| RVA/Human-wt/BRA/IAL-R1000/2012/G2P[6] | 2 months | F | São Paulo | SP | Short | IVa | I-a | V | V | IVa | V | IVa | V | V | - | IVa |
| RVA/Human-wt/BRA/IAL-R1007/2012/G2P[6] | 4 months | F | São Paulo | SP | Short | IVa | I-a | V | V | IVa | V | IVa | V | V | - | IVa |
| RVA/Human-wt/BRA/IAL-R1142/2012/G2P[6] | 2 months | M | São Paulo | SP | Short | IVa | I-a | V | V | IVa | V | IVa | V | V | - | IVa |
| RVA/Human-wt/BRA/IAL-R1175/2012/G2P[6] | 1 year | F | São Paulo | SP | Short | IVa | I-a | V | V | IVa | V | - | V | V | - | IVa |
| RVA/Human-wt/BRA/IAL-R1228/2012/G2P[6] | 10 months | M | Nova Laranjeiras | PR | Short | IVa | I-a | V | V | IVa | V | IVa | V | V | - | IVa |
| RVA/Human-wt/BRA/IAL-R126/2013/G2P[6] | 4 years | F | São Paulo | SP | Short | IVa | I-a | V | V | IVa | V | IVa | V | V | - | IVa |
| Strain | Age | Gender | City | State | Profile | VP7 | VP4 | VP6 | VP1 | VP2 | VP3 | NSP1 | NSP2 | NSP3 | NSP4 | NSP5 |
| G2 | P[6] | I2 | R2 | C2 | M2 | A2 | N2 | T2 | E2 | H2 | ||||||
| RVA/Human-wt/BRA/IAL-R50/2014/G2P[6] | 26 days | U | São Paulo | SP | Short | V | I-a | V | V | IVa | V | IVa | V | V | VI | IVa |
| RVA/Human-wt/BRA/IAL-R52/2014/G2P[6] | 22 days | M | São Paulo | SP | Short | V | I-a | V | V | IVa | V | IVa | V | V | VI | IVa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.C.M.; França, Y.; Azevedo, L.S.d.; Guiducci, R.; Villela, E.F.d.M.; Luchs, A. Unveiling the Genomic Landscape of G2P[6] Rotavirus a Strains in Brazil: Evolutionary and Epidemiological Perspectives. Viruses 2025, 17, 1103. https://doi.org/10.3390/v17081103
Silva VCM, França Y, Azevedo LSd, Guiducci R, Villela EFdM, Luchs A. Unveiling the Genomic Landscape of G2P[6] Rotavirus a Strains in Brazil: Evolutionary and Epidemiological Perspectives. Viruses. 2025; 17(8):1103. https://doi.org/10.3390/v17081103
Chicago/Turabian StyleSilva, Vanessa Cristina Martins, Yasmin França, Lais Sampaio de Azevedo, Raquel Guiducci, Edlaine Faria de Moura Villela, and Adriana Luchs. 2025. "Unveiling the Genomic Landscape of G2P[6] Rotavirus a Strains in Brazil: Evolutionary and Epidemiological Perspectives" Viruses 17, no. 8: 1103. https://doi.org/10.3390/v17081103
APA StyleSilva, V. C. M., França, Y., Azevedo, L. S. d., Guiducci, R., Villela, E. F. d. M., & Luchs, A. (2025). Unveiling the Genomic Landscape of G2P[6] Rotavirus a Strains in Brazil: Evolutionary and Epidemiological Perspectives. Viruses, 17(8), 1103. https://doi.org/10.3390/v17081103









