Bovine Viral Diarrhea Virus-1 (Pestivirus bovis) Associated with Stillborn and Mummified Fetuses in Farmed White-Tailed Deer (Odocoileus virginianus) in Florida
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical History and Specimen Collection
2.2. Mycobacterium PCR, Toxoplasma gondii PCR, and BTV & EHDV RT-qPCR Detection
2.3. Cell Culture
2.4. Virus Isolation in Cell Culture
2.5. BVDV-1 RT-PCR
2.6. Sanger Sequencing
3. Results
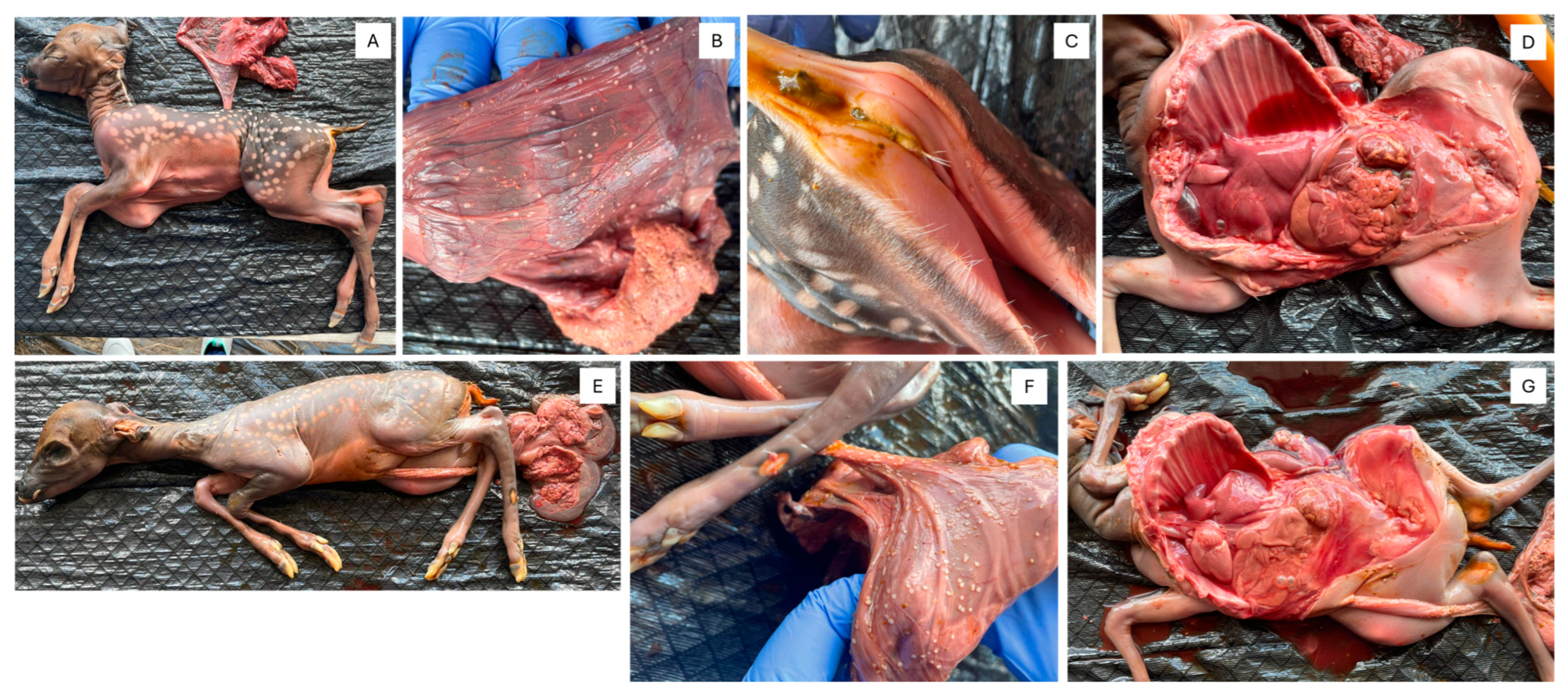
3.1. Gross Observations
3.2. Mycobacterium PCR, Toxoplasma gondii PCR, and BTV & EHDV RT-qPCR Detection
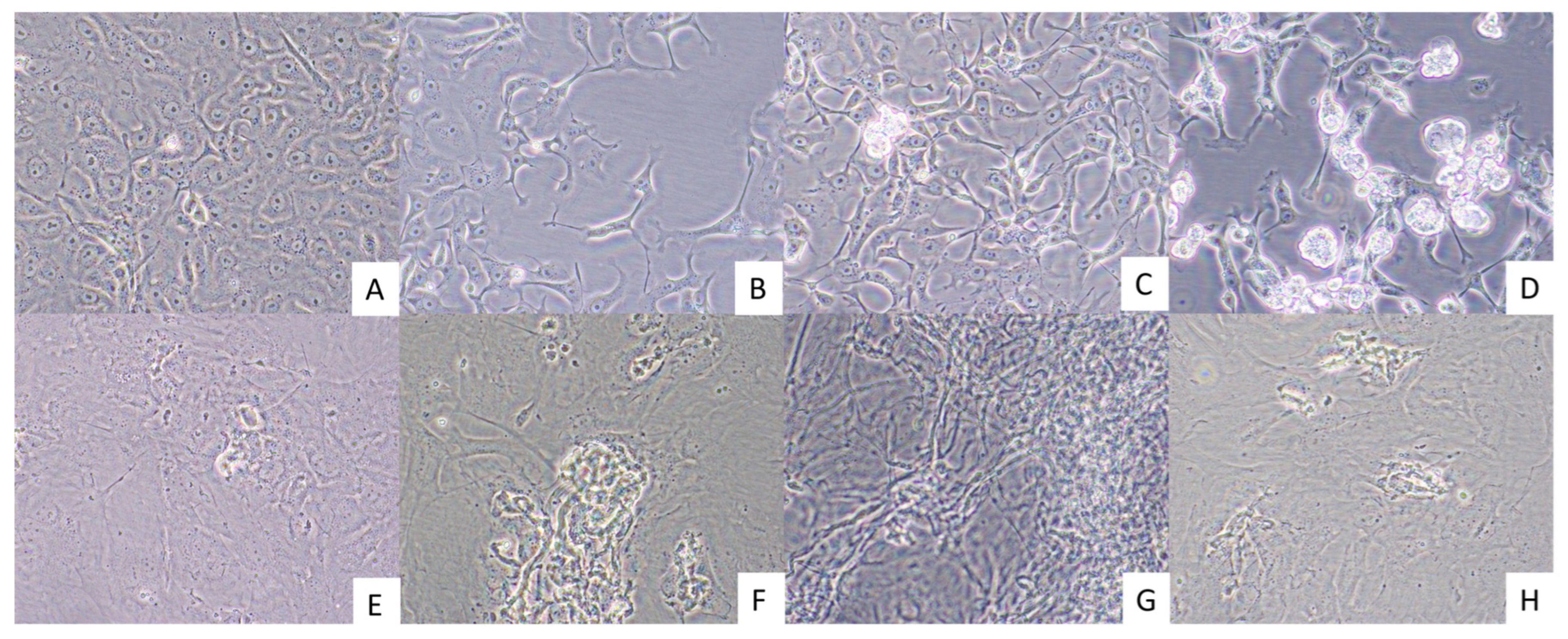
3.3. Evidence of Virus Isolation in Cultured Cells
3.4. BVDV-1 RT-PCR
3.5. Sanger Sequencing
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| BTV | Bluetongue virus |
| BVDV | Bovine viral diarrhea virus |
| dpi | Day post-inoculation |
| CHeRI | Cervidae Health Research Initiative |
| CP | Cytopathic |
| CPE | Cytopathic effects |
| EHDV | Epizootic hemorrhagic disease virus |
| FBS | Fetal bovine serum |
| HT | Hepatic tissues |
| KT | Kidney tissues |
| NCBI | National Center for Biotechnology Information |
| NCP | Non-cytopathic |
| PBS | Phosphate-buffered saline |
| PI | Persistently infected |
| PT | Placenta tissue |
| RT-PCR | Reverse transcription PCR |
| ST | Spleen tissues |
| UF | University of Florida |
| WB | Whole blood |
| WTD | White-tailed deer |
References
- Olafson, P.; MacCallum, A.D.; Fox, F.H. An Apparently New Transmissible Disease of Cattle. Cornell Vet. 1946, 36, 205–213. [Google Scholar]
- Pellerin, C.J.; van den, H.; Lecomte, J.; Tijssen, P. Identification of a New Group of Bovine Viral Diarrhea Virus Strains Associated with Severe Outbreaks and High Mortalities. Virology 1994, 203, 260–268. [Google Scholar] [CrossRef]
- Ridpath, J.F.; Bolin, S.R.; Dubovi, E.J. Segregation of Bovine Viral Diarrhea Virus into Genotypes. Virology 1994, 205, 66–74. [Google Scholar] [CrossRef]
- Donoso, A.; Inostroza, F.; Celedon, M.; Pizarro-Lucero, J. Genetic Diversity of Bovine Viral Diarrhea Virus from Cattle in Chile between 2003 and 2007. BMC Vet. Res. 2018, 14, 314. [Google Scholar] [CrossRef]
- Hause, B.M.; Pillatzki, A.; Clement, T.; Bragg, T.; Ridpath, J.; Chase, C.C.L. Persistent Infection of American Bison (Bison bison) with Bovine Viral Diarrhea Virus and Bosavirus. Vet. Microbiol. 2021, 252, 108949. [Google Scholar] [CrossRef]
- Newcomer, B.W. 75 Years of Bovine Viral Diarrhea Virus: Current Status and Future Applications of the Use of Directed Antivirals. Antiviral Res. 2021, 196, 105205. [Google Scholar] [CrossRef]
- Chakraborty, A.K.; Mukherjee, P.; Karam, A.; Das, S.; Barkalita, L.; Puro, K.; Sanjukta, R.; Ghatak, S.; Sakuntala, I.; Laha, R.G.; et al. Evidence of Bvdv in Pigs from North Eastern Part of India- Genetic Profiling and Characterisation. Open Virol. J. 2018, 12, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Aniță, D.C.; Popa, E.; Aniță, A.; Oșlobanu, L.E.; Savuța, G. Pestivirus Spillover Effect: Molecular Detection of Bovine Viral Diarrhea Virus in Domestic and Feral Pigs. Pesqui. Veterinária Bras. 2020, 40, 479–483. [Google Scholar] [CrossRef]
- Cantu, A.; Ortega-S, J.A.; Mosqueda, J.; Garcia-Vazquez, Z.; Henke, S.E.; George, J.E. Prevalence of Infectious Agents in Free-Ranging White-Tailed Deer in Northeastern Mexico. J. Wildl. Dis. 2008, 44, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Niskanen, R.; Lindberg, A. Transmission of Bovine Viral Diarrhoea Virus by Unhygienic Vaccination Procedures, Ambient Air, and from Contaminated Pens. Vet. J. 2003, 165, 125–130. [Google Scholar] [CrossRef]
- Chamorro, M.F.; Passler, T.; Givens, M.D.; Edmondson, M.A.; Wolfe, D.F.; Walz, P.H. Evaluation of Transmission of Bovine Viral Diarrhea Virus (Bvdv) between Persistently Infected and Naive Cattle by the Horn Fly (Haematobia irritans). Vet. Res. Commun. 2011, 35, 123–129. [Google Scholar] [CrossRef]
- Botner, A.; Belsham, G.J. Virus Survival in Slurry: Analysis of the Stability of Foot-and-Mouth Disease, Classical Swine Fever, Bovine Viral Diarrhoea and Swine Influenza Viruses. Vet. Microbiol. 2012, 157, 41–49. [Google Scholar] [CrossRef]
- Passler, T.; Walz, P.H.; Ditchkoff, S.S.; Brock, K.V.; DeYoung, R.W.; Foley, A.M.; Daniel Givens, M. Cohabitation of Pregnant White-Tailed Deer and Cattle Persistently Infected with Bovine Viral Diarrhea Virus Results in Persistently Infected Fawns. Vet. Microbiol. 2009, 134, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Negrón, M.E.; Pogranichniy, R.M.; Van Alstine, W.; Hilton, W.M.; Lévy, M.; Raizman, E.A. Evaluation of Horizontal Transmission of Bovine Viral Diarrhea Virus Type 1a from Experimentally Infected White-Tailed Deer Fawns (Odocoileus virginianus) to Colostrum-Deprived Calves. Am. J. Vet. Res. 2012, 73, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Passler, T.; Ditchkoff, S.S.; Givens, M.D.; Brock, K.V.; Deyoung, R.W.; Walz, P.H. Transmission of Bovine Viral Diarrhea Virus among White-Tailed Deer (Odocoileus virginianus). Vet. Res. 2010, 41, 20. [Google Scholar] [CrossRef]
- Van Campen, H.; Williams, E.S.; Edwards, J.; Cook, W.; Stout, G. Experimental Infection of Deer with Bovine Viral Diarrhea Virus. J. Wildl. Dis. 1997, 33, 567–573. [Google Scholar] [CrossRef]
- Passler, T.; Walz, P.H.; Ditchkoff, S.S.; Givens, M.D.; Maxwell, H.S.; Brock, K.V. Experimental Persistent Infection with Bovine Viral Diarrhea Virus in White-Tailed Deer. Vet. Microbiol. 2007, 122, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Ridpath, J.F.; Mark, C.S.; Chase, C.C.L.; Ridpath, A.C.; Neill, J.D. Febrile Response and Decrease in Circulating Lymphocytes Following Acute Infection of White-Tailed Deer Fawns with Either a Bvdv1 or a Bvdv2 Strain. J. Wildl. Dis. 2007, 43, 653–659. [Google Scholar] [CrossRef]
- Ridpath, J.F.; Driskell, E.A.; Chase, C.C.L.; Neill, J.D.; Palmer, M.V.; Brodersen, B.W. Reproductive Tract Disease Associated with Inoculation of Pregnant White-Tailed Deer with Bovine Viral Diarrhea Virus. Am. J. Vet. Res. 2008, 69, 1630–1636. [Google Scholar] [CrossRef]
- Ridpath, J.F.; Neill, J.D.; Chase, C.C.L. Impact of Bvdv Infection of White-Tailed Deer During Second and Third Trimesters of Pregnancy. J. Wildl. Dis. 2012, 48, 758–762. [Google Scholar] [CrossRef]
- Passler, T.; Ditchkoff, S.S.; Walz, P.H. Bovine Viral Diarrhea Virus (Bvdv) in White-Tailed Deer (Odocoileus virginianus). Front. Microbiol. 2016, 7, 945. [Google Scholar] [CrossRef] [PubMed]
- Khodakaram-Tafti, A.; Farjanikish, G.H. Persistent Bovine Viral Diarrhea Virus (Bvdv) Infection in Cattle Herds. Iran. J. Vet. Res. 2017, 18, 154–163. [Google Scholar]
- Gillespie, J.H.; Madin, S.H.; Darby, N.B. Cellular Resistance in Tissue Culture, Induced by Noncytopathogenic Strains, to a Cytopathogenic Strain of Virus Diarrhea Virus of Cattle. Proc. Soc. Exp. Biol. Med. 1962, 110, 248–250. [Google Scholar] [CrossRef]
- Brownlie, J. Pathogenesis of Mucosal Disease and Molecular Aspects of Bovine Virus Diarrhoea Virus. Vet. Microbiol. 1990, 23, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Peterhans, E.; Bachofen, C.; Stalder, H.; Schweizer, M. Cytopathic Bovine Viral Diarrhea Viruses (Bvdv): Emerging Pestiviruses Doomed to Extinction. Vet. Res. 2010, 41, 44. [Google Scholar] [CrossRef]
- Donis, R.O. Molecular Biology of Bovine Viral Diarrhea Virus and Its Interactions with the Host. Vet. Clin. North. Am. Food Anim. Pract. 1995, 11, 393–423. [Google Scholar] [CrossRef]
- Ahasan, M.S.; Subramaniam, K.; Sayler, K.A.; Loeb, J.C.; Popov, V.L.; Lednicky, J.A.; Wisely, S.M.; Krauer, J.M.C.; Waltzek, T.B. Molecular Characterization of a Novel Reassortment Mammalian Orthoreovirus Type 2 Isolated from a Florida White-Tailed Deer Fawn. Virus Res. 2019, 270, 197642. [Google Scholar] [CrossRef]
- Ford, A.K.; Niedringhaus, K.D.; Anderson, A.N.; LaCour, J.M.; Nemeth, N.M. Disseminated Mycobacterium Kansasii Infection in a White-Tailed Deer and Implications for Public and Livestock Health. J. Vet. Diagn. Investig. 2020, 32, 147–151. [Google Scholar] [CrossRef]
- Silva, M.S.; Uzêda, R.S.; Costa, K.S.; Santos, S.L.; Macedo, A.C.; Abe-Sandes, K.; Gondim, L.F.P. Detection of Hammondia Heydorni and Related Coccidia (Neospora caninum and Toxoplasma gondii) in Goats Slaughtered in Bahia, Brazil. Vet. Parasitol. 2009, 162, 156–159. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New Capabilities and Interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
| Sample ID | Mycobacterium PCR | Toxoplasma gondii PCR | BTV and EHDV RT-qPCR | BVDV1 RT-PCR | Sanger Sequencing |
|---|---|---|---|---|---|
| OV1659-WB | - | - | Negative | Positive | - |
| OV1660-ST | - | - | Negative | Negative | - |
| OV1660-HT | - | - | - | Negative | - |
| OV1660-PT | Negative | Negative | - | - | - |
| OV1660-ST_Vero E6 | - | - | - | Positive | - |
| OV1660-HT_Vero E6 | - | - | - | Positive | BVDV-1 |
| OV1660-ST_BT | - | - | - | Negative | - |
| OV1660-HT_BT | - | - | - | Positive | - |
| OV1660-KT_BT | - | - | - | Positive | - |
| OV1661-ST | - | - | Negative | Negative | - |
| OV1661-PT | Negative | Negative | - | - | - |
| OV1661-ST_BT | - | - | - | Positive | - |
| OV1661-HT_BT | - | - | - | Positive | - |
| OV1661-KT_BT | - | - | - | Positive | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, A.-C.; DeRuyter, E.; de Oliveira Viadanna, P.H.; White, Z.S.; Lednicky, J.A.; Wisely, S.M.; Subramaniam, K.; Campos Krauer, J.M. Bovine Viral Diarrhea Virus-1 (Pestivirus bovis) Associated with Stillborn and Mummified Fetuses in Farmed White-Tailed Deer (Odocoileus virginianus) in Florida. Viruses 2025, 17, 1104. https://doi.org/10.3390/v17081104
Cheng A-C, DeRuyter E, de Oliveira Viadanna PH, White ZS, Lednicky JA, Wisely SM, Subramaniam K, Campos Krauer JM. Bovine Viral Diarrhea Virus-1 (Pestivirus bovis) Associated with Stillborn and Mummified Fetuses in Farmed White-Tailed Deer (Odocoileus virginianus) in Florida. Viruses. 2025; 17(8):1104. https://doi.org/10.3390/v17081104
Chicago/Turabian StyleCheng, An-Chi, Emily DeRuyter, Pedro H. de Oliveira Viadanna, Zoe S. White, John A. Lednicky, Samantha M. Wisely, Kuttichantran Subramaniam, and Juan M. Campos Krauer. 2025. "Bovine Viral Diarrhea Virus-1 (Pestivirus bovis) Associated with Stillborn and Mummified Fetuses in Farmed White-Tailed Deer (Odocoileus virginianus) in Florida" Viruses 17, no. 8: 1104. https://doi.org/10.3390/v17081104
APA StyleCheng, A.-C., DeRuyter, E., de Oliveira Viadanna, P. H., White, Z. S., Lednicky, J. A., Wisely, S. M., Subramaniam, K., & Campos Krauer, J. M. (2025). Bovine Viral Diarrhea Virus-1 (Pestivirus bovis) Associated with Stillborn and Mummified Fetuses in Farmed White-Tailed Deer (Odocoileus virginianus) in Florida. Viruses, 17(8), 1104. https://doi.org/10.3390/v17081104








