PCV2 Infection Upregulates SOCS3 Expression to Facilitate Viral Replication in PK-15 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells Virus and Reagents
2.2. Plasmid Constructions
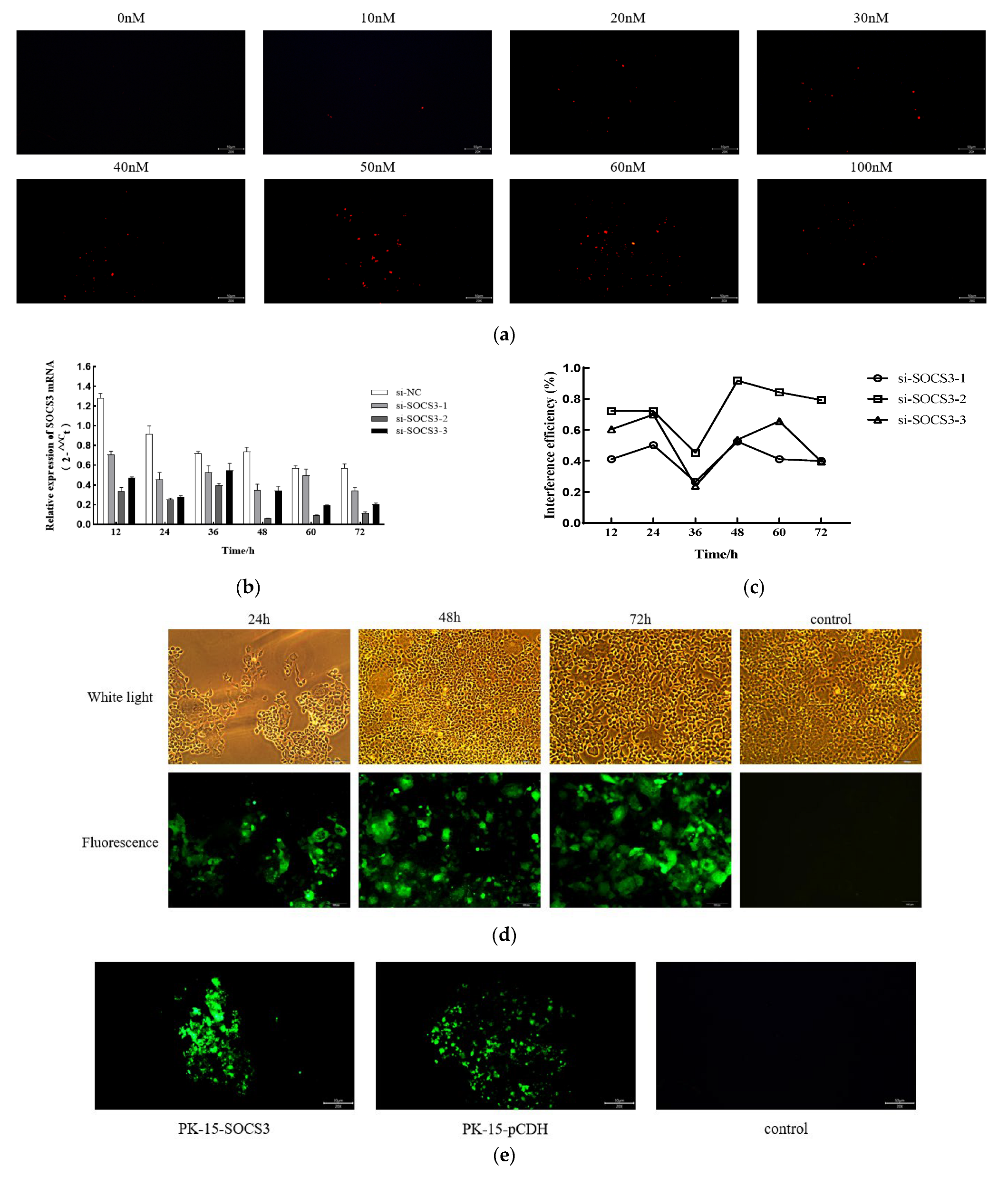
2.3. siRNA and Transfection
2.4. Construction of the SOCS3-Overexpressing PK-15 Cell Line
2.5. Cell Counting Kit-8 Assay
2.6. Viral Infection Assay
2.7. Quantitative Real-Time PCR Analysis
2.8. Western Blot Analysis
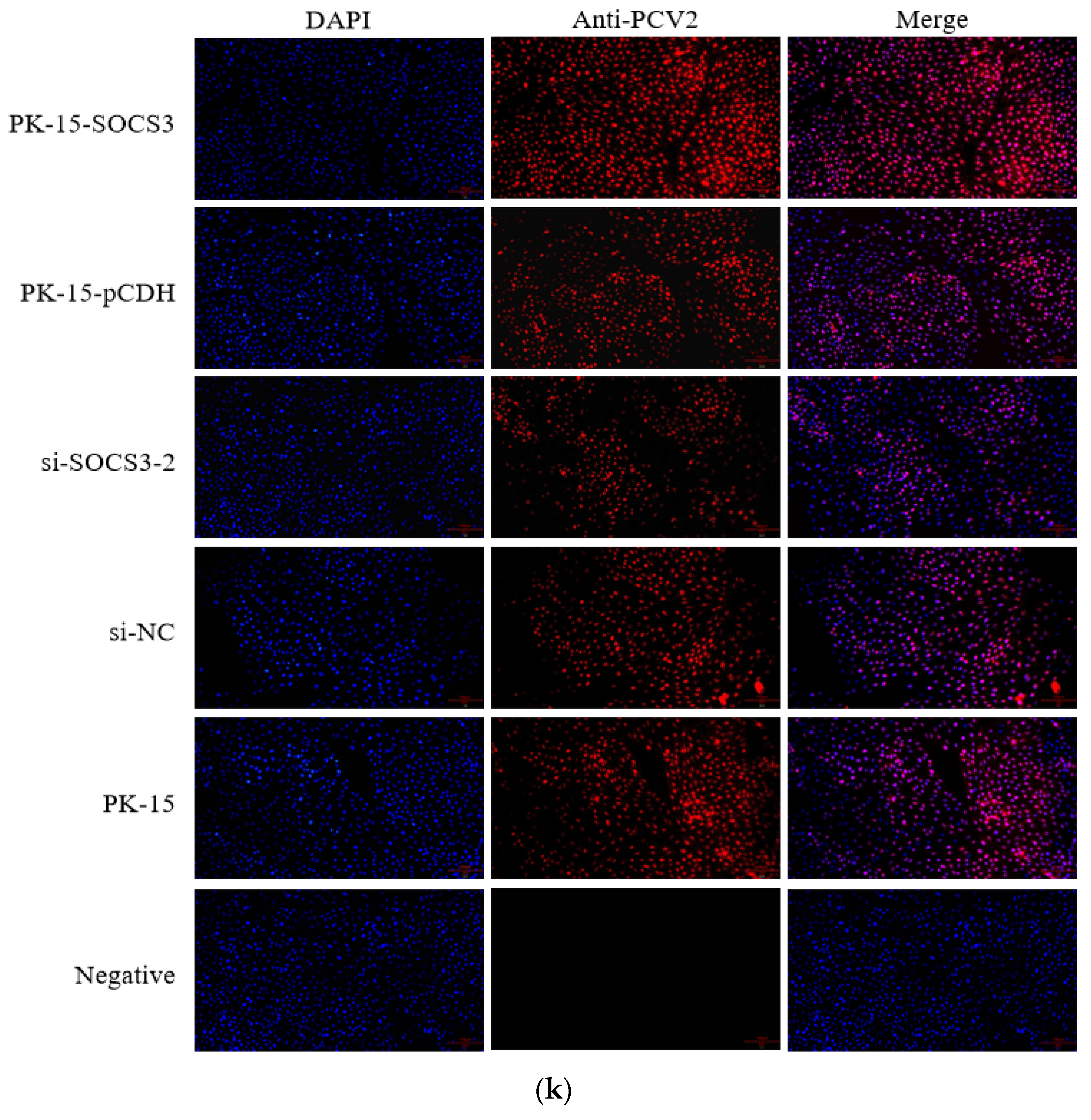
2.9. Indirect Immunofluorescence Assay (IFA)
3. Results
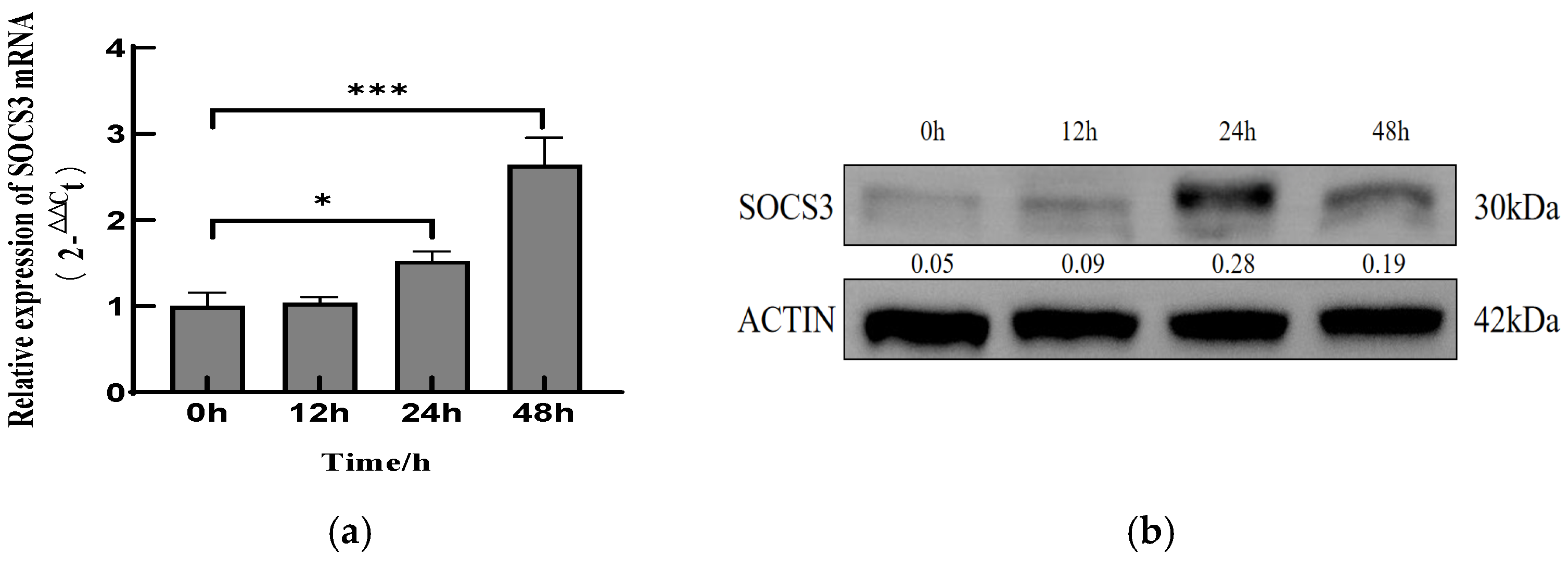
3.1. PCV2 Infection Upregulates SOCS3 Expression in PK-15 Cells
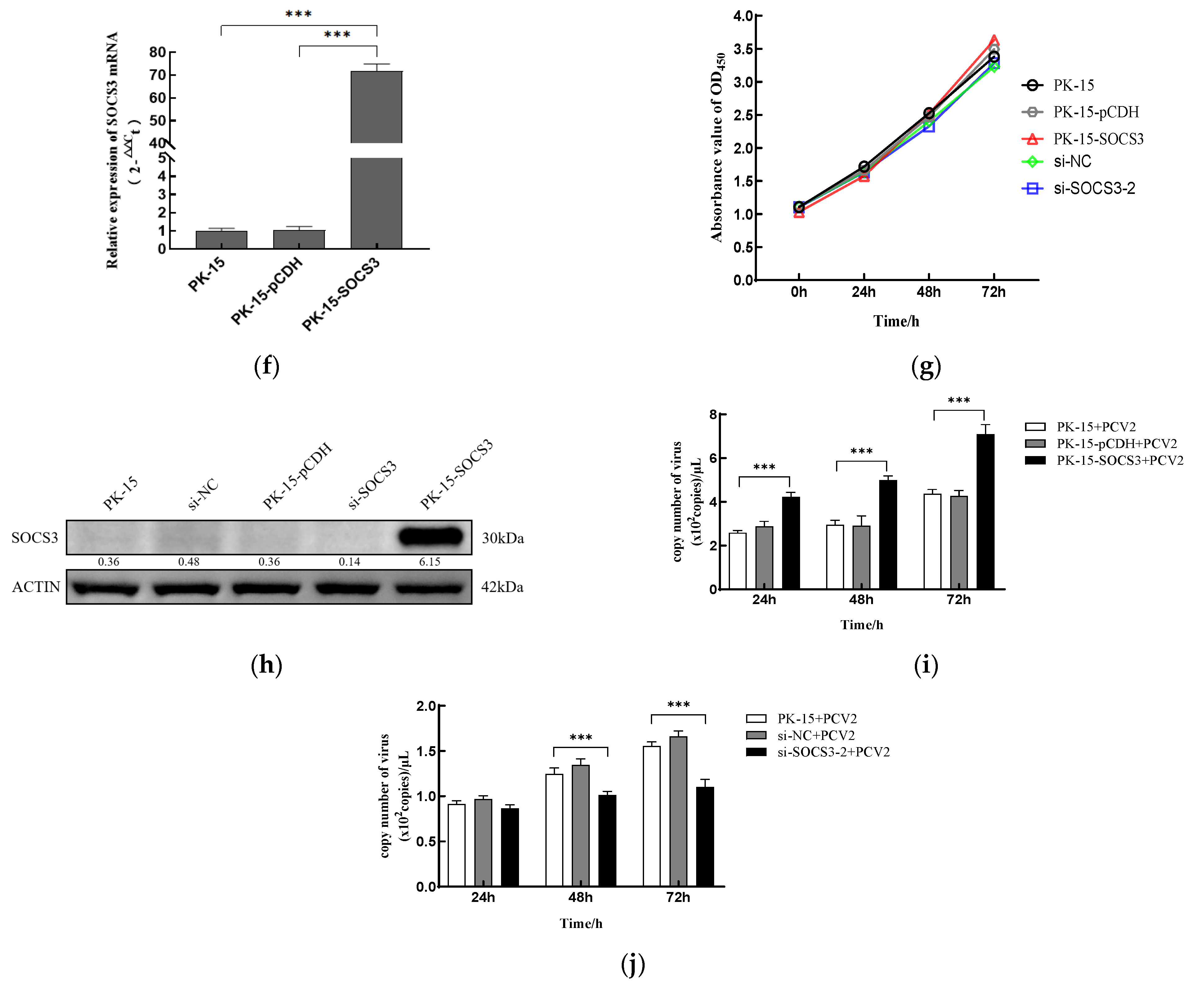
3.2. SOCS3 Expression Facilitates PCV2 Replication
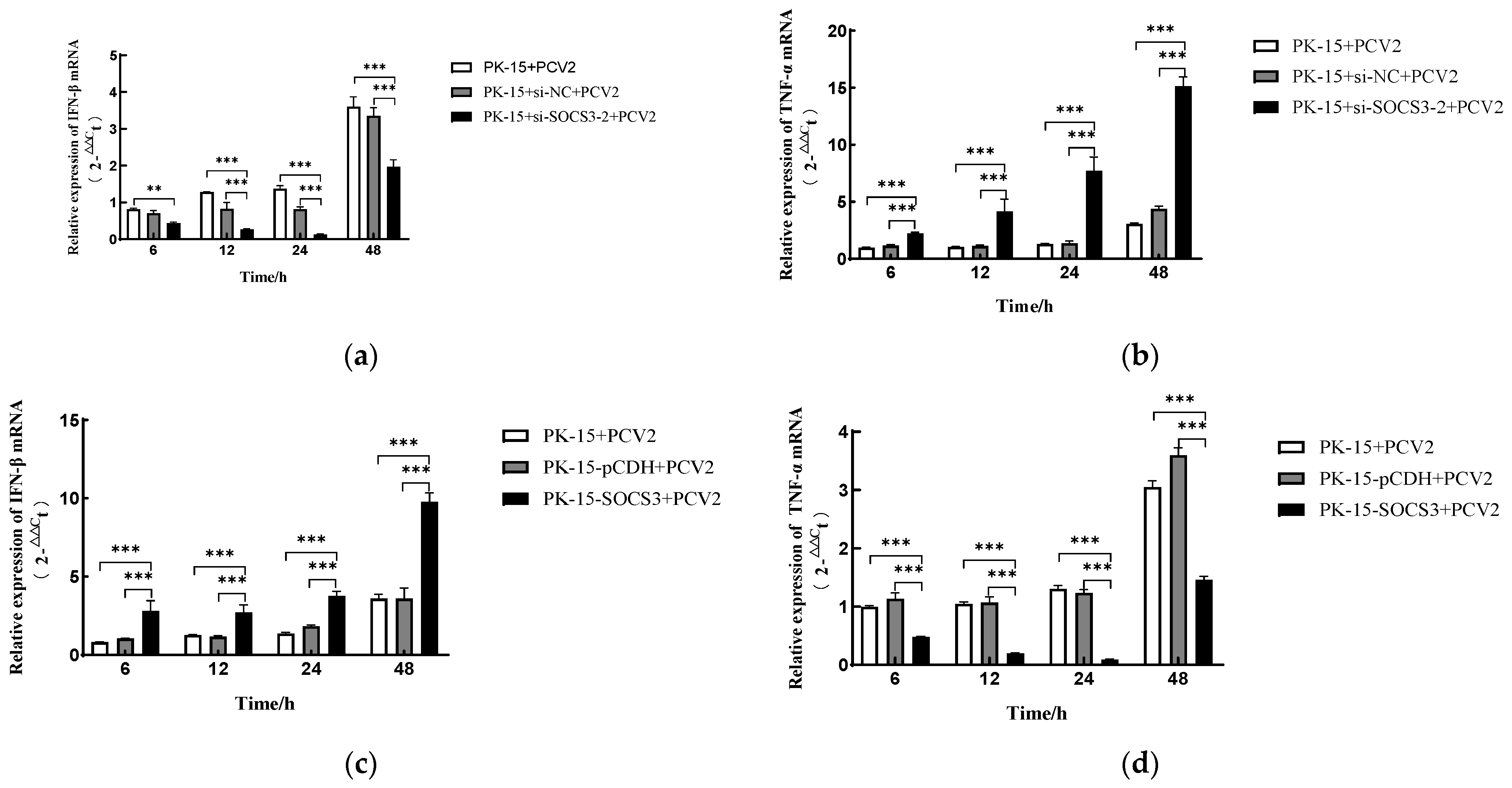
3.3. SOCS3 Modulates PCV2-Induced Expression of IFN-β and TNF-α mRNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.S.; Hou, L.; Zhou, J.W.; Wang, D.D.; Cui, Y.Q.; Feng, X.F.; Liu, J. Porcine Circovirus Type 2 Vaccines: Commercial Application and Research Advances. Viruses 2022, 14, 2005. [Google Scholar] [CrossRef] [PubMed]
- Fehér, E.; Jakab, F.; Bányai, K. Mechanisms of circovirus immunosuppression and pathogenesis with a focus on porcine circovirus 2: A review. Vet. Q. 2023, 43, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.W.; Zhao, J.; Sun, H.Y.; Dai, B.N.; Zhu, N.; Dai, Q.H.; Qiu, Y.H.; Wang, D.D.; Cui, Y.Q.; Guo, J.S.; et al. DEAD-box RNA helicase 21 interacts with porcine circovirus type 2 Cap protein and facilitates viral replication. Front. Microbiol. 2024, 15, 1298106. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.J.; Huang, Q.Y.; Shen, R.T.; Zhang, Y.N.; Zhou, L.; Ge, X.N.; Han, J.; Guo, X.; Yang, H.C. Foxp3 inhibits PCV2 replication by reducing the ATPase activity of Rep. Vet. Microbiol. 2025, 304, 110441. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.B.; Li, X.D.; Yin, B.; Deng, J.H.; Tian, K.G.; Yuan, A. Structural roles of PCV2 capsid protein N-terminus in PCV2 particle assembly and identification of PCV2 type-specific neutralizing epitope. PLoS Pathog. 2019, 15, e1007562. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Park, I.B.; Kang, S.J.; Bae, J.; Chun, T. Interaction between host cell proteins and open reading frames of porcine circovirus type 2. J. Anim. Sci. Technol. 2023, 65, 698–719. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Segalés, J. Porcine Circovirus 2 Genotypes, Immunity and Vaccines: Multiple Genotypes but One Single Serotype. Pathogens 2020, 9, 1049. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.R.; Sun, Y.H. Genotypic diversity and immunological implications of porcine circovirus: Inspiration from PCV1 to PCV4. Microb. Pathog. 2024, 196, 106997. [Google Scholar] [CrossRef] [PubMed]
- Unterweger, C.; Brunthaler, R.; Auer, A.; Fux, R.; Weissenbacher-Lang, C.; Ladinig, A. Reconsideration of the diagnostic criteria required for PCV2 reproductive disease. Vet. J. 2021, 272, 105660. [Google Scholar] [CrossRef] [PubMed]
- Saade, G.; Deblanc, C.; Bougon, J.; Marois-Créhan, C.; Fablet, C.; Auray, G.; Belloc, C.; Leblanc-Maridor, M.; Gagnon, C.A.; Zhu, J.Z.; et al. Coinfections and their molecular consequences in the porcine respiratory tract. Vet. Res. 2020, 51, 80. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Chen, J.; Wu, X.C.; Ma, D.; Zhang, X.H.; Li, R.Z.; Han, C.; Liu, H.X.; Yin, X.R.; Du, Q.; et al. PCV2 targets cGAS to inhibit type I interferon induction to promote other DNA virus infection. PLoS Pathog. 2021, 17, e1009940. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.N.; Li, J.S.; Wang, Z.J.; Jiang, P.; Bai, J.; Li, Y.F.; Wang, X.W. Vimentin promotes porcine circovirus type 2 (PCV2) replication in pig alveolar macrophage. Virus Res. 2022, 318, 198842. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.R.; Li, Z.A.; Liang, W.L.; Hu, W.H.; Zhou, S.L.; Yang, Z.; Tao, Y.R.; Hou, X.L.; Xing, Z.; Mao, J.C.; et al. SOCS proteins and their roles in the development of glioblastoma. Oncol. Lett. 2022, 23, 5. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Z.; Liu, K.; Cheng, A.C.; Wang, M.S.; Cui, M.; Huang, J.; Zhu, D.K.; Chen, S.; Liu, M.F.; Zhao, X.X.; et al. SOCS Proteins Participate in the Regulation of Innate Immune Response Caused by Viruses. Front. Immunol. 2020, 11, 558341. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Aki, D.; Ito, M. SOCS, SPRED, and NR4a: Negative regulators of cytokine signaling and transcription in immune tolerance. Proc. Jpn. Acad. Ser. B-Phys. Biol. Sci. 2021, 97, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.Y.; Wang, M.S.; Cheng, A.C.; Jia, R.Y.; Zhu, D.K.; Liu, M.F.; Chen, S.; Zhao, X.X.; Yang, Q.; Wu, Y.; et al. The role of SOCS proteins in the development of virus- induced hepatocellular carcinoma. Virol. J. 2021, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Sun, Z.Y.; Zhang, S.; Yang, G.X.; Jiang, X.; Wang, G.L.; Li, R.; Wang, Q.L.; Tian, X.W. SOCS modulates JAK-STAT pathway as a novel target to mediate the occurrence of neuroinflammation: Molecular details and treatment options. Brain Res. Bull. 2024, 213, 110988. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Bakay, M.; Hakonarson, H. SOCS-JAK-STAT inhibitors and SOCS mimetics as treatment options for autoimmune uveitis, psoriasis, lupus, and autoimmune encephalitis. Front. Immunol. 2023, 14, 1271102. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Rauf, M.; Tahir, F.; Manzoor, S. A comparative analysis of interferons and direct-acting antivirals on the expression of genes involved in hepatitis C pathogenesis. J. Med. Virol. 2021, 93, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Du, L.Y.; Cheng, X.; Yuan, M.; Shang, J.; Shi, Y.; Yang, H.L.; Tang, H. CpG Island Methylation of Suppressor of Cytokine Signaling-1 Gene Induced by HCV Is Associated With HCV-Related Hepatocellular Carcinoma. Front. Microbiol. 2022, 13, 679593. [Google Scholar] [CrossRef] [PubMed]
- Lungu, P.; Mushota, K.; Njelesani, E.; Sukwa, T.; Lakhi, S.; Mwaba, P. Expression of mycobacterium tuberculosis induced SOCS3 and STAT3 and the implications on innate immunity in TB patients vs healthy contacts in high TB/HIV endemic setting: A cross-sectional analytical study. PLoS ONE 2022, 17, e0263624. [Google Scholar] [CrossRef] [PubMed]
- Hersberger, M.; Schlaepfer, E.; Buehler, M.; Bochud, P.Y.; Vernazza, P.; Marti-Jaun, J.; Nemeth, J.; Zwahlen, M.; Schmidlin, K.; Speck, R.F. Polymorphisms of SOCS-1 Are Associated With a Rapid HIV Progression Rate. JAIDS-J. Acquir. Immune Defic. Syndr. 2020, 84, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Ferrando, S.; Segalés, J.; López-Soria, S.; Callén, A.; Merdy, O.; Joisel, F.; Sibila, M. Evaluation of natural porcine circovirus type 2 (PCV2) subclinical infection and seroconversion dynamics in piglets vaccinated at different ages. Vet. Res. 2016, 47, 121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.S.; Qi, B.Z.; Gu, Y.X.; Xu, F.; Du, H.H.; Li, X.L.; Fang, W.H. Porcine Circovirus 2 Deploys PERK Pathway and GRP78 for Its Enhanced Replication in PK-15 Cells. Viruses 2016, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Yan, W.D.; Gu, W.; He, Q.G. The ubiquitin-proteasome system is required for the early stages of porcine circovirus type 2 replication. Virology 2014, 456, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, H.C.; Wang, Q.; Cui, M.; Zhang, C.; Wang, Q.; Liu, X.Y.; Chen, K.P. SOCSs: Important regulators of host cell susceptibility or resistance to viral infection. Z. Naturforschung Sect. C-A J. Biosci. 2023, 78, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Jia, Y.Q.; Ren, J.; Huo, N.; Liu, H.J.; Xiao, S.; Wang, X.L.; Yang, Z.Q. Newcastle Disease Virus Nonstructural V Protein Upregulates SOCS3 Expression to Facilitate Viral Replication Depending on the MEK/ERK Pathway. Front. Cell. Infect. Microbiol. 2019, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.Y.; Wang, M.S.; Cheng, A.C.; Zhao, X.X.; Liu, M.F.; Zhu, D.K.; Chen, S.; Jia, R.Y.; Yang, Q.; Wu, Y.; et al. DHAV-1 Inhibits Type I Interferon Signaling to Assist Viral Adaption by Increasing the Expression of SOCS3. Front. Immunol. 2019, 10, 731. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.G.; Chen, X.X.; Qiao, S.L.; Li, R.; Lu, Q.X.; Geng, R.; Wang, L.; Zhou, E.M.; Zhang, G.P. Porcine reproductive and respiratory syndrome virus increases SOCS3 production via activation of p38/AP-1 signaling pathway to promote viral replication. Vet. Microbiol. 2021, 257, 109075. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.H.; Hong, H.; Yu, H.Z.; Yuan, J.F.; Guo, C.Y.; Cao, H.Y.; Li, W.B. MiR-340 affects gastric cancer cell proliferation, cycle, and apoptosis through regulating SOCS3/JAK-STAT signaling pathway. Immunopharmacol. Immunotoxicol. 2018, 40, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.Y.; Zhao, M.L.; Li, X.Q.; Gao, L.; Cao, H.; Wang, Y.Q.; Zheng, S.J.J. gga-miR-27b-3p enhances type I interferon expression and suppresses infectious bursal disease virus replication via targeting cellular suppressors of cytokine signaling 3 and 6 (SOCS3 and 6). Virus Res. 2020, 281, 197910. [Google Scholar] [CrossRef] [PubMed]
- Maimon, A.; Levi-Yahid, V.; Ben-Meir, K.; Halpern, A.; Talmi, Z.; Priya, S.; Mizraji, G.; Mistriel-Zerbib, S.; Berger, M.; Baniyash, M.; et al. Myeloid cell-derived PROS1 inhibits tumor metastasis by regulating inflammatory and immune responses via IL-10. J. Clin. Investig. 2021, 131, e126089. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Hu, H.; Li, H.S.; Yu, J.; Xiao, Y.; Brittain, G.C.; Zou, Q.; Cheng, X.; Mallette, F.A.; Watowich, S.S.; et al. Noncanonical NF-κB Pathway Controls the Production of Type I Interferons in Antiviral Innate Immunity. Immunity 2014, 40, 342–354. [Google Scholar] [CrossRef] [PubMed]
| Gene Product | Sense Primer (5′ to 3′) | Antisense Primer (5′ to 3′) |
|---|---|---|
| PCV2 | ACCGTTACCGCTGGAGAAGGAAAAA | TGGTTACACGGATATTGTAGTCCTG |
| SOCS3 | TCTAGAATGGTCACCCACAGCAAGTT | GCGGCCGCTTAAAGTGGGGCATCGTACT |
| qSOCS3 | CGAGGCGAACCTGCTGCTT | ATCTTCAACACCCGCCTCT |
| qPCV2 | GATGCGCAGGTTCTTGGTC | CAGGGCCAGAATTCAACCTT |
| β-actin | CTGTCCCTGTATGCCTCTG | ATGTCACGCACGATTTCC |
| qIFN-β | GCTAACAAGTGCATCCTCCAAA | AGCACATCATAGCTCATGGAAAGA |
| qTNF-α | CCTACTGCACTTCGAGGTTATC | GCATACCCACTCTGCCATT |
| Name | Sequence |
|---|---|
| si-SOCS3-1 | GCUUCUCGCUGCAGAGUGAtt |
| UCACUCUGCAGCGAGAAGCtt | |
| si-SOCS3-2 | GAAGAGCCUAUUACAUCUtt |
| UAGAUGUAAUAGGCUCUUCtt | |
| si-SOCS3-3 | CCUGGACUCCUAUGAGAAAtt |
| UUUCUCAUAGGAGUCCAGGtt |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, H.; Wu, Y.; Zhang, X.; Geng, J.; Wu, X.; Li, W.; Zhang, Z.; Song, J.; Zhang, Y.; et al. PCV2 Infection Upregulates SOCS3 Expression to Facilitate Viral Replication in PK-15 Cells. Viruses 2025, 17, 1081. https://doi.org/10.3390/v17081081
Li Y, Liu H, Wu Y, Zhang X, Geng J, Wu X, Li W, Zhang Z, Song J, Zhang Y, et al. PCV2 Infection Upregulates SOCS3 Expression to Facilitate Viral Replication in PK-15 Cells. Viruses. 2025; 17(8):1081. https://doi.org/10.3390/v17081081
Chicago/Turabian StyleLi, Yiting, Hongmei Liu, Yi Wu, Xiaomei Zhang, Juan Geng, Xin Wu, Wengui Li, Zhenxing Zhang, Jianling Song, Yifang Zhang, and et al. 2025. "PCV2 Infection Upregulates SOCS3 Expression to Facilitate Viral Replication in PK-15 Cells" Viruses 17, no. 8: 1081. https://doi.org/10.3390/v17081081
APA StyleLi, Y., Liu, H., Wu, Y., Zhang, X., Geng, J., Wu, X., Li, W., Zhang, Z., Song, J., Zhang, Y., & Chai, J. (2025). PCV2 Infection Upregulates SOCS3 Expression to Facilitate Viral Replication in PK-15 Cells. Viruses, 17(8), 1081. https://doi.org/10.3390/v17081081






