Hemophagocytic Lymphohistiocytosis Triggered by Dengue: A Narrative Review and Individual Patient Data Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
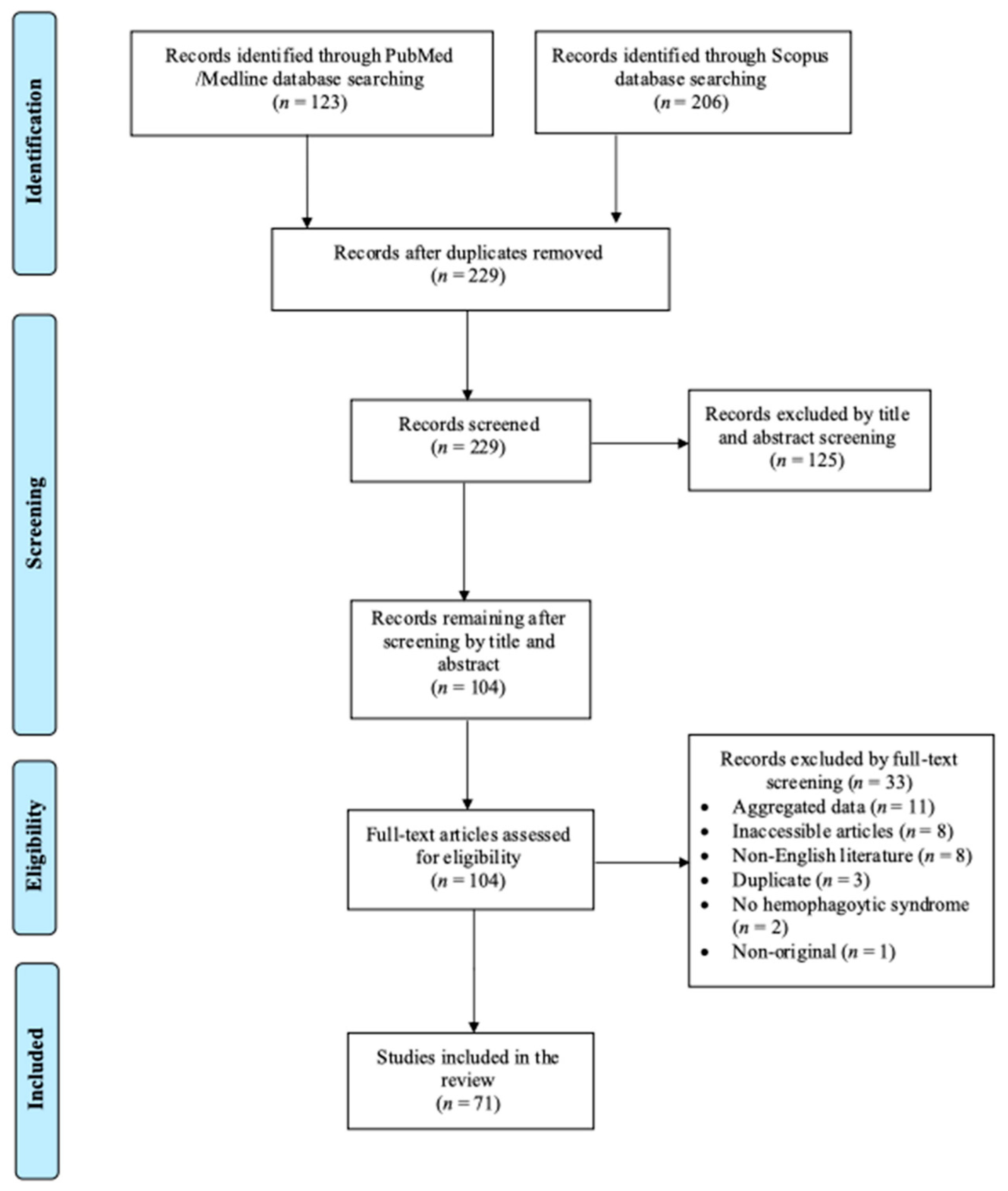
3.1. Included Studies’ Characteristics
3.2. Epidemiology and Clinical Characteristics of HLH Due to Dengue
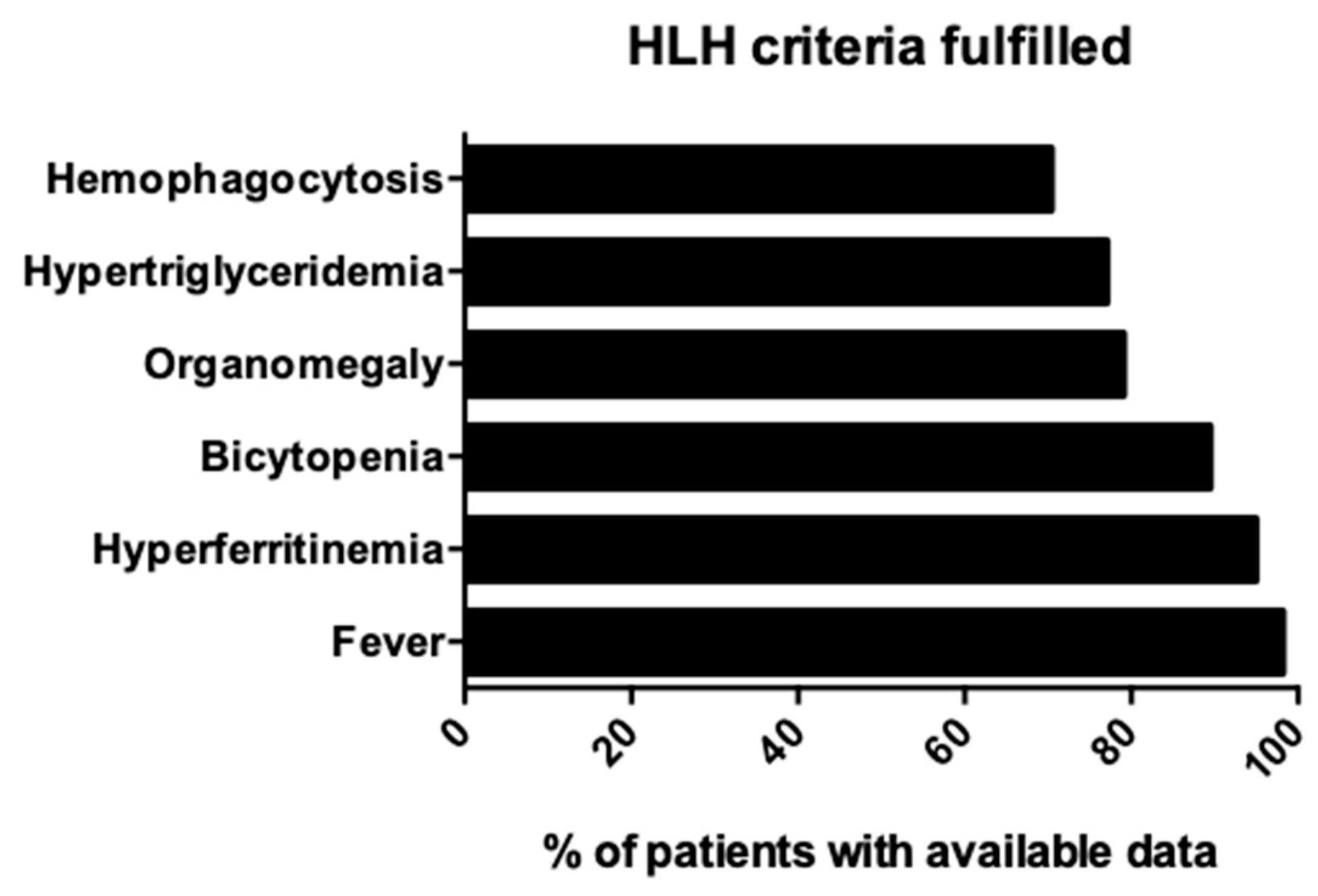
3.3. Microbiology and Diagnosis of HLH Due to Dengue
3.4. Treatment and Outcomes of HLH Due to Dengue
3.5. Statistical Analysis of HLH Due to Dengue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sen, E.S.; Clarke, S.L.N.; Ramanan, A.V. Macrophage Activation Syndrome. Indian J. Pediatr. 2016, 83, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Otrock, Z.K.; Daver, N.; Kantarjian, H.M.; Eby, C.S. Diagnostic Challenges of Hemophagocytic Lymphohistiocytosis. Clin. Lymphoma Myeloma Leuk. 2017, 17, S105–S110. [Google Scholar] [CrossRef]
- Machowicz, R.; Janka, G.; Wiktor-Jedrzejczak, W. Similar but Not the Same: Differential Diagnosis of HLH and Sepsis. Crit. Rev. Oncol. Hematol. 2017, 114, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Berliner, N. Hemophagocytic Lymphohistiocytosis. Annu. Rev. Pathol. 2018, 13, 27–49. [Google Scholar] [CrossRef]
- Ponnatt, T.S.; Lilley, C.M.; Mirza, K.M. Hemophagocytic Lymphohistiocytosis. Arch. Pathol. Lab. Med. 2022, 146, 507–519. [Google Scholar] [CrossRef]
- Ong, L.T.; Balasubramaniam, R. Prevalence and Mortality of Haemophagocytic Lymphohistiocytosis in Dengue Fever: A Systematic Review and Meta-Analysis. Trans. R. Soc. Trop. Med. Hyg. 2024, 118, 711–719. [Google Scholar] [CrossRef]
- Roy, S.K.; Bhattacharjee, S. Dengue Virus: Epidemiology, Biology, and Disease Aetiology. Can. J. Microbiol. 2021, 67, 687–702. [Google Scholar] [CrossRef]
- Kothari, D.; Patel, N.; Bishoyi, A.K. Dengue: Epidemiology, Diagnosis Methods, Treatment Options, and Prevention Strategies. Arch. Virol. 2025, 170, 48. [Google Scholar] [CrossRef]
- Henter, J.-I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and Therapeutic Guidelines for Hemophagocytic Lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef]
- Henter, J.-I. Hemophagocytic Lymphohistiocytosis. N. Engl. J. Med. 2025, 392, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.F.; Chan, J.K.C.; Chan, J.C.W.; Lim, W.W.L.; Wong, W.K. Letter to the Editor: Dengue Virus Infection-associated Hemophagocytic Syndrome. Am. J. Hematol. 1991, 38, 339–340. [Google Scholar] [CrossRef]
- Lu, P.; Hsiao, H.; Tsai, J.; Chen, T.; Chen, T.; Lin, S.; Feng, M. Dengue Virus-Associated Hemophagocytic Syndrome and Dyserythropoiesis: A Case Report. Kaohsiung J. Med. Sci. 2005, 21, 34–39. [Google Scholar] [CrossRef]
- Jain, D.; Singh, T. Dengue Virus Related Hemophagocytosis: A Rare Case Report. Hematology 2008, 13, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Srichaikul, T.; Punyagupta, S.; Kanchanapoom, T.; Chanokovat, C.; Likittanasombat, K.; Leelasiri, A. Hemophagocytic Syndrome in Dengue Hemorrhagic Fever with Severe Multiorgan Complications. J. Med. Assoc. Thai. 2008, 91, 104–109. [Google Scholar] [PubMed]
- Vijayalakshmi, A.M.; Ganesh, V.R.R. Hemophagocytic Syndrome Associated with Dengue Hemorrhagic Fever. Indian Pediatr. 2009, 46, 545. [Google Scholar]
- Joshi, R.; Phatarpekar, A.; Currimbhoy, Z.; Desai, M. Haemophagocytic Lymphohistiocytosis: A Case Series from Mumbai. Ann. Trop. Paediatr. 2011, 31, 135–140. [Google Scholar] [CrossRef]
- Ray, S.; Kundu, S.; Saha, M.; Chakrabarti, P. Hemophagocytic Syndrome in Classic Dengue Fever. J. Glob. Infect. Dis. 2011, 3, 399–401. [Google Scholar] [CrossRef]
- Kapdi, M.; Shah, I. Dengue and Haemophagocytic Lymphohistiocytosis. Scand. J. Infect. Dis. 2012, 44, 708–709. [Google Scholar] [CrossRef]
- Tangnararatchakit, K.; Tirapanich, W.; Tapaneya-Olarn, W.; Sumethkul, V.; Sirachainan, N.; Watcharananan, S.; Leenanupunth, C.; Yoksan, S.; Chuansumrit, A. Severe Nonfebrile Dengue Infection in an Adolescent After Postoperative Kidney Transplantation: A Case Report. Transpl. Proc. 2012, 44, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.H.; Lum, L.C.S.; Omar, S.F.S.; Kan, F.K. Hemophagocytosis in Dengue: Comprehensive Report of Six Cases. J. Clin. Virol. 2012, 55, 79–82. [Google Scholar] [CrossRef]
- Nair, V.; Das, S.; Sharma, A.; Sharma, S.; Sharma, P.; Ray, S.; Bhattacharya, S. A Clinicopathological Analysis of 26 Patients with Infection-Associated Haemophagocytic Lymphohistiocytosis and the Importance of Bone Marrow Phagocytosis for the Early Initiation of Immunomodulatory Treatment. Postgrad. Med. J. 2013, 89, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Morel, Z.; Ramírez, A. Respuesta autoinmune en niños con dengue. Reporte de casos. Reumatol. Clínica 2014, 10, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.M.; Gaul, L.; Muehlenbachs, A.; Hunsperger, E.; Bhatnagar, J.; Lueptow, R.; Santiago, G.A.; Muñoz-Jordan, J.L.; Blau, D.M.; Ettestad, P.; et al. Fatal Hemophagocytic Lymphohistiocytosis Associated with Locally Acquired Dengue Virus Infection—New Mexico and Texas, 2012. MMWR. Morb. Mortal. Wkly. Rep. 2014, 63, 49–54. [Google Scholar] [CrossRef][Green Version]
- Mitra, S.; Bhattacharyya, R. Hemophagocytic Syndrome in Severe Dengue Fever: A Rare Presentation. Indian J. Hematol. Blood Transfus. 2014, 30, 97–100. [Google Scholar] [CrossRef][Green Version]
- De Koninck, A.-S.; Dierick, J.; Steyaert, S.; Taelman, P. Hemophagocytic Lymphohistiocytosis and Dengue Infection: Rare Case Report. Acta Clin. Belg. 2014, 69, 210–213. [Google Scholar] [CrossRef]
- Ribeiro, E.; Kassab, S.; Pistone, T.; Receveur, M.-C.; Fialon, P.; Malvy, D. Primary Dengue Fever Associated with Hemophagocytic Syndrome: A Report of Three Imported Cases, Bordeaux, France. Intern. Med. 2014, 53, 899–902. [Google Scholar] [CrossRef][Green Version]
- Kodan, P.; Chakrapani, M.; Shetty, M.; Pavan, R.; Bhat, P. Hemophagocytic Lymphohistiocytosis Secondary to Infections: A Tropical Experience! J. Postgrad. Med. 2015, 61, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Hein, N.; Bergara, G.H.; Moura, N.B.V.; Cardoso, D.M.; Hirose, M.; Ferronato, A.E.; Pastorino, A.C.; Lo, D.S.; Gilio, A.E. Dengue Fever as a Cause of Hemophagocytic Lymphohistiocytosis. ACR 2015, 5, 33–36. [Google Scholar] [CrossRef]
- Arshad, U.; Ahmad, S.Q.; Khan, F. Hemophagocytic Lymphohistiocytosis in a Patient with Dengue Infection. Hematol. Oncol. Stem Cell Ther. 2015, 8, 189–190. [Google Scholar] [CrossRef]
- Khurram, M.; Faheem, M.; Umar, M.; Yasin, A.; Qayyum, W.; Ashraf, A.; Zahid Khan, J.; Hasnain Yasir, A.; Ansari, Y.; Asad, M.; et al. Hemophagocytic Lymphohistiocytosis Complicating Dengue and Plasmodium vivax Coinfection. Case Rep. Med. 2015, 2015, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Phuakpet, K.; Sanpakit, K.; Vathana, N.; Takpradit, C.; Chokephaibulkit, K.; Viprakasit, V. Hemophagocytic Lymphohistiocytosis Following Dengue Hemorrhagic Fever in H b H/H b Constant Spring Patient. Pediatr. Int. 2015, 57, 763–765. [Google Scholar] [CrossRef] [PubMed]
- Wan Jamaludin, W.F.; Periyasamy, P.; Wan Mat, W.R.; Abdul Wahid, S.F. Dengue Infection Associated Hemophagocytic Syndrome: Therapeutic Interventions and Outcome. J. Clin. Virol. 2015, 69, 91–95. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hikone, M.; Sakamoto, N.; Iwabuchi, S.; Kashiura, M.; Takasaki, T.; Fujita, H.; Ohnishi, K. Dengue-Associated Hemophagocytic Syndrome in a Japanese Traveler: A Case Report. J. Travel. Med. 2015, 22, 64–66. [Google Scholar] [CrossRef][Green Version]
- Ab-Rahman, H.A.; Wong, P.-F.; Rahim, H.; Abd-Jamil, J.; Tan, K.-K.; Sulaiman, S.; Lum, C.-S.; Syed-Omar, S.-F.; AbuBakar, S. Dengue Death with Evidence of Hemophagocytic Syndrome and Dengue Virus Infection in the Bone Marrow. SpringerPlus 2015, 4, 665. [Google Scholar] [CrossRef]
- Ellis, E.M.; Sharp, T.M.; Pérez-Padilla, J.; González, L.; Poole-Smith, B.K.; Lebo, E.; Baker, C.; Delorey, M.J.; Torres-Velasquez, B.; Ochoa, E.; et al. Incidence and Risk Factors for Developing Dengue-Associated Hemophagocytic Lymphohistiocytosis in Puerto Rico, 2008–2013. PLoS Negl. Trop. Dis. 2016, 10, e0004939. [Google Scholar] [CrossRef]
- Lakhotia, M.; Pahadiya, H.R.; Gandhi, R.; Prajapati, G.R.; Choudhary, A. Stuck with Pancytopenia in Dengue Fever: Evoke for Hemophagocytic Syndrome. Indian J. Crit. Care Med. 2016, 20, 55–56. [Google Scholar] [CrossRef]
- Koshy, M.; Mishra, A.K.; Agrawal, B.; Kurup, A.R.; Hansdak, S.G. Dengue Fever Complicated by Hemophagocytosis. Oxf. Med. Case Rep. 2016, 2016, 121–124. [Google Scholar] [CrossRef]
- Nandi, M.; Roy, S.; Das, M.; Datta, C. Neonatal-Onset Hemophagocytic Lymphohistiocytosis Associated with Primary Dengue Infection. Med. J. DY Patil. Univ. 2016, 9, 639. [Google Scholar] [CrossRef]
- Anam, A.M.; Rabbani, R.; Shumy, F. Expanded Dengue Syndrome: Three Concomitant Uncommon Presentations in the Same Patient. Trop. Doct 2017, 47, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.M.; Song, J.Y.; Kim, W.; Choi, M.J.; Jeon, J.H.; Kang, S.; Jung, E.; Noh, J.Y.; Cheong, H.J.; Kim, W.J. Dengue-Associated Hemophagocyticlymphohistiocytosis in an Adult: A Case Report and Literature Review. Medicine 2017, 96, e6159. [Google Scholar] [CrossRef] [PubMed]
- Ray, U.; Dutta, S.; Mondal, S.; Bandyopadhyay, S. Severe Dengue Due to Secondary Hemophagocytic Lymphohistiocytosis: A Case Study. IDCases 2017, 8, 50–53. [Google Scholar] [CrossRef]
- Krithika, M.; Amboiram, P.; Latha, S.M.; Ninan, B.; Suman, F.R.; Scott, J. Neonate with Haemophagocytic Lymphohistiocytosis Secondary to Dengue Infection: A Case Report. Trop. Dr. 2017, 47, 253–255. [Google Scholar] [CrossRef]
- Jasmine, Y.S.Y.; Lee, S.L.; Kan, F.K. Infection Associated Haemophagocytic Syndrome in Severe Dengue Infection—A Case Series in a District Hospital. Med. J. Malays. 2017, 72, 62–64. [Google Scholar]
- Kam, K.; Soh, S.Y.; Bhattacharyya, R. Dengue-Associated Hemophagocytic Lymphohistiocytosis: A Rare Complication of a Common Infection in Singapore. J. Pediatr. Hematol. Oncol. 2018, 40, e377–e379. [Google Scholar] [CrossRef]
- Ray, U.; Dutta, S.; Bandyopadhyay, S.; Mondal, S. Infections and HLH—Experience from a Tertiary Care Centre. J. Assoc. Physicians India 2019, 67, 54–57. [Google Scholar]
- Mushtaque, R.S.; Ahmad, S.M.; Mushtaque, R.; Baloch, S. A Curious Case of Dengue Fever: A Case Report of Unorthodox Manifestations. Case Rep. Med. 2020, 2020, 1–4. [Google Scholar] [CrossRef]
- Thadchanamoorthy, V.; Dayasiri, K. Dengue Fever Associated Haemophagocytic Lymphohistiocytosis: A Report of Two Children. Cureus 2020. [Google Scholar] [CrossRef]
- Narayanasami, E.; Umakanth, M.; Suganthan, N. Dengue Hemorrhagic Fever Complicated with Hemophagocytic Lymphohistiocytosis in an Adult with Diabetic Ketoacidosis. Cureus 2020, 12, e10172. [Google Scholar] [CrossRef] [PubMed]
- Ishak, S.H.; Yaacob, L.H.; Ishak, A. Severe Dengue with Hemophagocytosis Syndrome. Malays. Fam. Physician 2020, 15, 47–49. [Google Scholar]
- Agrawal, G.; Wazir, S.; Sachdeva, A.; Kumar, S. Primary Dengue Infection Triggered Haemophagocytic Lymphohistiocytosis in a Neonate. BMJ Case Rep. 2020, 13, e236881. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Rajappan, M.; Zaid, M.; Ong, E.L.C. Dengue Fever Complicated by Hemophagocytic Lymphohistiocytosis: Report of 2 Cases and Bone Marrow Findings. Clin. Case Rep. 2020, 8, 3426–3430. [Google Scholar] [CrossRef]
- Kanitkar, T.; Richardson, C.; Scobie, A.; Ireson, A.; Singh, A.; Jacobs, M.; Buckley, J.; Spiro, M. Fatal Primary Dengue-Induced Haemophagocytic Lymphohistiocytosis (HLH) in a Returning Traveller from India Treated with Anakinra for the First Time. Clin. Infect. Pract. 2020, 7–8, 100043. [Google Scholar] [CrossRef]
- Takkinsatian, P.; Sowithayasakul, P.; Prommalikit, O. Dengue Associated Haemophagocytic Lymphohystiocytosis: An Often-Missed Complication of a Common Infection. Med. J. Malays. 2020, 75, 588–590. [Google Scholar]
- Islam, Q.T.; Sagor, H.B.; Tuli, T.C. Haemophagocytic Lymphohistiocytosis Associated with Dengue Fever—A Case Series. J. Med. 2020, 21, 123–126. [Google Scholar] [CrossRef]
- Munshi, A.; Alsuraihi, A.; Balubaid, M.; Althobaiti, M.; Althaqafi, A. Dengue-Induced Hemophagocytic Lymphohistiocytosis: A Case Report and Literature Review. Cureus 2021, 13, e20172. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y. A Fatal Case of Dengue-Associated Hemophagocytic Lymphohistiocytosis and Retroperitoneal Hematoma in a Patient With Autoimmune Hemolytic Anemia. Cureus 2021, 13, e15001. [Google Scholar] [CrossRef]
- Krishnappa, A.; Munusamy, J.; Ray, S.; Rameshbabu, M.; Bhatia, P.; Roy, P.S.; Sundaram, V.; Kumar, P. Neonatal Dengue with HLH: Perks of Early Diagnosis and Management. J. Pediatr. Hematol. Oncol. 2021, 43, e770–e773. [Google Scholar] [CrossRef]
- Devi, K.; Ali, N. Case Report: Primary Hemophagocytic Syndrome Triggered by Dengue Infection. IDCases 2021, 26, e01275. [Google Scholar] [CrossRef]
- Kumar, H.C.K.; Kumar, K.J.; Balaji, S. Hemophagocytic Lymphohistiocytosis Associated with Coinfection of Scrub Typhus and Dengue Fever in a Child: A Case Report. Mediterr. J. Infect. Microb. Antimicrob. 2021, 10, 20. [Google Scholar] [CrossRef]
- Cheo, S.; Abdul Rashid, W.; Ho, C.; Ahmad Akhbar, R.Z.; Low, Q.; Rajahram, G.S. Haemophagocytic Lymphohistiocytosis Secondary to Dengue Fever: A Case Report. Hong Kong Med. J. 2021, 27, 287–289. [Google Scholar] [CrossRef]
- Jose, P.-M.M.; Paola, Z.-S.; Eduardo, D.-G.; Arturo, S.-M.M.O.; Fernando, B.-G. A Case of Coinfection of a Pediatric Patient with Acute SARS-CoV-2 with MIS-C and Severe DENV-2 in Mexico: A Case Report. BMC Infect. Dis. 2021, 21, 1072. [Google Scholar] [CrossRef] [PubMed]
- Kamineni, M.; Pai, T.; D’Sa, S.; Bhat, K. Acute Dengue Fever in a Neonate Secondary to Perinatal Transmission. Indian J. Nephrol. 2020, 12, 100–103. [Google Scholar] [CrossRef]
- Saw, Y.T.; Lee, H.G. Concurrent COVID-19 and Dengue with Hyperferritinaemia: A Case Report. Med. J. Malays. 2021, 76, 918–920. [Google Scholar]
- Ren, D.; Ong, S.W.X.; Batac, J.A.L.; Fan, B.E.; Vasoo, S. Haemophagocytic Lymphohistiocytosis in Dengue Fever. Lancet Infect. Dis. 2021, 21, 437. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Shukla, S.; Sontakke, T.; Vs, I.; Bagga, C.; Dronamraju, S.; Giri, A. A Case Report of Hemophagocytic Lymphohistiocytosis (HLH)—An Unusual Complication of Dengue Infection. Cureus 2022, 14, e26504. [Google Scholar] [CrossRef] [PubMed]
- Srivatsav, S.; Mahalingam, S.; Ramineni, P.; Manya, S. Dengue and Plasmodium Falciparum Coinfection with Secondary Hemophagocytic Lymphohistiocytosis in a 3-Year-Old Boy: A Clinical Conundrum. J. Pediatr. Hematol. Oncol. 2022, 44, e253–e254. [Google Scholar] [CrossRef]
- Jha, V.K.; Khurana, H.; Balakrishnan, A. Prolonged Fever and Pancytopenia in a Case of Severe Dengue May Be Secondary Hemophagocytic Lymphohistiocytosis. Med. J. Armed Forces India 2022, 78, S300–S302. [Google Scholar] [CrossRef]
- Porel, R.; Kumar, V.; Agarwal, K.; Biswas, R.; Ojha, V.S. Secondary Hemophagocytic Lymphohistiocytosis: A Series of Three Cases. Cureus 2023, 15, e46044. [Google Scholar] [CrossRef]
- Ray, S.; Kumar, M.; Mahajan, N.; Khatri, A. Paediatric Hemophagocytic Lymphohistiocytosis: A Case Series with a Diverse Spectrum from a Resource-Limited Setting. Cureus 2023, 15, e45140. [Google Scholar] [CrossRef]
- Krishnan, G.; Gosavi, S.; Gujral, M.; Basheer, N.; Kumar, B.; Jain, P. Hemophagocytic Lymphohistiocytosis: A Scourge for the Physician and Bane to the Bone Marrow. Ann. Afr. Med. 2023, 22, 532–536. [Google Scholar] [CrossRef]
- Arora, A.; Verma, S.; Khot, N.; Chalipat, S.; Agarkhedkar, S.; Kiruthiga, K.G. A Case Report on CNS Hemophagocytic Lymphohistiocytosis in an Infant with Dengue Hemorrhagic Fever. Cureus 2023, 15, e34773. [Google Scholar] [CrossRef]
- Mizutani, N.; Kenzaka, T.; Nishisaki, H. Dengue Fever Complicated with Hemophagocytic Lymphohistiocytosis: A Case Report of Resolution with Steroid-Sparing Supportive Care. TropicalMed 2023, 8, 497. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, C.; Karunathilake, P.; Abeyagunawardena, S.; Ralapanawa, U.; Jayalath, T. Hemophagocytic Lymphohistiocytosis as a Rare Complication of Dengue Haemorrhagic Fever: A Case Report. J. Med. Case Rep. 2023, 17, 224. [Google Scholar] [CrossRef] [PubMed]
- Shankar, M.; Gurusiddiah, S.C.; Nayaka, M.; Aralapuram, K. An Uncommon Complication of a Common Tropical Infection in a Kidney Transplant Recipient—A Case Report. IJN 2023, 34, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Kazi, A.N.; Ahmed, M.; Wasim, M.A.; Abbasi, L.I.; Herekar, F.F.; Patel, M.J. A Vector Borne, Airborne and Food Borne Infection with Secondary Hemophagocytic Lymphohistocytosis: Case of Triple Infections in an Immuno-Competent Patient. Indian J. Med. Microbiol. 2024, 52, 100752. [Google Scholar] [CrossRef] [PubMed]
- Dinkar, A.; Singh, J.; Kumar, N.; Kumar, K.; Singh, S.K. Dengue-Related Hemophagocytic Lymphohistiocytosis in an Adult: A Case Report and Brief Update. Avicenna J. Med. 2024, 14, 175–178. [Google Scholar] [CrossRef]
- Raza, M.; Ali, S. Hemophagocytic Lymphohistiocytosis (HLH): A Rare Complication of Dengue Hemorrhagic Fever. Cureus 2024, 16, e70895. [Google Scholar] [CrossRef]
- Ramamoorthy, L.; Sivakumar, N.; Murugesan, L.; Kumar, A. Hemophagocytic Lymphohistiocytosis Secondary to Dengue Fever in a Pediatric Patient: A Case Report. Cureus 2024, 16, e59165. [Google Scholar] [CrossRef]
- Mayurathan, P. Dengue Hemorrhagic Fever Causing Postpartum Hemorrhage and Hemophagocytic Lymphohistiocytosis in a Young Woman: A Case Report. Cureus 2024, 16, e53841. [Google Scholar] [CrossRef]
- Mateen, S.; Mishra, A.; Singh, S.; Jabeen, F. Severe Dengue, Aneurysmal Sub-Arachnoid Hemorrhage, and Hemophagocytic Lymphohistiocytosis: A Rare Case Combination. Einstein 2025, 23, eRC1209. [Google Scholar] [CrossRef]
- Sharma, R.; Ray, S.; Chattopadhyay, A.; Khatri, A. Dengue-Associated Hemophagocytic Lymphohistiocytosis: The Many Faces of Expanded Dengue Syndrome. Clin. Pediatr. 2024, 64, 00099228241309192. [Google Scholar] [CrossRef] [PubMed]
- La Marle, S.; Richard-Colmant, G.; Fauvernier, M.; Ghesquières, H.; Hot, A.; Sève, P.; Jamilloux, Y. Mortality and Associated Causes in Hemophagocytic Lymphohistiocytosis: A Multiple-Cause-of-Death Analysis in France. JCM 2023, 12, 1696. [Google Scholar] [CrossRef]
- Annan, E.; Treviño, J.; Zhao, B.; Rodriguez-Morales, A.J.; Haque, U. Direct and Indirect Effects of Age on Dengue Severity: The Mediating Role of Secondary Infection. PLoS Negl. Trop. Dis. 2023, 17, e0011537. [Google Scholar] [CrossRef]
- Sohail, A.; Zhong, S.; Nguyen, P.-Y.; McGuinness, S.L.; Leder, K. Dengue Fever in Immunocompromised Patients: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2024, 149, 107272. [Google Scholar] [CrossRef]
- See, K.C. Dengue-Associated Hemophagocytic Lymphohistiocytosis: A Narrative Review of Its Identification and Treatment. Pathogens 2024, 13, 332. [Google Scholar] [CrossRef]
- Tuiskunen Bäck, A.; Lundkvist, Å. Dengue Viruses—An Overview. Infect. Ecol. Epidemiol. 2013, 3, 19839. [Google Scholar] [CrossRef]
- Murray, N.E.A.; Quam, M.B.; Wilder-Smith, A. Epidemiology of Dengue: Past, Present and Future Prospects. Clin. Epidemiol. 2013, 5, 299–309. [Google Scholar] [CrossRef]
- Cattaneo, P.; Salvador, E.; Manica, M.; Barzon, L.; Castilletti, C.; Di Gennaro, F.; Huits, R.; Merler, S.; Poletti, P.; Riccardo, F.; et al. Transmission of Autochthonous Aedes-Borne Arboviruses and Related Public Health Challenges in Europe 2007–2023: A Systematic Review and Secondary Analysis. Lancet Reg. Health—Eur. 2025, 51, 101231. [Google Scholar] [CrossRef]
- Buchs, A.; Conde, A.; Frank, A.; Gottet, C.; Hedrich, N.; Lovey, T.; Shindleman, H.; Schlagenhauf, P. The Threat of Dengue in Europe. New Microbes New Infect. 2022, 49–50, 101061. [Google Scholar] [CrossRef] [PubMed]
- Masood, M.; Siddique, A.; Krishnamoorthi, R.; Kozarek, R.A. Liver Dysfunction in Adult Hemophagocytic Lymphohistiocytosis: A Narrative Review. Adv. Ther. 2024, 41, 553–566. [Google Scholar] [CrossRef]
- Kohli, S.; Chadha, R.; Rastogi, N.; Yadav, S.P. High Serum Ferritin Alone as a Predictor of Mortality and Hemophagocytic Lymphohistiocytosis. eJHaem 2021, 2, 136–138. [Google Scholar] [CrossRef] [PubMed]
- La Rosée, P.; La Rosée, F. HLH: Diagnostics Revisited and Improved. Blood 2024, 144, 2274–2275. [Google Scholar] [CrossRef] [PubMed]
- Raafat, N.; Blacksell, S.D.; Maude, R.J. A Review of Dengue Diagnostics and Implications for Surveillance and Control. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.B.; Allen, C.E.; Weitzman, S.; Filipovich, A.H.; McClain, K.L. How I Treat Hemophagocytic Lymphohistiocytosis. Blood 2011, 118, 4041–4052. [Google Scholar] [CrossRef]
- La Rosée, P.; Horne, A.; Hines, M.; Von Bahr Greenwood, T.; Machowicz, R.; Berliner, N.; Birndt, S.; Gil-Herrera, J.; Girschikofsky, M.; Jordan, M.B.; et al. Recommendations for the Management of Hemophagocytic Lymphohistiocytosis in Adults. Blood 2019, 133, 2465–2477. [Google Scholar] [CrossRef]
- Adrizain, R.; Setiabudi, D.; Chairulfatah, A. The Inappropriate Use of Antibiotics in Hospitalized Dengue Virus-Infected Children with Presumed Concurrent Bacterial Infection in Teaching and Private Hospitals in Bandung, Indonesia. PLoS Negl. Trop. Dis. 2019, 13, e0007438. [Google Scholar] [CrossRef]
- Giang, H.T.N.; Banno, K.; Minh, L.H.N.; Trinh, L.T.; Loc, L.T.; Eltobgy, A.; Tai, L.L.T.; Khan, A.; Tuan, N.H.; Reda, Y.; et al. Dengue Hemophagocytic Syndrome: A Systematic Review and Meta-Analysis on Epidemiology, Clinical Signs, Outcomes, and Risk Factors. Rev. Med. Virol. 2018, 28, e2005. [Google Scholar] [CrossRef]
- Kan, F.K.; Tan, C.C.; Von Bahr Greenwood, T.; Khalid, K.E.; Supramaniam, P.; Hed Myrberg, I.; Tan, L.H.; Henter, J.-I. Dengue Infection Complicated by Hemophagocytic Lymphohistiocytosis: Experiences from 180 Patients with Severe Dengue. Clin. Infect. Dis. 2020, 70, 2247–2255. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | All Patients (n = 133) * | Lived (n = 88) * | Died (n = 21) * | p-Value |
|---|---|---|---|---|
| Age, years, median (IQR) | 18 (8–33.8) | 25 (14–36.5) | 19 (6.5–40.5) | 0.4653 |
| Male gender, n (%) | 75/132 (56.8) | 54 (61.4) | 9 (42.9) | 0.1447 |
| Predisposing factors | ||||
| Immunosuppression, n (%) | 3/105 (2.9) | 1/64 (1.6) | 2/17 (11.8) | 0.11 |
| Previous HLH, n (%) | 1/105 (0.9) | 1/64 (1.6) | 0/17 (0) | 1 |
| Clinical characteristics | ||||
| Fever, n (%) | 125/129 (96.9) | 80/84 (95.2) | 21 (100) | 0.5814 |
| Poor feeding/anorexia, n (%) | 17/93 (18.3) | 12/73 (16.4) | 5/18 (27.8) | 0.3141 |
| Jaundice, n (%) | 11/93 (11.8) | 4/73 (5.5) | 7/18 (38.9) | 0.0008 |
| Organomegaly, n (%) | 62/95 (65.3) | 50/75 (66.7) | 11/18 (61.1) | 0.7832 |
| Lymphadenopathy, n (%) | 6/95 (6.3) | 5/75 (6.7) | 1/18 (5.6) | 1 |
| Lethargy/drowsiness, n (%) | 18/93 (19.4) | 11/73 (15.1) | 7/18 (38.9) | 0.0425 |
| Respiratory symptoms, n (%) | 31/93 (33.3) | 22/73 (30.1) | 8/18 (44.4) | 0.2722 |
| Fatigue/weakness, n (%) | 29/93 (31.2) | 24/73 (32.9) | 5/18 (27.8) | 1 |
| Bleeding, n (%) | 23/93 (24.7) | 16/73 (21.9) | 5/18 (27.8) | 0.7828 |
| Skin rash, n (%) | 28/93 (30.1) | 23/73 (31.5) | 4/18 (22.2) | 0.5697 |
| Neurological symptoms, n (%) | 11/93 (11.8) | 7/73 (9.6) | 4/18 (22.2) | 0.2180 |
| Gastrointestinal symptoms, n (%) | 35/93 (37.6) | 30/73 (41.1) | 5/18 (27.8) | 0.4188 |
| Abdominal pain, n (%) | 31/93 (33.3) | 27/73 (37) | 4/18 (22.2) | 0.2794 |
| Laboratory characteristics | ||||
| White blood cells K/mL, median (IQR) | 2900 (1800–4500) | 2500 (1700–4350) | 3600 (2950–9450) | 0.0459 |
| Hemoglobin g/dL, median (IQR) | 8.9 (7.4–10.9) | 9 (7.7–11.4) | 8.1 (7–9.9) | 0.1996 |
| Platelets K/mL, median (IQR) | 30,000 (19,000–44,500) | 28,000 (18,000–40,000) | 44,500 (21,950–68,675) | 0.0995 |
| AST U/mL, median (IQR) | 966 (188.5–2144) | 919 (198–1886) | 2390 (363–11,921) | 0.1586 |
| ALT U/mL, median (IQR) | 408.5 (197.8–1220) | 382 (186.5–777) | 3074 (1283–7834) | 0.0002 |
| LDH U/mL, median (IQR) | 1118 (500.8–2073) | 1082 (470–1822) | 5166 (390.3–7585) | 0.3891 |
| Triglycerides md/dL, median (IQR) | 295 (234–398.3) | 295 (217.5–385.8) | 283.4 (217.8–590) | 0.9116 |
| C-reactive protein mg/dL, median (IQR) | 33 (5–86.4) | 24 (4.1–54) | 52.2 (12.3–92) | 0.6018 |
| Fibrinogen mg/dL, median (IQR) | 155.9 (109.3–205.8) | 170.8 (120.8–203.3) | 100 (67.6–135) | 0.0045 |
| Ferritin ng/dL, median (IQR) | 28,060 (9270–75,031) | 34,951 (9600–93,026) | 40,000 (18,250–93,273) | NA |
| Dengue type | ||||
| DENV-1, n (%) | 27/47 (57.4) | 16/22 (72.7) | 2/8 (25) | 0.0342 |
| DENV-2, n (%) | 3/47 (6.4) | 2/22 (9.1) | 1/8 (12.5) | 1 |
| DENV-3, n (%) | 8/47 (17) | 6/22 (18.2) | 4/8 (50) | 0.1580 |
| DENV-4, n (%) | 9/47 (19.1) | 0/22 (0) | 1/8 (12.5) | 0.2667 |
| Polymicrobial, n (%) | 15/112 (13.4) | 9/71 (12.7) | 6/18 (33.3) | 0.0708 |
| Complications | ||||
| Sepsis, n (%) | 56/104 (53.8) | 37/81 (45.7) | 18 (85.7) | 0.0011 |
| Organ dysfunction, n (%) | 54/105 (51.4) | 36/82 (43.9) | 17 (81) | 0.003 |
| Shock, n (%) | 30/98 (30.6) | 16/77 (20.8) | 13/19 (68.4) | 0.0001 |
| Need for ICU, n (%) | 21/90 (23.3) | 8/72 (11.1) | 13/16 (81.3) | <0.0001 |
| Treatment | ||||
| Empirical antibiotic therapy, n (%) | 31/108 (28.7) | 21/87 (24.1) | 9/19 (47.4) | 0.0522 |
| IVIG, n (%) | 24/110 (21.8) | 22/88 (25) | 2 (9.5) | 0.1523 |
| Cyclosporin A, n (%) | 3/106 (2.8) | 3/84 (3.6) | 0 (0) | 1 |
| Etoposide, n (%) | 7/106 (6.6) | 5/84 (6) | 2 (9.5) | 0.625 |
| Steroids, n (%) | 68/106 (64.2) | 56/84 (66.7) | 11 (52.4) | 0.4361 |
| Fresh frozen plasma, n (%) | 19/104 (18.3) | 13/82 (15.9) | 6 (28.6) | 0.2105 |
| Plasma exchange, n (%) | 5/104 (4.8) | 2/82 (2.4) | 3 (14.3) | 0.0563 |
| Outcomes | ||||
| Deaths overall, n (%) | 21/109 (19.3) | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sourris, A.; Vorria, A.; Kypraiou, D.; Tsantes, A.G.; Ioannou, P. Hemophagocytic Lymphohistiocytosis Triggered by Dengue: A Narrative Review and Individual Patient Data Meta-Analysis. Viruses 2025, 17, 1047. https://doi.org/10.3390/v17081047
Sourris A, Vorria A, Kypraiou D, Tsantes AG, Ioannou P. Hemophagocytic Lymphohistiocytosis Triggered by Dengue: A Narrative Review and Individual Patient Data Meta-Analysis. Viruses. 2025; 17(8):1047. https://doi.org/10.3390/v17081047
Chicago/Turabian StyleSourris, Angelos, Alexandra Vorria, Despoina Kypraiou, Andreas G. Tsantes, and Petros Ioannou. 2025. "Hemophagocytic Lymphohistiocytosis Triggered by Dengue: A Narrative Review and Individual Patient Data Meta-Analysis" Viruses 17, no. 8: 1047. https://doi.org/10.3390/v17081047
APA StyleSourris, A., Vorria, A., Kypraiou, D., Tsantes, A. G., & Ioannou, P. (2025). Hemophagocytic Lymphohistiocytosis Triggered by Dengue: A Narrative Review and Individual Patient Data Meta-Analysis. Viruses, 17(8), 1047. https://doi.org/10.3390/v17081047







