Enterovirus Detection Trends Based on Respiratory Specimens from a Single Tertiary Hospital in Korea (2018–2024): A Retrospective Study Using Multiplex PCR Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. PCR Testing and Enterovirus Detection
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Annual Trends
3.2. Monthly and Seasonal Distribution
3.3. Distribution by Sex
3.4. Age and Demographic Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EV | Enterovirus |
| COVID-19 | Coronavirus disease-2019 |
| NPI | Non-pharmaceutical intervention |
| SOP | Standard operating procedure |
| LIS | Laboratory information system |
| ICH | International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use |
| IQR | Interquartile range |
| ICU | Intensive care unit |
| RSV | Respiratory syncytial virus |
References
- Jartti, M.; Flodström-Tullberg, M.; Hankaniemi, M.M. Enteroviruses: Epidemic potential, challenges and opportunities with vaccines. J. Biomed. Sci. 2024, 31, 73. [Google Scholar] [CrossRef] [PubMed]
- Grizer, C.S.; Messacar, K.; Mattapallil, J.J. Enterovirus-D68-A reemerging non-polio enterovirus that causes severe respiratory and neurological disease in children. Front. Virol. 2024, 4, 1328457. [Google Scholar] [CrossRef]
- Whitehouse, E.R.; Lopez, A.; English, R.; Getachew, H.; Ng, T.F.F.; Emery, B.; Rogers, S.; Kidd, S. Surveillance for acute flaccid myelitis—United States, 2018–2022. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, C.K.; Egeskov-Cavling, A.M.; Jepsen, M.P.G.; Lange, T.; Krause, T.G.; Nygaard, U.; Fischer, T.K. Changing rates but persisting seasons: Patterns of enterovirus infections in hospitalizations and outpatient visits in Denmark 2015–2022. Front. Virol. 2024, 4, 1346352. [Google Scholar] [CrossRef]
- Choi, Q.; Kim, H.J. Respiratory viruses co-detection on a multiplex RT-PCR panel: A comparative clinical study. Clin. Lab. 2024, 70, 1294. [Google Scholar] [CrossRef] [PubMed]
- Kriger, O.; Weil, M.; Fratty, S.I.; Leshem, E.; Gueta, I.; Sofer, D.; Amit, S. Separating the wheat from the chaff—Optimizing the diagnosis of enterovirus-associated meningitis. J. Clin. Virol. 2023, 165, 105522. [Google Scholar] [CrossRef] [PubMed]
- Fourgeaud, J.; Toubiana, J.; Chappuy, H.; Delacourt, C.; Moulin, F.; Parize, P.; Scemla, A.; Abid, H.; Leruez-Ville, M.; Frange, P. Impact of public health measures on the post-COVID-19 respiratory syncytial virus epidemics in France. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Teo, K.W.; Patel, D.; Sisodia, S.; Roland, D.; Gaillard, E.A.; Tang, J.W. Rhinovirus persistence during the COVID-19 pandemic-Impact on pediatric acute wheezing presentations. J. Med. Virol. 2022, 94, 5547–5552. [Google Scholar] [CrossRef] [PubMed]
- Pariani, E.; Piralla, A.; Pellegrinelli, L.; Giardina, F.; Porrello, V.N.; Romano, G.; Galli, C.; Sandri, L.; Ferrari, G.; Binda, S.; et al. Enhanced laboratory surveillance of respiratory infection disclosed the rapid rise of enterovirus D68 cases, northern Italy, August to September 2024. Eurosurveillance 2024, 29, 2400645. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, J.; Nie, W.; Tian, D.; Ye, Q. Study on the epidemiological characteristics of enterovirus among pediatric patients in Hangzhou, China: A comparison between the pre-COVID-19, COVID-19 pandemic, and post-COVID-19 periods. J. Med. Virol. 2024, 96, e29412. [Google Scholar] [CrossRef] [PubMed]
- Käding, N.; Waldeck, F.; Meier, B.; Boutin, S.; Borsche, M.; Balck, A.; Föh, B.; Kramer, J.; Klein, C.; Katalinic, A.; et al. Influence of non-pharmaceutical interventions during the COVID-19 pandemic on respiratory viral infections-A prospective population-based cohort study. Front. Public Health 2024, 12, 1415778. [Google Scholar] [CrossRef] [PubMed]
- Smedberg, J.R.; DiBiase, L.M.; Hawken, S.E.; Allen, A.; Mohan, S.; Santos, C.; Smedberg, T.; Barzin, A.H.; Wohl, D.A.; Miller, M.B. Reduction and persistence of co-circulating respiratory viruses during the SARS-CoV-2 pandemic. Am. J. Infect. Control. 2022, 50, 1064–1066. [Google Scholar] [CrossRef] [PubMed]
- Rankin, D.A.; Spieker, A.J.; Perez, A.; Stahl, A.L.; Rahman, H.K.; Stewart, L.S.; Schuster, J.E.; Lively, J.Y.; Haddadin, Z.; Probst, V.; et al. Circulation of rhinoviruses and/or enteroviruses in pediatric patients with acute respiratory illness before and during the COVID-19 pandemic in the US. JAMA Netw. Open 2023, 6, e2254909. [Google Scholar] [CrossRef] [PubMed]
- Han, J.S.; Chung, Y.N.; Kim, J.K. Retrospective Single-Center Study on the Epidemiological Characteristics of Influenza B Infections in Korea (2007–2024): Analysis of Sex, Age, and Seasonal Patterns. Microorganisms 2025, 13, 1141. [Google Scholar] [CrossRef] [PubMed]
- Han, J.S.; Kim, H.H.; Jeon, J.S.; Kim, J.K. Resurgence and seasonal patterns of RSV-B during the COVID-19 era: An 18-year retrospective hospital-based study. Eur. J. Clin. Microbiol. Infect. Dis. 2025, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Benschop, K.S.; Albert, J.; Anton, A.; Andrés, C.; Aranzamendi, M.; Armannsdóttir, B.; Bailly, J.L.; Baldanti, F.; Baldvinsdóttir, G.E.; Beard, S.; et al. Re-emergence of enterovirus D68 in Europe after easing the COVID-19 lockdown, September 2021. Eurosurveillance 2021, 26, 2100998. [Google Scholar] [CrossRef] [PubMed]
- Fall, A.; Gallagher, N.; Morris, C.P.; Norton, J.M.; Pekosz, A.; Klein, E.; Mostafa, H.H. Circulation of Enterovirus D68 during period of increased influenza-like illness, Maryland, USA, 2021. Emerg. Infect. Dis. 2022, 28, 1525–1527. [Google Scholar] [CrossRef] [PubMed]
- Simoes, M.P.; Hodcroft, E.B.; Simmonds, P.; Albert, J.; Alidjinou, E.K.; Ambert-Balay, K.; Andrés, C.; Antón, A.; Auvray, C.; Bailly, J.L.; et al. Epidemiological and clinical insights into the Enterovirus D68 upsurge in Europe 2021–2022 and emergence of novel B3-derived lineages, ENPEN multicentre study. J. Infect. Dis. 2024, 230, e917–e928. [Google Scholar] [CrossRef] [PubMed]
- Clopper, B.R.; Lopez, A.S.; Goldstein, L.A.; Ng, T.F.F.; Toepfer, A.P.; Staat, M.A.; Schlaudecker, E.P.; Sahni, L.C.; Boom, J.A.; Schuster, J.E.; et al. Enterovirus D68–associated respiratory illness in children. JAMA Netw. Open 2025, 8, e259131. [Google Scholar] [CrossRef] [PubMed]
- Uprety, P.; Curtis, D.; Elkan, M.; Fink, J.; Rajagopalan, R.; Zhao, C.; Bittinger, K.; Mitchell, S.; Ulloa, E.R.; Hopkins, S.; et al. Association of Enterovirus D68 with acute flaccid myelitis, Philadelphia, Pennsylvania, USA, 2009–2018. Emerg. Infect. Dis. 2019, 25, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, Y.; Li, L.; Li, Y.; Zhou, H.; Li, W. Epidemiology of childhood enterovirus infections in Hangzhou, China, 2019–2023. Virol. J. 2024, 21, 198. [Google Scholar] [CrossRef] [PubMed]
- Messali, S.; Bertelli, A.; Dotta, L.; Giovanetti, M.; Sclavi, L.; Venneri, G.; Ciccozzi, M.; Badolato, R.; Caruso, A.; Caccuri, F. Outbreak of Enterovirus D68 in young children, Brescia, Italy, August to November 2024. J. Med. Virol. 2025, 97, e70372. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, H.; Lizarazo-Forero, E.; Schuele, L.; Van Leer-Buter, C.; Niesters, H.G. Off-season circulation and characterization of enterovirus D68 with respiratory and neurological presentation using whole-genome sequencing. Front. Microbiol. 2023, 13, 1088770. [Google Scholar] [CrossRef] [PubMed]
- Dudman, S.; Klundby, I.; Øverbø, J.; Numanovic, S.; Nilsen, M.; Lind, A.; Holberg-Petersen, M.; Landaas, E.T. Trends in the enterovirus surveillance in Oslo, Norway before and during the COVID-19 pandemic. Front. Virol. 2024, 3, 1343781. [Google Scholar] [CrossRef]
- Brouwer, L.; Moreni, G.; Wolthers, K.C.; Pajkrt, D. World-wide prevalence and genotype distribution of enteroviruses. Viruses 2021, 13, 434. [Google Scholar] [CrossRef] [PubMed]
- Pidjadee, C.; Soh, K.L.; Attharos, T.; Soh, K.G. The effect of infection prevention and control programme for childcare workers in daycare centres: A systematic review. J. Pediatr. Nurs. 2025, 79, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, H.; van Leer-Buter, C.; Niesters, H.G. Enterovirus infections in solid organ transplant recipients: A clinical comparison from a regional university hospital in the Netherlands. Microbiol. Spectr. 2022, 10, e0221521. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, F.; Chang, L.; Lai, S.; Teng, J.; Duan, J.; Jian, H.; Liu, T.; Che, G. Molecular epidemiology and clinical characteristics of enteroviruses associated HFMD in Chengdu, China, 2013–2022. Virol. J. 2023, 20, 202. [Google Scholar] [CrossRef] [PubMed]
- de Schrijver, S.; Vanhulle, E.; Ingenbleek, A.; Alexakis, L.; Johannesen, C.K.; Broberg, E.K.; Harvala, H.; Fischer, T.K.; Benschop, K.S. ENPEN Study Collaborators. Epidemiological and clinical insights into enterovirus circulation in Europe, 2018–2023: A multi-center retrospective surveillance study. J. Infect. Dis. 2025, 231, jiaf179. [Google Scholar] [CrossRef] [PubMed]
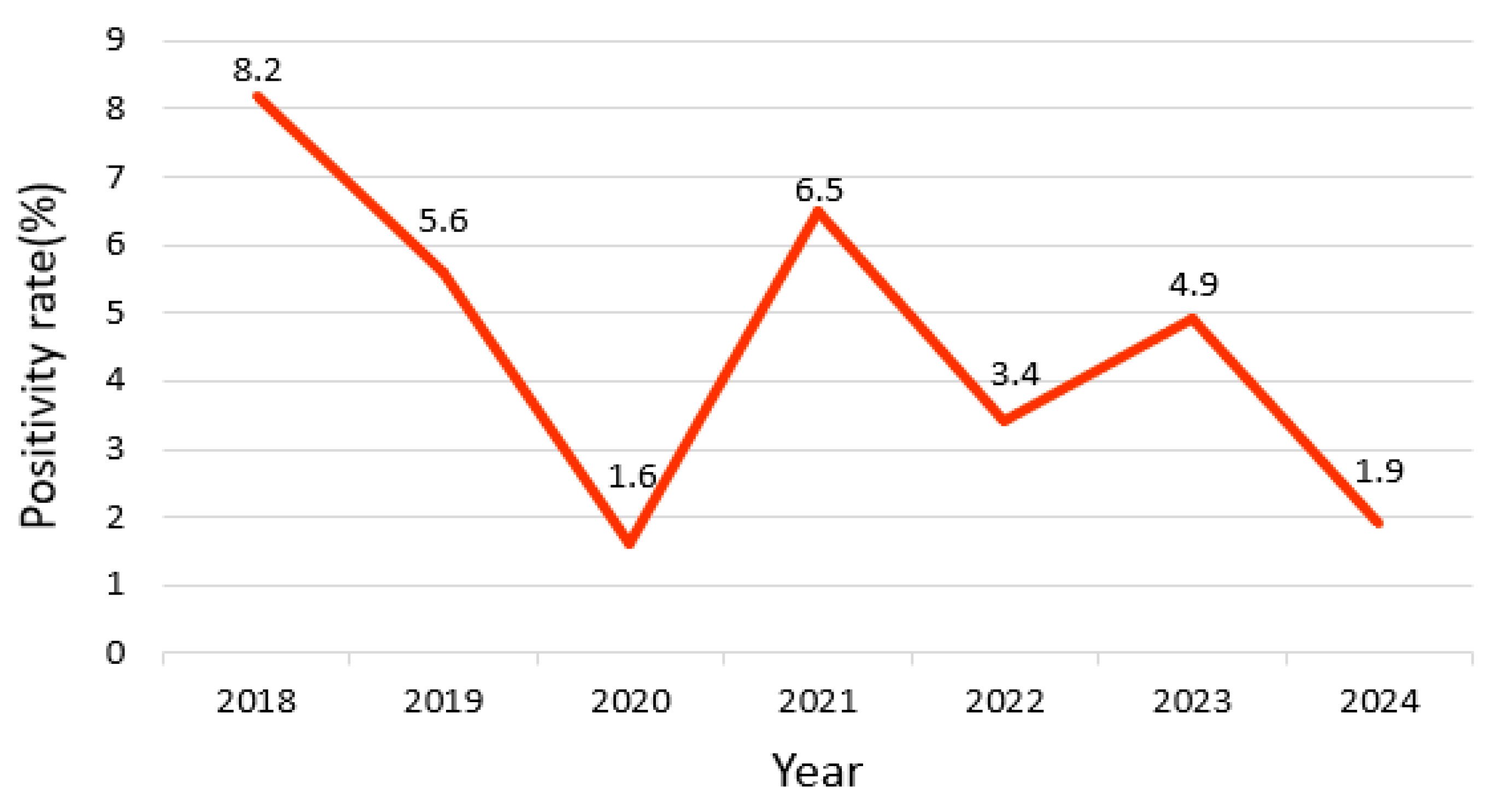
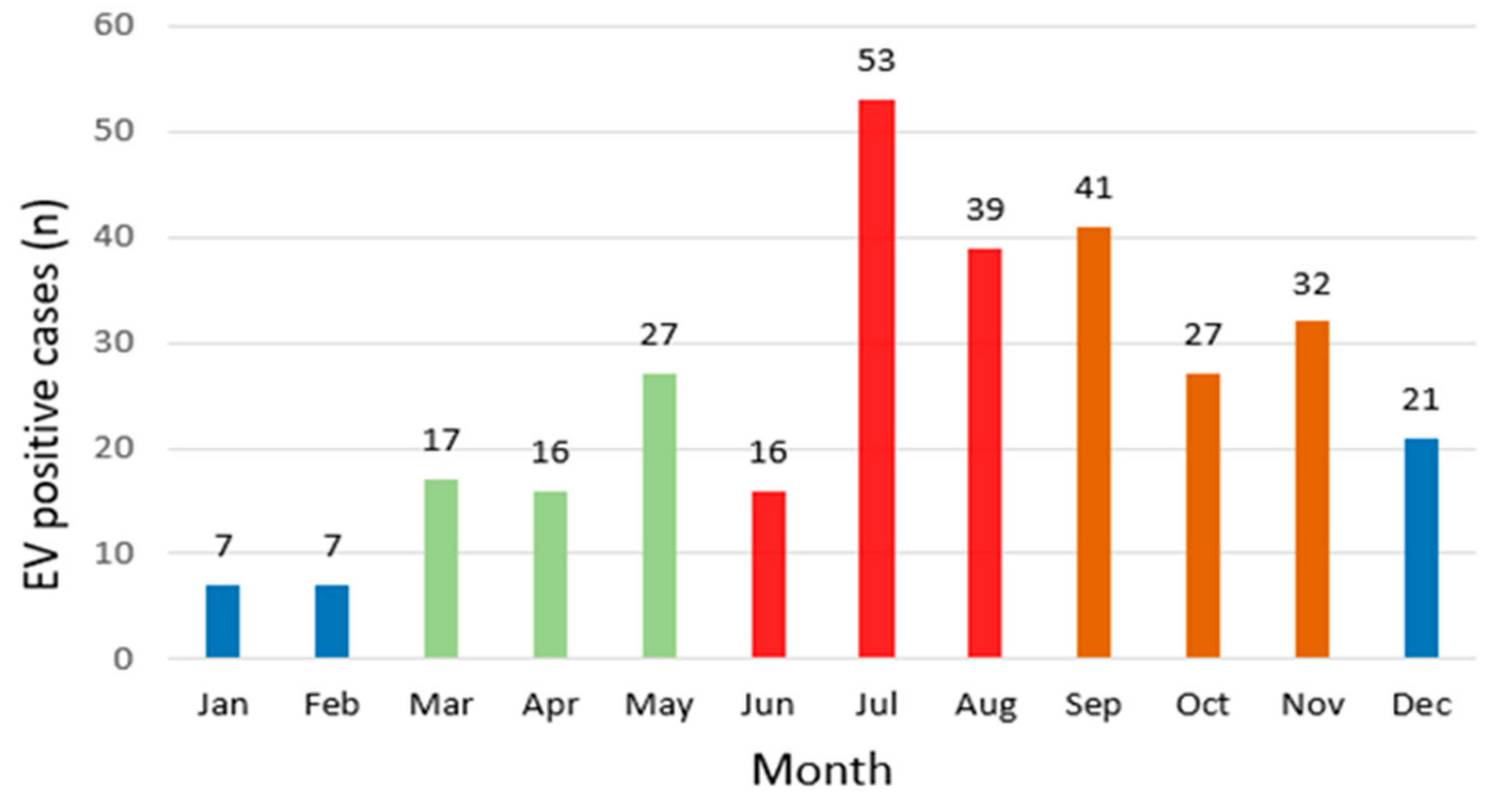
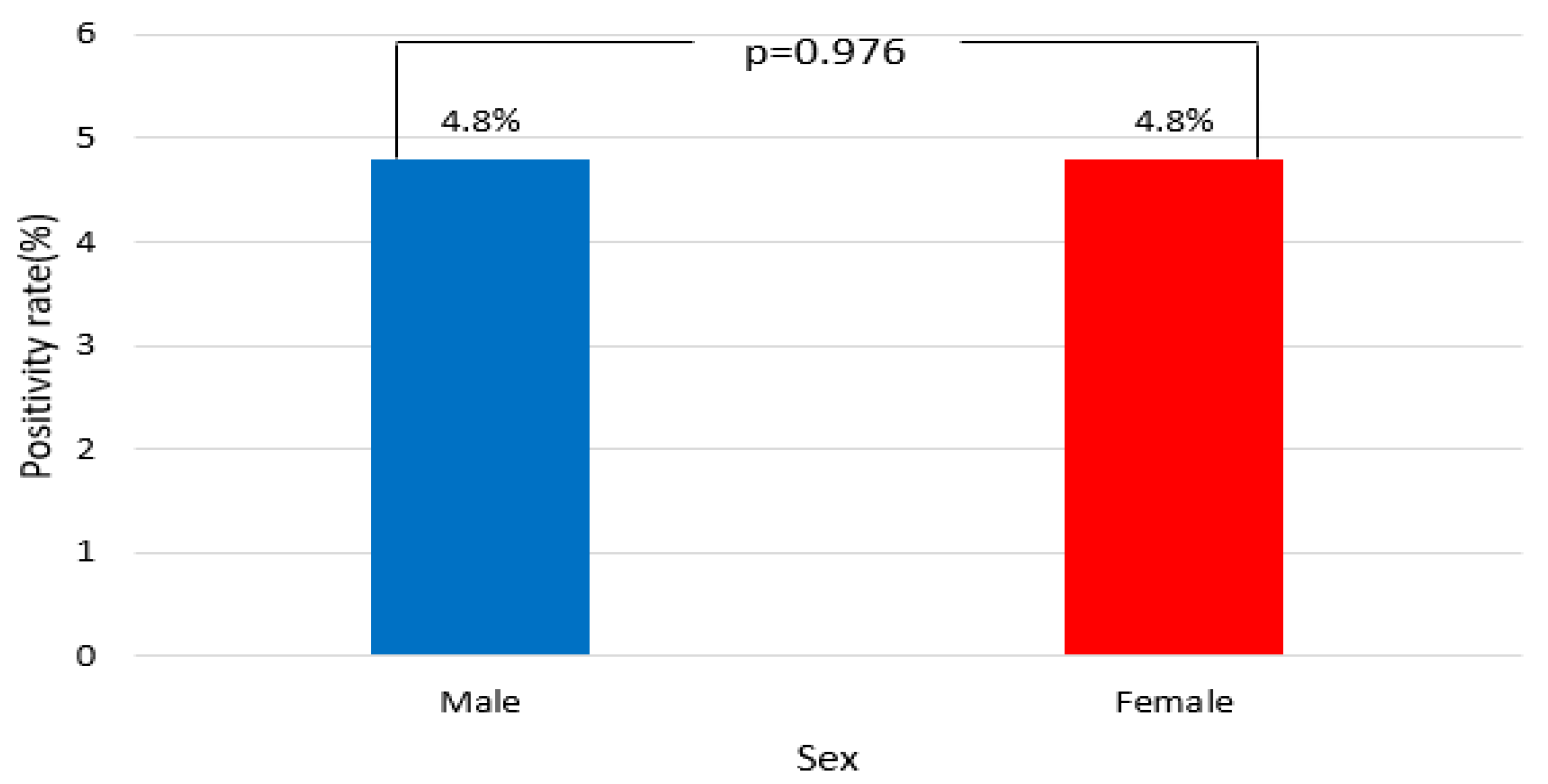
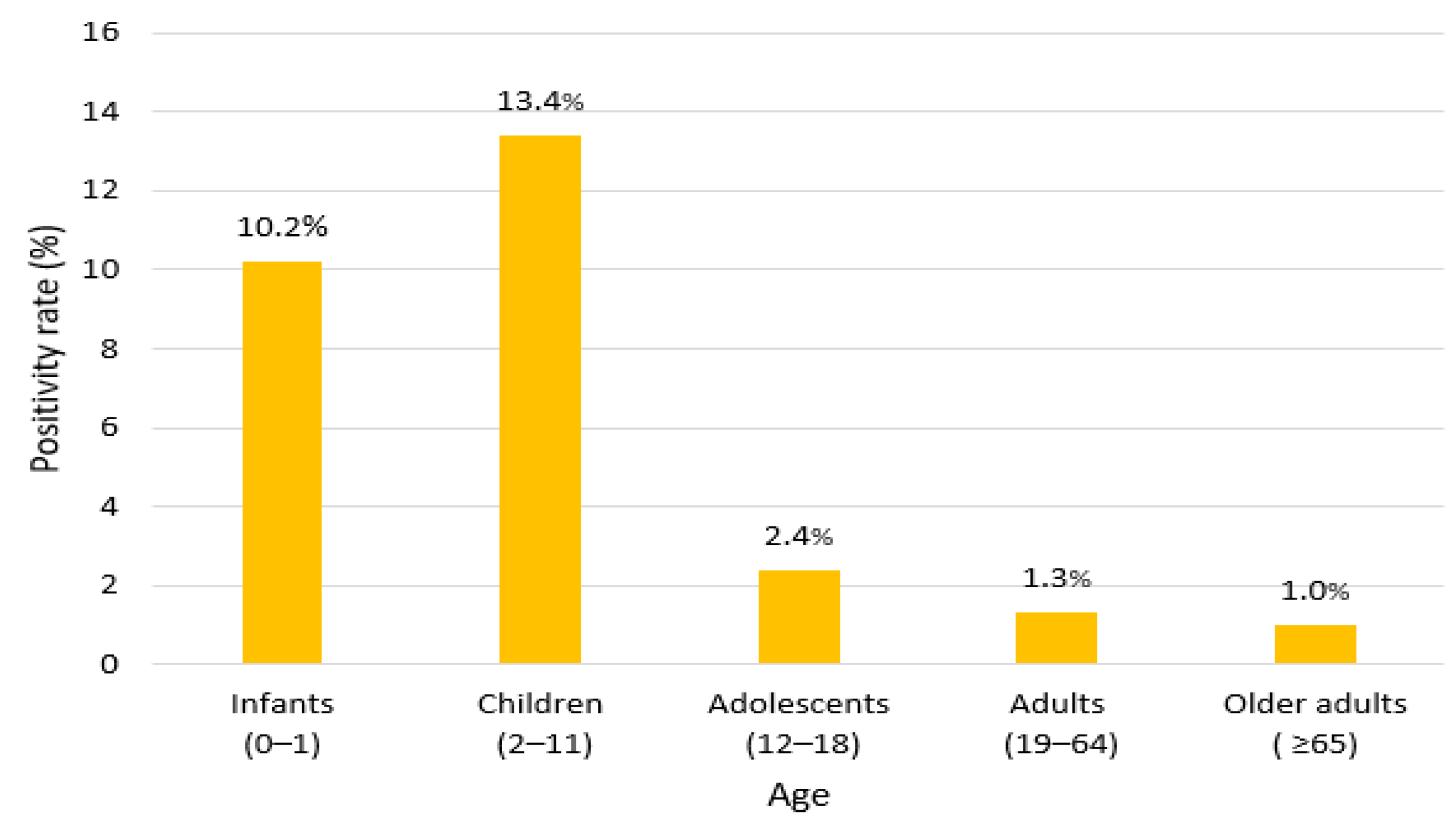
| Year | Total | Positive (Observed) | Positive (Expected) | Negative (Observed) | Negative (Expected) | Positivity Rate (%) | χ2 | p |
|---|---|---|---|---|---|---|---|---|
| 2018 | 926 | 76 | 44.4 | 850 | 881.4 | 8.2 | 61.2 | <0.001 |
| 2019 | 1432 | 81 | 68.9 | 1351 | 1363 | 5.6 | ||
| 2020 | 792 | 13 | 38.1 | 779 | 753.8 | 1.6 | ||
| 2021 | 613 | 40 | 29.5 | 573 | 583.4 | 6.5 | ||
| 2022 | 860 | 30 | 41.4 | 830 | 818.5 | 3.4 | ||
| 2023 | 1016 | 50 | 48.9 | 966 | 967 | 4.9 | ||
| 2024 | 653 | 13 | 31.4 | 640 | 621.5 | 1.9 |
| Season | Total | Positive (Observed) | Positive (Expected) | Negative (Observed) | Negative (Expected) | Positivity Rate (%) | χ2 | p |
|---|---|---|---|---|---|---|---|---|
| Spring | 1298 | 60 | 62.5 | 1238 | 1235.4 | 4.6 | 47.2 | <0.001 |
| Summer | 1620 | 108 | 78 | 1512 | 1541.9 | 6.6 | ||
| Autumn | 1644 | 100 | 79.1 | 1544 | 1564.8 | 6.0 | ||
| Winter | 1730 | 35 | 83.3 | 1695 | 1646.6 | 2.0 |
| Sex | Total | Positive (Observed) | Positive (Expected) | Negative (Observed) | Negative (Expected) | Positivity Rate (%) | χ2 | p |
|---|---|---|---|---|---|---|---|---|
| Male | 4003 | 193 | 192.7 | 3810 | 3810.2 | 4.8 | 0.001 | 0.976 |
| Female | 2289 | 110 | 110.2 | 2179 | 2178.7 | 4.8 |
| Age | Total | Positive (Observed) | Positive (Expected) | Negative (Observed) | Negative (Expected) | Positivity Rate (%) | χ2 | p |
|---|---|---|---|---|---|---|---|---|
| Infants (0–1) | 1276 | 131 | 61.4 | 1145 | 1214.5 | 10.2 | 348.5 | 0.001 |
| Children (2–11) | 917 | 123 | 44.1 | 794 | 872.8 | 13.4 | ||
| Adolescents (12–18) | 162 | 4 | 7.8 | 158 | 154.1 | 2.4 | ||
| Adults (19–64) | 1314 | 18 | 63.2 | 1296 | 1250.7 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.S.; Jang, S.H.; Jeon, J.-S.; Kim, J.K. Enterovirus Detection Trends Based on Respiratory Specimens from a Single Tertiary Hospital in Korea (2018–2024): A Retrospective Study Using Multiplex PCR Data. Viruses 2025, 17, 991. https://doi.org/10.3390/v17070991
Han JS, Jang SH, Jeon J-S, Kim JK. Enterovirus Detection Trends Based on Respiratory Specimens from a Single Tertiary Hospital in Korea (2018–2024): A Retrospective Study Using Multiplex PCR Data. Viruses. 2025; 17(7):991. https://doi.org/10.3390/v17070991
Chicago/Turabian StyleHan, Jeong Su, Sung Hun Jang, Jae-Sik Jeon, and Jae Kyung Kim. 2025. "Enterovirus Detection Trends Based on Respiratory Specimens from a Single Tertiary Hospital in Korea (2018–2024): A Retrospective Study Using Multiplex PCR Data" Viruses 17, no. 7: 991. https://doi.org/10.3390/v17070991
APA StyleHan, J. S., Jang, S. H., Jeon, J.-S., & Kim, J. K. (2025). Enterovirus Detection Trends Based on Respiratory Specimens from a Single Tertiary Hospital in Korea (2018–2024): A Retrospective Study Using Multiplex PCR Data. Viruses, 17(7), 991. https://doi.org/10.3390/v17070991





