Retinal Epithelial Neutralization Assay Optimizes AAV Serotype Selection for Ocular Gene Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Recombinant AAV Production
2.2. Human Serum Samples
2.3. Ethical Statement
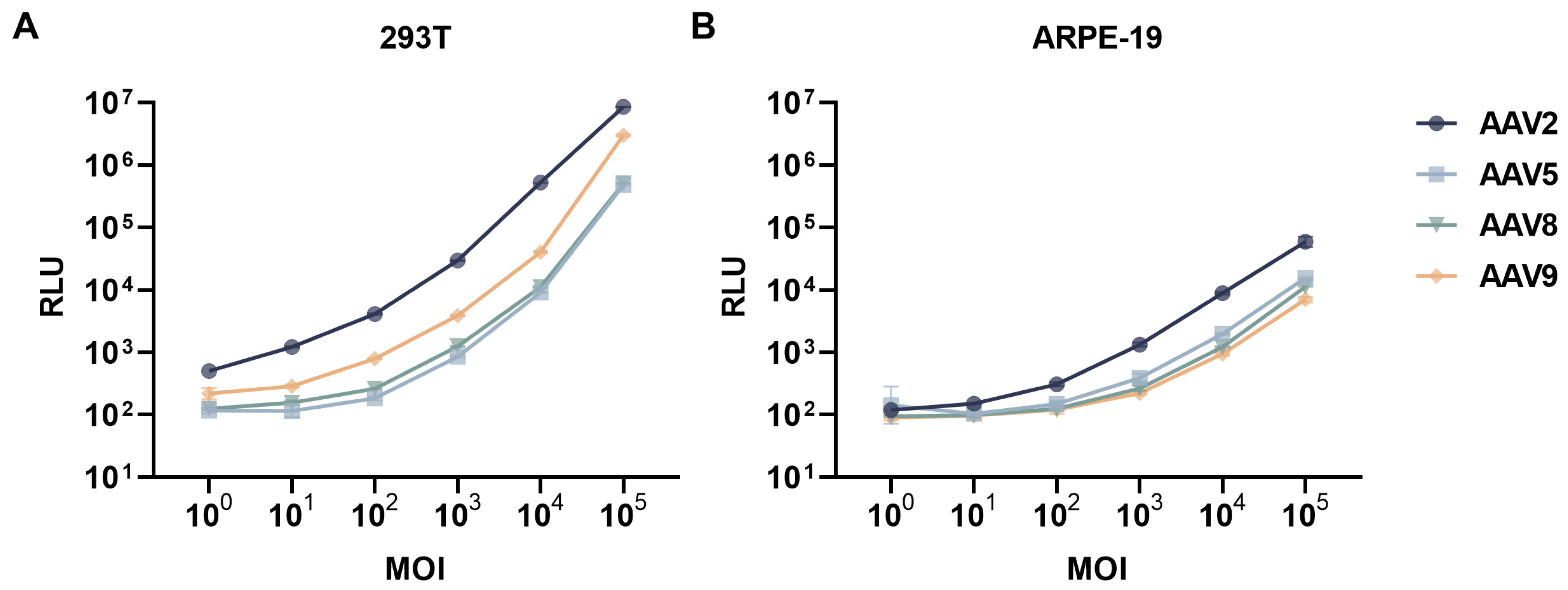
2.4. Neutralizing Antibody Assay
2.5. Statistical Analysis
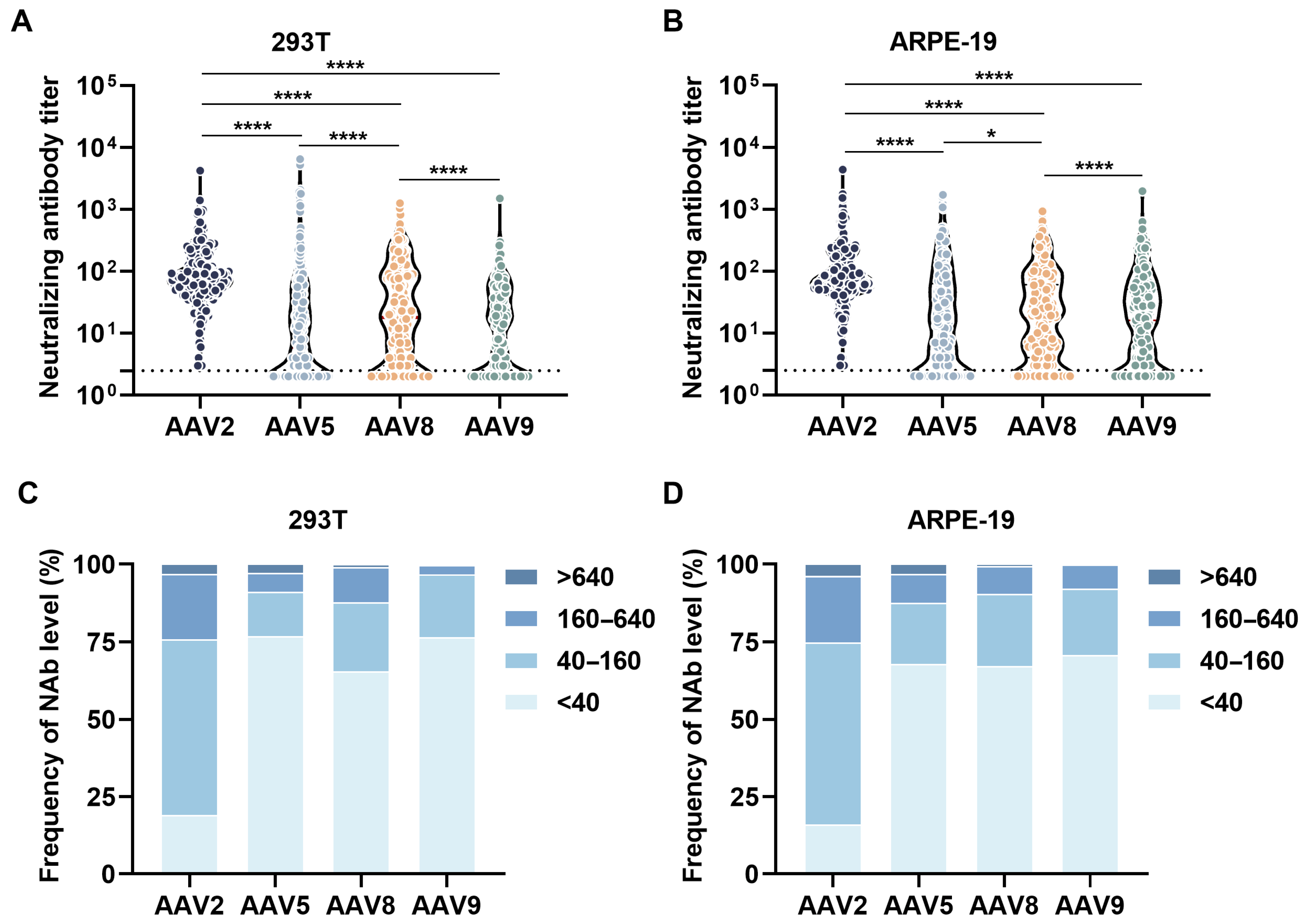
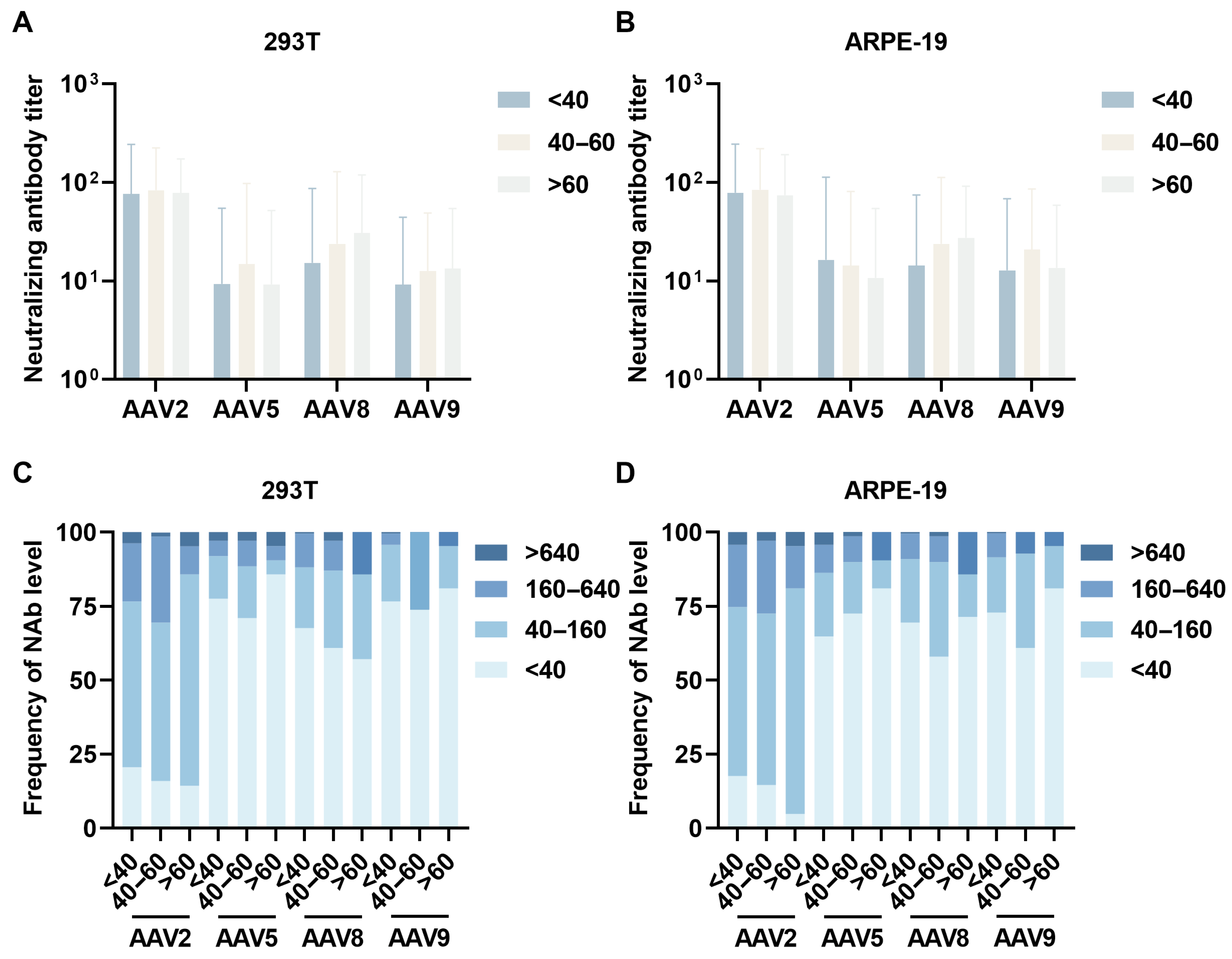
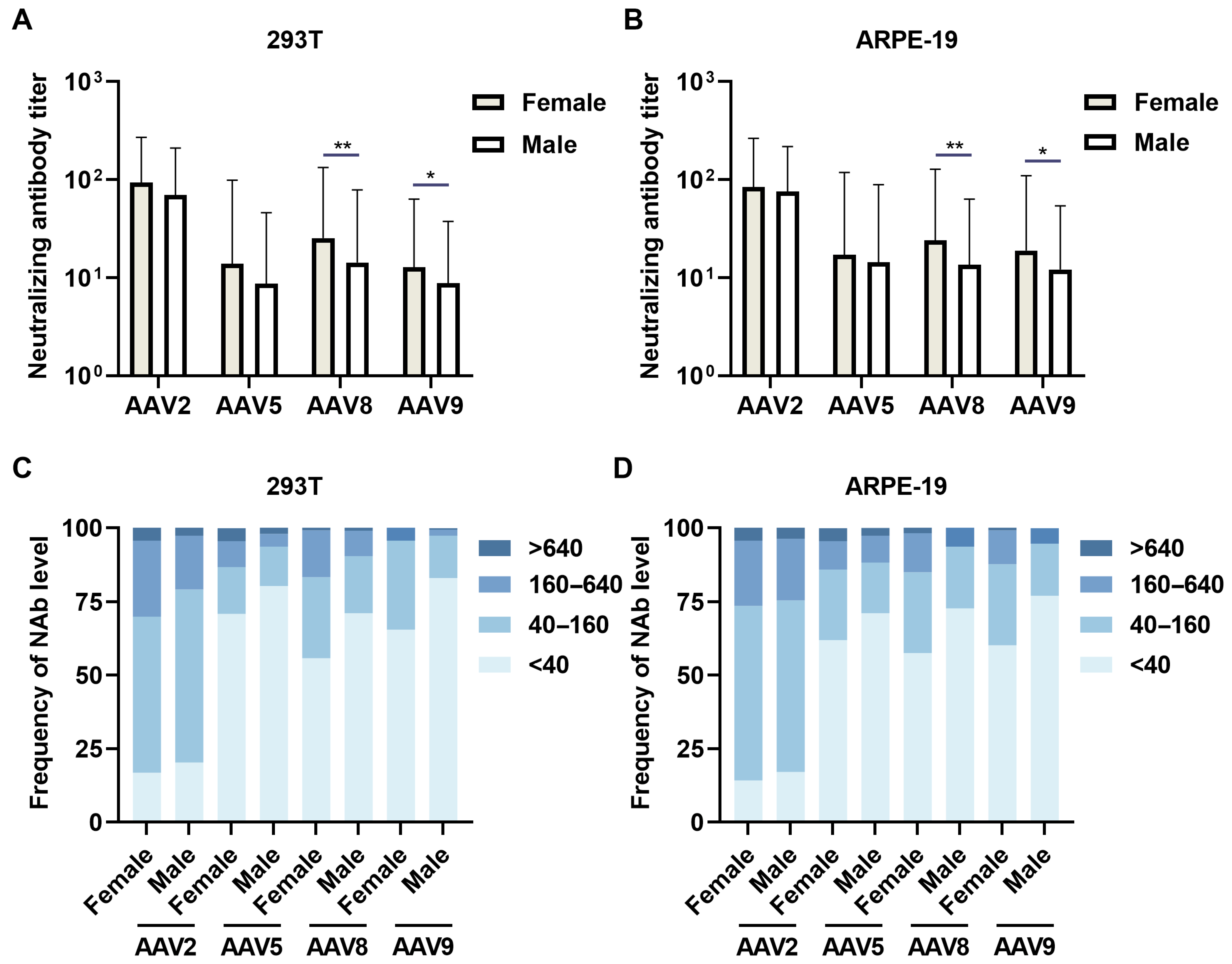
3. Results
3.1. Overall Neutralizing Antibody Profiles
3.2. Age-Stratified Neutralizing Antibody Profiles
3.3. Sex-Specific Neutralizing Antibody Prevalence
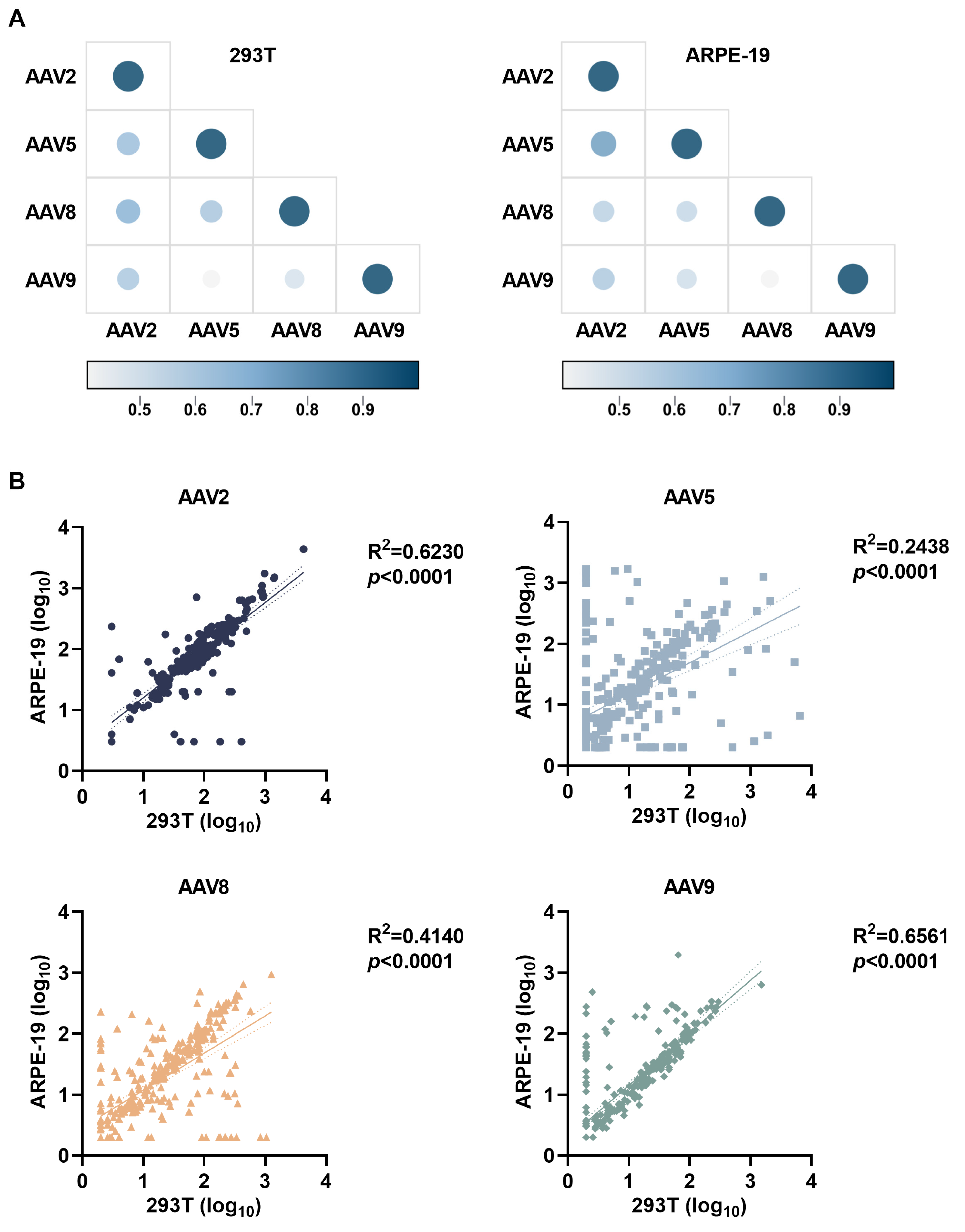
3.4. Cell Platform-Dependent AAV Neutralization Serotype Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.H.; Zhan, W.; Gallagher, T.L.; Gao, G. Recombinant adeno-associated virus as a delivery platform for ocular gene therapy: A comprehensive review. Mol. Ther. 2024, 32, 4185–4207. [Google Scholar] [CrossRef]
- Le Meur, G.; Lebranchu, P.; Billaud, F.; Adjali, O.; Schmitt, S.; Bezieau, S.; Pereon, Y.; Valabregue, R.; Ivan, C.; Darmon, C.; et al. Safety and Long-Term Efficacy of AAV4 Gene Therapy in Patients with RPE65 Leber Congenital Amaurosis. Mol. Ther. 2018, 26, 256–268. [Google Scholar] [CrossRef]
- Michaelides, M.; Besirli, C.G.; Yang, Y.; TAC, D.E.G.; Wong, S.C.; Huckfeldt, R.M.; Comander, J.I.; Sahel, J.A.; Shah, S.M.; Tee, J.J.L.; et al. Phase 1/2 AAV5-hRKp.RPGR (Botaretigene Sparoparvovec) Gene Therapy: Safety and Efficacy in RPGR-Associated X-Linked Retinitis Pigmentosa. Am. J. Ophthalmol. 2024, 267, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jing, Q.; She, K.; Wang, Q.; Jin, X.; Zhao, Q.; Su, J.; Song, L.; Fu, J.; Wu, X.; et al. Split AAV8 Mediated ABCA4 Expression for Gene Therapy of Mouse Stargardt Disease (STGD1). Hum. Gene Ther. 2023, 34, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.J.; Russell, K.N.; Melzer, T.R.; Gray, S.J.; Heap, S.J.; Palmer, D.N.; Mitchell, N.L. Intravitreal gene therapy protects against retinal dysfunction and degeneration in sheep with CLN5 Batten disease. Exp. Eye Res. 2021, 207, 108600. [Google Scholar] [CrossRef]
- Kruzik, A.; Fetahagic, D.; Hartlieb, B.; Dorn, S.; Koppensteiner, H.; Horling, F.M.; Scheiflinger, F.; Reipert, B.M.; de la Rosa, M. Prevalence of Anti-Adeno-Associated Virus Immune Responses in International Cohorts of Healthy Donors. Mol. Ther. Methods Clin. Dev. 2019, 14, 126–133. [Google Scholar] [CrossRef]
- Boutin, S.; Monteilhet, V.; Veron, P.; Leborgne, C.; Benveniste, O.; Montus, M.F.; Masurier, C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Impli-cations for gene therapy using AAV vectors. Hum. Gene Ther. 2010, 21, 704–712. [Google Scholar] [CrossRef]
- Mingozzi, F.; High, K.A. Therapeutic in vivo gene transfer for genetic disease using AAV: Progress and challenges. Nat. Rev. Genet. 2011, 12, 341–355. [Google Scholar] [CrossRef]
- Louis Jeune, V.; Joergensen, J.A.; Hajjar, R.J.; Weber, T. Pre-existing anti-adeno-associated virus antibodies as a chal-lenge in AAV gene therapy. Hum. Gene Ther. Methods 2013, 24, 59–67. [Google Scholar] [CrossRef]
- Schulz, M.; Levy, D.I.; Petropoulos, C.J.; Bashirians, G.; Winburn, I.; Mahn, M.; Somanathan, S.; Cheng, S.H.; Byrne, B.J. Binding and neutralizing anti-AAV antibodies: Detection and implications for rAAV-mediated gene therapy. Mol. Ther. 2023, 31, 616–630. [Google Scholar] [CrossRef]
- D’Alessio, A.M.; Boffa, I.; De Stefano, L.; Soria, L.R.; Brunetti-Pierri, N. Liver gene transfer for metabolite detoxification in inherited metabolic diseases. FEBS Lett. 2024, 598, 2372–2384. [Google Scholar] [CrossRef]
- Reichel, F.F.; Peters, T.; Wilhelm, B.; Biel, M.; Ueffing, M.; Wissinger, B.; Bartz-Schmidt, K.U.; Klein, R.; Michalakis, S.; Fischer, M.D.; et al. Humoral Immune Response After Intravitreal But Not After Subretinal AAV8 in Primates and Patients. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1910–1915. [Google Scholar] [CrossRef]
- Bennett, J.; Wellman, J.; Marshall, K.A.; McCague, S.; Ashtari, M.; DiStefano-Pappas, J.; Elci, O.U.; Chung, D.C.; Sun, J.; Wright, J.F.; et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: A fol-low-on phase 1 trial. Lancet 2016, 388, 661–672. [Google Scholar] [CrossRef]
- Desrosiers, M.; Dalkara, D. Neutralizing Antibodies Against Adeno-Associated Virus (AAV): Measurement and Influence on Retinal Gene Delivery. Methods Mol. Biol. 2018, 1715, 225–238. [Google Scholar] [PubMed]
- Meyer, N.L.; Chapman, M.S. Adeno-associated virus (AAV) cell entry: Structural insights. Trends Microbiol. 2022, 30, 432–451. [Google Scholar] [CrossRef] [PubMed]
- Kaludov, N.; Brown, K.E.; Walters, R.W.; Zabner, J.; Chiorini, J.A. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J. Virol. 2001, 75, 6884–6893. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Bryant, K.D.; Brown, S.M.; Randell, S.H.; Asokan, A. Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. J. Biol. Chem. 2011, 286, 13532–13540. [Google Scholar] [CrossRef]
- Summerford, C.; Samulski, R.J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 1998, 72, 1438–1445. [Google Scholar] [CrossRef]
- Cehajic-Kapetanovic, J.; Le Goff, M.M.; Allen, A.; Lucas, R.J.; Bishop, P.N. Glycosidic enzymes enhance retinal transduction following intravitreal delivery of AAV2. Mol. Vis. 2011, 17, 1771–1783. [Google Scholar]
- Nakamura, H.; Tanaka, T.; Zheng, C.; Afione, S.A.; Warner, B.M.; Noguchi, M.; Atsumi, T.; Chiorini, J.A. Correction of LAMP3-associated salivary gland hypofunction by aquaporin gene therapy. Sci. Rep. 2022, 12, 18570. [Google Scholar] [CrossRef]
- Large, E.E.; Chapman, M.S. Adeno-associated virus receptor complexes and implications for adeno-associated virus immune neutralization. Front. Microbiol. 2023, 14, 1116896. [Google Scholar] [CrossRef]
- Klamroth, R.; Hayes, G.; Andreeva, T.; Gregg, K.; Suzuki, T.; Mitha, I.H.; Hardesty, B.; Shima, M.; Pollock, T.; Slev, P.; et al. Global Seroprevalence of Pre-existing Immunity Against AAV5 and Other AAV Serotypes in People with Hemophilia A. Hum. Gene Ther. 2022, 33, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Calcedo, R.; Morizono, H.; Wang, L.; McCarter, R.; He, J.; Jones, D.; Batshaw, M.L.; Wilson, J.M. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccine Immunol. 2011, 18, 1586–1588. [Google Scholar] [CrossRef] [PubMed]
- Ertl, H.C.J. T Cell-Mediated Immune Responses to AAV and AAV Vectors. Front. Immunol. 2021, 12, 666666. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Wang, Y.; Feng, Y.L.; Zhang, Y.N.; Li, J.; Hu, X.R.; Wang, L.N.; Zhong, M.F.; Zhai, X.F.; Zolotukhin, I.; et al. Prevalence of neutralizing antibodies against liver-tropic adeno-associated virus serotype vectors in 100 healthy Chinese and its potential relation to body constitutions. J. Integr. Med. 2015, 13, 341–346. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, W.; Zhao, C.; Zhang, L.; Meng, S.; Gao, D.; Wang, Y. The prevalence of neutralizing antibodies against AAV serotype 1 in healthy subjects in China: Implications for gene therapy and vaccines using AAV1 vector. J. Med. Virol. 2013, 85, 1550–1556. [Google Scholar] [CrossRef]
- Dhungel, B.P.; Winburn, I.; Pereira, C.D.F.; Huang, K.; Chhabra, A.; Rasko, J.E.J. Understanding AAV vector im-munogenicity: From particle to patient. Theranostics 2024, 14, 1260–1288. [Google Scholar] [CrossRef]
- Gardner, M.R.; Mendes, D.E.; Muniz, C.P.; Martinez-Navio, J.M.; Fuchs, S.P.; Gao, G.; Desrosiers, R.C. High con-cordance of ELISA and neutralization assays allows for the detection of antibodies to individual AAV serotypes. Mol. Ther. Methods Clin. Dev. 2022, 24, 199–206. [Google Scholar] [CrossRef]
- O’Leary, F.; Campbell, M. The blood-retina barrier in health and disease. FEBS J. 2023, 290, 878–891. [Google Scholar] [CrossRef]
- Finnemann, S.C.; Bonilha, V.L.; Marmorstein, A.D.; Rodriguez-Boulan, E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc. Natl. Acad. Sci. USA 1997, 94, 12932–12937. [Google Scholar] [CrossRef]
| Variables | Tested | ||
|---|---|---|---|
| Age group (years) | <40 | 40–60 | >60 |
| Overall (N = 300) | 210 | 69 | 21 |
| Mean | 28 | 51 | 64 |
| Min, max | 18, 40 | 41, 60 | 61, 70 |
| Biological sex | Female | Male | |
| Overall (N = 300) | 113 | 187 | |
| AAV Serotypes | AAV2 | AAV5 | AAV8 | AAV9 |
|---|---|---|---|---|
| 293T | 77.8 ± 3.0 | 10.3 ± 6.0 | 17.7 ± 5.5 | 10.1 ± 4.6 |
| ARPE-19 | 79.1 ± 3.0 | 15.3 ± 6.5 | 16.8 ± 5.0 | 14.3 ± 5.0 |
| ARPE-19/293T | 1.02 | 1.48 | 0.95 | 1.42 |
| p | ns | **** | * | **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, Y.; Huo, N.; Jia, Z.; Huang, H.; Zhao, Z.; Wu, S.; Hou, L. Retinal Epithelial Neutralization Assay Optimizes AAV Serotype Selection for Ocular Gene Therapy. Viruses 2025, 17, 988. https://doi.org/10.3390/v17070988
Li Y, Chen Y, Huo N, Jia Z, Huang H, Zhao Z, Wu S, Hou L. Retinal Epithelial Neutralization Assay Optimizes AAV Serotype Selection for Ocular Gene Therapy. Viruses. 2025; 17(7):988. https://doi.org/10.3390/v17070988
Chicago/Turabian StyleLi, Yao, Yujia Chen, Nan Huo, Zuyuan Jia, He Huang, Zhenghao Zhao, Shipo Wu, and Lihua Hou. 2025. "Retinal Epithelial Neutralization Assay Optimizes AAV Serotype Selection for Ocular Gene Therapy" Viruses 17, no. 7: 988. https://doi.org/10.3390/v17070988
APA StyleLi, Y., Chen, Y., Huo, N., Jia, Z., Huang, H., Zhao, Z., Wu, S., & Hou, L. (2025). Retinal Epithelial Neutralization Assay Optimizes AAV Serotype Selection for Ocular Gene Therapy. Viruses, 17(7), 988. https://doi.org/10.3390/v17070988





