mRNA Vaccine Development in the Fight Against Zoonotic Viral Diseases
Abstract
1. Introduction
2. Vaccines: From Discovery to Global Utilization
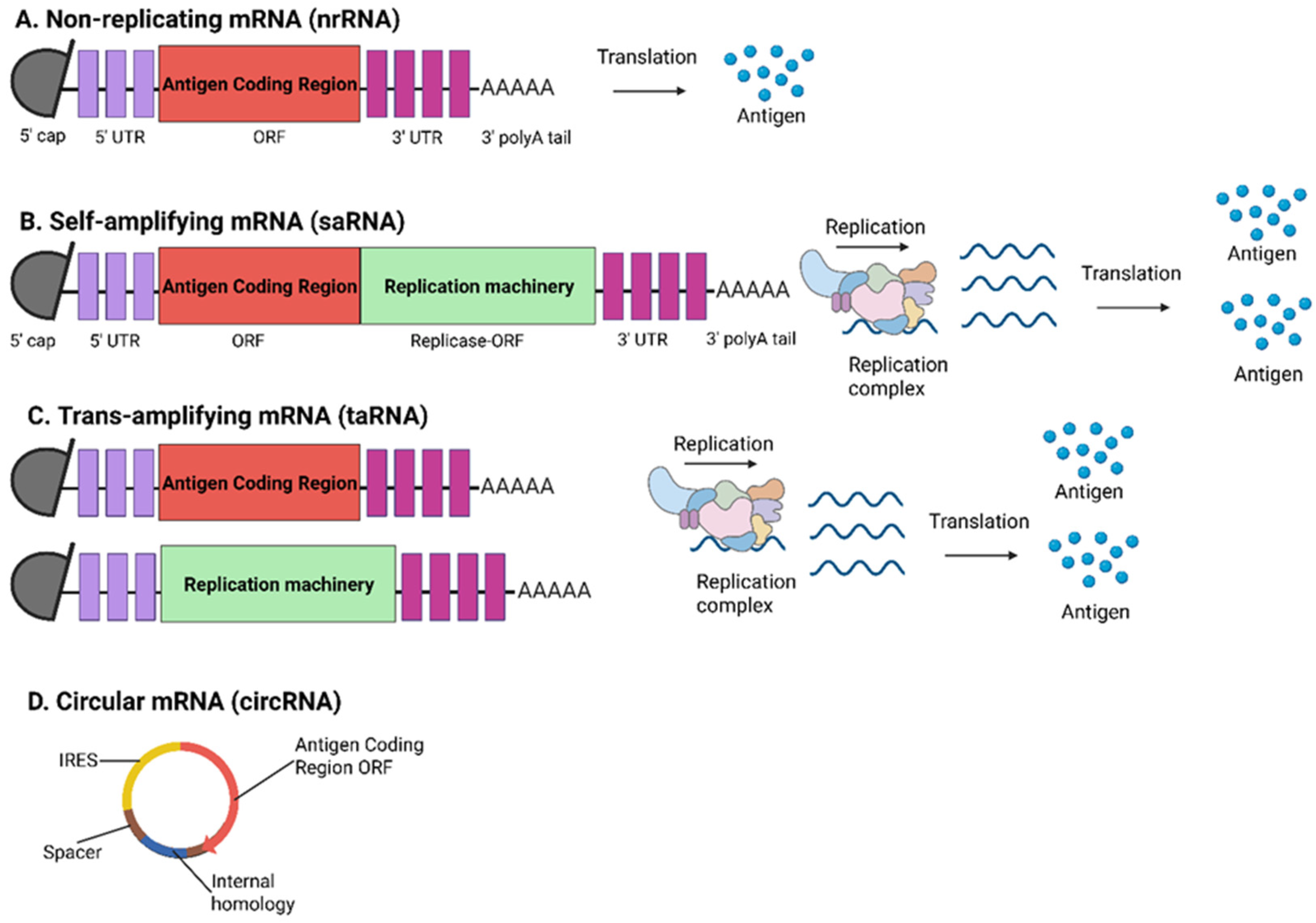
3. Advances of mRNA Vaccines
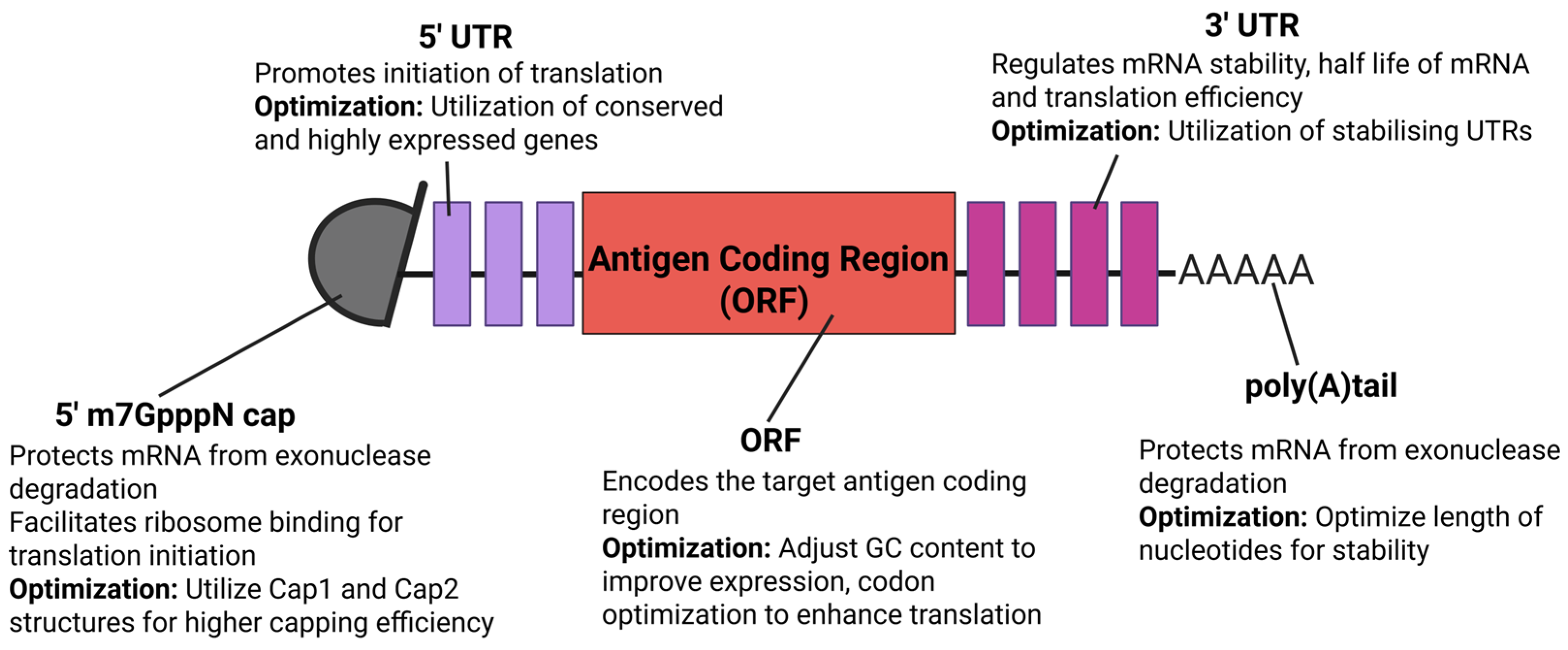
4. Optimization Approaches for mRNA Vaccines
5. mRNA Vaccines Against Emerging and Re-Emerging Viral Zoonoses
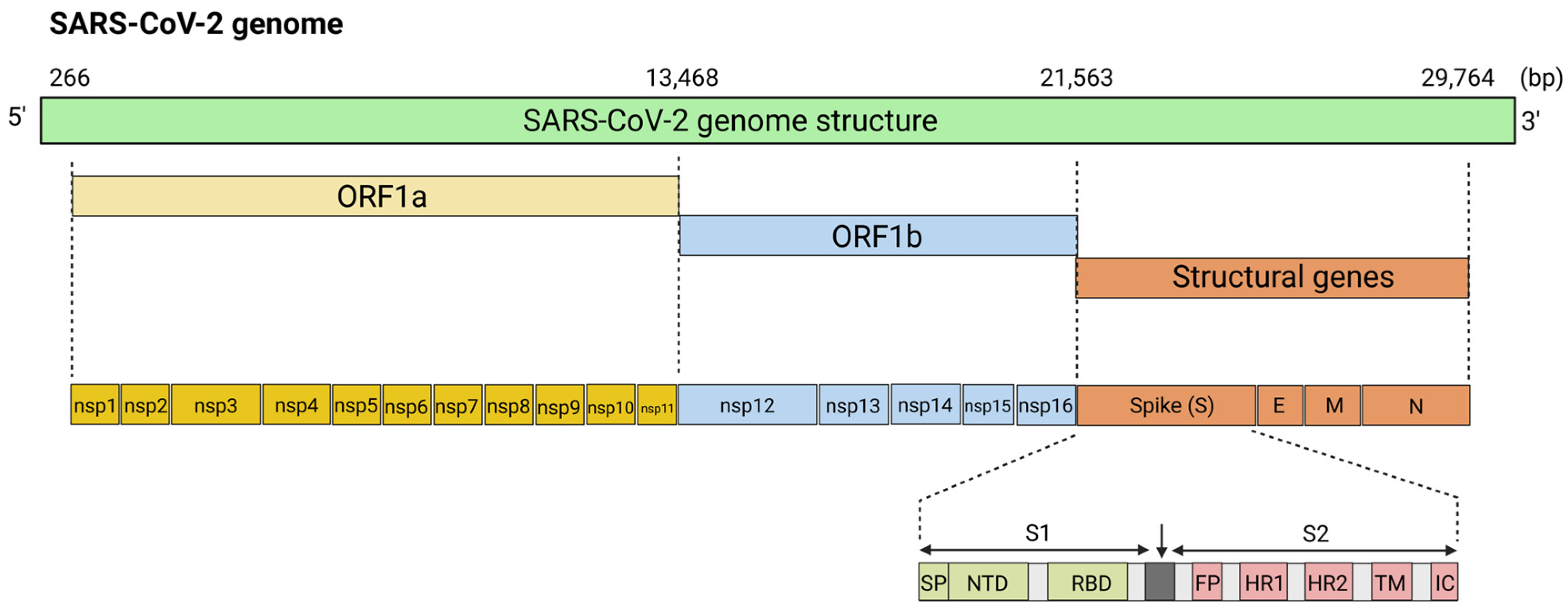
5.1. mRNA Vaccines for Coronaviruses
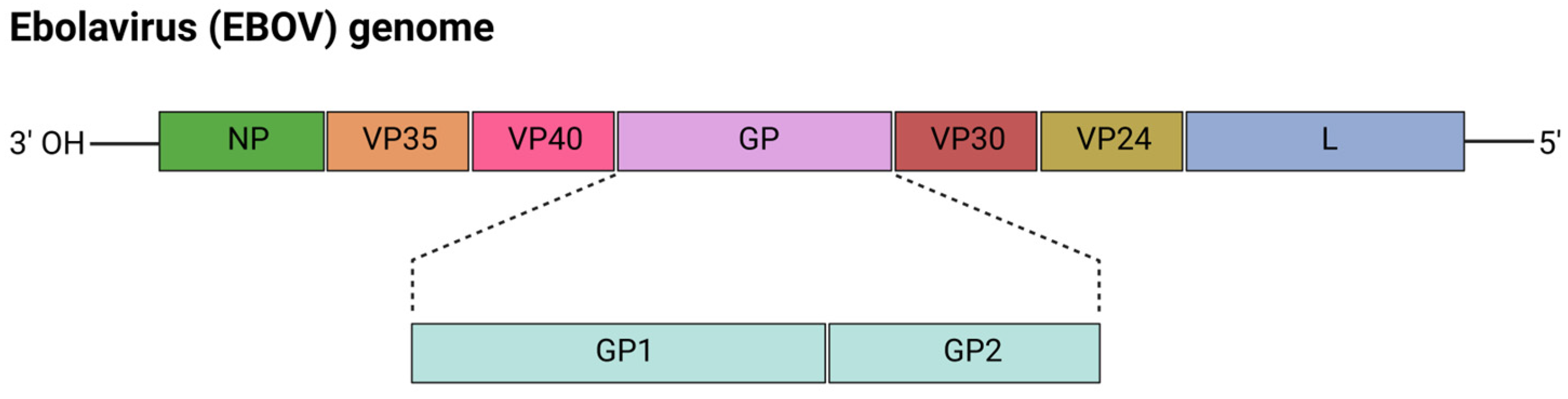
5.2. mRNA Vaccines for Ebolavirus (EBOV)
5.3. mRNA Vaccines for Nipah Virus (NiV)
5.4. mRNA Vaccines for Influenza Virus
5.5. mRNA Vaccines for Rabies Virus (RABV)
5.6. mRNA Vaccines for Zoonotic Arthropod-Borne Viruses
5.6.1. Zika Virus (ZIKV)
5.6.2. Dengue Virus (DENV)
5.6.3. Rift Valley Fever Virus (RVFV)
5.6.4. Powassan Virus (POWV)
5.6.5. Crimean-Congo Hemorrhagic Fever (CCHF) Virus
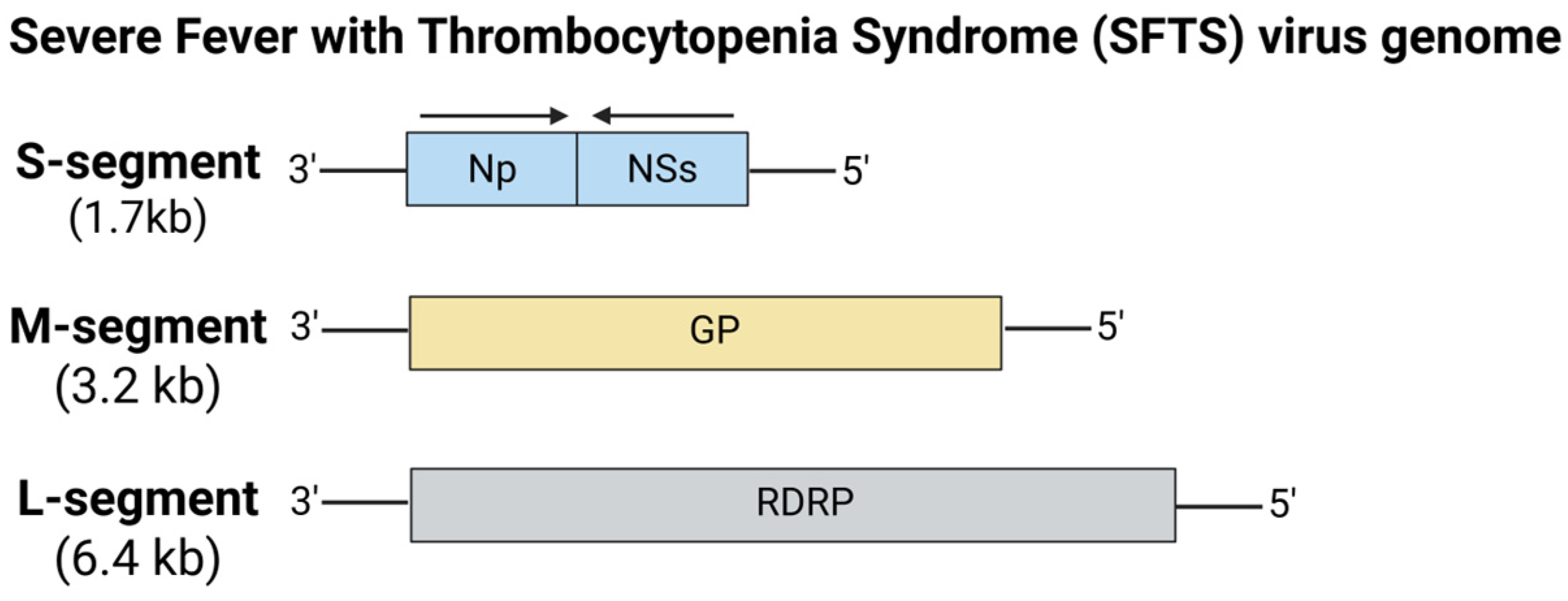
5.6.6. Severe Fever with Thrombocytopenia Syndrome (SFTS) Virus
5.7. mRNA Vaccines for Lassa Virus (LASV)
6. Future Directions in Preventing Infections Caused by Emerging and Re-Emerging Pathogens
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slingenbergh, J.; Gilbert, M.; Balogh, K.d.; Wint, W. Ecological sources of zoonotic diseases. Rev. Sci. Tech.-Off. Int. Des Épizoot. 2004, 23, 467–484. [Google Scholar] [CrossRef] [PubMed]
- WHO. Newsroom-Zoonoses. Fact Sheets, 29 July 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/zoonoses (accessed on 13 May 2025).
- WHO. Zoonotic Diseases: Emerging Public Health Threats in the Region; WHO: Geneva, Switzerland, 2025; Available online: https://www.emro.who.int/about-who/rc61/zoonotic-diseases.html (accessed on 13 May 2025).
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Cleaveland, S.; Laurenson, M.K.; Taylor, L.H. Diseases of humans and their domestic mammals: Pathogen characteristics, host range and the risk of emergence. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2001, 356, 991–999. [Google Scholar] [CrossRef]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef]
- Tahmo, N.B.; Wirsiy, F.S.; Nnamdi, D.-B.; Tongo, M.; Lawler, J.V.; Broadhurst, M.J.; Wondji, C.S.; Brett-Major, D.M. An epidemiological synthesis of emerging and re-emerging zoonotic disease threats in Cameroon, 2000–2022: A systematic review. IJID Reg. 2023, 7, 84–109. [Google Scholar] [CrossRef]
- Saba Villarroel, P.M.; Gumpangseth, N.; Songhong, T.; Yainoy, S.; Monteil, A.; Leaungwutiwong, P.; Missé, D.; Wichit, S. Emerging and re-emerging zoonotic viral diseases in Southeast Asia: One Health challenge. Front. Public Health 2023, 11, 1141483. [Google Scholar] [CrossRef]
- Wolfe, N.D.; Daszak, P.; Kilpatrick, A.M.; Burke, D.S. Bushmeat hunting, deforestation, and prediction of zoonotic disease. Emerg. Infect. Dis. 2005, 11, 1822. [Google Scholar] [CrossRef]
- Hassan, L. Emerging Zoonoses in Domesticated Livestock of Southeast Asia. Encycl. Agric. Food Syst. 2014, 68, 68–81. [Google Scholar]
- Naicker, P.R. The impact of climate change and other factors on zoonotic diseases. Arch. Clin. Microbiol. 2011, 2, 4. [Google Scholar]
- Davidson, T. Vaccines: History, Science, and Issues, 1st ed.; Bloomsbury Publishing: New York, NY, USA, 2017. [Google Scholar]
- Delany, I.; Rappuoli, R.; De Gregorio, E. Vaccines for the 21st century. EMBO Mol. Med. 2014, 6, 708–720. [Google Scholar] [CrossRef]
- Riedel, S. Edward Jenner and the history of smallpox and vaccination. In Baylor University Medical Center Proceedings; Taylor & Francis: Oxfordshire, UK, 2005; Volume 18, pp. 21–25. [Google Scholar]
- Stewart, G.T. Limitations of the germ theory. Lancet 1968, 291, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Calmette, A.; Guérin, C.; Boquet, A.; Nègre, L. La Vaccination Préventive Contre La Tuberculose Par Le “BCG”; Masson et Cie: Paris, France, 1927. [Google Scholar]
- Saleh, A.; Qamar, S.; Tekin, A.; Singh, R.; Kashyap, R. Vaccine development throughout history. Cureus 2021, 13, 16635. [Google Scholar] [CrossRef]
- Sabin, A.B. Oral poliovirus vaccine: History of its development and use and current challenge to eliminate poliomyelitis from the world. J. Infect. Dis. 1985, 151, 420–436. [Google Scholar] [CrossRef]
- Baicus, A. History of polio vaccination. World J. Virol. 2012, 1, 108–114. [Google Scholar] [CrossRef]
- Ghattas, M.; Dwivedi, G.; Lavertu, M.; Alameh, M.-G. Vaccine technologies and platforms for infectious diseases: Current progress, challenges, and opportunities. Vaccines 2021, 9, 1490. [Google Scholar] [CrossRef]
- Kozak, M.; Hu, J. The integrated consideration of vaccine platforms, adjuvants, and delivery routes for successful vaccine development. Vaccines 2023, 11, 695. [Google Scholar] [CrossRef]
- Bouazzaoui, A.; Abdellatif, A.A.; Al-Allaf, F.A.; Bogari, N.M.; Al-Dehlawi, S.; Qari, S.H. Strategies for vaccination: Conventional vaccine approaches versus new-generation strategies in combination with adjuvants. Pharmaceutics 2021, 13, 140. [Google Scholar] [CrossRef]
- Gote, V.; Bolla, P.K.; Kommineni, N.; Butreddy, A.; Nukala, P.K.; Palakurthi, S.S.; Khan, W. A comprehensive review of mRNA vaccines. Int. J. Mol. Sci. 2023, 24, 2700. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Karikó, K.; Megyeri, K.; Xiao, Q.; Barnathan, E.S. Lipofectin-aided cell delivery of ribozyme targeted to human urokinase receptor mRNA. FEBS Lett. 1994, 352, 41–44. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Jirikowski, G.F.; Sanna, P.P.; Maciejewski-Lenoir, D.; Bloom, F. Reversal of diabetes insipidus in Brattleboro rats: Intrahypothalamic injection of vasopressin mRNA. Science 1992, 255, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magné, R.; Gomard, E.; Guillet, J.G.; Lévy, J.P.; Meulien, P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993, 23, 1719–1722. [Google Scholar] [CrossRef]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef]
- Jackson, N.A.; Kester, K.E.; Casimiro, D.; Gurunathan, S.; DeRosa, F. The promise of mRNA vaccines: A biotech and industrial perspective. NPJ Vaccines 2020, 5, 11. [Google Scholar] [CrossRef]
- Yogurtcu, O.N.; Funk, P.R.; Forshee, R.A.; Anderson, S.A.; Marks, P.W.; Yang, H. Benefit-risk assessment of COVID-19 vaccine, MRNA (MRNA-1273) for males age 18–64 years. Vaccine X 2023, 14, 100325. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Weissman, D. Recent advances in mRNA vaccine technology. Curr. Opin. Immunol. 2020, 65, 14–20. [Google Scholar] [CrossRef]
- Hogan, M.J.; Pardi, N. mRNA vaccines in the COVID-19 pandemic and beyond. Annu. Rev. Med. 2022, 73, 17–39. [Google Scholar] [CrossRef]
- Sun, N.; Su, Z.; Zheng, X. Research progress of mosquito-borne virus mRNA vaccines. Mol. Ther. Methods Clin. Dev. 2025, 12, 101398. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Kumari, M.; Chen, G.-H.; Hong, M.-H.; Yuan, J.P.-Y.; Tsai, J.-L.; Wu, H.-C. mRNA-based vaccines and therapeutics: An in-depth survey of current and upcoming clinical applications. J. Biomed. Sci. 2023, 30, 84. [Google Scholar] [CrossRef]
- Bloom, K.; van den Berg, F.; Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021, 28, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Beissert, T.; Perkovic, M.; Vogel, A.; Erbar, S.; Walzer, K.C.; Hempel, T.; Brill, S.; Haefner, E.; Becker, R.; Türeci, Ö.; et al. A trans-amplifying RNA vaccine strategy for induction of potent protective immunity. Mol. Ther. 2020, 28, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Sun, C.; Chang, J.; Zhang, G.; Yin, X. mRNA vaccine development for emerging animal and zoonotic diseases. Viruses 2022, 14, 401. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Leong, K.Y.; Tham, S.K.; Poh, C.L. Revolutionizing immunization: A comprehensive review of mRNA vaccine technology and applications. Virol. J. 2025, 22, 71. [Google Scholar] [CrossRef]
- Zhang, G.; Tang, T.; Chen, Y.; Huang, X.; Liang, T. mRNA vaccines in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 365. [Google Scholar] [CrossRef]
- Liu, H.; Yin, J.; Xiao, M.; Gao, C.; Mason, A.S.; Zhao, Z.; Liu, Y.; Li, J.; Fu, D. Characterization and evolution of 5′ and 3′ untranslated regions in eukaryotes. Gene 2012, 507, 106–111. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). 8 Zoonotic Diseases Shared Between Animals and People of Most Concern in the U.S.; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019. Available online: https://archive.cdc.gov/www_cdc_gov/media/releases/2019/s0506-zoonotic-diseases-shared.html (accessed on 13 May 2025).
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.; Urbani, C.; Comer, J.A.; Lim, W.; et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [Google Scholar] [CrossRef]
- Zaki, A.M.; Van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Gralinski, L.E.; Baric, R.S. Molecular pathology of emerging coronavirus infections. J. Pathol. 2015, 235, 185–195. [Google Scholar] [CrossRef]
- Mole, B. Deadly pig virus slips through US borders. Nature 2013, 499, 388. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virol. J. 2015, 12, 1–16. [Google Scholar] [CrossRef]
- Halaji, M.; Heiat, M.; Faraji, N.; Ranjbar, R. Epidemiology of COVID-19: An updated review. J. Res. Med. Sci. 2021, 26, 82. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Barchitta, M.; Battiato, S.; Agodi, A. Estimation of unreported novel coronavirus (SARS-CoV-2) infections from reported deaths: A susceptible–exposed–infectious–recovered–dead model. J. Clin. Med. 2020, 9, 1350. [Google Scholar] [CrossRef] [PubMed]
- WHO. COVID-19 Epidemiological Update-22 December 2023, 162nd ed.; WHO: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update---22-december-2023 (accessed on 13 May 2025).
- He, F.; Deng, Y.; Li, W. Coronavirus disease 2019: What We Know? J. Med. Virol. 2020, 92, 719–725. [Google Scholar] [CrossRef]
- Li, F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef]
- Singhal, T. A review of coronavirus disease-2019 (COVID-19). Indian. J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef]
- Alanagreh, L.a.; Alzoughool, F.; Atoum, M. The human coronavirus disease COVID-19: Its origin, characteristics, and insights into potential drugs and its mechanisms. Pathogens 2020, 9, 331. [Google Scholar] [CrossRef]
- He, Y.; Zhou, Y.; Liu, S.; Kou, Z.; Li, W.; Farzan, M.; Jiang, S. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: Implication for developing subunit vaccine. Biochem. Biophys. Res. Commun. 2004, 324, 773–781. [Google Scholar] [CrossRef]
- Xia, X. Domains and functions of spike protein in SARS-CoV-2 in the context of vaccine design. Viruses 2021, 13, 109. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.-W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Sharif-Yakan, A.; Kanj, S.S.; Spindler, K.R. Emergence of MERS-CoV in the Middle East: Origins, transmission, treatment, and perspectives. PLoS Pathog. 2014, 10, e1004457. [Google Scholar] [CrossRef]
- Cauchemez, S.; Nouvellet, P.; Cori, A.; Jombart, T.; Garske, T.; Clapham, H.; Moore, S.; Mills, H.L.; Salje, H.; Collins, C.; et al. Unraveling the drivers of MERS-CoV transmission. Proc. Natl. Acad. Sci. USA 2016, 113, 9081–9086. [Google Scholar] [CrossRef]
- Yoon, I.-K.; Kim, J.H. First clinical trial of a MERS coronavirus DNA vaccine. Lancet Infect. Dis. 2019, 19, 924. [Google Scholar] [CrossRef]
- Chao, C.W.; Sprouse, K.R.; Miranda, M.C.; Catanzaro, N.J.; Hubbard, M.L.; Addetia, A.; Stewart, C.; Brown, J.T.; Dosey, A.; Valdez, A.; et al. Protein nanoparticle vaccines induce potent neutralizing antibody responses against MERS-CoV. Cell Rep. 2024, 43, 115036. [Google Scholar] [CrossRef]
- Yong, C.Y.; Ong, H.K.; Yeap, S.K.; Ho, K.L.; Tan, W.S. Recent advances in the vaccine development against Middle East respiratory syndrome-coronavirus. Front. Microbiol. 2019, 10, 1781. [Google Scholar] [CrossRef]
- Jung, B.-K.; An, Y.; Park, J.-E.; Chang, K.-S.; Jang, H. Development of a recombinant vaccine containing a spike S1-Fc fusion protein induced protection against MERS-CoV in human DPP4 knockin transgenic mice. J. Virol. Methods 2022, 299, 114347. [Google Scholar] [CrossRef]
- Tai, W.; Zhang, X.; Yang, Y.; Zhu, J.; Du, L. Advances in mRNA and other vaccines against MERS-CoV. Transl. Res. 2022, 242, 20–37. [Google Scholar] [CrossRef]
- Tai, W.; Zheng, J.; Zhang, X.; Shi, J.; Wang, G.; Guan, X.; Zhu, J.; Perlman, S.; Du, L. MERS-CoV RBD-mRNA vaccine induces potent and broadly neutralizing antibodies with protection against MERS-CoV infection. Virus Res. 2023, 334, 199156. [Google Scholar] [CrossRef]
- WHO. Transmission of SARS-CoV-2: Implications for Infection Prevention Precautions; Scientific Brief; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions (accessed on 13 May 2025).
- Meyerowitz, E.A.; Richterman, A. SARS-CoV-2 transmission and prevention in the era of the Delta variant. Infect. Dis. Clin. North Am. 2022, 36, 267. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Jia, W.; Qian, H.; Xiao, S.; Miao, T.; Yen, H.-L.; Tan, H.; Kang, M.; Cowling, B.J.; Li, Y. Lack of cross-transmission of SARS-CoV-2 between passenger’s cabins on the Diamond Princess cruise ship. Build. Environ. 2021, 198, 107839. [Google Scholar] [CrossRef] [PubMed]
- Adedokun, K.A.; Kamorudeen, R.T.; Bello, I.O. Authorization of the first COVID-19 emergency vaccines: The matters arising. EXCLI J. 2021, 20, 655. [Google Scholar]
- Echaide, M.; Chocarro de Erauso, L.; Bocanegra, A.; Blanco, E.; Kochan, G.; Escors, D. mRNA vaccines against SARS-CoV-2: Advantages and caveats. Int. J. Mol. Sci. 2023, 24, 5944. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Malvy, D.; McElroy, A.K.; de Clerck, H.; Günther, S.; van Griensven, J. Ebola virus disease. Lancet 2019, 393, 936–948. [Google Scholar] [CrossRef]
- Ohimain, E.I.; Silas-Olu, D. The 2013–2016 Ebola virus disease outbreak in West Africa. Curr. Opin. Pharmacol. 2021, 60, 360–365. [Google Scholar] [CrossRef]
- Kabami, Z.; Ario, A.R.; Harris, J.R.; Ninsiima, M.; Ahirirwe, S.R.; Ocero, J.R.A.; Atwine, D.; Mwebesa, H.G.; Kyabayinze, D.J.; Muruta, A.N.; et al. Ebola disease outbreak caused by the Sudan virus in Uganda, 2022: A descriptive epidemiological study. Lancet Glob. Health 2024, 12, e1684–e1692. [Google Scholar] [CrossRef]
- Baseler, L.; Chertow, D.S.; Johnson, K.M.; Feldmann, H.; Morens, D.M. The pathogenesis of Ebola virus disease. Annu. Rev. Pathol. 2017, 12, 387–418. [Google Scholar] [CrossRef]
- WHO. Ebola virus disease. Fact Sheets, 24 April 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/ebola-disease (accessed on 13 May 2025).
- Woolsey, C.; Geisbert, T.W.; Dutch, R.E. Current state of Ebola virus vaccines: A snapshot. PLoS Pathog. 2021, 17, e1010078. [Google Scholar] [CrossRef]
- Meyer, M.; Huang, E.; Yuzhakov, O.; Ramanathan, P.; Ciaramella, G.; Bukreyev, A. Modified mRNA-based vaccines elicit robust immune responses and protect guinea pigs from Ebola virus disease. J. Infect. Dis. 2018, 217, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Shariff, M. Nipah virus infection: A review. Epidemiol. Infect. 2019, 147, e95. [Google Scholar]
- Looi, L.M.; Chua, K.B. Lessons from the Nipah virus outbreak in Malaysia. Malaysian J. Pathol. 2007, 29, 63–67. [Google Scholar]
- Ang, B.S.; Lim, T.C.; Wang, L. Nipah virus infection. J. Clin. Microbiol. 2018, 56, e01875-17. [Google Scholar] [CrossRef]
- Nikolay, B.; Salje, H.; Hossain, M.J.; Khan, A.D.; Sazzad, H.M.; Rahman, M.; Daszak, P.; Ströher, U.; Pulliam, J.R.; Kilpatrick, A.M.; et al. Transmission of Nipah virus—14 years of investigations in Bangladesh. N. Engl. J. Med. 2019, 380, 1804–1814. [Google Scholar] [CrossRef]
- Tan, F.H.; Sukri, A.; Idris, N.; Ong, K.C.; Schee, J.P.; Tan, C.T.; Tan, S.H.; Wong, K.T.; Wong, L.P.; Tee, K.K.; et al. A systematic review on Nipah virus: Global molecular epidemiology and medical countermeasures development. Virus Evol. 2024, 10, veae048. [Google Scholar] [CrossRef]
- WHO. Newsroom-Nipah virus. Fact Sheets, 30 May 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/nipah-virus (accessed on 13 May 2025).
- Liew, Y.J.M.; Ibrahim, P.A.S.; Ong, H.M.; Chong, C.N.; Tan, C.T.; Schee, J.P.; Gómez Román, R.; Cherian, N.G.; Wong, W.F.; Chang, L.-Y. The immunobiology of Nipah virus. Microorganisms 2022, 10, 1162. [Google Scholar] [CrossRef]
- Harcourt, B.H.; Tamin, A.; Ksiazek, T.G.; Rollin, P.E.; Anderson, L.J.; Bellini, W.J.; Rota, P.A. Molecular characterization of Nipah virus, a newly emergent paramyxovirus. Virology 2000, 271, 334–349. [Google Scholar] [CrossRef]
- Bossart, K.N.; Rockx, B.; Feldmann, F.; Brining, D.; Scott, D.; LaCasse, R.; Geisbert, J.B.; Feng, Y.-R.; Chan, Y.-P.; Hickey, A.C.; et al. A Hendra virus G glycoprotein subunit vaccine protects African green monkeys from Nipah virus challenge. Sci. Transl. Med. 2012, 4, 146ra107. [Google Scholar] [CrossRef]
- van Doremalen, N.; Lambe, T.; Sebastian, S.; Bushmaker, T.; Fischer, R.; Feldmann, F.; Haddock, E.; Letko, M.; Avanzato, V.A.; Rissanen, I.; et al. A single-dose ChAdOx1-vectored vaccine provides complete protection against Nipah Bangladesh and Malaysia in Syrian golden hamsters. PLoS Neglected Trop. Dis. 2019, 13, e0007462. [Google Scholar] [CrossRef]
- Lo, M.K.; Spengler, J.R.; Welch, S.R.; Harmon, J.R.; Coleman-McCray, J.D.; Scholte, F.E.M.; Shrivastava-Ranjan, P.; Montgomery, J.M.; Nichol, S.T.; Weissman, D.; et al. Evaluation of a single-dose nucleoside-modified messenger RNA vaccine encoding Hendra virus-soluble glycoprotein against lethal Nipah virus challenge in Syrian hamsters. J. Infect. Dis. 2020, 221, S493–S498. [Google Scholar] [CrossRef] [PubMed]
- Loomis, R.J.; DiPiazza, A.T.; Falcone, S.; Ruckwardt, T.J.; Morabito, K.M.; Abiona, O.M.; Chang, L.A.; Caringal, R.T.; Presnyak, V.; Narayanan, E.; et al. Chimeric fusion (F) and attachment (G) glycoprotein antigen delivery by mRNA as a candidate Nipah vaccine. Front. Immunol. 2021, 12, 772864. [Google Scholar] [CrossRef]
- Pedrera, M.; McLean, R.K.; Medfai, L.; Thakur, N.; Todd, S.; Marsh, G.; Bailey, D.; Donofrio, G.; Muramatsu, H.; Pardi, N.; et al. Evaluation of the immunogenicity of an mRNA vectored Nipah virus vaccine candidate in pigs. Front. Immunol. 2024, 15, 1384417. [Google Scholar] [CrossRef] [PubMed]
- Brandys, P.; Albariño, C.G.; Jain, S.; Merenkova, I.; Shork, N.J.; Deng, A.; Valière, M.; Herold, J. A mRNA Vaccine Encoding for a 60-mer G Glycoprotein Nanoparticle Elicits a Robust Neutralizing Antibodies Response Against the Nipah Virus. bioRxiv 2024. [Google Scholar] [CrossRef]
- Moderna. Moderna Announces First Participant Dosed in a Phase 1 Trial of Its Nipah Virus mRNA Vaccine, mRNA-1215; Moderna: Cambridge, MA, USA, 2022; Available online: https://investors.modernatx.com/news/news-details/2022/Moderna-Announces-First-Participant-Dosed-in-a-Phase-1-Trial-of-its-Nipah-Virus-mRNA-Vaccine-mRNA-1215/default.aspx (accessed on 13 May 2025).
- WHO. Newsroom-Influenza (seasonal). Fact Sheets, 28 February 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 13 May 2025).
- Suarez, D.L. Influenza A virus. In Animal Influenza, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 1–30. [Google Scholar]
- Abdelwhab, E.M.; Mettenleiter, T.C. Zoonotic animal influenza virus and potential mixing vessel hosts. Viruses 2023, 15, 980. [Google Scholar] [CrossRef]
- Monto, A.S.; Fukuda, K. Lessons from influenza pandemics of the last 100 years. Clin. Infect. Dis. 2020, 70, 951–957. [Google Scholar] [CrossRef]
- Yamamoto, S.; Mizoue, T.; Ujiie, M.; Horii, K.; Takeuchi, J.S.; Konishi, M.; Sugiura, W.; Ohmagari, N. Low Levels of Postvaccination Hemagglutination Inhibition Antibodies and Their Correlation With Influenza Protection Among Healthcare Workers During the 2024–2025 A/H1N1 Outbreak in Japan. J. Infect. Dis. 2025, jiaf183. [Google Scholar] [CrossRef]
- WHO. Newsroom-Influenza (avian and other zoonotic). Fact Sheets, 3 October 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(avian-and-other-zoonotic) (accessed on 13 May 2025).
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26, D49–D53. [Google Scholar] [CrossRef]
- Szewczyk, B.; Bieńkowska-Szewczyk, K.; Król, E. Introduction to molecular biology of influenza a viruses. Acta Biochim. Pol. 2014, 61, 397–401. [Google Scholar] [CrossRef]
- Li, Y.-T.; Linster, M.; Mendenhall, I.H.; Su, Y.C.; Smith, G.J. Avian influenza viruses in humans: Lessons from past outbreaks. Br. Med. Bull. 2019, 132, 81–95. [Google Scholar] [CrossRef]
- Pappas, C.; Aguilar, P.V.; Basler, C.F.; Solórzano, A.; Zeng, H.; Perrone, L.A.; Palese, P.; García-Sastre, A.; Katz, J.M.; Tumpey, T.M. Single gene reassortants identify a critical role for PB1, HA, and NA in the high virulence of the 1918 pandemic influenza virus. Proc. Natl. Acad. Sci. USA 2008, 105, 3064–3069. [Google Scholar] [CrossRef] [PubMed]
- Petsch, B.; Schnee, M.; Vogel, A.B.; Lange, E.; Hoffmann, B.; Voss, D.; Schlake, T.; Thess, A.; Kallen, K.-J.; Stitz, L.; et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 2012, 30, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef] [PubMed]
- Magini, D.; Giovani, C.; Mangiavacchi, S.; Maccari, S.; Cecchi, R.; Ulmer, J.B.; De Gregorio, E.; Geall, A.J.; Brazzoli, M.; Bertholet, S.; et al. Self-amplifying mRNA vaccines expressing multiple conserved influenza antigens confer protection against homologous and heterosubtypic viral challenge. PLoS ONE 2016, 11, e0161193. [Google Scholar] [CrossRef]
- Vogel, A.B.; Lambert, L.; Kinnear, E.; Busse, D.; Erbar, S.; Reuter, K.C.; Wicke, L.; Perkovic, M.; Beissert, T.; Haas, H.; et al. Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Mol. Ther. 2018, 26, 446–455. [Google Scholar] [CrossRef]
- Pardi, N.; Parkhouse, K.; Kirkpatrick, E.; McMahon, M.; Zost, S.J.; Mui, B.L.; Tam, Y.K.; Karikó, K.; Barbosa, C.J.; Madden, T.D.; et al. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nat. Commun. 2018, 9, 3361. [Google Scholar] [CrossRef]
- Freyn, A.W.; da Silva, J.R.; Rosado, V.C.; Bliss, C.M.; Pine, M.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Ferreira, L.C.d.S.; Weissman, D.; et al. A multi-targeting, nucleoside-modified mRNA influenza virus vaccine provides broad protection in mice. Mol. Ther. 2020, 28, 1569–1584. [Google Scholar] [CrossRef]
- Chivukula, S.; Plitnik, T.; Tibbitts, T.; Karve, S.; Dias, A.; Zhang, D.; Goldman, R.; Gopani, H.; Khanmohammed, A.; Sarode, A.; et al. Development of multivalent mRNA vaccine candidates for seasonal or pandemic influenza. NPJ Vaccines 2021, 6, 153. [Google Scholar] [CrossRef]
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.; Furey, C.; Muramatsu, H.; Alameh, M.-G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 2022, 378, 899–904. [Google Scholar] [CrossRef]
- van de Ven, K.; Lanfermeijer, J.; van Dijken, H.; Muramatsu, H.; de Melo, C.V.B.; Lenz, S.; Peters, F.; Beattie, M.B.; Lin, P.J.C.; Ferreira, J.A.; et al. A universal influenza mRNA vaccine candidate boosts T cell responses and reduces zoonotic influenza virus disease in ferrets. Sci. Adv. 2022, 8, eadc9937. [Google Scholar] [CrossRef]
- Kackos, C.M.; DeBeauchamp, J.; Davitt, C.J.H.; Lonzaric, J.; Sealy, R.E.; Hurwitz, J.L.; Samsa, M.M.; Webby, R.J. Seasonal quadrivalent mRNA vaccine prevents and mitigates influenza infection. NPJ Vaccines 2023, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Leonard, R.A.; Burke, K.N.; Spreng, R.L.; Macintyre, A.N.; Tam, Y.; Alameh, M.-G.; Weissman, D.; Heaton, N.S. Improved influenza vaccine responses after expression of multiple viral glycoproteins from a single mRNA. Nat. Commun. 2024, 15, 8712. [Google Scholar] [CrossRef] [PubMed]
- Sanofi. Sanofi and Translate Bio Initiate Phase 1 Clinical Trial of mRNA Influenza Vaccine; Sanofi: Paris, France, 2021; Available online: https://www.sanofi.com/en/media-room/press-releases/2021/2021-06-22-05-00-00-2250633 (accessed on 13 May 2025).
- Moderna. Moderna Announces Interim Phase 3 Safety and Immunogenicity Results for mRNA-1010, a Seasonal Influenza Vaccine Candidate; Moderna: Cambridge, MA, USA, 2023; Available online: https://investors.modernatx.com/news/news-details/2023/Moderna-Announces-Interim-Phase-3-Safety-and-Immunogenicity-Results-for-mRNA-1010-a-Seasonal-Influenza-Vaccine-Candidate/default.aspx (accessed on 13 May 2025).
- Soens, M.; Ananworanich, J.; Hicks, B.; Lucas, K.J.; Cardona, J.; Sher, L.; Livermore, G.; Schaefers, K.; Henry, C.; Choi, A.; et al. A phase 3 randomized safety and immunogenicity trial of mRNA-1010 seasonal influenza vaccine in adults. Vaccine 2025, 50, 126847. [Google Scholar] [CrossRef] [PubMed]
- Moderna. Moderna Announces First Participants Dosed in Phase 1/2 Study with mRNA-1020 and mRNA-1030 Seasonal Influenza Vaccine Candidates; Moderna: Cambridge, MA, USA, 2022; Available online: https://investors.modernatx.com/news/news-details/2022/Moderna-Announces-First-Participants-Dosed-in-Phase-12-Study-with-mRNA-1020-and-mRNA-1030-Seasonal-Influenza-Vaccine-Candidates/default.aspx (accessed on 13 May 2025).
- Pfizer. Pfizer and BioNTech Provide Update on mRNA-based Combination Vaccine Program Against Influenza and COVID-19 in Individuals 18–64 Years of Age; Pfizer: New York, NY, USA, 2024; Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-provide-update-mrna-based-combination (accessed on 13 May 2025).
- Jackson, A.C. Update on rabies. Res. Rep. Trop. Med. 2011, 2, 31–43. [Google Scholar] [CrossRef]
- WHO. Newsroom-Rabies. Fact Sheets, 5 June 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/rabies (accessed on 13 May 2025).
- Albertini, A.A.; Ruigrok, R.W.; Blondel, D. Rabies virus transcription and replication. Adv. Virus Res. 2011, 79, 1–22. [Google Scholar]
- Faber, M.; Pulmanausahakul, R.; Hodawadekar, S.S.; Spitsin, S.; McGettigan, J.P.; Schnell, M.J.; Dietzschold, B. Overexpression of the rabies virus glycoprotein results in enhancement of apoptosis and antiviral immune response. J. Virol. 2002, 76, 3374–3381. [Google Scholar] [CrossRef]
- Armbruster, N.; Jasny, E.; Petsch, B. Advances in RNA vaccines for preventive indications: A case study of a vaccine against rabies. Vaccines 2019, 7, 132. [Google Scholar] [CrossRef]
- Fang, Z.; Yu, P.; Zhu, W. Development of mRNA rabies vaccines. Hum. Vaccines Immunother. 2024, 20, 2382499. [Google Scholar] [CrossRef]
- Schnee, M.; Vogel, A.B.; Voss, D.; Petsch, B.; Baumhof, P.; Kramps, T.; Stitz, L. An mRNA vaccine encoding rabies virus glycoprotein induces protection against lethal infection in mice and correlates of protection in adult and newborn pigs. PLoS Negl. Trop. Dis. 2016, 10, e0004746. [Google Scholar] [CrossRef]
- Li, J.; Liu, Q.; Liu, J.; Wu, X.; Lei, Y.; Li, S.; Zhao, D.; Li, Z.; Luo, L.; Peng, S.; et al. An mRNA-based rabies vaccine induces strong protective immune responses in mice and dogs. Virol. J. 2022, 19, 184. [Google Scholar] [CrossRef]
- Li, J.; Yu, P.; Liu, Q.; Xu, L.; Chen, Y.; Li, Y.; Zhang, F.; Zhu, W.; Peng, Y. Safety and efficacy assessment of an mRNA rabies vaccine in dogs, rodents, and cynomolgus macaques. NPJ Vaccines 2024, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, F.; Cagigi, A.; Cerveira, R.A.; Ols, S.; Kern, T.; Lin, A.; Eriksson, B.; Dodds, M.G.; Jasny, E.; Schwendt, K.; et al. Unmodified rabies mRNA vaccine elicits high cross-neutralizing antibody titers and diverse B cell memory responses. Nat. Commun. 2023, 14, 3713. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Bai, H.; Yu, X.; Liu, Q.; Hu, R. Immunogenicity of Rabies Virus G-Protein mRNA Formulated with Muscle-Targeting Lipid Nanoparticles in Mice. Vaccines 2025, 13, 217. [Google Scholar] [CrossRef]
- Alberer, M.; Gnad-Vogt, U.; Hong, H.S.; Mehr, K.T.; Backert, L.; Finak, G.; Gottardo, R.; Bica, M.A.; Garofano, A.; Koch, S.D.; et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: An open-label, non-randomized, prospective, first-in-human phase 1 clinical trial. Lancet 2017, 390, 1511–1520. [Google Scholar] [CrossRef]
- Aldrich, C.; Leroux–Roels, I.; Huang, K.B.; Bica, M.A.; Loeliger, E.; Schoenborn-Kellenberger, O.; Walz, L.; Leroux-Roels, G.; von Sonnenburg, F.; Oostvogels, L. Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: A phase 1 trial. Vaccine 2021, 39, 1310–1318. [Google Scholar] [CrossRef]
- Socha, W.; Kwasnik, M.; Larska, M.; Rola, J.; Rozek, W. Vector-borne viral diseases as a current threat for human and animal health—One Health perspective. J. Clin. Med. 2022, 11, 3026. [Google Scholar] [CrossRef]
- WHO. Vector-borne diseases. Fact Sheets, 26 September 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 13 May 2025).
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA vaccines protect against Zika virus infection. Cell 2017, 168, 1114–1125.e10. [Google Scholar] [CrossRef]
- Essink, B.; Chu, L.; Seger, W.; Barranco, E.; Le Cam, N.; Bennett, H.; Faughnan, V.; Pajon, R.; Paila, Y.D.; Bollman, B.; et al. The safety and immunogenicity of two Zika virus mRNA vaccine candidates in healthy flavivirus baseline seropositive and seronegative adults: The results of two randomised, placebo-controlled, dose-ranging, phase 1 clinical trials. Lancet Infect. Dis. 2023, 23, 621–633. [Google Scholar] [CrossRef]
- Bollman, B.; Nunna, N.; Bahl, K.; Hsiao, C.J.; Bennett, H.; Butler, S.; Foreman, B.; Burgomaster, K.E.; Aleshnick, M.; Kong, W.-P.; et al. An optimized messenger RNA vaccine candidate protects non-human primates from Zika virus infection. NPJ Vaccines 2023, 8, 58. [Google Scholar] [CrossRef]
- Pintado Silva, J.; Fernandez-Sesma, A. Challenges on the development of a dengue vaccine: A comprehensive review of the state of the art. J. Gen. Virol. 2023, 104, 001831. [Google Scholar] [CrossRef]
- Mady, B.J.; Erbe, D.V.; Kurane, I.; Fanger, M.W.; Ennis, F.A. Antibody-dependent enhancement of dengue virus infection mediated by bispecific antibodies against cell surface molecules other than Fc gamma receptors. J. Immunol. 1991, 147, 3139–3144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, J.; Li, M.; Jin, X. Modified mRNA-LNP vaccines confer protection against experimental DENV-2 infection in mice. Mol. Ther. Methods Clin. Dev. 2020, 18, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Wollner, C.J.; Richner, M.; Hassert, M.A.; Pinto, A.K.; Brien, J.D.; Richner, J.M.; Heise, M.T. A dengue virus serotype 1 mRNA-LNP vaccine elicits protective immune responses. J. Virol. 2021, 95, 10–1128. [Google Scholar] [CrossRef]
- He, L.; Sun, W.; Yang, L.; Liu, W.; Li, J. A multiple-target mRNA-LNP vaccine induces protective immunity against experimental multi-serotype DENV in mice. Virol. Sin. 2022, 37, 746–757. [Google Scholar] [CrossRef]
- Alkan, C.; Jurado-Cobena, E.; Ikegami, T. Advancements in Rift Valley fever vaccines: A historical overview and prospects for next generation candidates. NPJ Vaccines 2023, 8, 171. [Google Scholar] [CrossRef]
- Islam, M.R.; Ahmed, I.; Urmi, T.J. The pathogenicity and risk evaluation of Rift Valley virus to cause mysterious “Disease X”: An update on recent evidences. Ann. Med. Surg. 2024, 86, 1243–1246. [Google Scholar] [CrossRef]
- Bian, T.; Hao, M.; Zhao, X.; Zhao, C.; Luo, G.; Zhang, Z.; Fu, G.; Yang, L.; Chen, Y.; Wang, Y.; et al. A Rift Valley fever mRNA vaccine elicits strong immune responses in mice and rhesus macaques. NPJ Vaccines 2023, 8, 164. [Google Scholar] [CrossRef]
- Kitandwe, P.K.; Rogers, P.; Hu, K.; Nayebare, O.; Blakney, A.K.; McKay, P.F.; Kaleebu, P.; Shattock, R.J. A Lipid Nanoparticle-Formulated Self-Amplifying RNA Rift Valley Fever Vaccine Induces a Robust Humoral Immune Response in Mice. Vaccines 2024, 12, 1088. [Google Scholar] [CrossRef]
- Hermance, M.E.; Thangamani, S. Powassan virus: An emerging arbovirus of public health concern in North America. Vector-Borne Zoonotic Dis. 2017, 17, 453–462. [Google Scholar] [CrossRef]
- Ebel, G.D. Update on Powassan virus: Emergence of a North American tick-borne flavivirus. Annu. Rev. Entomol. 2010, 55, 95–110. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Powassan Virus; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2024.
- VanBlargan, L.A.; Himansu, S.; Foreman, B.M.; Ebel, G.D.; Pierson, T.C.; Diamond, M.S. An mRNA vaccine protects mice against multiple tick-transmitted flavivirus infections. Cell Rep. 2018, 25, 3382–3392.e3. [Google Scholar] [CrossRef]
- Hoogstraal, H. Review Article: The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J. Med. Entomol. 1979, 15, 307–417. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Pigott, D.M.; Golding, N.; Duda, K.A.; Brownstein, J.S.; Weiss, D.J.; Gibson, H.; Robinson, T.P.; Gilbert, M.; Wint, G.R.W.; et al. The global distribution of Crimean-Congo hemorrhagic fever. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 503–513. [Google Scholar] [CrossRef] [PubMed]
- WHO. Newsroom-Crimean-Congo Haemorrhagic Fever; WHO: Geneva, Switzerland, 2025; Available online: https://www.who.int/news-room/fact-sheets/detail/crimean-congo-haemorrhagic-fever (accessed on 13 May 2025).
- Shayan, S.; Bokaean, M.; Shahrivar, M.R.; Chinikar, S. Crimean-Congo hemorrhagic fever. Lab. Med. 2015, 46, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.E.; Gilbride, C.; Dowall, S.; Morris, S.; Ulaszewska, M.; Spencer, A.J.; Rayner, E.; Graham, V.A.; Kennedy, E.; Thomas, K.; et al. Adenoviral vectored vaccination protects against Crimean-Congo Haemorrhagic Fever disease in a lethal challenge model. eBioMedicine 2023, 90, 104523. [Google Scholar] [CrossRef]
- Appelberg, S.; John, L.; Pardi, N.; Végvári, Á.; Bereczky, S.; Ahlén, G.; Monteil, V.; Abdurahman, S.; Mikaeloff, F.; Beattie, M.; et al. Nucleoside-modified mRNA vaccines protect IFNAR–/–mice against Crimean-Congo hemorrhagic fever virus infection. J. Virol. 2022, 96, e01568-21. [Google Scholar] [CrossRef]
- Leventhal, S.S.; Shaia, C.; Rao, D.; Lewis, M.; Meade-White, K.; Erasmus, J.H.; Feldmann, H.; Hawman, D.W. Replicating RNA vaccine confers durable immunity against Crimean Congo hemorrhagic fever virus challenge in mice. NPJ Vaccines 2024, 9, 249. [Google Scholar] [CrossRef]
- Hawman, D.W.; Leventhal, S.S.; Meade-White, K.; Khandhar, A.; Murray, J.; Lovaglio, J.; Shaia, C.; Saturday, G.; Hinkley, T.; Erasmus, J.; et al. A replicating RNA vaccine confers protection in a rhesus macaque model of Crimean-Congo hemorrhagic fever. NPJ Vaccines 2024, 9, 86. [Google Scholar] [CrossRef]
- Chen, T.; Ding, Z.; Li, X.; Li, Y.; Lan, J.; Wong, G. A mRNA Vaccine for Crimean–Congo Hemorrhagic Fever Virus Expressing Non-Fusion GnGc Using NSm Linker Elicits Unexpected Immune Responses in Mice. Viruses 2024, 16, 378. [Google Scholar] [CrossRef]
- González-Cueto, E.; De La Fuente, J.; Lopez-Camacho, C. Potential of mRNA-based vaccines for the control of tick-borne pathogens in one health perspective. Front. Immunol. 2024, 15, 1384442. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Yang, L.; Cao, P.; Lu, J. Severe fever with thrombocytopenia syndrome virus: A highly lethal bunyavirus. Crit. Rev. Microbiol. 2021, 47, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-J.; Liang, M.-F.; Zhang, S.-Y.; Liu, Y.; Li, J.-D.; Sun, Y.-L.; Zhang, L.; Zhang, Q.-F.; Popov, V.L.; Li, C.; et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Tran, X.C.; Yun, Y.; Van An, L.; Kim, S.-H.; Thao, N.T.P.; Man, P.K.C.; Yoo, J.R.; Heo, S.T.; Cho, N.-H.; Lee, K.H. Endemic severe fever with thrombocytopenia syndrome, Vietnam. Emerg. Infect. Dis. 2019, 25, 1029. [Google Scholar] [CrossRef]
- Rattanakomol, P.; Khongwichit, S.; Linsuwanon, P.; Lee, K.H.; Vongpunsawad, S.; Poovorawan, Y. Severe fever with thrombocytopenia syndrome virus infection, Thailand, 2019–2020. Emerg. Infect. Dis. 2022, 28, 2572. [Google Scholar] [CrossRef]
- Ai, L.; Wang, W.; Teng, Z. Advancements in the worldwide detection of severe fever with thrombocytopenia syndrome virus infection from 2009 to 2023. China CDC Wkly. 2023, 5, 687. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, S.; Jiang, M.; Bi, Z.; Liang, M.; Ding, S.; Wang, S.; Liu, J.; Zhou, S.; Zhang, X.; et al. A cluster of person-to-person transmission cases caused by SFTS virus in Penglai, China. Clin. Microbiol. Infect. 2015, 21, 274–279. [Google Scholar] [CrossRef]
- Kim, D.; Lai, C.-J.; Cha, I.; Jung, J.U. Current Progress of Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV) Vaccine Development. Viruses 2024, 16, 128. [Google Scholar] [CrossRef]
- Wormser, G.P.; McKenna, D.; Piedmonte, N.; Vinci, V.; Egizi, A.M.; Backenson, B.; Falco, R.C. First recognized human bite in the United States by the Asian longhorned tick, Haemaphysalis longicornis. Clin. Infect. Dis. 2020, 70, 314–316. [Google Scholar] [CrossRef]
- Pritt, B.S. Haemaphysalis longicornis is in the United States and biting humans: Where Do We Go From Here? Clin. Infect. Dis. 2019, 70, 317–318. [Google Scholar] [CrossRef]
- Kim, D.; Lai, C.; Cha, I.; Kang, S.; Yang, W.; Choi, Y.; Jung, J.U. SFTSV Gn-Head mRNA vaccine confers efficient protection against lethal viral challenge. J. Med. Virol. 2023, 95, e29203. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Jeon, K.; Park, S.-I.; Bang, Y.-J.; Park, H.-J.; Kwak, H.W.; Kim, D.-H.; Lee, S.-Y.; Choi, E.-J.; Cho, N.-H.; et al. mRNA vaccine encoding Gn provides protection against severe fever with thrombocytopenia syndrome virus in mice. NPJ Vaccines 2023, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, J.; Wu, Y.; He, X.; Gao, X.; Chen, X.; Chen, S.; Zhu, X.; Peng, Y.; Xiao, G.; et al. A full-length glycoprotein mRNA vaccine confers complete protection against severe fever with thrombocytopenia syndrome virus, with broad-spectrum protective effects against bandaviruses. J. Virol. 2024, 98, e00769-24. [Google Scholar] [CrossRef] [PubMed]
- WHO. Newsroom-Lassa fever. Fact Sheets, 5 December 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/lassa-fever (accessed on 13 May 2025).
- Ly, H. Progress toward the development of Lassa vaccines. Expert Rev. Vaccines 2024, 23, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Aloke, C.; Obasi, N.A.; Aja, P.M.; Emelike, C.U.; Egwu, C.O.; Jeje, O.; Edeogu, C.O.; Onisuru, O.O.; Orji, O.U.; Achilonu, I. Combating Lassa fever in West African sub-region: Progress, challenges, and future perspectives. Viruses 2023, 15, 146. [Google Scholar] [CrossRef]
- Warner, B.M.; Safronetz, D.; Stein, D.R. Current perspectives on vaccines and therapeutics for Lassa Fever. Virol. J. 2024, 21, 320. [Google Scholar] [CrossRef]
- Grant, D.S.; Engel, E.J.; Yerkes, N.R.; Kanneh, L.; Koninga, J.; Gbakie, M.A.; Alhasan, F.; Kanneh, F.B.; Kanneh, I.M.; Kamara, F.K.; et al. Seroprevalence of anti-Lassa Virus IgG antibodies in three districts of Sierra Leone: A cross-sectional, population-based study. PLoS Neglected Trop. Dis. 2023, 17, e0010938. [Google Scholar] [CrossRef]
- Ronk, A.J.; Lloyd, N.M.; Zhang, M.; Atyeo, C.; Perrett, H.R.; Mire, C.E.; Hastie, K.M.; Sanders, R.W.; Brouwer, P.J.M.; Saphire, E.O.; et al. A Lassa virus mRNA vaccine confers protection but does not require neutralizing antibody in a guinea pig model of infection. Nat. Commun. 2023, 14, 5603. [Google Scholar] [CrossRef]
- Hashizume, M.; Takashima, A.; Iwasaki, M.; Gallagher, T. An mRNA-LNP-based Lassa virus vaccine induces protective immunity in mice. J. Virol. 2024, 98, e00578-24. [Google Scholar] [CrossRef]
- Chu, L.; Vrbicky, K.; Montefiori, D.; Huang, W.; Nestorova, B.; Chang, Y.; Carfi, A.; Edwards, D.K.; Oestreicher, J.; Legault, H.; et al. Immune response to SARS-CoV-2 after a booster of mRNA-1273: An open-label phase 2 trial. Nat. Med. 2022, 28, 1042–1049. [Google Scholar] [CrossRef]
- Schultz, M. Rudolf Virchow. Emerg. Infect. Dis. 2008, 14, 1480. [Google Scholar] [CrossRef][Green Version]
- WHO. Newsroom-One Health; WHO: Geneva, Switzerland, 2017; Available online: https://www.who.int/news-room/questions-and-answers/item/one-health (accessed on 13 May 2025).
- Erkyihun, G.A.; Alemayehu, M.B. One Health approach for the control of zoonotic diseases. Zoonoses 2022, 2, 963. [Google Scholar] [CrossRef]
- Carpenter, A.; Waltenburg, M.A.; Hall, A.; Kile, J.; Killerby, M.; Knust, B.; Negron, M.; Nichols, M.; Wallace, R.M.; Behravesh, C.B.; et al. Vaccine preventable zoonotic diseases: Challenges and opportunities for public health progress. Vaccines 2022, 10, 993. [Google Scholar] [CrossRef] [PubMed]
- Rabozzi, G.; Bonizzi, L.; Crespi, E.; Somaruga, C.; Sokooti, M.; Tabibi, R.; Vellere, F.; Brambilla, G.; Colosio, C. Emerging zoonoses: The “one health approach”. Saf. Health Work 2012, 3, 77–83. [Google Scholar] [CrossRef]
- WHO. Blueprint for R&D preparedness and response to public health emergencies due to highly infectious pathogens. In Proceedings of the Workshop on Prioritization of Pathogens, Geneva, Switzerland, 15 December 2015; Available online: https://www.who.int/publications/m/item/blueprint-for-r-d-preparedness-and-response-to-public-health-emergencies-due-to-highly-infectious-pathogens (accessed on 13 May 2025).
- Skyle, D. WHO Pathogen X Conference. Lancet Infect. Dis. 2022, 22, 1541. [Google Scholar] [CrossRef]
- Horefti, E. The importance of the One Health concept in combating zoonoses. Pathogens 2023, 12, 977. [Google Scholar] [CrossRef]
- Banji, A.F.; Adekola, A.D.; Dada, S.A. mRNA-based vaccines for rapid response to emerging infectious disease outbreaks. Int. J. Front. Med. Surg. Res. 2024, 6, 97–105. [Google Scholar] [CrossRef]
- Kowalzik, F.; Schreiner, D.; Jensen, C.; Teschner, D.; Gehring, S.; Zepp, F. mRNA-based vaccines. Vaccines 2021, 9, 390. [Google Scholar] [CrossRef]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef]
- Uddin, M.N.; Roni, M.A. Challenges of storage and stability of mRNA-based COVID-19 vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef]
- Li, M.; Jia, L.; Xie, Y.; Ma, W.; Yan, Z.; Liu, F.; Deng, J.; Zhu, A.; Siwei, X.; Su, W.; et al. Lyophilization process optimization and molecular dynamics simulation of mRNA-LNPs for SARS-CoV-2 vaccine. NPJ Vaccines 2023, 8, 153. [Google Scholar] [CrossRef]
- Brito, L.A.; Chan, M.; Shaw, C.A.; Hekele, A.; Carsillo, T.; Schaefer, M.; Archer, J.; Seubert, A.; Otten, G.R.; Beard, C.W. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol. Ther. 2014, 22, 2118–2129. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Ai, X.; Remant-Bahadur, K.; Dick, T.A.; Yan, B.; Lu, T.; Zhou, X.; Luo, R.; Liu, M. Safe and effective delivery of mRNA using modified PEI-based lipopolymers. Pharmaceutics 2023, 15, 410. [Google Scholar] [CrossRef] [PubMed]
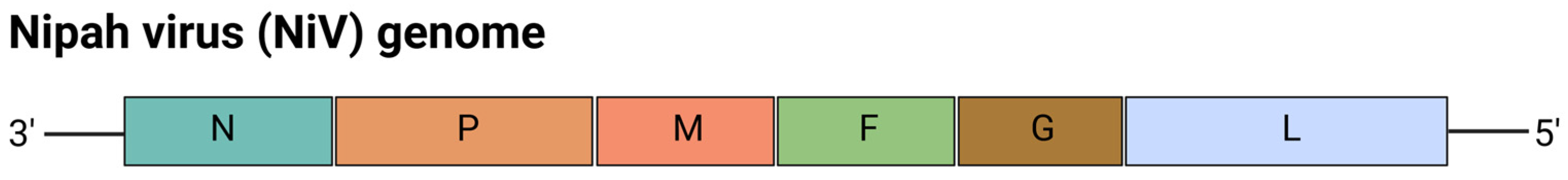

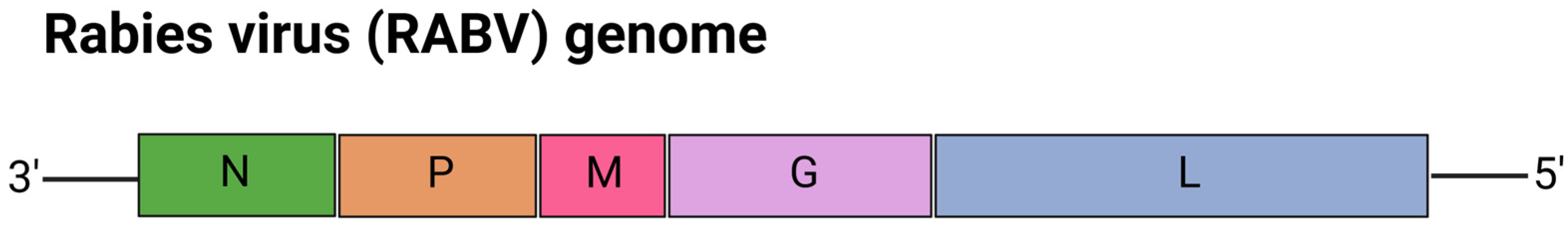

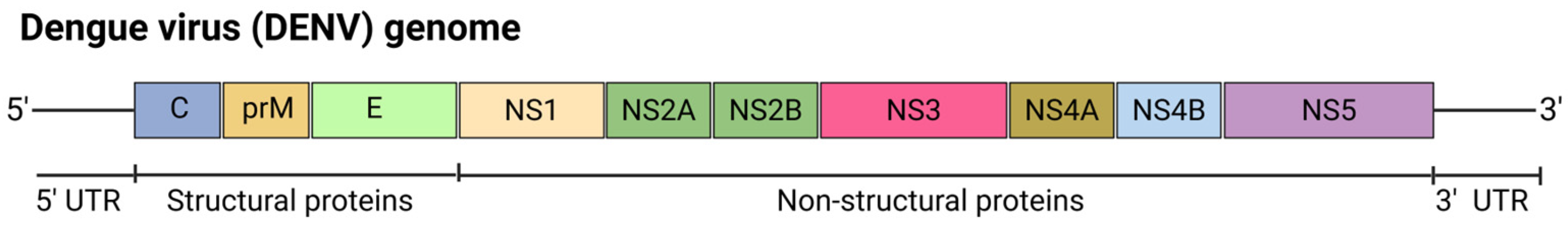


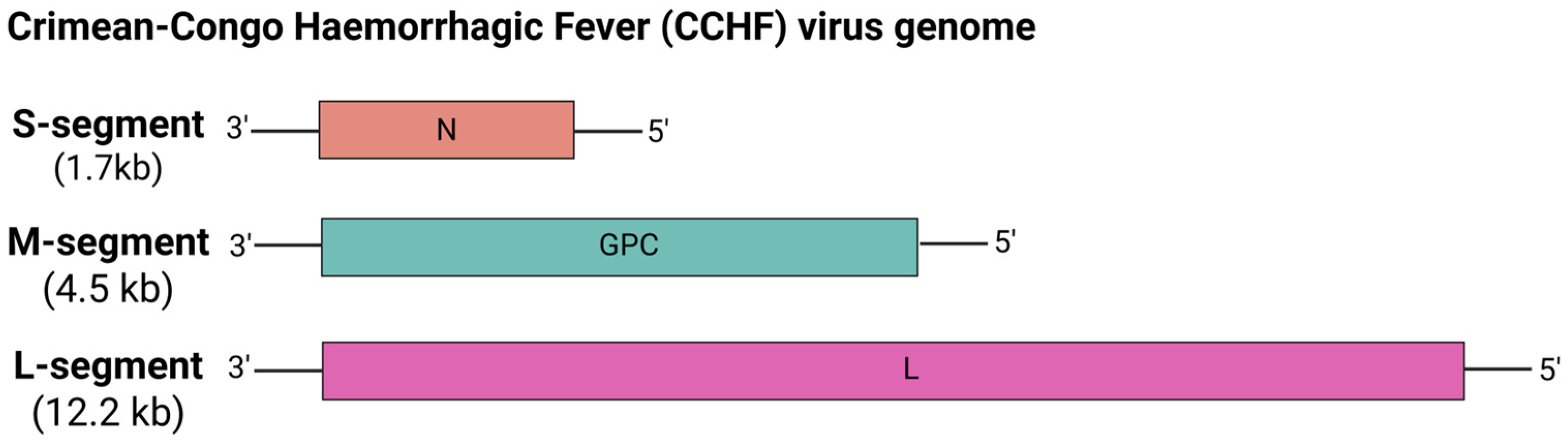

| Vaccine Name | Target Virus | Target | Vaccine Design | Findings | Development Stage | Ref. |
|---|---|---|---|---|---|---|
| MERS-COV RBD-mRNA vaccine | MERS-CoV | RBD domain of S protein | Nucleoside-modified mRNA-LNP vaccine candidate | Elicited durable, potent, and broad cross-neutralization antibodies against multiple MERS-CoV strains Induced robust cellular immune responses Conferred complete protection against MERS-CoV challenge in mice | Pre-clinical BALB/c mice | [67] |
| BNT162b2 (Cominarty by Pfizer-BioNTech) | SARS-CoV-2 | Full-length S protein of SARS-CoV-2 | Nucleoside-modified mRNA-LNP containing the full-length S protein of SARS-CoV-2 with S2 subunit containing two proline substitutions (amino acid positions 986 and 987) in prefusion conformation | Elicited a robust immune response characterized by the production of high levels of SARS-CoV-2-specific neutralizing antibodies and strong T-cell responses Two doses conferred 95% protection against COVID-19 in individuals aged ≥ 16 years or older | Approved for use in December 2020 | [72,73] |
| mRNA-1273 (Spikevax by Moderna) | SARS-CoV-2 | Full-length S protein of SARS-CoV-2 | Nucleoside-modified mRNA-LNP containing full-length S protein of SARS-CoV-2 with two proline substitutions within the S2 subunit in prefusion conformation and an intact furin cleavage site | Elicited robust and durable immune response against SARS-CoV-2 following vaccination with two doses of the mRNA-1273 A third dose significantly increases neutralizing antibody levels Demonstrated 94.1% efficacy against symptomatic COVID-19 following Day 64 post-vaccination follow-up | Approved for use in January 2021 | [182] |
| Nucleoside- modified mRNA-LNP vaccine | EBOV | Ebola virus glycoprotein (EBOV-GP) | Two nucleoside-modified mRNA-LNP vaccine candidates, EBOV-GP with wild type EBOV-GP signal peptide (Vaccine A) and EBOV-GP with human Igκ signal peptide (Vaccine B) | Vaccine B elicited higher GP-specific IgG and neutralizing antibody titers when compared to Vaccine A Two doses of either vaccine conferred 100% protection in guinea pigs against a lethal guinea pig-adapted EBOV challenge | Pre-clinical Guinea pigs strain Harley | [80] |
| sHeVG mRNA-LNP vaccine | NiV | Soluble Hendra virus glycoprotein (sHeVG) | Nucleoside-modified mRNA-LNP vaccine candidate encoding the sHEVG | No detectable pre-challenge neutralizing antibodies in the plasma of immunized hamsters A single dose of the mRNA-LNP vaccine protected 70% of the immunized Syrian hamsters against the lethal NiV challenge Surviving hamsters displayed robust NiV-specific IgG and neutralizing antibodies post-challenge infection | Pre-clinical Syrian hamsters (Mesocricetus auratus) | [91] |
| Nucleoside- modified mRNA-LNP vaccine | NiV | NiV prefusion-stabilized fusion glycoprotein (pre-F+G) | Nucleoside-modified mRNA-LNP vaccine candidate encoding the pre-F+G | Elicited strong neutralizing antibodies and robust T-cell immunity by inducing T follicular helper (Tfh) and CD8+ T-cell responses | Pre-clinical CB6F1/J mice | [92] |
| NiV sG mRNA-LNP vaccine | NiV | NiV soluble glycoprotein (NiV sG) from a Malaysian NiV strain (NiV-M) | Nucleoside-modified mRNA-LNP vaccine candidate encoding the NiV-M sG | Elicited potent antigen-binding and virus-neutralizing antibodies following the second booster dose immunization Induced both CD4+ and CD8+ T-cell responses | Pre-clinical White-Landrace-Hampshire cross-bred pigs | [93] |
| mRNA NiV G-NP | NiV | 60 head domains of NiV glycoproteins | mRNA nanoparticle (NP) vaccine displaying 60 head domains of NiV glycoprotein | Elicited a robust anti-NiV G humoral response and NiV neutralizing antibody response with high serum NiV neutralizing titers using a pseudotyped NiV system | Pre-clinical CB6F1/J mice | [94] |
| mRNA-1215 (Moderna) | NiV | Secreted pre-F/G of the NiV-M | mRNA vaccine encoding the secreted pre-F/G of the NiV-M | Currently being evaluated for its safety and immunogenicity in healthy adult participants aged 18 to 60 years | Phase I NCT05398796 (Completed) | [95] |
| mRNA-LNP vaccine | Influenza virus | Hemagglutinin (HA) | mRNA-LNP vaccine candidate encoding the HA from the H1N1 influenza strain | Vaccinated mice elicited robust neutralizing antibodies and T-cell responses Conferred complete protection against a lethal viral challenge | Pre-clinical BALB/c mice | [106] |
| SAM M1-NP mRNA-LNP vaccine | Influenza virus | Nucleoprotein (NP) and matrix protein 1 (M1) | mRNA-LNP vaccine candidate encoding theH1N1 NP and M1 antigens | Induced robust NP-specific CD8+ T-cells and polyfunctional CD4+ Th1 cells 78% survival against lethal homologous (H1N1) challenge, whilst achieving 100% survival against lethal heterosubtypic (H3N2) challenge | Pre-clinical BALB/c mice | [108] |
| sa-RNA vaccine/non-replicating synthetic RNA vaccine | Influenza virus | HA from a model influenza strain (monovalent) HA from multiple influenza A and B strains (A/H1N1, A/H3N2, B/Massachusetts) (trivalent) | Two RNA vaccine candidates, saRNA and non-replicating RNA | Both monovalent sa-RNA and non-replicating RNA vaccines conferred equivalent protective immunity, but sa-RNA required a 64-fold lower dose to achieve protective immunity when compared to the non-replicating RNA vaccine Trivalent saRNA vaccine protected the immunized mice against sequential H1N1 and H3N2 lethal challenges | Pre-clinical BALB/c mice | [109] |
| Nucleoside-modified mRNA-LNP vaccine | Influenza virus | Fast protein liquid chromatography (FPLC)-purified full-length HA protein | Nucleoside-modified mRNA-LNP vaccine candidate encoding the full-length HA from H1N1 (A/Cal09) | Elicited potent antibody responses against the HA head and stalk domains in mice, rabbits, and ferrets Protected immunized mice against homologous (H1N1) and heterosubtypic (H5N1) influenza strains | Pre-clinical BALB/c mice Rabbits Ferrets | [110] |
| Nucleoside-modified mRNA-LNP vaccine | Influenza virus | HA stalk, matrix-2 ion channel (M2), neuraminidase (NA) and nucleoprotein (NP) | Multivalent nucleoside- modified mRNA-LNP vaccine candidate encoding a combination of conserved influenza virus antigens from different H1N1 strains | Single dose elicited a robust humoral immune response by eliciting antigen-specific antibodies, with NA-specific antibodies displaying strong neutralization activity Immunized mice were protected from a diverse range of influenza A viruses (H1N1, H5N8, cH6/1N5) | Pre-clinical BALB/c mice | [111] |
| Unmodified mRNA-LNP vaccines | Influenza virus | Full-length HA and NA antigens | Monovalent/multivalent mRNA-LNP vaccine candidate encoding full-length HA or NA, or both, from several seasonal and pandemic influenza strains | Mice immunized with the monovalent vaccine candidate elicited robust functional antibody responses and conferred sufficient protection against lethal viral challenge | Pre-clinical BALB/c mice Cynomolgus macaques | [112] |
| Nucleoside-modified mRNA-LNP vaccine | Influenza virus | 20 HA antigens | Nucleoside-modified mRNA-LNP vaccine candidate encoding 20 HA antigens from all known IAV and IBV subtypes | Elicited high levels of cross-reactive and subtype- specific antibodies in mice and ferrets Conferred protection in both mice and ferrets against matched and mismatched viral strains | Pre-clinical Mice Ferrets | [113] |
| Universal influenza mRNA-LNP vaccine (mRNA-Flu) | Influenza virus | NP, M1 and polymerase basic protein 1 (PB1) of the H1N1 | Universal influenza mRNA-LNP vaccine candidate targeting conserved influenza proteins | Elicited robust and broad T-cell responses in blood, bone marrow and respiratory tract Vaccination with a booster dose enhanced protection against the zoonotic H7N9 avian influenza strain, especially in influenza-primed ferrets | Pre-clinical Ferrets | [114] |
| Quadrivalent nucleoside-modified mRNA-LNP vaccine | Influenza virus | HA antigens | Nucleoside-modified mRNA-LNP vaccine candidate targeting HA from four seasonal influenza strains (A/H1N1, A/H3N2, B/Victoria, B/Yamagata) | Elicited robust antibody responses against all four subtypes Provided complete protection against lethal H1N1 viral challenge | Pre-clinical C57BL/6 mice | [115] |
| Unmodified mRNA-LNP vaccine | Influenza virus | HA and NA antigens | Unmodified mRNA-LNP vaccine candidate expressing both HA and NA from a single ORF using an artificial furin cleavage site and 2A ribosome-skipping sequences | Induced high levels of functional neutralizing antibodies and protected mice from a lethal dose of H3N2 challenge Octavalent vaccine encoding 4 HA and 4 NA antigens (A/H1N1, A/H3N2, B/Victoria, B/Yamagata) demonstrated strong immunogenicity and completely protected mice against lethal challenge from three virus strains (A/H1N1, A/H3N2, B/Yamagata) | Pre-clinical Mice Ferrets | [116] |
| Monovalent mRNA-LNP influenza vaccine (Sanofi-Translate Bio) | Influenza virus | HA | Monovalent mRNA-LNP vaccine encoding the HAprotein of the A/H3N2 seasonal influenza strain | Not available | Phase I | [117] |
| mRNA-1010 (Moderna) | Influenza virus | HA | Quadrivalent mRNA vaccine expressing HA proteins from four seasonal influenza viruses (A/H1N1, A/H3N2, B/Victoria, B/Yamagata) | A single 50 µg dose of mRNA-1010 elicited superior HAI antibody responses in all four vaccine strains when compared to a licensed seasonal influenza vaccine Robust immunogenicity was observed across all age groups, especially in older adults (≥65 years) | Phase III NCT05827978 (Completed) | [118,119] |
| mRNA-1020 and mRNA-1030 (Moderna) | Influenza virus | HA and NA | mRNA vaccine encoding HA and NA glycoproteins of 8 influenza strains | Not available | Phase I/II NCT05333289 (completed) | [120] |
| Combined modified RNA vaccine (Pfizer-BioNTech) | Influenza virus and COVID-19 | Not available | Not available | Elicited robust immunogenicity against both influenza and SARS-CoV-2 with no safety concerns | Phase III NCT06178991 (Completed) | [121] |
| Trivalent influenza vaccine (tIRV) (Pfizer-BioNTech) | Influenza virus | Not available | Not available | Demonstrated strong immunogenicity in adults aged 18–64 years Elicited robust influenza A responses when compared to licensed vaccine Showed lower geometric mean titers (GMT) and seroconversion against the influenza B strain | Phase II NCT06436703 (Completed) | [121] |
| RABV mRNA vaccine | RABV | RABV glycoprotein (RABV-G) | Non-replicating mRNA vaccine encoding the RABV-G | Elicited high levels of neutralizing antibodies and antigen-specific CD4+ and CD8+ T-cell responses in mice Protected immunized mice against a lethal intracerebral challenge infection Induced protective immunity in both adult and newborn pigs with only a single dose | Pre-clinical BALB/c mice Female pregnant pigs (Susscrofa domesticus) Hungarian large white pig | [128] |
| LVRNA001 | RABV | RABV-G | Non-replicating mRNA vaccine encoding the RABV-G | Induced strong humoral and Th-1-derived cellular immune responses in mice Immunized mice and dogs were completely protected following an intracerebral challenge with a highly virulent RABV strain | Pre-clinical BALB/c mice Dogs | [129] |
| LVRNA001 | RABV | RABV-G | Non-replicating mRNA vaccine encoding the RABV-G | Two LVRNA001 doses elicited strong immune responses in pre- and post-exposure scenarios and conferred complete protection in dogs No significant adverse effects in immunized cynomolgus macaques No toxicity observed in immunized Sprague-Dawley rats | Pre-clinical Cynomolgus macaques Dogs Sprague-Dawley rats | [130] |
| mRNA-LNP vaccine | RABV | RABV-G | Unmodified mRNA-LNP vaccine encoding the RABV-G | Elicited higher levels of neutralizing antibodies, memory B-cells, plasma cells, and T-cells in comparison to the licensed inactivated RABV vaccine, Rabipur Provided broader and durable immune responses | Pre-clinical Rhesus macaques | [131] |
| mRNA-based vaccine | RABV | RABV-G | mRNA-LNP vaccine encoding the RABV-G, encapsulated in a novel muscle-targeting LNP based on proprietary STAR-002 LNP formulation | Elicited high virus-neutralizing antibody and IgG titers in a dose-dependent manner A single dose was sufficient to confer 100% and 60% protection in pre-exposure and post-exposure challenges, respectively | Pre-clinical BALB/c mice | [132] |
| CV7201 (CureVac) | RABV | RABV-G | Lyophilized, thermostable mRNA vaccine encoding the RABV-G and formulated with a cationic protein, protamine | Highly immunogenic, but the generation of a sufficient immune response was highly dependent on the mode of administration Deemed to be safe and well-tolerated in immunized participants | Phase I NCT02241135 (Completed) | [133] |
| CV7202 (CureVac) | RABV | RABV-G | Similar to CV7201 but utilizes a novel mRNA-LNP formulation | Elicited RABV-specific neutralizing antibody responses even at low doses (1 µg or 2 µg) A 5 µg dose of CV7202 resulted in unacceptable reactogenicity in vaccinees | Phase I NCT03713086 (Completed) | [134] |
| ZIKV mRNA-LNP vaccine | ZIKV | Pre-membrane (prM) and envelope (E) structural proteins | Nucleoside-modified mRNA-LNP vaccines expressing the prM and E proteins | Elicited high neutralizing antibody titers (~1/100,000) in all three mouse models Provided protection in all three-immunized mice models against lethal ZIKV challenge | Pre-clinical AG129 mice BALB/c mice C57BL/6 mice | [137] |
| mRNA-1325 (Moderna) (1st generation ZIKV mRNA vaccine) | ZIKV | prM and E proteins from a Micronesia 2017 ZIKV strain | mRNA vaccine encoding the prM and E proteins | Well-tolerated among the immunized participants, but elicited poor ZIKV-specific neutralizing antibodies | Phase I NCT03014089 (Completed) | [138,139] |
| mRNA-1893 (Moderna) (2nd generation ZIKV mRNA vaccine) | ZIKV | prM and E proteins from a contemporary ZIKV strain (RIO-U1) | mRNA vaccine encoding the prM and E proteins | Two doses of mRNA-1893 were well-tolerated and elicited robust neutralizing antibodies in immunized participants | Phase I NCT04064905 (Completed) Phase II NCT04917861 (Completed) | [138,139] |
| DENV-2 mRNA-LNP vaccine | DENV | prM-E, 80% envelope protein (E80), NS1 of DENV-2 serotype | Three nucleotide-modified mRNA-LNP vaccines encoding prME-mRNA, E80-mRNA, or NS1-mRNA of DENV-2 serotype | Each vaccine candidate demonstrated robust immunogenicity by eliciting strong neutralizing antibodies, DENV-2 specific IgG, and antigen-specific T-cell responses The E80-mRNA vaccine alone or combined with the NS1-mRNA vaccine elicited high levels of neutralizing antibodies and conferred complete protection against DENV-2 challenge | Pre-clinical BALB/c mice | [142] |
| prM/E mRNA-LNP vaccine | DENV | prM and E proteins of DENV-1 serotype | Nucleotide-modified mRNA-LNP vaccine encoding the DENV-1 prM and E proteins | Elicited cellular and humoral immunity with neutralizing antibody titers that were sufficient for protection against DENV-1 Induced elicited serotype-specific antibody responses with minimal cross-reactivity to other DENV serotypes, reducing the risk of ADE | Pre-clinical AG129 mice C57BL/6 mice | [143] |
| DENV E-DIII + NS1 mRNA-LNP vaccine | DENV | Envelope E-DIII domain and NS1 | DENV mRNA-LNP vaccine candidate containing DENV-a vaccine construct (DENV-1 E-DIII +DENV-2 NS1) and DENV-b vaccine construct (DENV-4 E-DIII + DENV-3 NS1) | Elicited high levels of neutralizing antibody titers against all four DENV serotypes with minimal ADE observed Induced elevated cytokine productions (TNF-α, IFN-γ), indicating Th-1 biased responses | Pre-clinical C57BL/6 mice | [144] |
| RVFV mRNA-LNP vaccines | RVFV | RVFV Gn and Gc glycoproteins | Six nucleoside-modified mRNA-LNP vaccine candidates encoding different regions of the RVFV Gn and Gc glycoproteins | mRNA-LNP vaccine candidate expressing the RVFV full-length Gn and Gc glycoproteins elicited robust humoral and cellular immune responses in mice, including conferring protection from lethal RVFV challenge Induced potent neutralizing antibodies, antigen-specific T-cell responses, and humoral memory B-cells in rhesus macaques | Pre-clinical BALB/c mice Rhesus macaques | [147] |
| RVFV mRNA-LNP VEEV genome-based vaccines | RVFV | RVFV Gn and Gc glycoproteins | Two mRNA-LNP VEEV genome-based vaccine candidates encoding the wild type RVFV Gn/Gc or modified (furin-T2A) Gn/Gc glycoproteins | Both vaccine candidates induced high levels of RVFV Gn-specific IgG antibodies in a dose-dependent manner Wild type RVFV vaccine candidate elicited pseudovirus-neutralizing activity whilst the modified vaccine candidate displayed reduced neutralization capacity | Pre-clinical BALB/c mice | [148] |
| POWV mRNA-LNP vaccine | POWV | POWV prM and E genes | Nucleoside-modified mRNA-LNP vaccine candidate encoding the POWV prM and E genes | Induced high titers of neutralizing antibody responses and protected mice from lethal challenges from several POWV strains Elicited cross-neutralizing antibodies against other tick-borne flaviviruses (TBFV), such as Langat virus Protected mice from Langat virus-induced disease | Pre-clinical C57BL/6 mice | [152] |
| CCHFV mRNA-LNP vaccines | CCHFV | CCHFV Gn/Gc glycoproteins or CCHFV nucleoprotein (N) | Two nucleoside-modified mRNA-LNP vaccine candidates expressing the CCHFV Gn/Gc glycoproteins or N | Induced robust humoral and cellular immune responses and provided complete protection against lethal CCHFV infection in A129 mice | Pre-clinical A129 IFNAR-/- mice 129S2 mice | [158] |
| repGc/repNP RNA vaccine | CCHFV | CCHFV Gc (repGc) and NP (repNP) | Alphavirus-based self-replicating RNA vaccine candidate expressing the CCHFV repGc and repNP proteins | Elicited non-neutralizing NP antibodies and Gc- specific T-cell responses Immunized mice were protected against lethal CCHFV challenge for a year, with 100% survival up to 9 months and 80% survival within 1 year post-vaccination | Pre-clinical C57BL/J mice | [159] |
| repGc/repNP RNA vaccine | CCHFV | CCHFV repGc and repNP | Alphavirus-based self- replicating RNA vaccine candidate expressing the CCHFV repGc and repNP proteins | Two-dose vaccinated rhesus macaques elicited robust non-neutralizing humoral and cellular immune responses Provided significant protection against CCHFV challenge with the CCHFV strain Hoti | Pre-clinical Rhesus macaques (Macca mulatta) | [160] |
| CCHFV mRNA-LNP vaccines | CCHFV | CCHFV Gn and Gc glycoproteins | Three nucleoside-modified mRNA-LNP vaccine candidates encoding the Gn (vLMn), Gc (vLMc) or with Gn linked to Gc with an NSm linker (vLMs) | vLMc vaccine candidate elicited stronger B-cell and T-cell immune responses when compared to vLMn and vLMs candidates vLMc elicited higher Gc-specific IgG titers and IFN-γ T-cell responses | Pre-clinical BALB/c mice C57BL/6J | [161] |
| SFTSV mRNA-LNP vaccines | SFTSV | SFTSV soluble Gn head region (sGn-H) and sGn-H fused with 24-mer ferritin (FT) (sGn-H-FT) | Two mRNA-LNP vaccine candidates encoding the sGn-H and sGn-H-FT | Both vaccine candidates induced potent and durable neutralizing antibody responses lasting up to 12 weeks following a booster dose, with sGn-H-FT displaying slightly higher immunogenicity Immunization with either vaccine candidate completely protected mice from lethal SFTSV challenge | Pre-clinical A129 IFNAR-/- mice BALB/c mice | [172] |
| SFTSV Gn mRNA-LNP vaccine | SFTSV | SFTSV Gn glycoprotein | mRNA-LNP vaccine candidate expressing the SFTSV Gn glycoprotein | Elicited robust neutralizing antibodies and Gn-specific T-cell responses, including increased frequency of Tfh cells Immunized mice were fully protected from lethal SFTSV challenge, with minimal weight loss, reduced liver and spleen damage observed | Pre-clinical C57BL/6 mice | [173] |
| Nucleoside- modified mRNA-LNP vaccine | SFTSV | SFTSV full-length Gn+Gc glycoproteins | Nucleoside-modified mRNA-LNP vaccine candidate encoding the SFTSV full-length Gn+Gc glycoproteins | Induced robust humoral response via generation of high titers of neutralizing antibodies and Th-1 biased responses in immunized mice Low or high doses of the vaccine provided complete protection in immunized mice from lethal SFTSV challenge and durable immunity for five months Conferred cross-protection against other bandaviruses like Heartland virus and Guertu virus | Pre-clinical BALB/c mice | [174] |
| LASV mRNA-LNP vaccines | LASV | LASV wild type or prefusion- stabilized glycoprotein complex (GPC) | Two nucleoside-modified mRNA-LNP vaccine candidates expressing the wild type or prefusion- stabilized GPC of LASV strain Josiah | Both vaccine candidates elicited robust binding antibody responses, specifically to the vaccine candidate containing the prefusion-stabilized GPC Neutralizing antibodies were interestingly detected in several of the guinea pigs Guinea pigs immunized with either vaccine candidate were protected from a lethal dose of the LASV strain Josiah without any severe disease manifestations or deaths, indicating that protection might be linked with Fc-mediated effects | Pre-clinical Hartley outbred guinea pigs | [180] |
| LASV mRNA-LNP vaccines | LASV | LASV glycoprotein precursor (LASgpc) or lymphocytic choriomeningitis virus nucleoprotein (LCMnp) | Two nucleoside-modified mRNA-LNP vaccine candidates expressing the LASgpc or LCMnp | Both vaccine candidates conferred protection from lethal lymphocytic choriomeningitis virus (LCMV), despite inducing negligible neutralizing antibodies Two doses of the vaccine candidates elicited robust humoral and cell-mediated immune responses, with the protective effect suggested to be associated with anti-LASV CD8+ T-cell responses | Pre-clinical C57BL/6N mice CBA/N mice | [181] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, B.E.K.; Tham, S.K.; Poh, C.L. mRNA Vaccine Development in the Fight Against Zoonotic Viral Diseases. Viruses 2025, 17, 960. https://doi.org/10.3390/v17070960
Tan BEK, Tham SK, Poh CL. mRNA Vaccine Development in the Fight Against Zoonotic Viral Diseases. Viruses. 2025; 17(7):960. https://doi.org/10.3390/v17070960
Chicago/Turabian StyleTan, Brandon E. K., Seng Kong Tham, and Chit Laa Poh. 2025. "mRNA Vaccine Development in the Fight Against Zoonotic Viral Diseases" Viruses 17, no. 7: 960. https://doi.org/10.3390/v17070960
APA StyleTan, B. E. K., Tham, S. K., & Poh, C. L. (2025). mRNA Vaccine Development in the Fight Against Zoonotic Viral Diseases. Viruses, 17(7), 960. https://doi.org/10.3390/v17070960






