Differential HIV-1 Proviral Defects in Children vs. Adults on Antiretroviral Therapy
Abstract
1. Introduction
2. Results
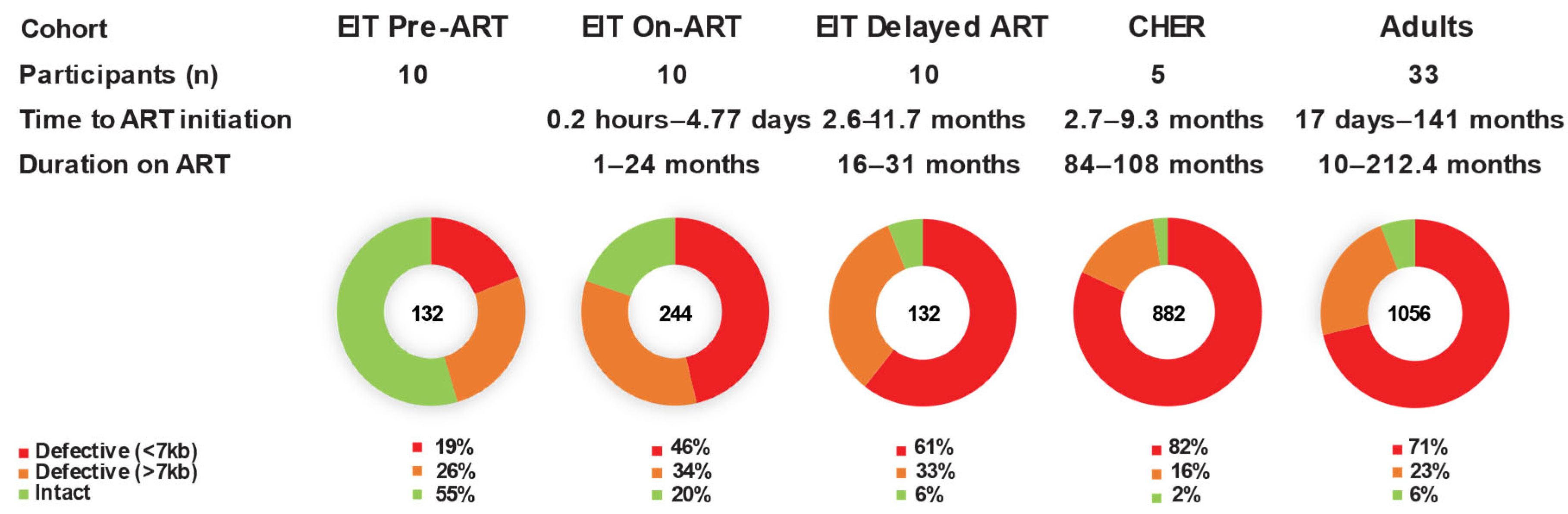
2.1. Fewer Proviruses in Children on Long-Term ART Are Sequence-Intact Compared to Children on Short-Term ART
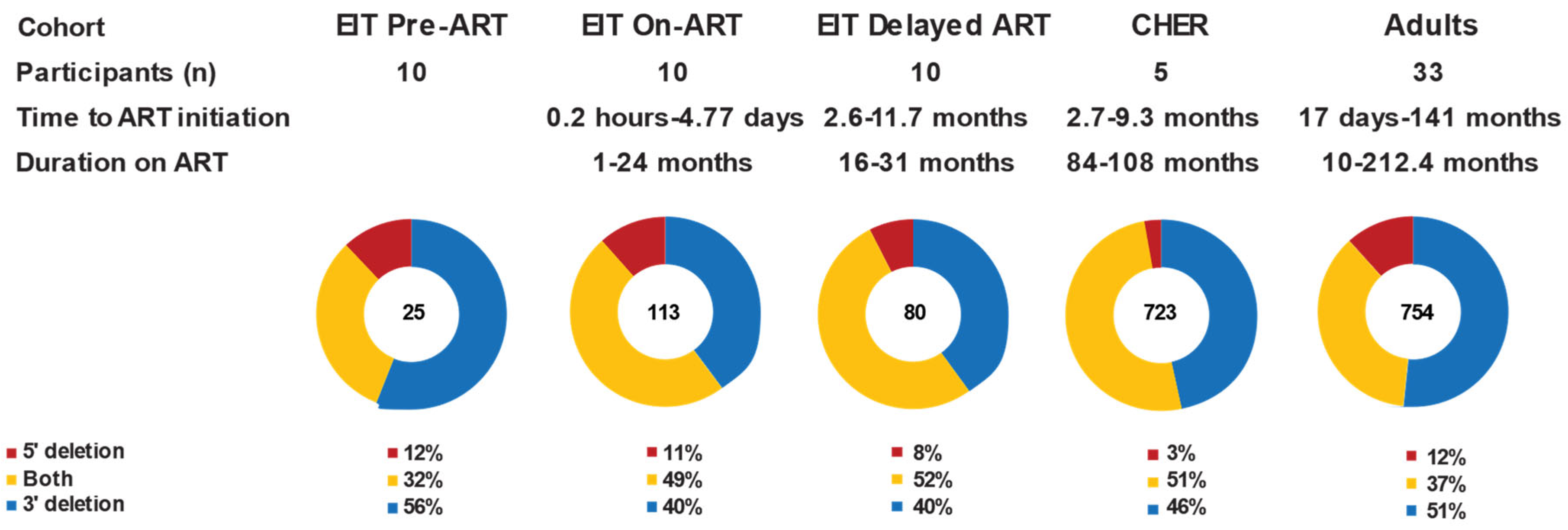
2.2. Partial or Complete Env Deletions Were Found in Most Defective Proviruses Regardless of ART Status or Duration
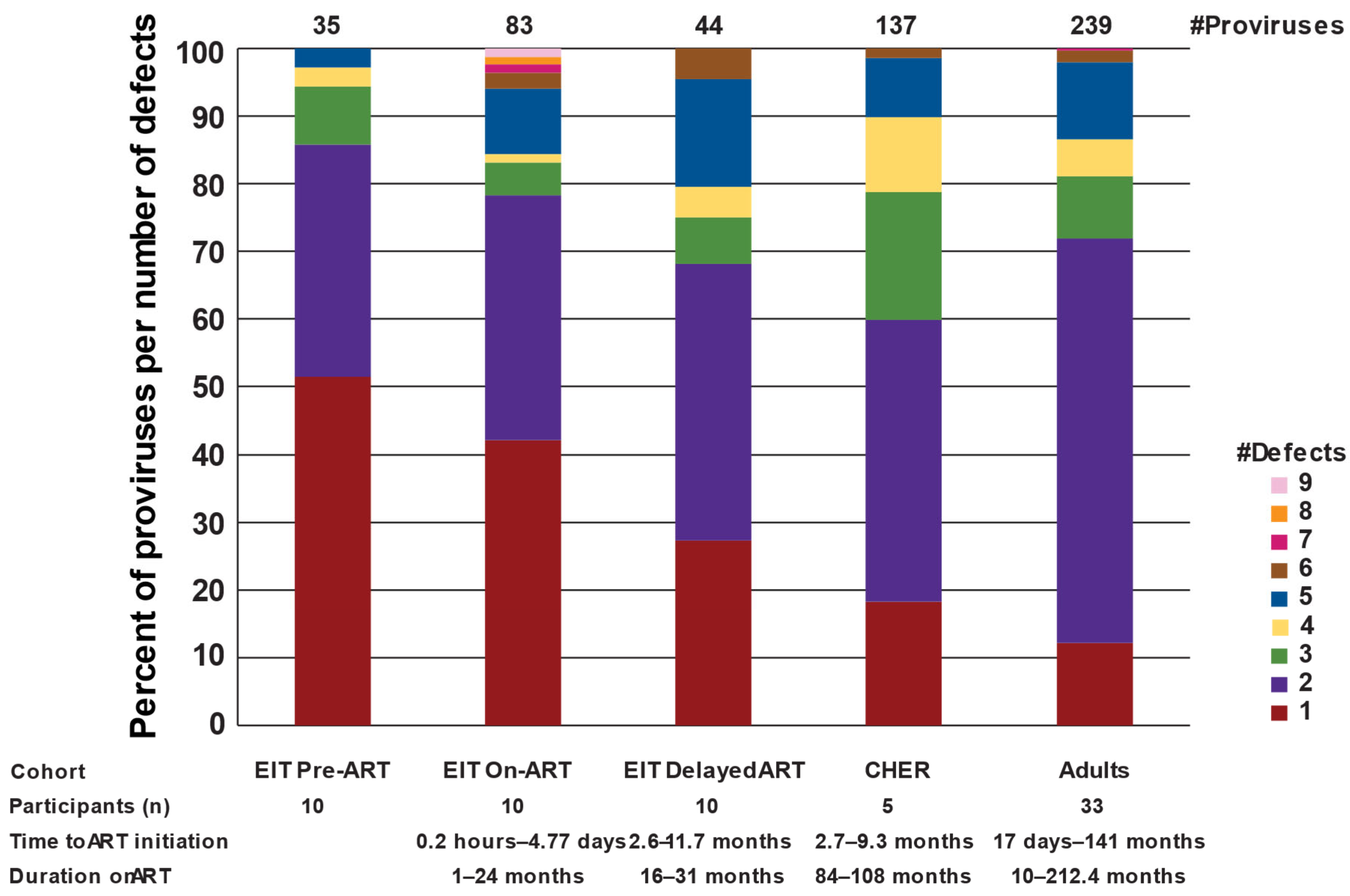
2.3. Most Proviruses > 7 kb in Children on Long-Term ART Contain Multiple Fatal Defects
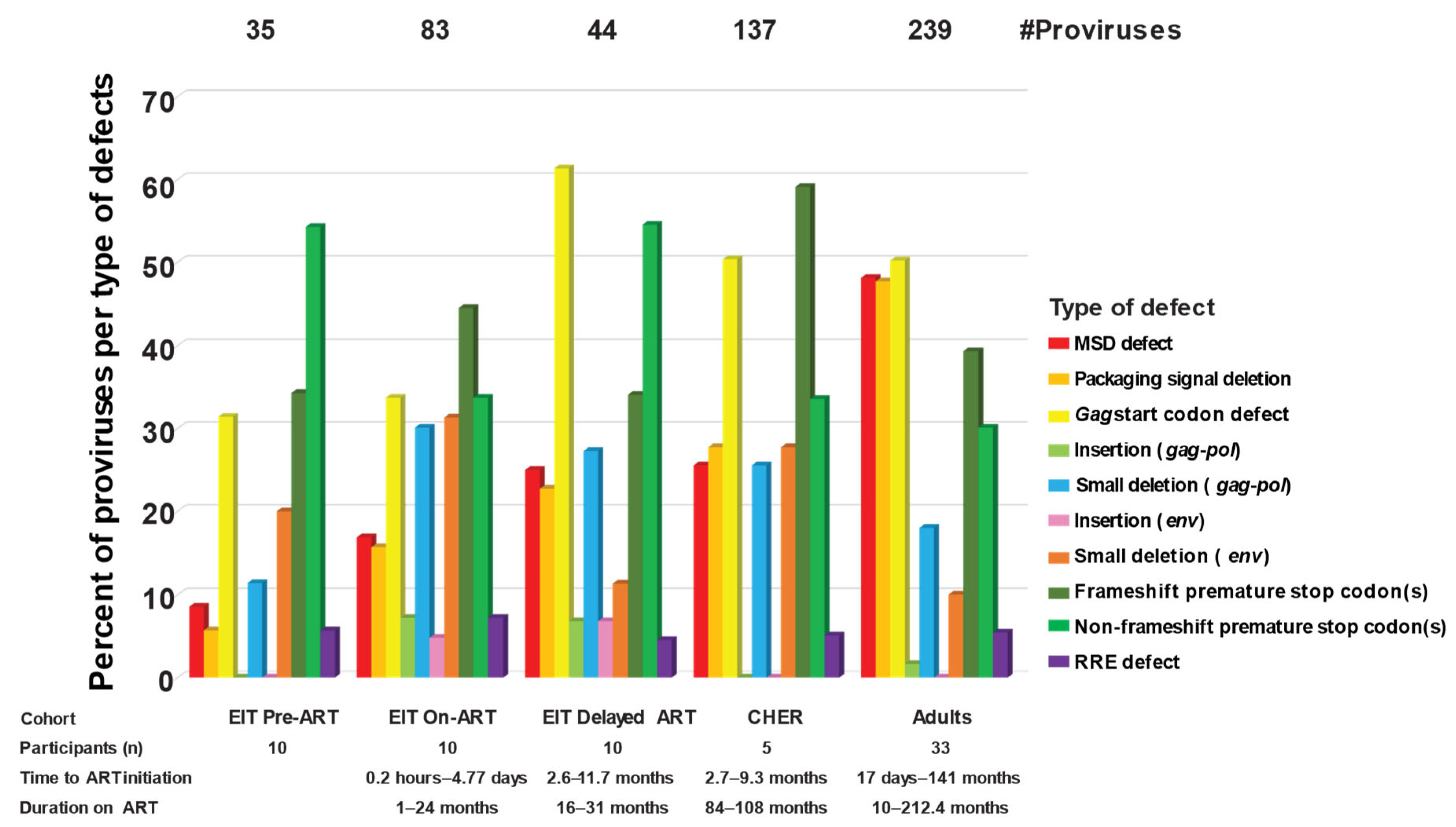
2.4. Different Defects in Proviruses That Are >7 kb in Children vs. Adults on ART
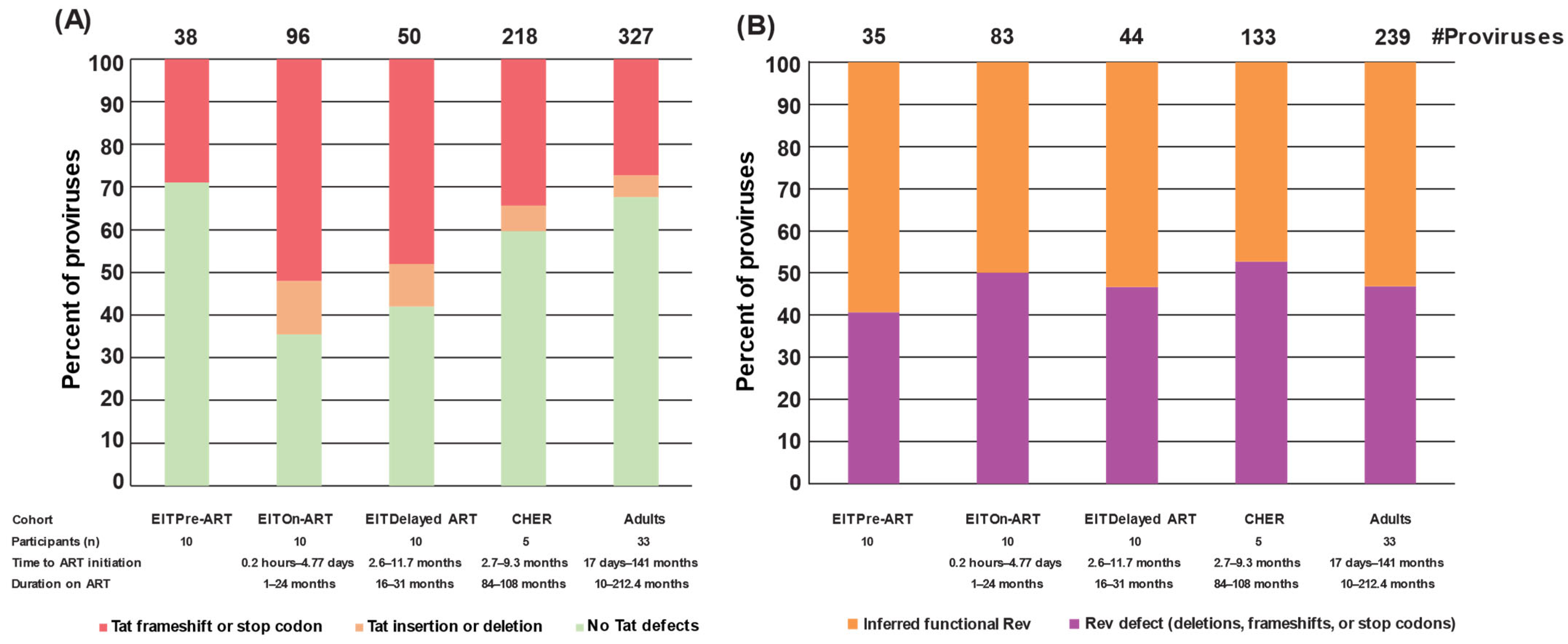
2.5. Defects of Tat Are Observed in Proviruses That Retain Env in Children
3. Discussion
4. Materials and Methods
4.1. Donor Samples
4.2. Genomic DNA Extraction
4.3. Near-Full-Length HIV Single-Genome Sequencing (NFL-SGS)
4.4. Illumina Sequencing
4.5. Sequence Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS. Fact Sheet 2024—Latest Global and Regional HIV Statistics on the Status of the AIDS. 2024. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 10 December 2024).
- Myburgh, D.; Rabie, H.; Slogrove, A.L.; Edson, C.; Cotton, M.F.; Dramowski, A. Horizontal HIV transmission to children of HIV-uninfected mothers: A case series and review of the global literature. Int. J. Infect. Dis. 2020, 98, 315–320. [Google Scholar] [PubMed]
- Newell, M.L.; Coovadia, H.; Cortina-Borja, M.; Rollins, N.; Gaillard, P.; Dabis, F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: A pooled analysis. Lancet 2004, 364, 1236–1243. [Google Scholar]
- Chawla, A.; Wang, C.; Patton, C.; Murray, M.; Punekar, Y.; de Ruiter, A.; Steinhart, C. A Review of Long-Term Toxicity of Antiretroviral Treatment Regimens and Implications for an Aging Population. Infect. Dis. Ther. 2018, 7, 183–195. [Google Scholar]
- Guaraldi, G.; Milic, J.; Mussini, C. Aging with HIV. Curr. HIV/AIDS Rep. 2019, 16, 475–481. [Google Scholar] [PubMed]
- Webel, A.R.; Schexnayder, J.; Cioe, P.A.; Zuñiga, J.A. A Review of Chronic Comorbidities in Adults Living with HIV: State of the Science. J. Assoc. Nurses AIDS Care 2021, 32, 322–346. [Google Scholar] [PubMed]
- Abrams, E.J.; Wiener, J.; Carter, R.; Kuhn, L.; Palumbo, P.; Nesheim, S.; Lee, F.; Vink, P.; Bulterys, M. Maternal health factors and early pediatric antiretroviral therapy influence the rate of perinatal HIV-1 disease progression in children. Aids 2003, 17, 867–877. [Google Scholar]
- Ioannidis, J.P.; Tatsioni, A.; Abrams, E.J.; Bulterys, M.; Coombs, R.W.; Goedert, J.J.; Korber, B.T.; Mayaux, M.J.; Mofenson, L.M.; Moye, J., Jr.; et al. Maternal viral load and rate of disease progression among vertically HIV-1-infected children: An international meta-analysis. Aids 2004, 18, 99–108. [Google Scholar]
- Russell, E.S.; Kwiek, J.J.; Keys, J.; Barton, K.; Mwapasa, V.; Montefiori, D.C.; Meshnick, S.R.; Swanstrom, R. The genetic bottleneck in vertical transmission of subtype C HIV-1 is not driven by selection of especially neutralization-resistant virus from the maternal viral population. J. Virol. 2011, 85, 8253–8262. [Google Scholar]
- Martinez, D.R.; Tu, J.J.; Kumar, A.; Mangold, J.F.; Mangan, R.J.; Goswami, R.; Giorgi, E.E.; Chen, J.; Mengual, M.; Douglas, A.O.; et al. Maternal Broadly Neutralizing Antibodies Can Select for Neutralization-Resistant, Infant-Transmitted/Founder HIV Variants. mBio 2020, 11, e00176-20. [Google Scholar]
- Centers for Disease Control and Prevention; HIV Medicine Association of the Infectious Diseases Society of America; Pediatric Infectious Diseases Society; HHS Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission—A Working Group of the Office of AIDS Research Advisory Council (OARAC). Recommendations for the Use of Antiretroviral Drugs During Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States; Department of Health and Human Services: Washington, DC, USA, 2024. [Google Scholar]
- HHS Panel on Antiretroviral Therapy; Medical Management of Children Living with HIV. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection; Department of Health and Human Services: Washington, DC, USA, 2024. [Google Scholar]
- Laanani, M.; Ghosn, J.; Essat, A.; Melard, A.; Seng, R.; Gousset, M.; Panjo, H.; Mortier, E.; Girard, P.M.; Goujard, C.; et al. Impact of the Timing of Initiation of Antiretroviral Therapy During Primary HIV-1 Infection on the Decay of Cell-Associated HIV-DNA. Clin. Infect. Dis. 2015, 60, 1715–1721. [Google Scholar]
- Jain, V.; Hartogensis, W.; Bacchetti, P.; Hunt, P.W.; Hatano, H.; Sinclair, E.; Epling, L.; Lee, T.H.; Busch, M.P.; McCune, J.M.; et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J. Infect. Dis. 2013, 208, 1202–1211. [Google Scholar] [PubMed]
- Bachmann, N.; von Siebenthal, C.; Vongrad, V.; Turk, T.; Neumann, K.; Beerenwinkel, N.; Bogojeska, J.; Fellay, J.; Roth, V.; Kok, Y.L.; et al. Determinants of HIV-1 reservoir size and long-term dynamics during suppressive ART. Nat. Commun. 2019, 10, 3193. [Google Scholar]
- Hill, A.L.; Rosenbloom, D.I.; Goldstein, E.; Hanhauser, E.; Kuritzkes, D.R.; Siliciano, R.F.; Henrich, T.J. Real-Time Predictions of Reservoir Size and Rebound Time during Antiretroviral Therapy Interruption Trials for HIV. PLoS Pathog. 2016, 12, e1005535. [Google Scholar]
- Ananworanich, J.; Puthanakit, T.; Suntarattiwong, P.; Chokephaibulkit, K.; Kerr, S.J.; Fromentin, R.; Bakeman, W.; Intasan, J.; Mahanontharit, A.; Sirivichayakul, S.; et al. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. Aids 2014, 28, 1015–1020. [Google Scholar]
- van Zyl, G.U.; Bedison, M.A.; van Rensburg, A.J.; Laughton, B.; Cotton, M.F.; Mellors, J.W. Early Antiretroviral Therapy in South African Children Reduces HIV-1-Infected Cells and Cell-Associated HIV-1 RNA in Blood Mononuclear Cells. J. Infect. Dis. 2015, 212, 39–43. [Google Scholar]
- Luzuriaga, K.; Tabak, B.; Garber, M.; Chen, Y.H.; Ziemniak, C.; McManus, M.M.; Murray, D.; Strain, M.C.; Richman, D.D.; Chun, T.W.; et al. HIV type 1 (HIV-1) proviral reservoirs decay continuously under sustained virologic control in HIV-1-infected children who received early treatment. J. Infect. Dis. 2014, 210, 1529–1538. [Google Scholar]
- Vela, L.C.; Carrere, L.; Naasz, C.; Kalavacherla, S.; Tan, T.S.; de Armas, L.; Gao, C.; Yu, X.G.; Pahwa, S.G.; Luzuriaga, K.; et al. Profound reduction of HIV-1 reservoir cells over 3 decades of antiretroviral therapy started in early infancy. JCI Insight 2024, 10, e186550. [Google Scholar] [PubMed]
- Persaud, D.; Patel, K.; Karalius, B.; Rainwater-Lovett, K.; Ziemniak, C.; Ellis, A.; Chen, Y.H.; Richman, D.; Siberry, G.K.; Van Dyke, R.B.; et al. Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA Pediatr. 2014, 168, 1138–1146. [Google Scholar]
- Persaud, D.; Palumbo, P.E.; Ziemniak, C.; Hughes, M.D.; Alvero, C.G.; Luzuriaga, K.; Yogev, R.; Capparelli, E.V.; Chadwick, E.G. Dynamics of the resting (CD4+) T-cell latent HIV reservoir in infants initiating HAART less than 6 months of age. Aids 2012, 26, 1483–1490. [Google Scholar]
- Persaud, D.; Pierson, T.; Ruff, C.; Finzi, D.; Chadwick, K.R.; Margolick, J.B.; Ruff, A.; Hutton, N.; Ray, S.; Siliciano, R.F. A stable latent reservoir for HIV-1 in resting (CD4+) T lymphocytes in infected children. J. Clin. Investig. 2000, 105, 995–1003. [Google Scholar]
- Besson, G.J.; Lalama, C.M.; Bosch, R.J.; Gandhi, R.T.; Bedison, M.A.; Aga, E.; Riddler, S.A.; McMahon, D.K.; Hong, F.; Mellors, J.W. HIV-1 DNA decay dynamics in blood during more than a decade of suppressive antiretroviral therapy. Clin. Infect. Dis. 2014, 59, 1312–1321. [Google Scholar] [PubMed]
- Zanchetta, M.; Walker, S.; Burighel, N.; Bellanova, D.; Rampon, O.; Giaquinto, C.; De Rossi, A. Long-term decay of the HIV-1 reservoir in HIV-1-infected children treated with highly active antiretroviral therapy. J. Infect. Dis. 2006, 193, 1718–1727. [Google Scholar] [PubMed]
- Ajibola, G.; Garcia-Broncano, P.; Maswabi, K.; Bennett, K.; Hughes, M.D.; Moyo, S.; Mohammed, T.; Jean-Philippe, P.; Sakoi, M.; Batlang, O.; et al. Viral Reservoir in Early-Treated Human Immunodeficiency Virus-Infected Children and Markers for Sustained Viral Suppression. Clin. Infect. Dis. 2021, 73, e997–e1003. [Google Scholar] [PubMed]
- Koofhethile, C.K.; Rinaldi, S.; Rassadkina, Y.; Dinh, V.B.; Gao, C.; Pallikkuth, S.; Garcia-Broncano, P.; de Armas, L.R.; Pahwa, R.; Cotugno, N.; et al. HIV-1 reservoir evolution in infants infected with clade C from Mozambique. Int. J. Infect. Dis. 2023, 127, 129–136. [Google Scholar]
- Reeves, D.B.; Litchford, M.; Fish, C.S.; Farrell-Sherman, A.; Poindexter, M.; Ahmed, N.; Cassidy, N.A.J.; Neary, J.; Wamalwa, D.; Langat, A.; et al. Intact HIV DNA decays in children with and without complete viral load suppression. PLoS Pathog. 2025, 21, e1013003. [Google Scholar]
- Pankau, M.D.; Wamalwa, D.; Benki-Nugent, S.; Tapia, K.; Ngugi, E.; Langat, A.; Otieno, V.; Moraa, H.; Maleche-Obimbo, E.; Overbaugh, J.; et al. Decay of HIV DNA in the Reservoir and the Impact of Short Treatment Interruption in Kenyan Infants. Open Forum Infect. Dis. 2018, 5, ofx268. [Google Scholar]
- Violari, A.; Cotton, M.F.; Gibb, D.M.; Babiker, A.G.; Steyn, J.; Madhi, S.A.; Jean-Philippe, P.; McIntyre, J.A. Early antiretroviral therapy and mortality among HIV-infected infants. N. Engl. J. Med. 2008, 359, 2233–2244. [Google Scholar]
- Van Zyl, G.U.; Katusiime, M.G.; Wiegand, A.; McManus, W.R.; Bale, M.J.; Halvas, E.K.; Luke, B.; Boltz, V.F.; Spindler, J.; Laughton, B.; et al. No evidence of HIV replication in children on antiretroviral therapy. J. Clin. Investig. 2017, 127, 3827–3834. [Google Scholar]
- Katusiime, M.G.; Halvas, E.K.; Wright, I.; Joseph, K.; Bale, M.J.; Kirby-McCullough, B.; Engelbrecht, S.; Shao, W.; Hu, W.S.; Cotton, M.F.; et al. Intact HIV Proviruses Persist in Children Seven to Nine Years after Initiation of Antiretroviral Therapy in the First Year of Life. J. Virol. 2020, 94, jvi.01519-19. [Google Scholar]
- Bruner, K.M.; Murray, A.J.; Pollack, R.A.; Soliman, M.G.; Laskey, S.B.; Capoferri, A.A.; Lai, J.; Strain, M.C.; Lada, S.M.; Hoh, R.; et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat. Med. 2016, 22, 1043–1049. [Google Scholar]
- Hiener, B.; Horsburgh, B.A.; Eden, J.S.; Barton, K.; Schlub, T.E.; Lee, E.; von Stockenstrom, S.; Odevall, L.; Milush, J.M.; Liegler, T.; et al. Identification of Genetically Intact HIV-1 Proviruses in Specific CD(4+) T Cells from Effectively Treated Participants. Cell Rep. 2017, 21, 813–822. [Google Scholar] [PubMed]
- Ho, Y.C.; Shan, L.; Hosmane, N.N.; Wang, J.; Laskey, S.B.; Rosenbloom, D.I.; Lai, J.; Blankson, J.N.; Siliciano, J.D.; Siliciano, R.F. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013, 155, 540–551. [Google Scholar] [PubMed]
- Bale, M.J.; Katusiime, M.G.; Wells, D.; Wu, X.; Spindler, J.; Halvas, E.K.; Cyktor, J.C.; Wiegand, A.; Shao, W.; Cotton, M.F.; et al. Early Emergence and Long-Term Persistence of HIV-Infected T-Cell Clones in Children. mBio 2021, 12, e00568-21. [Google Scholar]
- Botha, J.C.; Demirov, D.; Gordijn, C.; Katusiime, M.G.; Bale, M.J.; Wu, X.; Wells, D.; Hughes, S.H.; Cotton, M.F.; Mellors, J.W.; et al. The largest HIV-1-infected T cell clones in children on long-term combination antiretroviral therapy contain solo LTRs. mBio 2023, 14, e0111623. [Google Scholar]
- Garcia-Broncano, P.; Maddali, S.; Einkauf, K.B.; Jiang, C.; Gao, C.; Chevalier, J.; Chowdhury, F.Z.; Maswabi, K.; Ajibola, G.; Moyo, S.; et al. Early antiretroviral therapy in neonates with HIV-1 infection restricts viral reservoir size and induces a distinct innate immune profile. Sci. Transl. Med. 2019, 11, eaax7350. [Google Scholar]
- National Cancer Institute. Proviral Sequence Annotation and Intactness Tool. Available online: https://psd.cancer.gov/tools/pvs_annot.php (accessed on 10 May 2024).
- Bruner, K.M.; Wang, Z.; Simonetti, F.R.; Bender, A.M.; Kwon, K.J.; Sengupta, S.; Fray, E.J.; Beg, S.A.; Antar, A.A.R.; Jenike, K.M.; et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019, 566, 120–125. [Google Scholar]
- Gandhi, R.T.; Cyktor, J.C.; Bosch, R.J.; Mar, H.; Laird, G.M.; Martin, A.; Collier, A.C.; Riddler, S.A.; Macatangay, B.J.; Rinaldo, C.R.; et al. Selective Decay of Intact HIV-1 Proviral DNA on Antiretroviral Therapy. J. Infect. Dis. 2021, 223, 225–233. [Google Scholar]
- Peluso, M.J.; Bacchetti, P.; Ritter, K.D.; Beg, S.; Lai, J.; Martin, J.N.; Hunt, P.W.; Henrich, T.J.; Siliciano, J.D.; Siliciano, R.F.; et al. Differential decay of intact and defective proviral DNA in HIV-1-infected individuals on suppressive antiretroviral therapy. JCI Insight 2020, 5, e132997. [Google Scholar] [PubMed]
- Fisher, K.; Wang, X.Q.; Lee, A.; Morcilla, V.; de Vries, A.; Lee, E.; Eden, J.S.; Deeks, S.G.; Kelleher, A.D.; Palmer, S. Plasma-Derived HIV-1 Virions Contain Considerable Levels of Defective Genomes. J. Virol. 2022, 96, e0201121. [Google Scholar]
- Calugi, G.; Montella, F.; Favalli, C.; Benedetto, A. Entire genome of a strain of human immunodeficiency virus type 1 with a deletion of nef that was recovered 20 years after primary infection: Large pool of proviruses with deletions of env. J. Virol. 2006, 80, 11892–11896. [Google Scholar]
- de Verneuil, A.; Migraine, J.; Mammano, F.; Molina, J.M.; Gallien, S.; Mouquet, H.; Hance, A.J.; Clavel, F.; Dutrieux, J. Genetically Intact but Functionally Impaired HIV-1 Env Glycoproteins in the T-Cell Reservoir. J. Virol. 2018, 92, e01684-17. [Google Scholar] [PubMed]
- Anderson, E.M.; Simonetti, F.R.; Gorelick, R.J.; Hill, S.; Gouzoulis, M.A.; Bell, J.; Rehm, C.; Pérez, L.; Boritz, E.; Wu, X.; et al. Dynamic Shifts in the HIV Proviral Landscape During Long Term Combination Antiretroviral Therapy: Implications for Persistence and Control of HIV Infections. Viruses 2020, 12, 136. [Google Scholar] [CrossRef]
- Thomas, J.; Perron, H.; Feschotte, C. Variation in proviral content among human genomes mediated by LTR recombination. Mob. DNA 2018, 9, 36. [Google Scholar] [PubMed]
- Jern, P.; Coffin, J.M. Effects of retroviruses on host genome function. Annu. Rev. Genet. 2008, 42, 709–732. [Google Scholar]
- Grandi, N.; Tramontano, E. Human Endogenous Retroviruses Are Ancient Acquired Elements Still Shaping Innate Immune Responses. Front. Immunol. 2018, 9, 2039. [Google Scholar]
- Imamichi, H.; Dewar, R.L.; Adelsberger, J.W.; Rehm, C.A.; O’Doherty, U.; Paxinos, E.E.; Fauci, A.S.; Lane, H.C. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2016, 113, 8783–8788. [Google Scholar]
- Pollack, R.A.; Jones, R.B.; Pertea, M.; Bruner, K.M.; Martin, A.R.; Thomas, A.S.; Capoferri, A.A.; Beg, S.A.; Huang, S.H.; Karandish, S.; et al. Defective HIV-1 Proviruses Are Expressed and Can Be Recognized by Cytotoxic T Lymphocytes, Which Shape the Proviral Landscape. Cell Host Microbe 2017, 21, 494–506.e4. [Google Scholar] [PubMed]
- Imamichi, H.; Smith, M.; Adelsberger, J.W.; Izumi, T.; Scrimieri, F.; Sherman, B.T.; Rehm, C.A.; Imamichi, T.; Pau, A.; Catalfamo, M.; et al. Defective HIV-1 proviruses produce viral proteins. Proc. Natl. Acad. Sci. USA 2020, 117, 3704–3710. [Google Scholar]
- Pollard, V.W.; Malim, M.H. The HIV-1 Rev protein. Annu. Rev. Microbiol. 1998, 52, 491–532. [Google Scholar]
- Jayaraman, B.; Fernandes, J.D.; Yang, S.; Smith, C.; Frankel, A.D. Highly Mutable Linker Regions Regulate HIV-1 Rev Function and Stability. Sci. Rep. 2019, 9, 5139. [Google Scholar]
- Kummet, N.; Mishra, N.; Diaz, A.; Cusick, N.; Klotz, S.; Ahmad, N. Genetic Characterization of HIV-1 tat Gene from Virologically Controlled Aging Individuals with HIV on Long-Term Antiretroviral Therapy. AIDS Res. Hum. Retroviruses 2025, 41, 143–154. [Google Scholar] [PubMed]
- Das, A.T.; Harwig, A.; Berkhout, B. The HIV-1 Tat protein has a versatile role in activating viral transcription. J. Virol. 2011, 85, 9506–9516. [Google Scholar]
- Harrich, D.; Ulich, C.; García-Martínez, L.F.; Gaynor, R.B. Tat is required for efficient HIV-1 reverse transcription. EMBO J. 1997, 16, 1224–1235. [Google Scholar] [PubMed]
- Karn, J.; Dingwall, C.; Finch, J.T.; Heaphy, S.; Gait, M.J. RNA binding by the tat and rev proteins of HIV-1. Biochimie 1991, 73, 9–16. [Google Scholar]
- Shuck-Lee, D.; Chang, H.; Sloan, E.A.; Hammarskjold, M.L.; Rekosh, D. Single-nucleotide changes in the HIV Rev-response element mediate resistance to compounds that inhibit Rev function. J. Virol. 2011, 85, 3940–3949. [Google Scholar][Green Version]
- Richman, D.D.; Wrin, T.; Little, S.J.; Petropoulos, C.J. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 2003, 100, 4144–4149. [Google Scholar] [PubMed][Green Version]
- Piantadosi, A.; Chohan, B.; Panteleeff, D.; Baeten, J.M.; Mandaliya, K.; Ndinya-Achola, J.O.; Overbaugh, J. HIV-1 evolution in gag and env is highly correlated but exhibits different relationships with viral load and the immune response. Aids 2009, 23, 579–587. [Google Scholar][Green Version]
- Troyer, R.M.; McNevin, J.; Liu, Y.; Zhang, S.C.; Krizan, R.W.; Abraha, A.; Tebit, D.M.; Zhao, H.; Avila, S.; Lobritz, M.A.; et al. Variable fitness impact of HIV-1 escape mutations to cytotoxic T lymphocyte (CTL) response. PLoS Pathog. 2009, 5, e1000365. [Google Scholar]
- Leal, E.; Janini, M.; Diaz, R.S. Selective pressures of human immunodeficiency virus type 1 (HIV-1) during pediatric infection. Infect. Genet. Evol. 2007, 7, 694–707. [Google Scholar][Green Version]
- Lynch, R.M.; Wong, P.; Tran, L.; O’Dell, S.; Nason, M.C.; Li, Y.; Wu, X.; Mascola, J.R. HIV-1 fitness cost associated with escape from the VRC01 class of CD4 binding site neutralizing antibodies. J. Virol. 2015, 89, 4201–4213. [Google Scholar][Green Version]
- Bar, K.J.; Tsao, C.Y.; Iyer, S.S.; Decker, J.M.; Yang, Y.; Bonsignori, M.; Chen, X.; Hwang, K.K.; Montefiori, D.C.; Liao, H.X.; et al. Early low-titer neutralizing antibodies impede HIV-1 replication and select for virus escape. PLoS Pathog. 2012, 8, e1002721. [Google Scholar]
- Cassidy, N.A.J.; Fish, C.S.; Levy, C.N.; Roychoudhury, P.; Reeves, D.B.; Hughes, S.M.; Schiffer, J.T.; Benki-Nugent, S.; John-Stewart, G.; Wamalwa, D.; et al. HIV reservoir quantification using cross-subtype multiplex ddPCR. iScience 2022, 25, 103615. [Google Scholar][Green Version]
- Gaebler, C.; Lorenzi, J.C.C.; Oliveira, T.Y.; Nogueira, L.; Ramos, V.; Lu, C.L.; Pai, J.A.; Mendoza, P.; Jankovic, M.; Caskey, M.; et al. Combination of quadruplex qPCR and next-generation sequencing for qualitative and quantitative analysis of the HIV-1 latent reservoir. J. Exp. Med. 2019, 216, 2253–2264. [Google Scholar] [PubMed][Green Version]
- Delporte, M.; Lambrechts, L.; Blomme, E.E.; van Snippenberg, W.; Rutsaert, S.; Verschoore, M.; De Smet, E.; Noppe, Y.; De Langhe, N.; De Scheerder, M.-A.; et al. Integrative Assessment of Total and Intact HIV-1 Reservoir by a 5-Region Multiplexed Rainbow DNA Digital PCR Assay. Clin. Chem. 2025, 71, 203–214. [Google Scholar][Green Version]
- Ferreira, F.A.; He, Q.; Banning, S.; Roberts-Sano, O.; Wilkins, O.; Kuritzkes, D.R.; Tsibris, A. HIV-1 proviral landscape characterization varies by pipeline analysis. J. Int. AIDS Soc. 2021, 24, e25725. [Google Scholar][Green Version]
- Veldsman, K.A.; Maritz, J.; Isaacs, S.; Katusiime, M.G.; Janse van Rensburg, A.; Laughton, B.; Mellors, J.W.; Cotton, M.F.; van Zyl, G.U. Rapid decline of HIV-1 DNA and RNA in infants starting very early antiretroviral therapy may pose a diagnostic challenge. Aids 2018, 32, 629–634. [Google Scholar][Green Version]
- White, J.A.; Kufera, J.T.; Bachmann, N.; Dai, W.; Simonetti, F.R.; Armstrong, C.; Lai, J.; Beg, S.; Siliciano, J.D.; Siliciano, R.F. Measuring the latent reservoir for HIV-1: Quantification bias in near full-length genome sequencing methods. PLoS Pathog. 2022, 18, e1010845. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [PubMed][Green Version]
- Rausch, J.W.; Le Grice, S.F. HIV Rev Assembly on the Rev Response Element (RRE): A Structural Perspective. Viruses 2015, 7, 3053–3075. [Google Scholar] [CrossRef]
- Shao, W.; Shan, J.; Hu, W.S.; Halvas, E.K.; Mellors, J.W.; Coffin, J.M.; Kearney, M.F. HIV Proviral Sequence Database: A New Public Database for Near Full-Length HIV Proviral Sequences and Their Meta-Analyses. AIDS Res. Hum Retroviruses 2020, 36, 1–3. [Google Scholar]
| Participant ID | Sex A | Age at ART Initiation (Months) | Time on Suppressive ART (Years) | HIV DNA Copies/106 PBMC on ART | ART Regimen B |
|---|---|---|---|---|---|
| ZA-001 | F | 10.9 | 8.80 | 86.3 | AZT/3TC/LPV/r |
| ZA-004 | M | 2.7 | 7.92 | 23.6 | AZT/3TC/LPV/r |
| ZA-006 | F | 9.0 | 7.45 | 46.7 | AZT/3TC/LPV/r |
| ZA-011 | F | 9.3 | 7.35 | 181.5 | AZT/3TC/LPV/r |
| ZA-012 | M | 5.1 | 8.60 | 11.9 | AZT/3TC/LPV/r |
| Participant ID | Number of NFL Proviral Sequences Obtained A | Number of Sequences | ||
|---|---|---|---|---|
| Intact B (%) | >7 kb Defective (%) | <7 kb Defective (%) | ||
| ZA-001 | 139 | 2 (1.4) | 14 (10.1) | 123 (88.5) |
| ZA-004 | 56 | 3 (5.4) | 21 (37.5) | 32 (57.1) |
| ZA-006 | 84 | 2 (2.4) | 21 (25.0) | 61 (72.6) |
| ZA-011 | 450 | 15 (3.4) | 62 (13.7) | 373 (82.9) |
| ZA-012 | 153 | 0 (<0.6) | 19 (12.4) | 134 (87.6) |
| Total | 882 | 22 (2.5) | 137 (15.5) | 723 (82.0) |
| Study | Age Group | Subtype | # Donors | Median Time of ART Initiation (Range) A | Median Time on Suppressive ART (Range) A |
|---|---|---|---|---|---|
| Ho et al. [35] | Adult | B | 8 | 73.5 m (6 m–141 m) | 68 m (28 m–108 m) |
| Bruner et al. [33] | Adult | B | 10 | 3.28 m (17 d–6 m) | 106.5 m (10 m–203 m) |
| Hiener et al. [34] | Adult | B | 6 | 9 m (6 m–12 m) | 125.4 m (38.4 m–212.4 m) |
| Garcia-Broncano et al. [38] | Infant B | C | 10 | 2.39 d (0.20 h–4.77 d) | 12.5 m (1 m–24 m) |
| Children C | C | 10 | 7.15 m (2.6 m–11.7 m) | 23.5 m (16 m–31 m) |
| Participant ID | Number of Proviral Sequences Obtained A | Number of Sequences | ||
|---|---|---|---|---|
| Intact B (%) | >7 kb Defective (%) | <7 kb Defective (%) | ||
| EIT Pre-ART | 132 | 72 (54.5) | 35 (18.0) | 25 (26.5) |
| EIT On-ART | 244 | 48 (19.7) | 83 (34.0) | 113 (46.3) |
| EIT Delayed-ART | 132 | 8 (6.1) | 44 (33.3) | 80 (60.6) |
| Adults | 1056 | 63 (6.0) | 239 (22.6) | 754 (71.4) |
| CHER | 882 | 22 (2.5) | 137 (15.5) | 723 (82.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasson, J.M.; Katusiime, M.G.; Capoferri, A.A.; Bale, M.J.; Luke, B.T.; Shao, W.; Cotton, M.F.; van Zyl, G.; Patro, S.C.; Kearney, M.F. Differential HIV-1 Proviral Defects in Children vs. Adults on Antiretroviral Therapy. Viruses 2025, 17, 961. https://doi.org/10.3390/v17070961
Hasson JM, Katusiime MG, Capoferri AA, Bale MJ, Luke BT, Shao W, Cotton MF, van Zyl G, Patro SC, Kearney MF. Differential HIV-1 Proviral Defects in Children vs. Adults on Antiretroviral Therapy. Viruses. 2025; 17(7):961. https://doi.org/10.3390/v17070961
Chicago/Turabian StyleHasson, Jenna M., Mary Grace Katusiime, Adam A. Capoferri, Michael J. Bale, Brian T. Luke, Wei Shao, Mark F. Cotton, Gert van Zyl, Sean C. Patro, and Mary F. Kearney. 2025. "Differential HIV-1 Proviral Defects in Children vs. Adults on Antiretroviral Therapy" Viruses 17, no. 7: 961. https://doi.org/10.3390/v17070961
APA StyleHasson, J. M., Katusiime, M. G., Capoferri, A. A., Bale, M. J., Luke, B. T., Shao, W., Cotton, M. F., van Zyl, G., Patro, S. C., & Kearney, M. F. (2025). Differential HIV-1 Proviral Defects in Children vs. Adults on Antiretroviral Therapy. Viruses, 17(7), 961. https://doi.org/10.3390/v17070961






