Homoharringtonine Inhibits CVS-11 and Clinical Isolates of Rabies Virus In Vitro: Identified via High-Throughput Screening of an FDA-Approved Drug Library
Abstract
1. Importance
2. Introduction
3. Materials and Methods
3.1. Cell Lines
3.2. RABV (CVS-11) and Clinical Isolates of RABV
3.3. FDA-Approved Drug Library
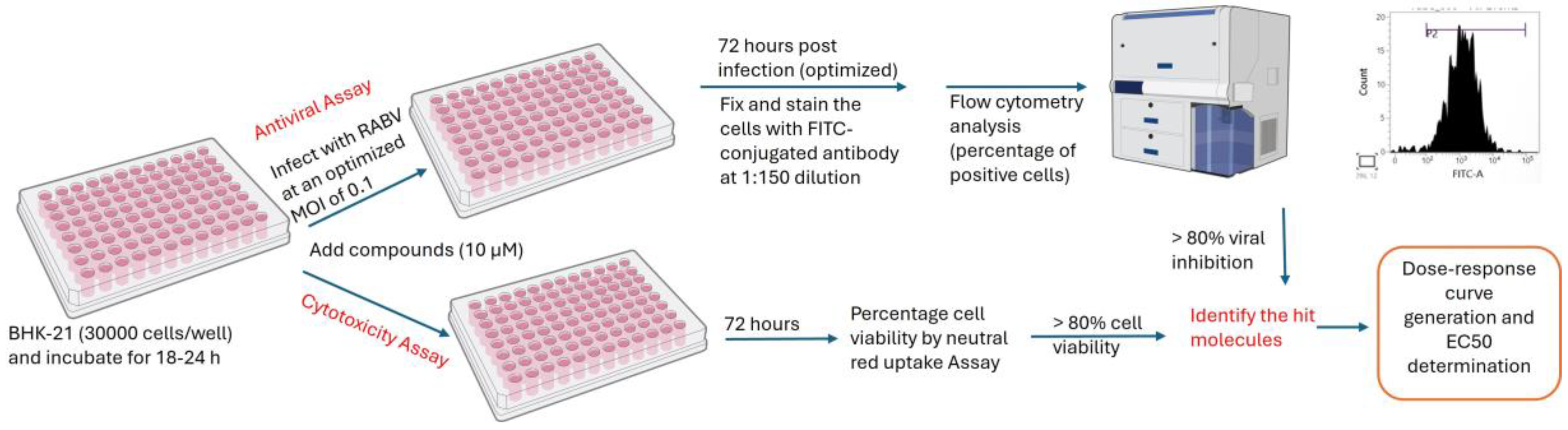
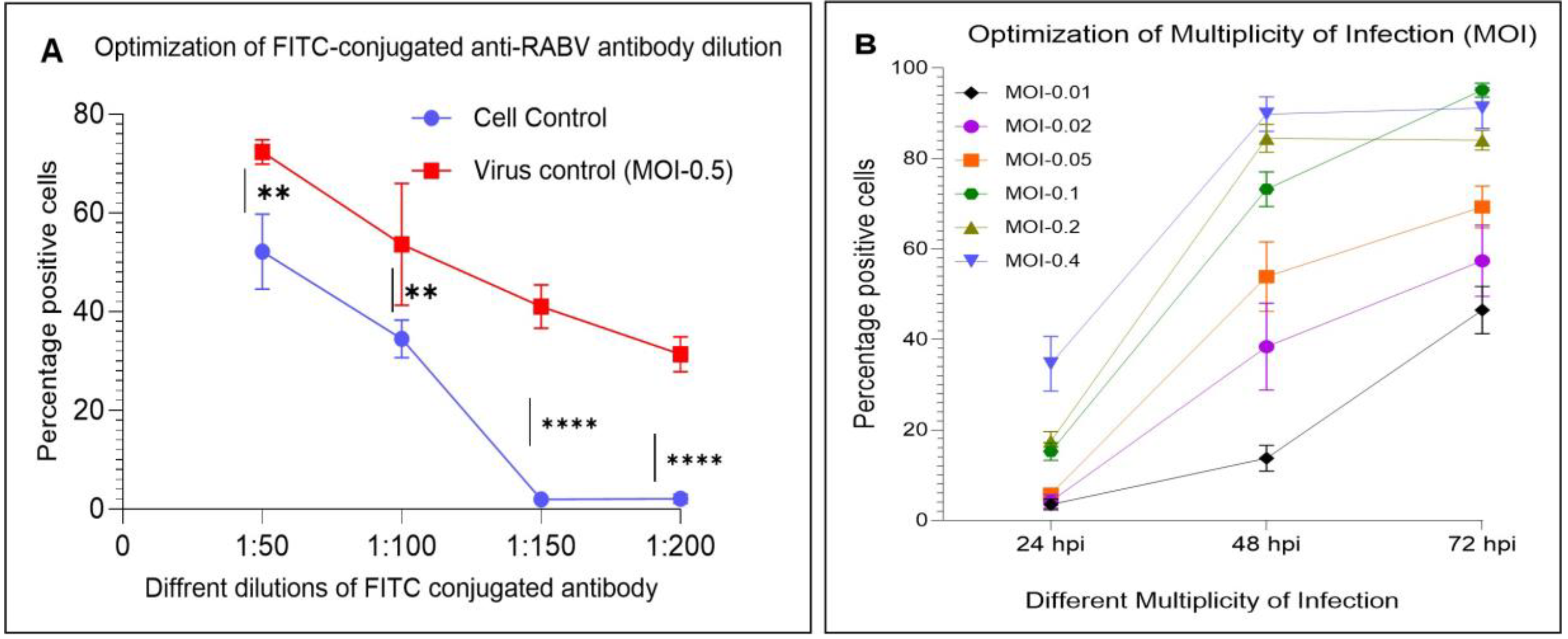
3.4. Optimization of a High-Throughput Flow Cytometry Assay
3.5. Development and Validation of a High-Throughput Antiviral Assay
3.6. Antiviral Screening of FDA-Approved Drug Library
3.7. Time of Addition (ToA) and Cell-to-Cell Infection Assays
3.8. Antiviral Activity of HHT Against Clinical Isolates in BHK-21 and Neural Cell Lines
3.9. Statistical Analysis
4. Results
4.1. Optimized Parameters of a High-Throughput Flow Cytometry Assay
4.2. FITC Antibody-Based High-Throughput Antiviral Assay
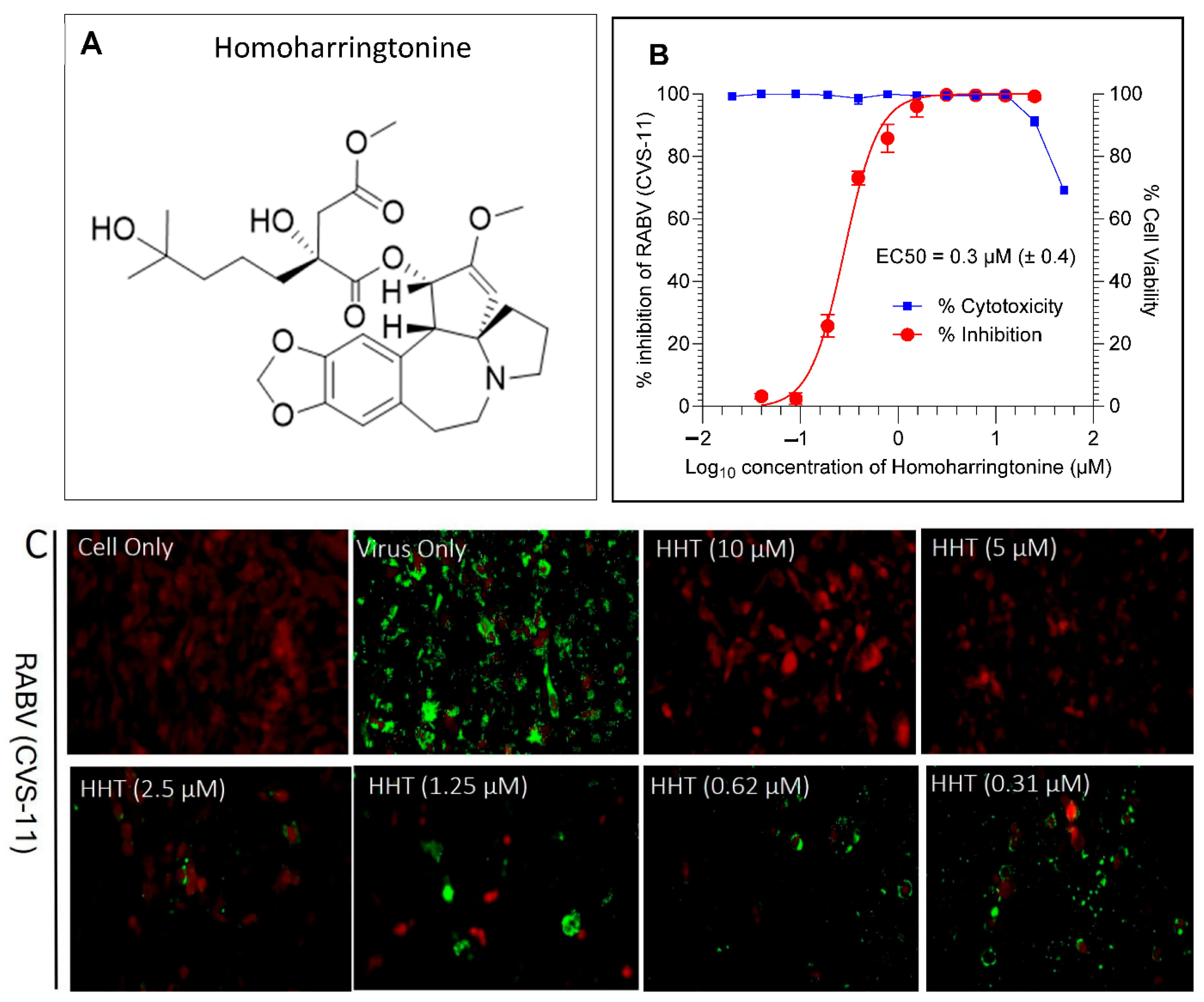
4.3. Antiviral Hits from FDA-Approved Drug Library
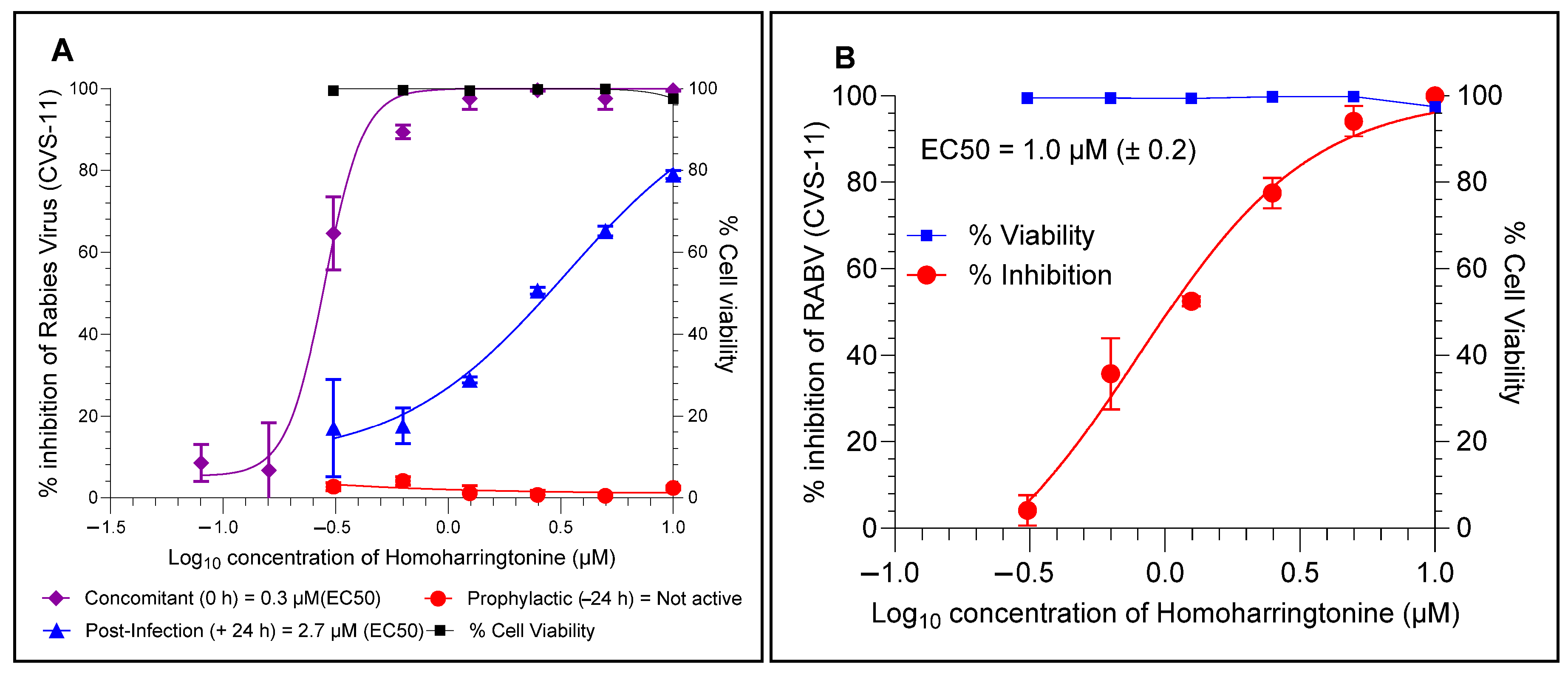
4.4. HHT Inhibits RABV at the Later Stages of Infection and Prevents Cell–Cell Spread
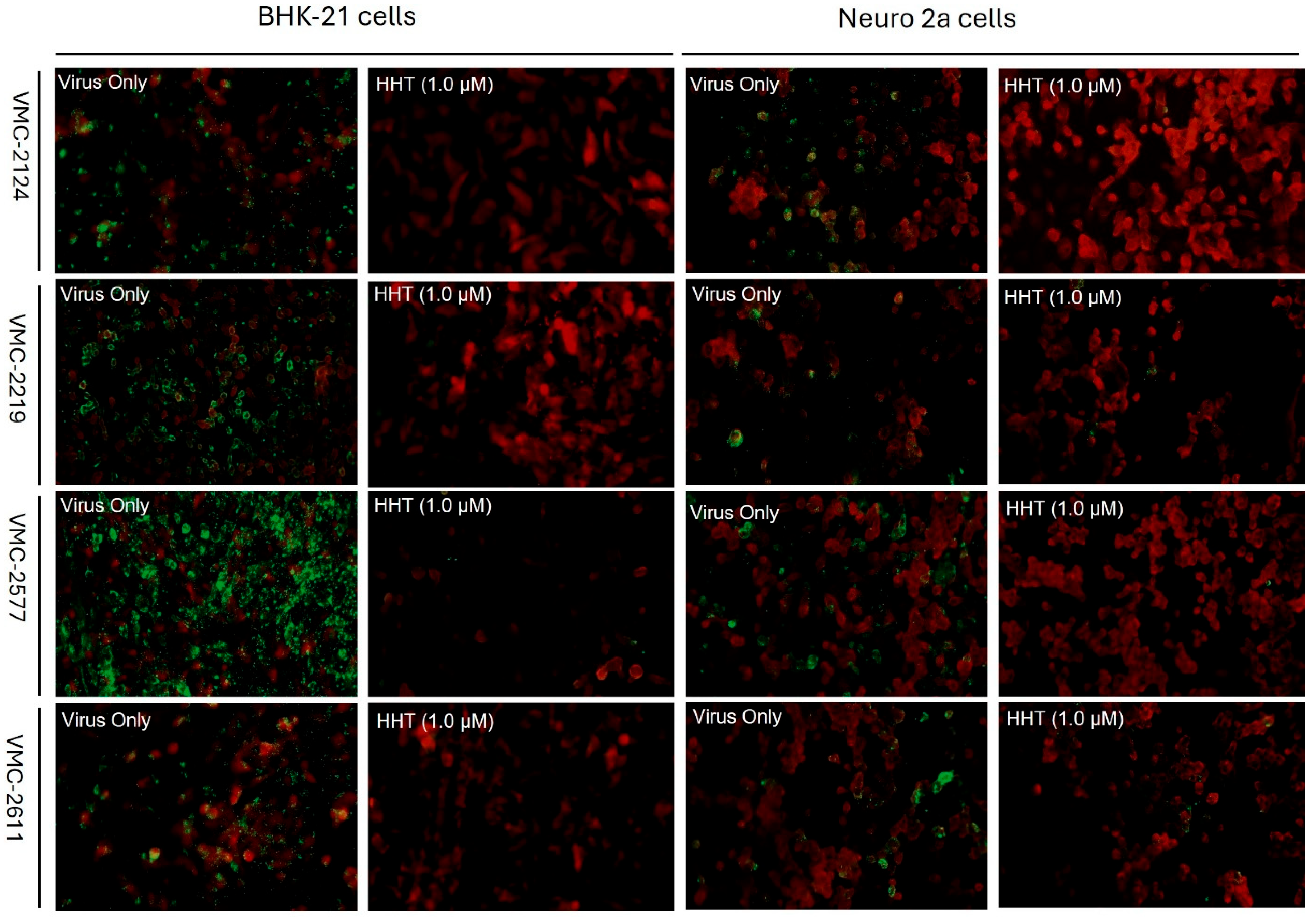
4.5. HHT Inhibits Clinical Isolates of RABV in BHK-21 and Neuro-2a Cell Lines
5. Discussion
6. Published Material
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cantaert, T.; Borand, L.; Kergoat, L.; Leng, C.; Ung, S.; In, S.; Peng, Y.; Phoeun, C.; Hing, C.; Taing, C.N.; et al. A 1-Week Intradermal Dose-Sparing Regimen for Rabies Post-Exposure Prophylaxis (RESIST-2): An Observational Cohort Study. Lancet Infect. Dis. 2019, 19, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Expert Consultation on Rabies: Third Report; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Zhu, S.; Guo, C. Rabies Control and Treatment: From Prophylaxis to Strategies with Curative Potential. Viruses 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Badrane, H.; Bahloul, C.; Perrin, P.; Tordo, N. Evidence of Two Lyssavirus Phylogroups with Distinct Pathogenicity and Immunogenicity. J. Virol. 2001, 75, 3268–3276. [Google Scholar] [CrossRef]
- Du Pont, V.; Plemper, R.K.; Schnell, M.J. Status of Antiviral Therapeutics against Rabies Virus and Related Emerging Lyssaviruses. Curr. Opin. Virol. 2019, 35, 1–13. [Google Scholar] [CrossRef]
- Jochmans, D.; Neyts, J. The Path towards Effective Antivirals against Rabies. Vaccine 2019, 37, 4660–4662. [Google Scholar] [CrossRef]
- Willoughby, R.E.; Tieves, K.S.; Hoffman, G.M.; Ghanayem, N.S.; Amlie-Lefond, C.M.; Schwabe, M.J.; Chusid, M.J.; Rupprecht, C.E. Survival after Treatment of Rabies with Induction of Coma. N. Engl. J. Med. 2005, 352, 2508–2514. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Jackson, A.C. Critical Appraisal of the Milwaukee Protocol for Rabies: This Failed Approach Should Be Abandoned. Can. J. Neurol. Sci. 2015, 43, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.C. Rabies: A Medical Perspective. Rev. Sci. Et Tech. (Int. Off. Epizoot.) 2018, 37, 569–580. [Google Scholar] [CrossRef]
- Ledesma, L.A.; Lemos, E.R.S.; Horta, M.A. Comparing Clinical Protocols for the Treatment of Human Rabies: The Milwaukee Protocol and the Brazilian Protocol (Recife). Rev. Da Soc. Bras. De Med. Trop. 2020, 53, e20200352. [Google Scholar] [CrossRef]
- Fooks, A.R.; Jackson, A.C. Rabies: Scientific Basis of the Disease and Its Management, 4th ed.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Jackson, A.C. Demise of the Milwaukee Protocol for Rabies. Clin. Infect. Dis. 2025, ciaf157. [Google Scholar] [CrossRef]
- Superti, F.; Seganti, L.; Pana, A.; Orsi, N. Effect of Amantidine on Rhabdovirus Infection. Drugs Under Exp. Clin. Res. 1985, 11, 69–74. [Google Scholar]
- Appolinário, C.M.; Prehaud, C.; Allendorf, S.D.; Antunes, J.M.A.P.; Peres, M.G.; Lafon, M.; Megid, J. Ribavirin Has an In Vitro Antiviral Effect in Rabies Virus Infected Neuronal Cells but Fails to Provide Benefit in Experimental Rabies in Mice. J. Virol. Antivir. Res. 2013, 2, 2. [Google Scholar] [CrossRef]
- Zamore, P.D.; Tuschl, T.; Sharp, P.A.; Bartel, D.P. RNAi: Double-Stranded RNA Directs the ATP-Dependent Cleavage of MRNA at 21 to 23 Nucleotide Intervals. Cell 2000, 101, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.A.D.; Taniwaki, S.A.; Brandão, P. Short Interfering RNAs Targeting a Vampire-Bat Related Rabies Virus Phosphoprotein MRNA. Braz. J. Microbiol. 2017, 48, 566–569. [Google Scholar] [CrossRef]
- Appolinario, C.M.; Allendorf, S.D.; Peres, M.G.; Fonseca, C.R.; Vicente, A.F.; Antunes, J.M.A.d.P.; Pantoja, J.C.F.; Megid, J. Evaluation of Short-Interfering RNAs Treatment in Experimental Rabies Due to Wild-Type Virus. Braz. J. Infect. Dis. 2015, 19, 453–458. [Google Scholar] [CrossRef]
- de Melo, G.D.; Sonthonnax, F.; Lepousez, G.; Jouvion, G.; Minola, A.; Zatta, F.; Larrous, F.; Kergoat, L.; Mazo, C.; Moigneu, C.; et al. A Combination of Two Human Monoclonal Antibodies Cures Symptomatic Rabies. EMBO Mol. Med. 2020, 12, e12628. [Google Scholar] [CrossRef] [PubMed]
- Du Pont, V.; Wirblich, C.; Yoon, J.-J.; Cox, R.M.; Schnell, M.J.; Plemper, R.K. Identification and Characterization of a Small-Molecule Rabies Virus Entry Inhibitor. J. Virol. 2020, 94, e00321-20. [Google Scholar] [CrossRef]
- Smith, T.G.; Jackson, F.R.; Morgan, C.N.; Carson, W.C.; Martin, B.E.; Gallardo-Romero, N.; Ellison, J.A.; Greenberg, L.; Hodge, T.; Squiquera, L.; et al. Antiviral Ranpirnase TMR-001 Inhibits Rabies Virus Release and Cell-to-Cell Infection in Vitro. Viruses 2020, 12, 177. [Google Scholar] [CrossRef]
- Yamada, K.; Noguchi, K.; Komeno, T.; Furuta, Y.; Nishizono, A. Efficacy of Favipiravir (T-705) in Rabies Postexposure Prophylaxis. J. Infect. Dis. 2016, 213, 1253–1261. [Google Scholar] [CrossRef]
- Wang, X.; Chiu, W.; Klaassen, H.; Marchand, A.; Chaltin, P.; Neyts, J.; Jochmans, D. A Robust Phenotypic High-Throughput Antiviral Assay for the Discovery of Rabies Virus Inhibitors. Viruses 2023, 15, 2292. [Google Scholar] [CrossRef]
- Rudd, R.J.; Trimarchi, C.V.; Abelseth, M.K. Tissue Culture Technique for Routine Isolation of Street Strain Rabies Virus. J. Clin. Microbiol. 1980, 12, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Yager, P.A.; Baer, G.M.; Meslin, F.X.; Kaplan, M.M.; Koprowski, H. Laboratory Techniques in Rabies; World Health Organization: Geneva, Switzerland, 1996; Volume 181. [Google Scholar]
- Zalan, E.; Wilson, C.; Pukitis, D. A Microtest for the Quantitation of Rabies Virus Neutralizing Antibodies. J. Biol. Stand. 1979, 7, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Anindita, P.D.; Sasaki, M.; Okada, K.; Ito, N.; Sugiyama, M.; Saito-Tarashima, N.; Minakawa, N.; Shuto, S.; Otsuguro, S.; Ichikawa, S.; et al. Ribavirin-Related Compounds Exert in Vitro Inhibitory Effects toward Rabies Virus. Antivir. Res. 2018, 154, 1–9. [Google Scholar] [CrossRef]
- Wallace, R.; Petersen, B.; Rabies, D.S. CDC Yellow Book 2024, Travel-Associated Infections & Diseases; CDC: Atlanta, GA, USA, 2023. [Google Scholar]
- Gluska, S.; Zahavi, E.E.; Chein, M.; Gradus, T.; Bauer, A.; Finke, S.; Perlson, E. Rabies Virus Hijacks and Accelerates the P75NTR Retrograde Axonal Transport Machinery. PLoS Pathog. 2014, 10, e1004348. [Google Scholar] [CrossRef] [PubMed]
- Potratz, M.; Zaeck, L.M.; Weigel, C.; Klein, A.; Freuling, C.M.; Müller, T.; Finke, S. Neuroglia Infection by Rabies Virus after Anterograde Virus Spread in Peripheral Neurons. Acta Neuropathol. Commun. 2020, 8, 199. [Google Scholar] [CrossRef]
- Boonsriroj, H.; Manalo, D.L.; Kimitsuki, K.; Shimatsu, T.; Shiwa, N.; Shinozaki, H.; Takahashi, Y.; Tanaka, N.; Inoue, S.; Park, C.H. A Pathological Study of the Salivary Glands of Rabid Dogs in the Philippines. J. Vet. Med. Sci. 2016, 78, 35–42. [Google Scholar] [CrossRef]
- McSharry, J.J.; Costantino, R.; McSharry, M.B.; Venezia, R.A.; Lehman, J.M. Rapid Detection of Herpes Simplex Virus in Clinical Samples by Flow Cytometry after Amplification in Tissue Culture. J. Clin. Microbiol. 1990, 28, 1864–1866. [Google Scholar] [CrossRef]
- Schols, D.; Snoeck, R.; Neyts, J.; De Clercq, E. Detection of Immediate Early, Early and Late Antigens of Human Cytomegalovirus by Flow Cytometry. J. Virol. Methods 1989, 26, 247–254. [Google Scholar] [CrossRef]
- Abad, F.X.; Pintó, R.M.; Bosch, A. Flow Cytometry Detection of Infectious Rotaviruses in Environmental and Clinical Samples. Appl Environ. Microbiol. 1998, 64, 2392–2396. [Google Scholar] [CrossRef]
- Bordignon, J.; Pires Ferreira, S.C.; Medeiros Caporale, G.M.; Carrieri, M.L.; Kotait, I.; Lima, H.C.; Zanetti, C.R. Flow Cytometry Assay for Intracellular Rabies Virus Detection. J. Virol. Methods 2002, 105, 181–186. [Google Scholar] [CrossRef]
- Bordignon, J.; Comin, F.; Ferreira, S.C.P.; Caporale, G.M.M.; Lima Filho, J.H.C.; Zanetti, C.R. Calculating Rabies Virus Neutralizing Antibodies Titres by Flow Cytometry. Rev. Do Inst. De Med. Trop. De São Paulo 2002, 44, 151–154. [Google Scholar] [CrossRef]
- Wu, J.; Cao, S.; Lei, S.; Liu, Q.; Li, Y.; Yu, Y.; Xie, H.; Li, Q.; Zhao, X.; Chen, R.; et al. Clofazimine: A Promising Inhibitor of Rabies Virus. Front. Pharmacol. 2021, 12, 598241. [Google Scholar] [CrossRef]
- Rogée, S.; Larrous, F.; Jochmans, D.; Ben-Khalifa, Y.; Neyts, J.; Bourhy, H. Pyrimethamine Inhibits Rabies Virus Replication in Vitro. Antivir. Res. 2019, 161, 1–9. [Google Scholar] [CrossRef]
- Zhou, D.C.; Zittoun, R.; Marie, J.P. Homoharringtonine: An Effective New Natural Product in Cancer Chemotherapy. Bull. Du Cancer 1995, 82, 987–995. [Google Scholar]
- Dong, H.J.; Wang, Z.H.; Meng, W.; Li, C.C.; Hu, Y.X.; Zhou, L.; Wang, X.J. The Natural Compound Homoharringtonine Presents Broad Antiviral Activity in Vitro and in Vivo. Viruses 2018, 10, 601. [Google Scholar] [CrossRef]
- Cao, J.; Forrest, J.C.; Zhang, X. A Screen of the NIH Clinical Collection Small Molecule Library Identifies Potential Anti-Coronavirus Drugs. Antivir. Res. 2015, 114, 1–10. [Google Scholar] [CrossRef]
- Gong, M.J.; Li, S.F.; Xie, Y.L.; Zhao, F.R.; Shao, J.J.; Zhang, Y.G.; Wang, W.H.; Chang, H.Y. Inhibitory Effects of Homoharringtonine on Foot and Mouth Disease Virus in Vitro. J. Med. Virol. 2019, 91, 1595–1601. [Google Scholar] [CrossRef]
- Montero, H.; García-Román, R.; Mora, S.I. EIF4E as a Control Target for Viruses. Viruses 2015, 7, 739–750. [Google Scholar] [CrossRef]
- Banerjee, S.; Narayanan, K.; Mizutani, T.; Makino, S. Murine Coronavirus Replication-Induced P38 Mitogen-Activated Protein Kinase Activation Promotes Interleukin-6 Production and Virus Replication in Cultured Cells. J. Virol. 2002, 76, 5937–5948. [Google Scholar] [CrossRef]
- Walsh, D.; Mohr, I. Phosphorylation of EIF4E by Mnk-1 Enhances HSV-1 Translation and Replication in Quiescent Cells. Genes Dev. 2004, 18, 660–672. [Google Scholar] [CrossRef]
- Connor, J.H.; Lyles, D.S. Vesicular Stomatitis Virus Infection Alters the EIF4F Translation Initiation Complex and Causes Dephosphorylation of the EIF4E Binding Protein 4E-BP1. J. Virol. 2002, 76, 10177–10187. [Google Scholar] [CrossRef] [PubMed]
- Burgui, I.; Yángüez, E.; Sonenberg, N.; Nieto, A. Influenza Virus MRNA Translation Revisited: Is the EIF4E Cap-Binding Factor Required for Viral MRNA Translation? J. Virol. 2007, 81, 12427–12438. [Google Scholar] [CrossRef] [PubMed]
- Komarova, A.V.; Real, E.; Borman, A.M.; Brocard, M.; England, P.; Tordo, N.; Hershey, J.W.B.; Kean, K.M.; Jacob, Y. Rabies Virus Matrix Protein Interplay with EIF3, New Insights into Rabies Virus Pathogenesis. Nucleic Acids Res. 2007, 35, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, G.R.; Harisha, K.R.; Kailaje, V.; Narayanan, S.; Shandil, R.K. Homoharringtonine Inhibits Rabies Virus In-Vitro. In Proceedings of the 38th International Conference on Antiviral Research (ICAR), Las Vegas, NV, USA, 17–21 March 2025. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harisha, K.R.; Kailaje, V.; Kondreddi, R.R.; Gudla, C.S.; Singh, S.; Ramakrishnaiah, S.; Isloor, S.; Narayanan, S.; Shandil, R.K.; Rudramurthy, G.R. Homoharringtonine Inhibits CVS-11 and Clinical Isolates of Rabies Virus In Vitro: Identified via High-Throughput Screening of an FDA-Approved Drug Library. Viruses 2025, 17, 945. https://doi.org/10.3390/v17070945
Harisha KR, Kailaje V, Kondreddi RR, Gudla CS, Singh S, Ramakrishnaiah S, Isloor S, Narayanan S, Shandil RK, Rudramurthy GR. Homoharringtonine Inhibits CVS-11 and Clinical Isolates of Rabies Virus In Vitro: Identified via High-Throughput Screening of an FDA-Approved Drug Library. Viruses. 2025; 17(7):945. https://doi.org/10.3390/v17070945
Chicago/Turabian StyleHarisha, Kalenahalli Rajappa, Varun Kailaje, Ravinder Reddy Kondreddi, Chandra Sekhar Gudla, Shraddha Singh, Sharada Ramakrishnaiah, Shrikrishna Isloor, Shridhar Narayanan, Radha Krishan Shandil, and Gudepalya Renukaiah Rudramurthy. 2025. "Homoharringtonine Inhibits CVS-11 and Clinical Isolates of Rabies Virus In Vitro: Identified via High-Throughput Screening of an FDA-Approved Drug Library" Viruses 17, no. 7: 945. https://doi.org/10.3390/v17070945
APA StyleHarisha, K. R., Kailaje, V., Kondreddi, R. R., Gudla, C. S., Singh, S., Ramakrishnaiah, S., Isloor, S., Narayanan, S., Shandil, R. K., & Rudramurthy, G. R. (2025). Homoharringtonine Inhibits CVS-11 and Clinical Isolates of Rabies Virus In Vitro: Identified via High-Throughput Screening of an FDA-Approved Drug Library. Viruses, 17(7), 945. https://doi.org/10.3390/v17070945







