Integrated In Silico, In Vitro, and In Vivo Studies Reveal Mangiferin as a Promising Antiviral Agent Against H1N1/pdm2009 Influenza Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Docking
2.2. Assessment of Mangiferin’s Anti-Neuraminidase Activity
2.3. Virus Strain and Propagation
2.4. Virus Titration
2.5. Cytotoxicity Assessment of Mangiferin
2.6. Evaluation of Mangiferin’s Antiviral Activity Against Influenza Virus A(H1N1)pdm09
2.7. In Vivo Assessment of Mangiferin’s Efficacy Against Influenza Virus A(H1N1)pdm09
2.8. Statistical Analysis
3. Results
3.1. Molecular Docking Analysis Revealed Mangiferin as a Promising Inhibitory Candidate Against Influenza Virus A(H1N1)pdm09 Neuraminidase
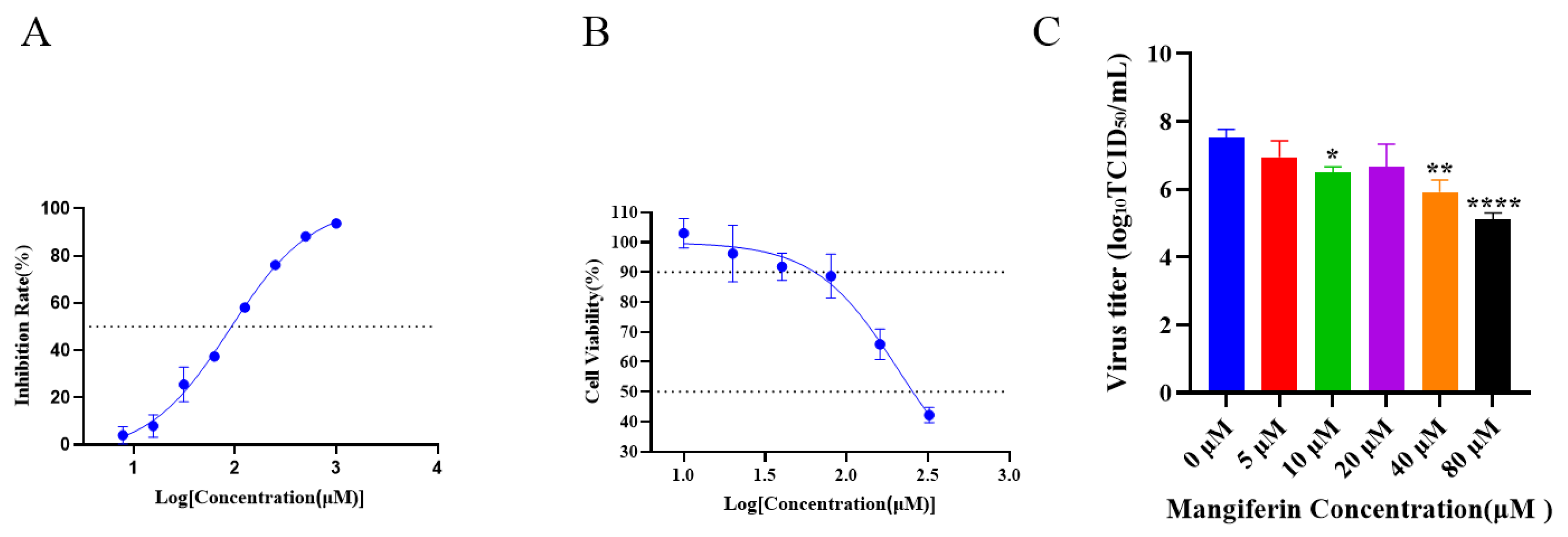
3.2. Mangiferin’s Anti-Neuraminidase Activity and Cytotoxicity In Vitro
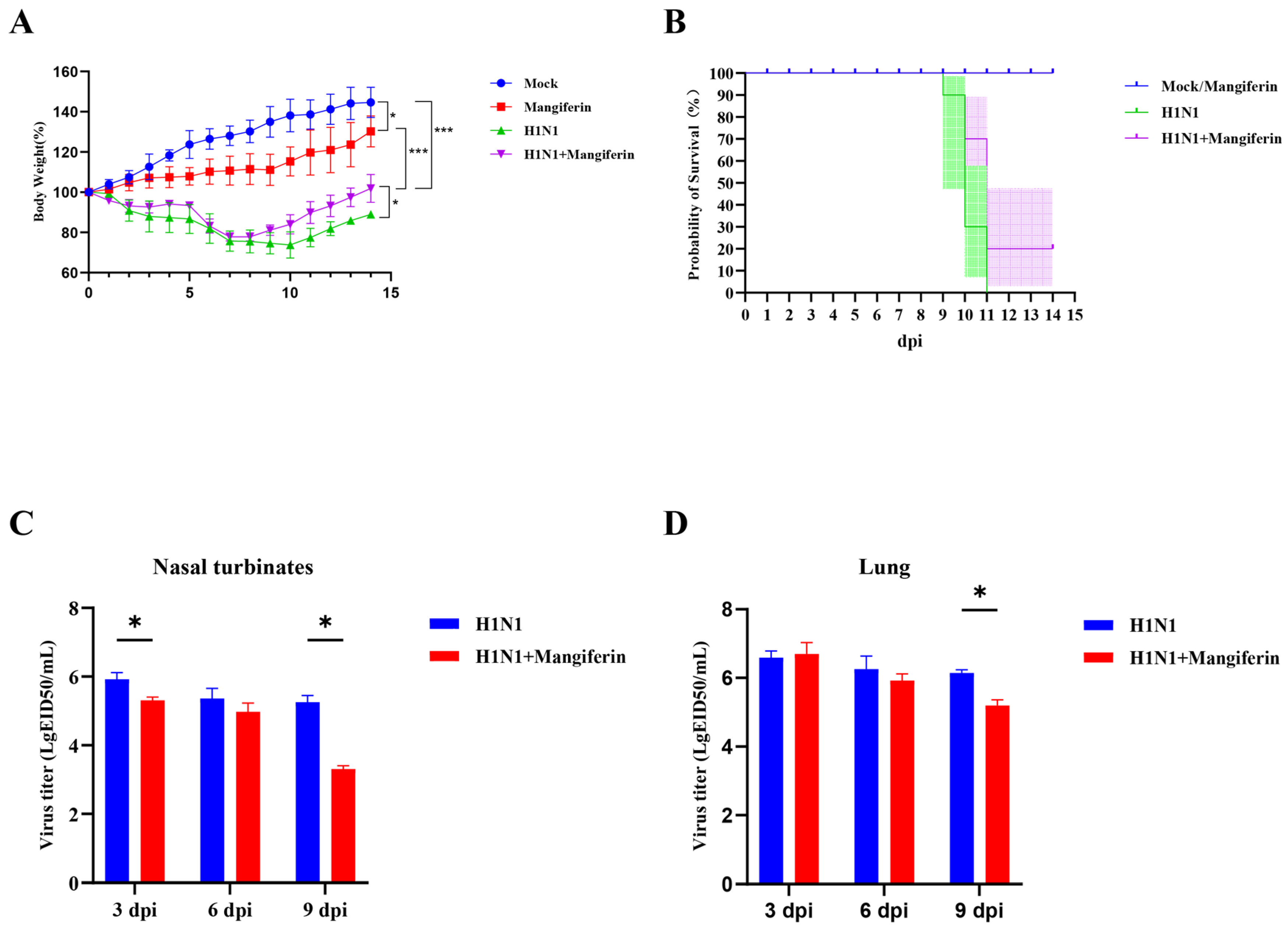
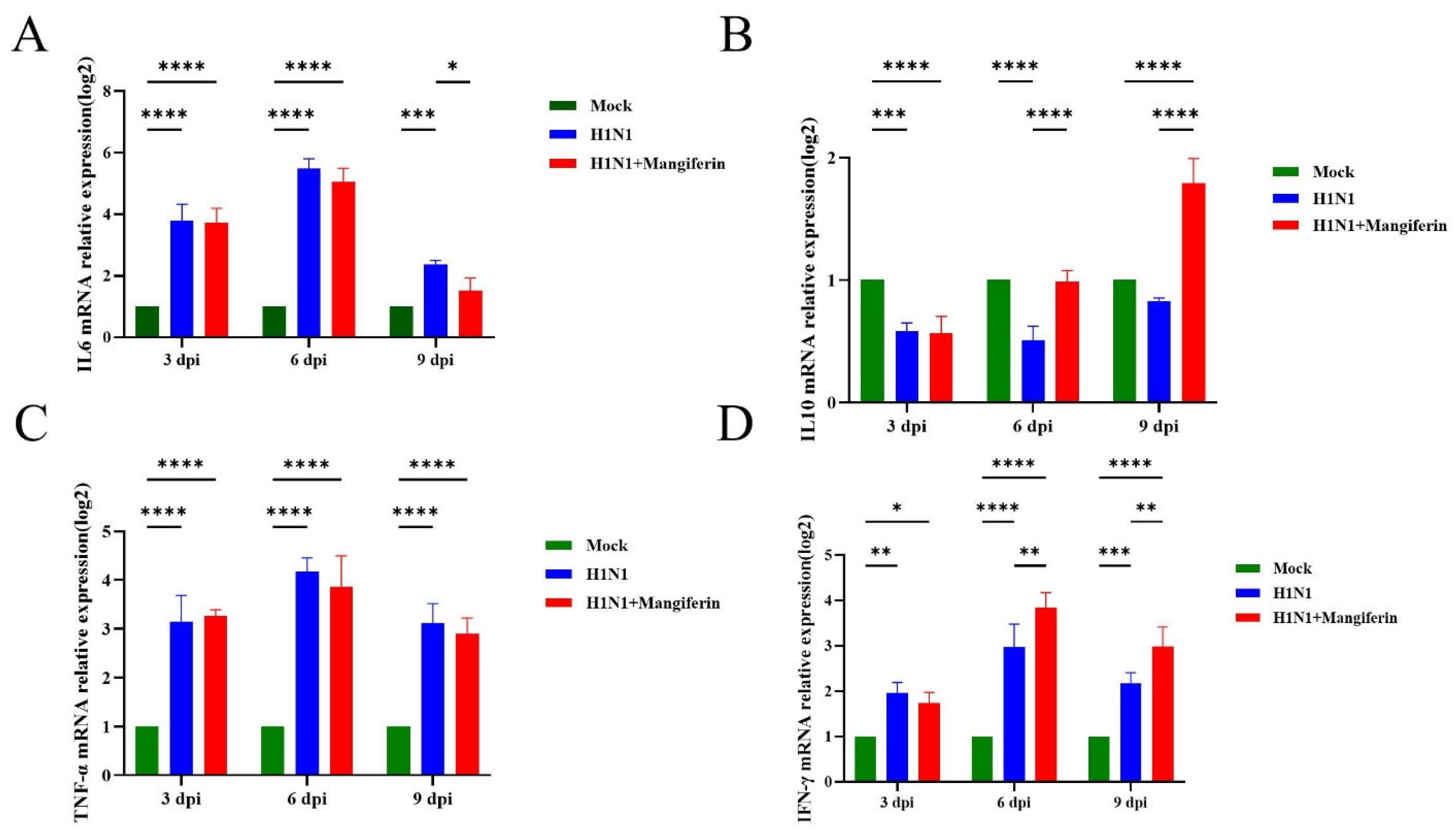
3.3. Effects of Mangiferin on Influenza Virus A(H1N1)pdm09 in a Murine Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vijaykrishna, D.; Poon, L.L.M.; Zhu, H.C.; Ma, S.K.; Li, O.T.W.; Cheung, C.L.; Smith, G.J.D.; Peiris, J.S.M.; Guan, Y. Reassortment of pandemic H1N1/2009 influenza A virus in swine. Science 2010, 328, 1529. [Google Scholar] [CrossRef] [PubMed]
- Lazniewski, M.; Dawson, W.K.; Szczepinska, T.; Plewczynski, D. The structural variability of the influenza A hemagglutinin receptor-binding site. Brief. Funct. Genom. 2018, 17, 415–427. [Google Scholar] [CrossRef]
- Emborg, H.; Botnen, A.B.; Nielsen, J.; Vestergaard, L.S.; Lomholt, F.K.; Munkstrup, C.; Moller, K.L.; Kjelso, C.; Rasmussen, S.H.; Trebbien, R. Age-dependent influenza infection patterns and subtype circulation in Denmark, in seasons 2015/16 to 2021/22. Eurosurveillance 2024, 29, 2300263. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Zhai, K.; Han, W.; Zeng, J.; Qiu, Z.; Chen, Y.; Tang, K.; Tang, J.; Long, H.; Jiang, T.; et al. Characterizing the antigenic evolution of pandemic influenza A (H1N1) pdm09 from 2009 to 2023. J. Med. Virol. 2024, 96, e29657. [Google Scholar] [CrossRef]
- van der Vries, E.; Collins, P.J.; Vachieri, S.G.; Xiong, X.; Liu, J.; Walker, P.A.; Haire, L.F.; Hay, A.J.; Schutten, M.; Osterhaus, A.D.M.E.; et al. H1N1 2009 pandemic influenza virus: Resistance of the I223R neuraminidase mutant explained by kinetic and structural analysis. PLoS Pathog. 2012, 8, e1002914. [Google Scholar] [CrossRef]
- Yang, J.; Liu, S.; Du, L.; Jiang, S. A new role of neuraminidase (NA) in the influenza virus life cycle: Implication for developing NA inhibitors with novel mechanism of action. Rev. Med. Virol. 2016, 26, 242–250. [Google Scholar] [CrossRef]
- Lackenby, A.; Besselaar, T.G.; Daniels, R.S.; Fry, A.; Gregory, V.; Gubareva, L.V.; Huang, W.; Hurt, A.C.; Leang, S.; Lee, R.T.C.; et al. Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors and status of novel antivirals, 2016–2017. Antivir. Res. 2018, 157, 38–46. [Google Scholar] [CrossRef]
- Takashita, E.; Daniels, R.S.; Fujisaki, S.; Gregory, V.; Gubareva, L.V.; Huang, W.; Hurt, A.C.; Lackenby, A.; Nguyen, H.T.; Pereyaslov, D.; et al. Global update on the susceptibilities of human influenza viruses to neuraminidase inhibitors and the cap-dependent endonuclease inhibitor baloxavir, 2017–2018. Antivir. Res. 2020, 175, 104718. [Google Scholar] [CrossRef]
- Pizzorno, A.; Bouhy, X.; Abed, Y.; Boivin, G. Generation and characterization of recombinant pandemic influenza A(H1N1) viruses resistant to neuraminidase inhibitors. J. Infect. Dis. 2011, 203, 25–31. [Google Scholar] [CrossRef]
- Ashtiwi, N.M.; Sarr, D.; Nagy, T.; Reneer, Z.B.; Tripp, R.A.; Rada, B. The Hypothiocyanite and Amantadine Combination Treatment Prevents Lethal Influenza A Virus Infection in Mice. Front. Immunol. 2022, 13, 859033. [Google Scholar] [CrossRef]
- Paggi, J.M.; Pandit, A.; Dror, R.O. The Art and Science of Molecular Docking. Annu. Rev. Biochem. 2024, 93, 389–410. [Google Scholar] [CrossRef] [PubMed]
- Sarukhanyan, E.; Shanmugam, T.A.; Dandekar, T. In Silico Studies Reveal Peramivir and Zanamivir as an Optimal Drug Treatment Even If H7N9 Avian Type Influenza Virus Acquires Further Resistance. Molecules 2022, 27, 5920. [Google Scholar] [CrossRef] [PubMed]
- Ivashchenko, A.A.; Jones, J.C.; Shkil, D.O.; Ivanenkov, Y.A.; Pascua, P.N.Q.; Penaflor, M.K.; Karapetian, R.N.; Govorkova, E.A.; Ivachtchenko, A.V. Resistance profiles for the investigational neuraminidase inhibitor AV5080 in influenza A and B viruses. Antivir. Res. 2023, 217, 105701. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Zhang, P.; Zhang, Z.; Youn, J.Y.; Wang, C.; Zhang, H.; Cai, H. Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: Efficacies and mechanisms. Pharmacol. Ther. 2021, 225, 107843. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, S. Research progress on antiviral constituents in traditional Chinese medicines and their mechanisms of action. Pharm. Biol. 2022, 60, 1063–1076. [Google Scholar] [CrossRef]
- Yin, J.; Ma, L.; Wang, H.; Yan, H.; Hu, J.; Jiang, W.; Li, Y. Chinese herbal medicine compound Yi-Zhi-Hao pellet inhibits replication of influenza virus infection through activation of heme oxygenase-1. Acta Pharm. Sin. B 2017, 7, 630–637. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, N.X.; Duan, N.; Liu, B.; Zhu, H.; Zhang, C.; Li, L.; Lu, C.; Huang, L. Traditional Chinese Medicine in Treating Influenza: From Basic Science to Clinical Applications. Front. Pharmacol. 2020, 11, 575803. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; He, J.; Yan, W.; Jiang, H.; Chen, Q.; Li, L.; Yang, Z. Lianhua-Qingwen Displays Antiviral and Anti-Inflammatory Activity and Synergistic Effects with Oseltamivir against Influenza B Virus Infection in the Mouse Model. Evid.-Based Complement. Altern. Med. 2020, 2020, 3196375. [Google Scholar] [CrossRef]
- Ji, L.; Song, T.; Ge, C.; Wu, Q.; Ma, L.; Chen, X.; Chen, T.; Chen, Q.; Chen, Z.; Chen, W. Identification of bioactive compounds and potential mechanisms of scutellariae radix-coptidis rhizoma in the treatment of atherosclerosis by integrating network pharmacology and experimental validation. Biomed. Pharmacother. 2023, 165, 115210. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Yin, L.; Huang, C.; Fan, S. Mangiferin relieves CCl4-induced liver fibrosis in mice. Sci. Rep. 2023, 13, 4172. [Google Scholar] [CrossRef]
- Zheng, M.S.; Lu, Z.Y. Antiviral effect of mangiferin and isomangiferin on herpes simplex virus. Chin. Med. J. 1990, 103, 160–165. [Google Scholar] [PubMed]
- Li, X.; Ohtsuki, T.; Shindo, S.; Sato, M.; Koyano, T.; Preeprame, S.; Kowithayakorn, T.; Ishibashi, M. Mangiferin identified in a screening study guided by neuraminidase inhibitory activity. Planta Med. 2007, 73, 1195–1196. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins 2009, 77 (Suppl. S9), 114–122. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminformatics 2011, 3, 33. [Google Scholar] [CrossRef]
- Schuttelkopf, A.W.; van Aalten, D.M.F. PRODRG: A tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 1355–1363. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Yang, X.; Long, F.; Jia, W.; Zhang, M.; Su, G.; Liao, M.; Zeng, Z.; Chen, W.; Chen, J. Artesunate inhibits PDE4 leading to intracellular cAMP accumulation, reduced ERK/MAPK signaling, and blockade of influenza A virus vRNP nuclear export. Antivir. Res. 2023, 215, 105635. [Google Scholar] [CrossRef]
- Bryan, W.R. Interpretation of host response in quantitative studies on animal viruses. Ann. N. Y. Acad. Sci. 1957, 69, 698–728. [Google Scholar] [CrossRef]
- Hamelin, M.; Baz, M.; Abed, Y.; Couture, C.; Joubert, P.; Beaulieu, E.; Bellerose, N.; Plante, M.; Mallett, C.; Schumer, G.; et al. Oseltamivir-resistant pandemic A/H1N1 virus is as virulent as its wild-type counterpart in mice and ferrets. PLoS Pathog. 2010, 6, e1001015. [Google Scholar] [CrossRef]
- Gao, J.; Couzens, L.; Burke, D.F.; Wan, H.; Wilson, P.; Memoli, M.J.; Xu, X.; Harvey, R.; Wrammert, J.; Ahmed, R.; et al. Antigenic Drift of the Influenza A(H1N1)pdm09 Virus Neuraminidase Results in Reduced Effectiveness of A/California/7/2009 (H1N1pdm09)-Specific Antibodies. Mbio 2019, 10, 2129–2161. [Google Scholar] [CrossRef]
- Martinez-Baz, I.; Fernandez-Huerta, M.; Navascues, A.; Pozo, F.; Trobajo-Sanmartin, C.; Casado, I.; Echeverria, A.; Ezpeleta, C.; Castilla, J. Influenza Vaccine Effectiveness in Preventing Laboratory-Confirmed Influenza Cases and Hospitalizations in Navarre, Spain, 2022–2023. Vaccines 2023, 11, 1478. [Google Scholar] [CrossRef] [PubMed]
- Westgeest, K.B.; de Graaf, M.; Fourment, M.; Bestebroer, T.M.; van Beek, R.; Spronken, M.I.J.; de Jong, J.C.; Rimmelzwaan, G.F.; Russell, C.A.; Osterhaus, A.D.M.E.; et al. Genetic evolution of the neuraminidase of influenza A (H3N2) viruses from 1968 to 2009 and its correspondence to haemagglutinin evolution. J. Gen. Virol. 2012, 93, 1996–2007. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Nelson-Sathi, S.; Wang, Y.; Prasad, R.; Rayen, S.; Nandel, V.; Hu, Y.; Zhang, W.; Nair, R.; Dharmaseelan, S.; et al. Evolutionary, genetic, structural characterization and its functional implications for the influenza A (H1N1) infection outbreak in India from 2009 to 2017. Sci. Rep. 2019, 9, 14690. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chan, C.; Su, Z.; Chen, C. Homology modeling, docking, and molecular dynamics reveal HR1039 as a potent inhibitor of 2009 A(H1N1) influenza neuraminidase. Biophys. Chem. 2010, 147, 74–80. [Google Scholar] [CrossRef]
- Vavricka, C.J.; Li, Q.; Wu, Y.; Qi, J.; Wang, M.; Liu, Y.; Gao, F.; Liu, J.; Feng, E.; He, J.; et al. Structural and functional analysis of laninamivir and its octanoate prodrug reveals group specific mechanisms for influenza NA inhibition. PLoS Pathog. 2011, 7, e1002249. [Google Scholar] [CrossRef]
- Liu, F.; Cao, W.; Deng, C.; Wu, Z.; Zeng, G.; Zhou, Y. Polyphenolic glycosides isolated from Pogostemon cablin (Blanco) Benth. as novel influenza neuraminidase inhibitors. Chem. Cent. J. 2016, 10, 51. [Google Scholar] [CrossRef][Green Version]
- McKimm-Breschkin, J.L. Influenza neuraminidase inhibitors: Antiviral action and mechanisms of resistance. Influenza Other Respir. Viruses 2013, 7 (Suppl. S1), 25–36. [Google Scholar] [CrossRef]
- Leang, S.; Hurt, A.C. Fluorescence-based Neuraminidase Inhibition Assay to Assess the Susceptibility of Influenza Viruses to The Neuraminidase Inhibitor Class of Antivirals. Jove-J. Vis. Exp. 2017, 7, e55570. [Google Scholar] [CrossRef]
- Goto, T.; Kawai, N.; Bando, T.; Sato, T.; Tani, N.; Chong, Y.; Ikematsu, H. In vitro neuraminidase inhibitory concentrations (IC(50)) of four neuraminidase inhibitors in the Japanese 2023-24 season: Comparison with the 2010-11 to 2022-23 seasons. J. Infect. Chemother. 2025, 31, 102602. [Google Scholar] [CrossRef]
- Zivkovic, J.; Kumar, K.A.; Rushendran, R.; Ilango, K.; Fahmy, N.M.; El-Nashar, H.A.S.; El-Shazly, M.; Ezzat, S.M.; Melgar-Lalanne, G.; Romero-Montero, A.; et al. Pharmacological properties of mangiferin: Bioavailability, mechanisms of action and clinical perspectives. Naunyn Schmiedeb. Arch. Pharmacol. 2024, 397, 763–781. [Google Scholar] [CrossRef]
- Kim, H.; Moon, J.Y.; Kim, H.; Lee, D.; Cho, M.; Choi, H.; Kim, Y.S.; Mosaddik, A.; Cho, S.K. Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem. 2010, 121, 429–436. [Google Scholar] [CrossRef]
- Yehia, R.S.; Altwaim, S.A. An Insight into In Vitro Antioxidant, Antimicrobial, Cytotoxic, and Apoptosis Induction Potential of Mangiferin, a Bioactive Compound Derived from Mangifera indica. Plants 2023, 12, 1539. [Google Scholar] [CrossRef] [PubMed]
- Morozkina, S.N.; Nhung Vu, T.H.; Generalova, Y.E.; Snetkov, P.P.; Uspenskaya, M.V. Mangiferin as New Potential Anti-Cancer Agent and Mangiferin-Integrated Polymer Systems—A Novel Research Direction. Biomolecules 2021, 11, 79. [Google Scholar] [CrossRef]
- Li, N.; Xiong, R.; He, R.; Liu, B.; Wang, B.; Geng, Q. Mangiferin Mitigates Lipopolysaccharide-Induced Lung Injury by Inhibiting NLRP3 Inflammasome Activation. J. Inflamm. Res. 2021, 14, 2289–2300. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Wang, F.; Li, Y.; Li, Y.; Wang, M.; Sun, D.; Sun, C. Pharmacokinetic study of mangiferin in human plasma after oral administration. Food Chem. 2012, 132, 289–294. [Google Scholar] [CrossRef]
- Mei, S.; Perumal, M.; Battino, M.; Kitts, D.D.; Xiao, J.; Ma, H.; Chen, X. Mangiferin: A review of dietary sources, absorption, metabolism, bioavailability, and safety. Crit. Rev. Food Sci. 2023, 63, 3046–3064. [Google Scholar] [CrossRef]
- Reddeman, R.A.; Glavits, R.; Endres, J.R.; Clewell, A.E.; Hirka, G.; Vertesi, A.; Beres, E.; Szakonyine, I.P. A Toxicological Evaluation of Mango Leaf Extract (Mangifera indica) Containing 60% Mangiferin. J. Toxicol. 2019, 2019, 4763015. [Google Scholar] [CrossRef]
- Lopes, S.C.; Da Silva, A.V.L.; Arruda, B.R.; Morais, T.C.; Rios, J.B.; Trevisan, M.T.S.; Rao, V.S.; Santos, F.A. Peripheral antinociceptive action of mangiferin in mouse models of experimental pain: Role of endogenous opioids, KATP-channels and adenosine. Pharmacol. Biochem. Behav. 2013, 110, 19–26. [Google Scholar] [CrossRef]
- Huynh, D.T.M.; Le, L.M.; Nguyen, L.T.; Nguyen, T.H.N.; Nguyen, M.H.; Nguyen, K.T.V.; Tran, K.Q.; Tran, T.L.Q.; Thi Le, M.; Mai, H.N. Investigation of acute, sub-chronic toxicity, effects of mangiferin and mangiferin solid dispersion (HPTR) on Triton WR1339-induced hyperlipidemia on Swiss albino mice. Pharmacia 2024, 71, 1–14. [Google Scholar] [CrossRef]
- Morris, G.; Bortolasci, C.C.; Puri, B.K.; Marx, W.; O’Neil, A.; Athan, E.; Walder, K.; Berk, M.; Olive, L.; Carvalho, A.F.; et al. The cytokine storms of COVID-19, H1N1 influenza, CRS and MAS compared. Can one sized treatment fit all? Cytokine 2021, 144, 155593. [Google Scholar] [CrossRef]
- Mahallawi, W.H.; Shrwani, K.J.; Alomar, S.Y. Association between H1N1 infection and pro-inflammatory Th-1 and Th-17 cytokines production. J. King Saud Univ.-Sci. 2024, 36, 103198. [Google Scholar] [CrossRef]
- Tsubaki, M.; Takeda, T.; Kino, T.; Itoh, T.; Imano, M.; Tanabe, G.; Muraoka, O.; Satou, T.; Nishida, S. Mangiferin suppresses CIA by suppressing the expression of TNF-alpha, IL-6, IL-1beta, and RANKL through inhibiting the activation of NF-kappaB and ERK1/2. Am. J. Transl. Res. 2015, 7, 1371–1381. [Google Scholar] [PubMed]
- Askari, V.R.; Saadat, S.; Rahimi, V.B.; Kamelnia, E.; Boskabady, M.H. Evaluation of the effects of Mangiferin on human lymphocytes of healthy individuals and the balance of Th1 and Th2. Food Chem. Toxicol. 2025, 202, 115495. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Rodriguez, A.; Ferino-Perez, A.; Rodriguez-Riera, Z.; Rodeiro Guerra, I.; Reha, D.; Minofar, B.; Jauregui-Haza, U.J. Explaining the interaction of mangiferin with MMP-9 and NF-kbeta: A computational study. J. Mol. Model. 2022, 28, 266. [Google Scholar] [CrossRef]
- Guo, H.; Yun, C.; Hou, G.; Du, J.; Huang, X.; Lu, Y.; Keller, E.T.; Zhang, J.; Deng, J. Mangiferin attenuates TH1/TH2 cytokine imbalance in an ovalbumin-induced asthmatic mouse model. PLoS ONE 2014, 9, e100394. [Google Scholar] [CrossRef]
- Jayasuriya, R.; Ganesan, K.; Ramkumar, K.M. Mangiferin Represses Inflammation in Macrophages Under a Hyperglycemic Environment Through Nrf2 Signaling. Int. J. Mol. Sci. 2024, 25, 11197. [Google Scholar] [CrossRef]
- Chen, H.; Jie, C.; Tang, L.; Meng, H.; Li, X.; Li, Y.; Chen, L.; Yan, C.; Kurihara, H.; Li, Y.; et al. New insights into the effects and mechanism of a classic traditional Chinese medicinal formula on influenza prevention. Phytomedicine 2017, 27, 52–62. [Google Scholar] [CrossRef]
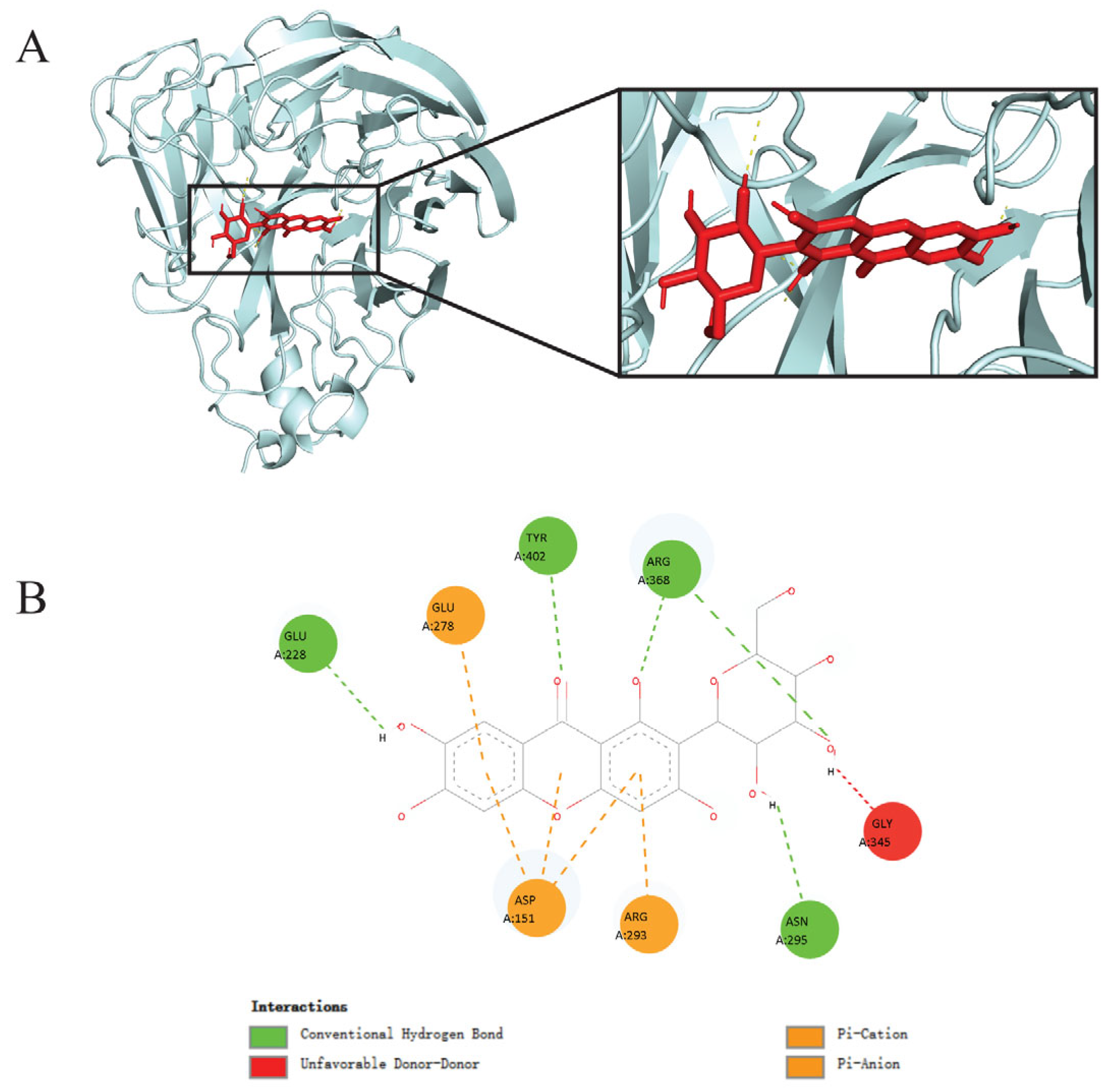
| Ligands | Binding Energy (kcal/mol) |
|---|---|
| Mangiferin | −8.1 |
| Oseltamivir (Drug) | −8.0 |
| Isobellidifolin | −7.6 |
| Swerchirin | −7.6 |
| Swertianin | −7.6 |
| Trachylobane | −7.4 |
| Anisotine | −7.3 |
| Gentiopicrin | −7.3 |
| Adhatodine | −7.3 |
| Lupeol | −7.2 |
| Stigmasterol | −7.2 |
| Betulin | −7.0 |
| Beta-sitosterol | −6.9 |
| Decussatin | −6.8 |
| Vasicoline | −6.6 |
| Clerosterol | −6.6 |
| Zanamivir (Drug) | −6.4 |
| Caryophyllene | −6.4 |
| Vasicine | −6.3 |
| Gentianine | −6.0 |
| Enicoflavine | −6.0 |
| Carvacrol | −5.8 |
| Cineole | −5.6 |
| Eugenol | −5.5 |
| Squalene | −5.2 |
| Catechol | −5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Y.; Guo, F.; Roy, A.; Wang, X.; Shen, Y. Integrated In Silico, In Vitro, and In Vivo Studies Reveal Mangiferin as a Promising Antiviral Agent Against H1N1/pdm2009 Influenza Virus. Viruses 2025, 17, 873. https://doi.org/10.3390/v17070873
Gan Y, Guo F, Roy A, Wang X, Shen Y. Integrated In Silico, In Vitro, and In Vivo Studies Reveal Mangiferin as a Promising Antiviral Agent Against H1N1/pdm2009 Influenza Virus. Viruses. 2025; 17(7):873. https://doi.org/10.3390/v17070873
Chicago/Turabian StyleGan, Yinde, Fucheng Guo, Ayan Roy, Xiao Wang, and Yongyi Shen. 2025. "Integrated In Silico, In Vitro, and In Vivo Studies Reveal Mangiferin as a Promising Antiviral Agent Against H1N1/pdm2009 Influenza Virus" Viruses 17, no. 7: 873. https://doi.org/10.3390/v17070873
APA StyleGan, Y., Guo, F., Roy, A., Wang, X., & Shen, Y. (2025). Integrated In Silico, In Vitro, and In Vivo Studies Reveal Mangiferin as a Promising Antiviral Agent Against H1N1/pdm2009 Influenza Virus. Viruses, 17(7), 873. https://doi.org/10.3390/v17070873








