Rift Valley Fever Outbreak Investigation Associated with a Dairy Farm Abortion Storm, Mbarara District, Western Uganda, 2023
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Detection and Field Investigation
2.2. Laboratory Methods
2.3. High-Throughput Sequencing, Bioinformatics, and Phylogenetics
2.4. Data Analysis and Management
3. Results
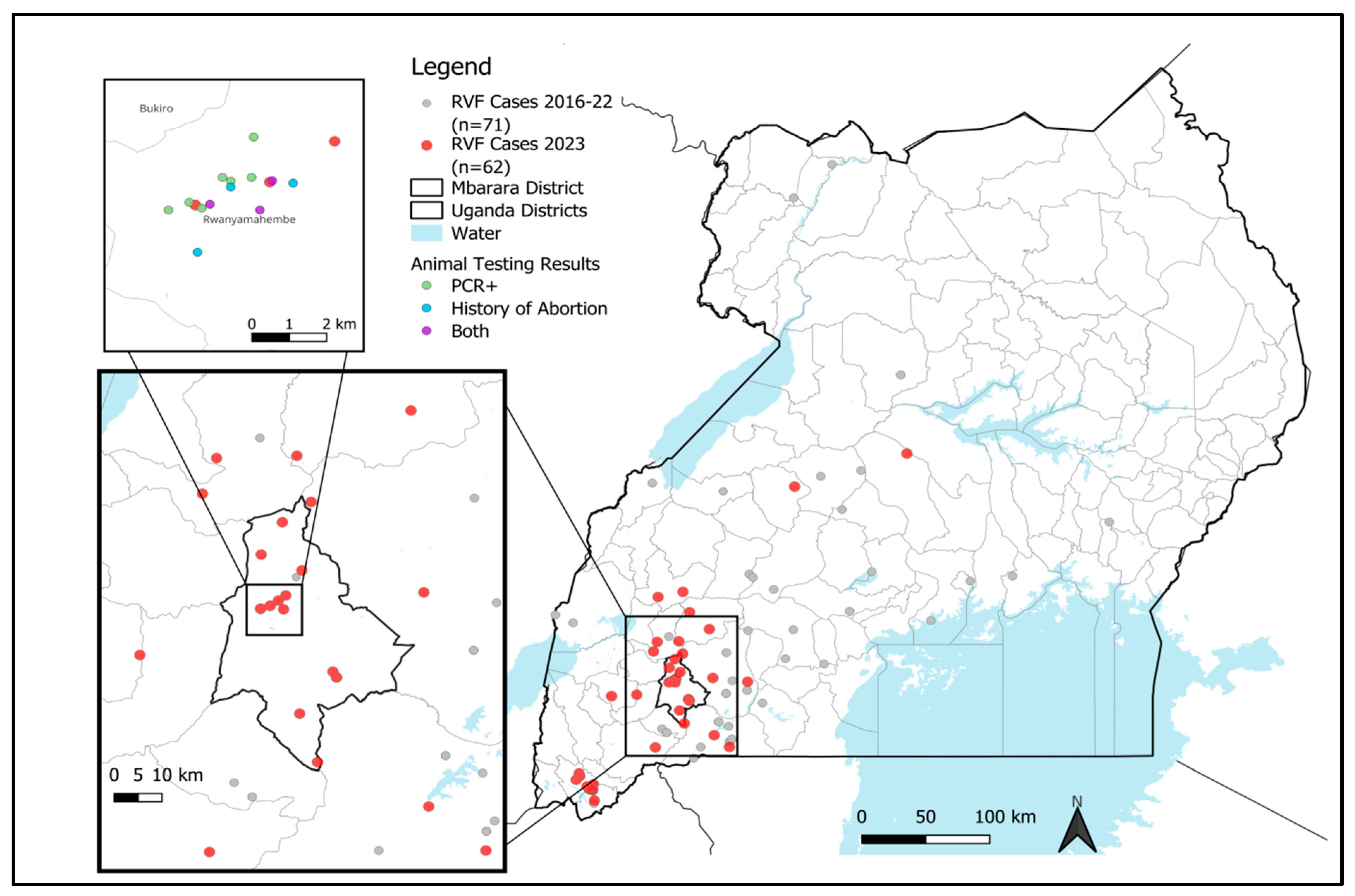
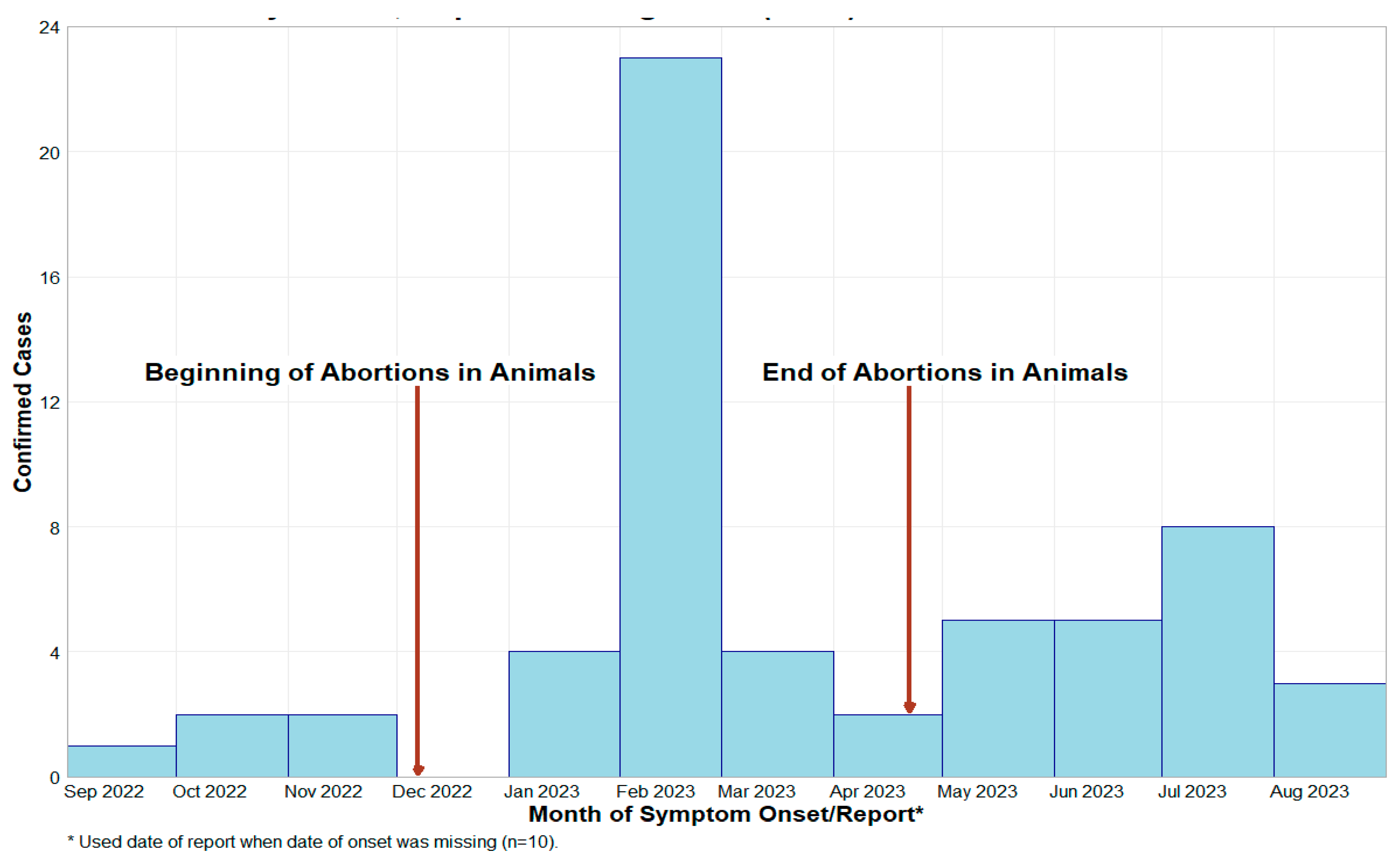
3.1. Investigation of Abortion Storms at Dairy Farm and Potential Link to Human Cases
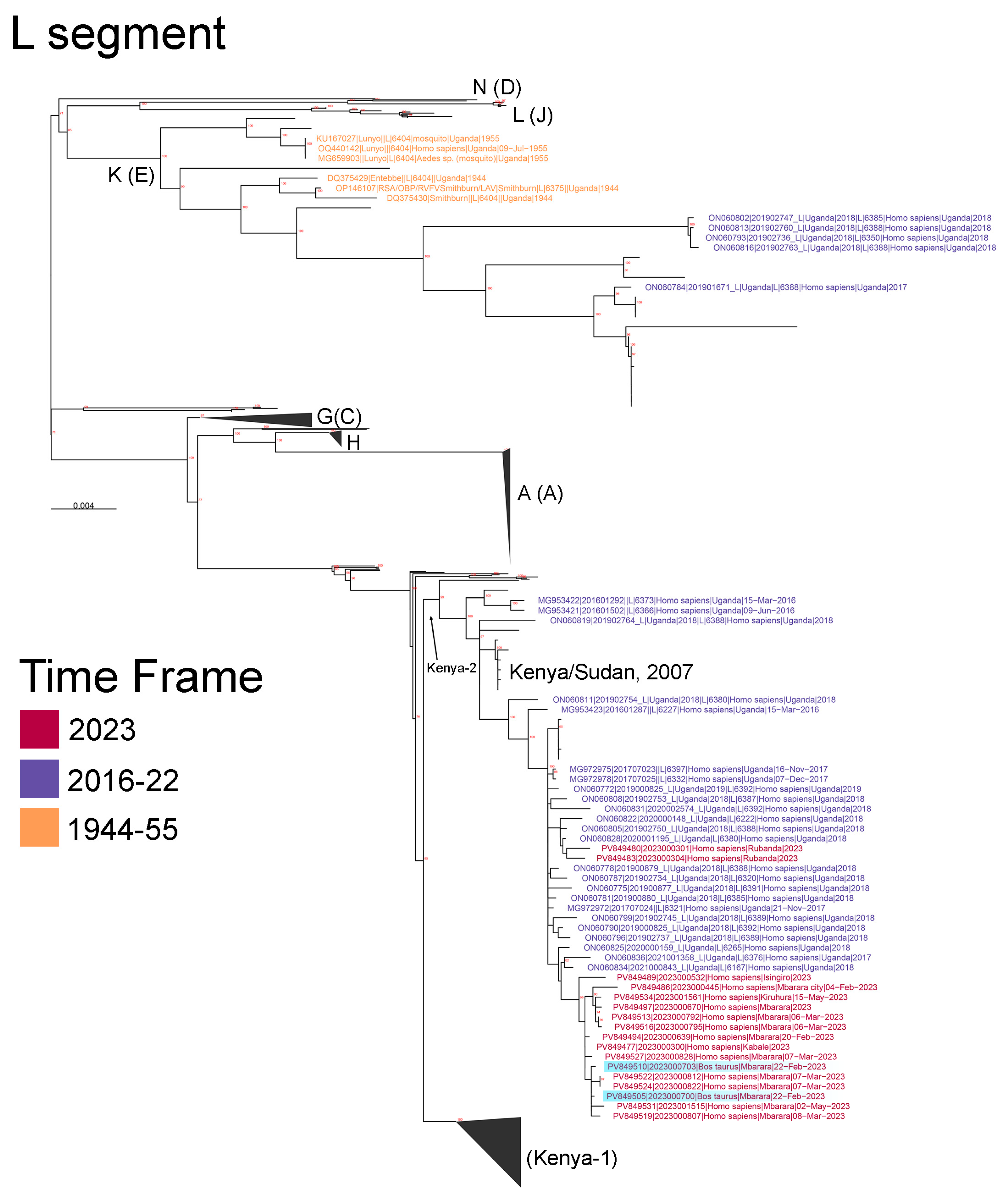
3.2. Results of the Next-Generation Sequencing and Phylogenetic Analysis of the Mbarara RVF Outbreak Cluster
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
CDC Disclaimer
References
- Shoemaker, T.R.; Nyakarahuka, L.; Balinandi, S.; Ojwang, J.; Tumusiime, A.; Mulei, S.; Kyondo, J.; Lubwama, B.; Sekamatte, M.; Namutebi, A.; et al. First Laboratory-Confirmed Outbreak of Human and Animal Rift Valley Fever Virus in Uganda in 48 Years. Am. J. Trop. Med. Hyg. 2019, 100, 659–671. [Google Scholar] [CrossRef]
- Nyakarahuka, L.; de St Maurice, A.; Purpura, L.; Ervin, E.; Balinandi, S.; Tumusiime, A.; Kyondo, J.; Mulei, S.; Tusiime, P.; Lutwama, J.; et al. Prevalence and Risk Factors of Rift Valley Fever in Humans and Animals from Kabale District in Southwestern Uganda, 2016. PLoS Negl. Trop. Dis. 2018, 12, e0006412. [Google Scholar] [CrossRef]
- Nanyingi, M.O.; Munyua, P.; Kiama, S.G.; Muchemi, G.M.; Thumbi, S.M.; Bitek, A.O.; Bett, B.; Muriithi, R.M.; Njenga, M.K. A Systematic Review of Rift Valley Fever Epidemiology 1931–2014. Infect. Ecol. Epidemiol. 2015, 5, 28024. [Google Scholar] [CrossRef]
- Nyakarahuka, L.; Whitmer, S.; Klena, J.; Balinandi, S.; Talundzic, E.; Tumusiime, A.; Kyondo, J.; Mulei, S.; Patel, K.; Baluku, J.; et al. Detection of Sporadic Outbreaks of Rift Valley Fever in Uganda through the National Viral Hemorrhagic Fever Surveillance System, 2017–2020. Am. J. Trop. Med. Hyg. 2023, 1, 995–1002. [Google Scholar] [CrossRef]
- Shoemaker, T.R.; Balinandi, S.; Tumusiime, A.; Nyakarahuka, L.; Lutwama, J.; Mbidde, E.; Kofman, A.; Klena, J.D.; Ströher, U.; Rollin, P.E.; et al. Impact of Enhanced Viral Haemorrhagic Fever Surveillance on Outbreak Detection and Response in Uganda. Lancet Infect. Dis. 2018, 18, 373–375. [Google Scholar] [CrossRef]
- Bird, B.H.; Bawiec, D.A.; Ksiazek, T.G.; Shoemaker, T.R.; Nichol, S.T. Highly Sensitive and Broadly Reactive Quantitative Reverse Transcription-PCR Assay for High-Throughput Detection of Rift Valley Fever Virus. J. Clin. Microbiol. 2007, 45, 3506–3513. [Google Scholar] [CrossRef]
- Mobile|Epi InfoTM|CDC. Available online: https://www.cdc.gov/epiinfo/mobile.html (accessed on 14 February 2024).
- Balinandi, S.; Whitmer, S.; Mulei, S.; Nassuna, C.; Pimundu, G.; Muyigi, T.; Kainulainen, M.; Shedroff, E.; Krapiunaya, I.; Scholte, F.; et al. Molecular Characterization of the 2022 Sudan Virus Disease Outbreak in Uganda. J. Virol. 2023, 97, e0059023. [Google Scholar] [CrossRef]
- Schafer, I.J.; Knudsen, E.; McNamara, L.A.; Agnihotri, S.; Rollin, P.E.; Islam, A. The Epi Info Viral Hemorrhagic Fever (VHF) Application: A Resource for Outbreak Data Management and Contact Tracing in the 2014–2016 West Africa Ebola Epidemic. J. Infect. Dis. 2016, 214, S122–S136. [Google Scholar] [CrossRef][Green Version]
- RStudio. RStudio: Integrated Development Environment for R; Available online: https://www.rstudio.com/products/rstudio/ (accessed on 18 March 2022).
- QGIS Welcome to the QGIS Project! Available online: https://qgis.org/en/site/ (accessed on 13 February 2023).
- Grobbelaar, A.A.; Weyer, J.; Leman, P.A.; Kemp, A.; Paweska, J.T.; Swanepoel, R. Molecular Epidemiology of Rift Valley Fever Virus. Emerg. Infect. Dis. 2011, 17, 2270–2276. [Google Scholar] [CrossRef]
- Samy, A.M.; Peterson, A.T.; Hall, M. Phylogeography of Rift Valley Fever Virus in Africa and the Arabian Peninsula. PLoS Neglected Trop. Dis. 2017, 11, e0005226. [Google Scholar] [CrossRef]
- Bron, G.M.; Strimbu, K.; Cecilia, H.; Lerch, A.; Moore, S.M.; Tran, Q.; Perkins, T.A.; Ten Bosch, Q.A. Over 100 Years of Rift Valley Fever: A Patchwork of Data on Pathogen Spread and Spillover. Pathogens 2021, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Anyamba, A.; Linthicum, K.J.; Small, J.L.; Collins, K.M.; Tucker, C.J.; Pak, E.W.; Britch, S.C.; Eastman, J.R.; Pinzon, J.E.; Russell, K.L. Climate Teleconnections and Recent Patterns of Human and Animal Disease Outbreaks. PLoS Neglected Trop. Dis. 2012, 6, e1465. [Google Scholar] [CrossRef]
- LaBeaud, A.D.; Muchiri, E.M.; Ndzovu, M.; Mwanje, M.T.; Muiruri, S.; Peters, C.J.; King, C.H. Interepidemic Rift Valley Fever Virus Seropositivity, Northeastern Kenya. Emerg. Infect. Dis. 2008, 14, 1240–1246. [Google Scholar] [CrossRef]
- Tumusiime, D.; Isingoma, E.; Tashoroora, O.B.; Ndumu, D.B.; Bahati, M.; Nantima, N.; Mugizi, D.R.; Jost, C.; Bett, B. Mapping the Risk of Rift Valley Fever in Uganda Using National Seroprevalence Data from Cattle, Sheep and Goats. PLoS Neglected Trop. Dis. 2023, 17, e0010482. [Google Scholar] [CrossRef]
- Bird, B.H.; Khristova, M.L.; Rollin, P.E.; Ksiazek, T.G.; Nichol, S.T. Complete Genome Analysis of 33 Ecologically and Biologically Diverse Rift Valley Fever Virus Strains Reveals Widespread Virus Movement and Low Genetic Diversity Due to Recent Common Ancestry. J. Virol. 2007, 81, 2805–2816. [Google Scholar] [CrossRef]
- Wang, Z.; Pei, S.; Ye, R.; Chen, J.; Cheng, N.; Zhao, M.; Cao, W.; Jia, Z. Increasing Evolution, Prevalence, and Outbreaks for Rift Valley Fever Virus in the Process of Breaking Geographical Barriers. Sci. Total Environ. 2024, 917, 170302. [Google Scholar] [CrossRef]
- Munyua, P.M.; Murithi, R.M.; Ithondeka, P.; Hightower, A.; Thumbi, S.M.; Anyangu, S.A.; Kiplimo, J.; Bett, B.; Vrieling, A.; Breiman, R.F.; et al. Predictive Factors and Risk Mapping for Rift Valley Fever Epidemics in Kenya. PLoS ONE 2016, 11, e0144570. [Google Scholar] [CrossRef]
- Budasha, N.H.; Gonzalez, J.-P.; Sebhatu, T.T.; Arnold, E. Rift Valley Fever Seroprevalence and Abortion Frequency among Livestock of Kisoro District, South Western Uganda (2016): A Prerequisite for Zoonotic Infection. BMC Vet. Res. 2018, 14, 271. [Google Scholar] [CrossRef]
- Telford, C.; Nyakarahuka, L.; Waller, L.; Kitron, U.; Shoemaker, T. Geostatistical Modeling and Prediction of Rift Valley Fever Seroprevalence among Livestock in Uganda. Am. J. Trop. Med. Hyg. 2023, 108, 712–721. [Google Scholar] [CrossRef]
- Majiwa, H.; Bukachi, S.A.; Omia, D.; Fèvre, E.M. Knowledge, Perceptions, and Practices around Zoonotic Diseases among Actors in the Livestock Trade in the Lake Victoria Crescent Ecosystem in East Africa. Front. Public Health 2024, 11, 1199664. [Google Scholar] [CrossRef]
- O’Neill, L.; Gubbins, S.; Reynolds, C.; Limon, G.; Giorgakoudi, K. The Socioeconomic Impacts of Rift Valley Fever: A Rapid Review. PLoS Neglected Trop. Dis. 2024, 18, e0012347. [Google Scholar] [CrossRef] [PubMed]
- Gerken, K.N.; LaBeaud, A.D.; Mandi, H.; L’Azou Jackson, M.; Breugelmans, J.G.; King, C.H. Paving the Way for Human Vaccination against Rift Valley Fever Virus: A Systematic Literature Review of RVFV Epidemiology from 1999 to 2021. PLoS Negl. Trop. Dis. 2022, 16, e0009852. [Google Scholar] [CrossRef] [PubMed]
- Eskew, E.A.; Clancey, E.; Singh, D.; Situma, S.; Nyakarahuka, L.; Njenga, M.K.; Nuismer, S.L. Projecting Climate Change Impacts on Inter-Epidemic Risk of Rift Valley Fever across East Africa. medRxiv 2025. [Google Scholar] [CrossRef]
- Cecilia, H.; Vriens, R.; Schreur, P.J.W.; de Wit, M.M.; Métras, R.; Ezanno, P.; Bosch, Q.A. Ten Heterogeneity of Rift Valley Fever Virus Transmission Potential across Livestock Hosts, Quantified through a Model-Based Analysis of Host Viral Load and Vector Infection. PLoS Comput. Biol. 2022, 18, e1010314. [Google Scholar] [CrossRef] [PubMed]
- Odinoh, R.; Dawa, J.; Situma, S.; Nyakarahuka, L.; Lepore, L.; Vanlerberghe, V.; Nasimiyu, C.; Makiala, S.; Ifufa, C.; Mukadi, D.; et al. Have You Heard of Rift Valley Fever? Findings from a Multi-Country Study in East and Central Africa. medRxiv 2024. [Google Scholar] [CrossRef]
- de St Maurice, A.; Nyakarahuka, L.; Purpura, L.; Ervin, E.; Tumusiime, A.; Balinandi, S.; Kyondo, J.; Mulei, S.; Tusiime, P.; Manning, C.; et al. Rift Valley Fever: A Survey of Knowledge, Attitudes, and Practice of Slaughterhouse Workers and Community Members in Kabale District, Uganda. PLoS Negl. Trop. Dis. 2018, 12, e0006175. [Google Scholar] [CrossRef]
- Chiuya, T.; Fevre, E.M.; Junglen, S.; Borgemeister, C. Understanding Knowledge, Attitude and Perception of Rift Valley Fever in Baringo South, Kenya: A Cross-Sectional Study. PLoS Glob. Public Health 2023, 3, e0002195. [Google Scholar] [CrossRef]
| Demographic Characteristics | N = 61 (%) | Clinical Symptoms | N = 61 (%) |
|---|---|---|---|
| RVF Cases | 61 (100%) | Fever | 42 (69%) |
| Female | 5 (8.2%) | Unexplained bleeding | 25 (41%) |
| Male | 56 (91.8%) | Fatigue/general weakness | 37 (61%) |
| Status | Anorexia | 30 (49%) | |
| Alive | 42 (68.9%) | Abdominal pain | 23 (38%) |
| Dead | 19 (31.1%) | Headache | 34 (56%) |
| Region | Vomiting/nausea | 28 (46%) | |
| Central | 2 (3.3%) | Diarrhea | 11 (18%) |
| Northern | 1 (1.6%) | Muscle pain | 30 (49%) |
| Western | 58 (95.1%) | Joint pain | 34 (56%) |
| Surveillance Type | Chest pain | 18 (30%) | |
| Active | 21 (34.4%) | Conjunctivitis | 12 (20%) |
| Passive | 40 (65.6%) | Sore throat | 4 (6.6%) |
| Contact with Livestock | 53 (86.9%) | Difficulty breathing | 10 (16%) |
| Travel history | 10 (16.4%) | Difficulty swallowing | 5 (8.2%) |
| Cattle Corridor | 50 (81.7%) | Hiccups | 4 (6.6%) |
| Age, years | Cough | 7 (11%) | |
| 0–19 | 4 (6.6%) | Skin rash | 2 (3.3%) |
| 20–49 | 46 (75.4%) | ||
| 50> | 11 (18%) |
| Characteristic | Positive (N = 61) | Negative (N = 63) | p-Value 1 | RR (95% CI) |
|---|---|---|---|---|
| Gender | <0.001 | |||
| Female | 5 (8.2%) | 22 (34.9%) | Reference | |
| Male | 56 (91.8%) | 41 (65.1%) | 2.67 (1.29–5.55) | |
| Age Group | 0.2 | |||
| <25 | 11 (18.0%) | 12 (19.0%) | Reference | |
| 25–39 | 15 (24.6%) | 18 (28.6%) | 0.95 (0.54–1.68) | |
| 40–54 | 24 (39.3%) | 14 (22.2%) | 1.32 (0.81–2.16) | |
| 55+ | 11 (18.0%) | 19 (30.2%) | 0.77 (0.41–1.45) | |
| Farmer | 23 (37.7%) | 23 (36.5%) | 0.9 | 1.03 (0.71–1.48) |
| Butcher | 10 (16.4%) | 17 (27.0%) | 0.2 | 0.70 (0.42–1.19) |
| Healthcare worker | 1 (1.6%) | 1 (1.6%) | 0.9 | 1.02 (0.25–4.11) |
| Fever | 42 (68.9%) | 10 (15.9%) | <0.001 | 3.06 (2.04–4.60) |
| Unexplained bleeding from any site | 25 (41.0%) | 3 (4.8%) | <0.001 | 8.5 (2.7–26.8) |
| Vomiting/Nausea | 28 (45.9%) | 2 (3.2%) | <0.001 | 14.2 (3.5–57.7) |
| Bleeding of the gums | 2 (3.3%) | 0 (0%) | 0.2 | ∞ (0.92–∞) |
| Diarrhea | 11 (18.0%) | 1 (1.6%) | 0.002 | 11.4 (1.5–86.0) |
| Bleeding from injection site | 2 (3.3%) | 0 (0%) | 0.2 | ∞ (0.92–∞) |
| Intense fatigue/General weakness | 37 (60.7%) | 7 (11.1%) | <0.001 | 2.80 (1.96–4.01) |
| Abdominal pain | 23 (37.7%) | 13 (20.6%) | 0.036 | 1.48 (1.05–2.09) |
| Chest pain | 18 (29.5%) | 2 (3.2%) | <0.001 | 9.2 (2.2–37.8) |
| Muscle pain | 30 (49.2%) | 6 (9.5%) | <0.001 | 2.37 (1.72–3.25) |
| Joint pain | 34 (55.7%) | 10 (15.9%) | <0.001 | 2.29 (1.62–3.24) |
| Headache | 34 (55.7%) | 17 (27.0%) | 0.001 | 1.80 (1.26–2.58) |
| Cough | 7 (11.5%) | 5 (7.9%) | 0.5 | 1.21 (0.72–2.03) |
| Contact with Livestock | 53 (86.9%) | 53 (84.1%) | 0.7 | 1.13 (0.65–1.95) |
| Characteristic | Positive on IgG ELISA, N = 27 | Negative on IgG ELISA, N = 20 | p-Value |
|---|---|---|---|
| Breed | |||
| Cross | 13 (48%) | 11 (55%) | 0.5 |
| Exotic | 12 (44%) | 6 (30%) | |
| Local | 2 (7%) | 3 (15%) | |
| Species | |||
| Cattle | 25 (93%) | 17 (85%) | 0.6 |
| Sheep | 2 (7%) | 3 (15%) | |
| Sex | |||
| Female | 27 (100%) | 16 (80%) | 0.032 |
| Male | 0 (0%) | 4 (20%) | |
| Age | |||
| Adult | 26 (96%) | 14 (70%) | 0.027 |
| Young | 1 (4%) | 6 (30%) | |
| Abortions | 5 (19%) | 1 (5%) | 0.2 |
| PCR Results | |||
| Negative | 21 (78%) | 15 (75%) | 0.9 |
| Positive | 6 (22%) | 5 (25%) | |
| Associated with human cases | 17 (63%) | 10 (50%) | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyakarahuka, L.; Whitmer, S.; Mulei, S.; Mutesi, J.; Baluku, J.; Kyondo, J.; Whitesell, A.; Telford, C.; Tumusiime, A.; Torach, C.R.; et al. Rift Valley Fever Outbreak Investigation Associated with a Dairy Farm Abortion Storm, Mbarara District, Western Uganda, 2023. Viruses 2025, 17, 1015. https://doi.org/10.3390/v17071015
Nyakarahuka L, Whitmer S, Mulei S, Mutesi J, Baluku J, Kyondo J, Whitesell A, Telford C, Tumusiime A, Torach CR, et al. Rift Valley Fever Outbreak Investigation Associated with a Dairy Farm Abortion Storm, Mbarara District, Western Uganda, 2023. Viruses. 2025; 17(7):1015. https://doi.org/10.3390/v17071015
Chicago/Turabian StyleNyakarahuka, Luke, Shannon Whitmer, Sophia Mulei, Joanita Mutesi, Jimmy Baluku, Jackson Kyondo, Amy Whitesell, Carson Telford, Alex Tumusiime, Calvin Richie Torach, and et al. 2025. "Rift Valley Fever Outbreak Investigation Associated with a Dairy Farm Abortion Storm, Mbarara District, Western Uganda, 2023" Viruses 17, no. 7: 1015. https://doi.org/10.3390/v17071015
APA StyleNyakarahuka, L., Whitmer, S., Mulei, S., Mutesi, J., Baluku, J., Kyondo, J., Whitesell, A., Telford, C., Tumusiime, A., Torach, C. R., Namanya, D., Nambuya, M., Muhereza, D., Kabami, Z., Nankya, A., Muwanguzi, D., Mugabi, F., Wandera, N., Muhindo, R., ... Shoemaker, T. R. (2025). Rift Valley Fever Outbreak Investigation Associated with a Dairy Farm Abortion Storm, Mbarara District, Western Uganda, 2023. Viruses, 17(7), 1015. https://doi.org/10.3390/v17071015








