The Renin–Angiotensin System Modulates SARS-CoV-2 Entry via ACE2 Receptor
Abstract
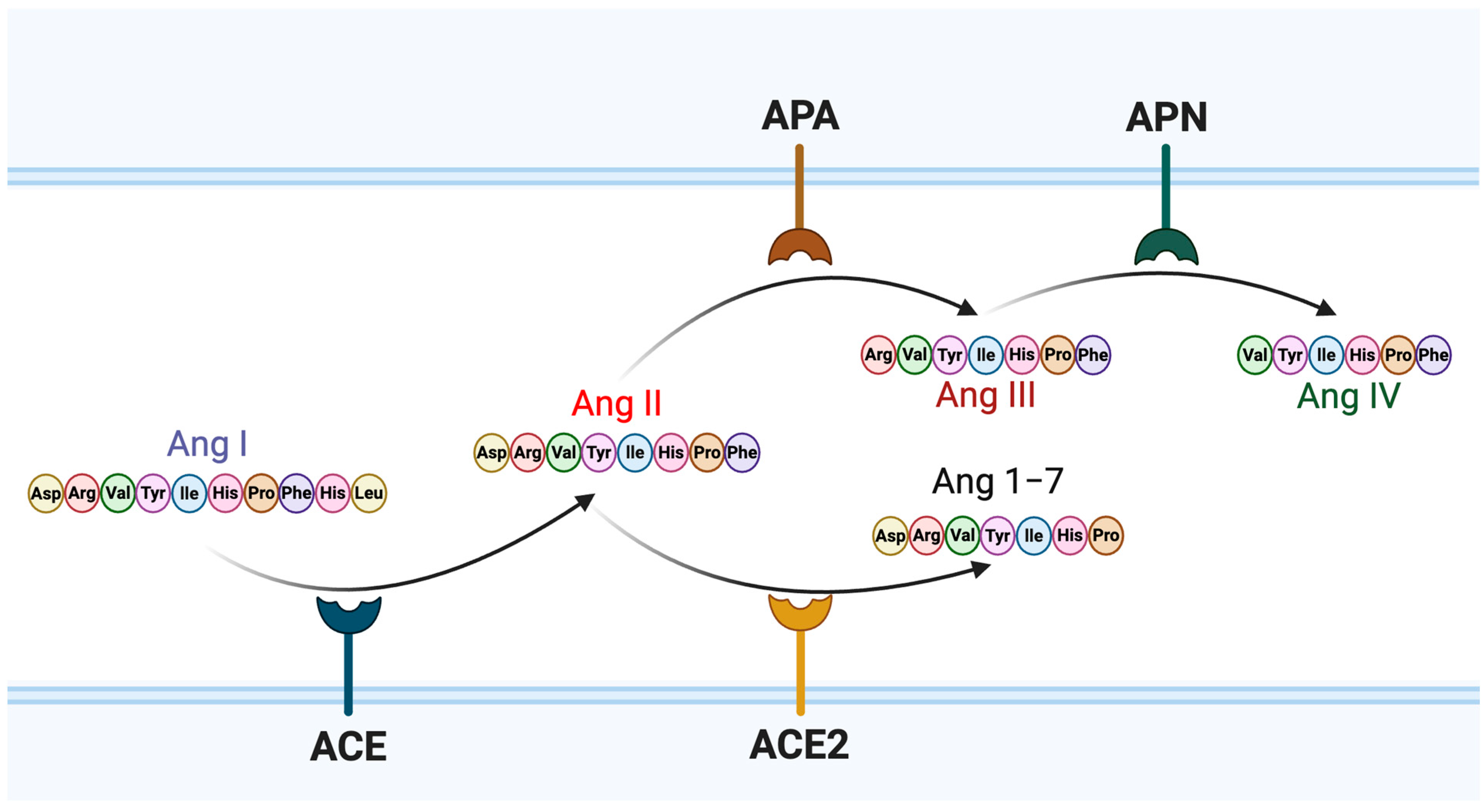
1. Introduction
2. Materials and Methods
2.1. In Silico Docking Simulation
2.2. Peptides
2.3. Plasmid DNAs
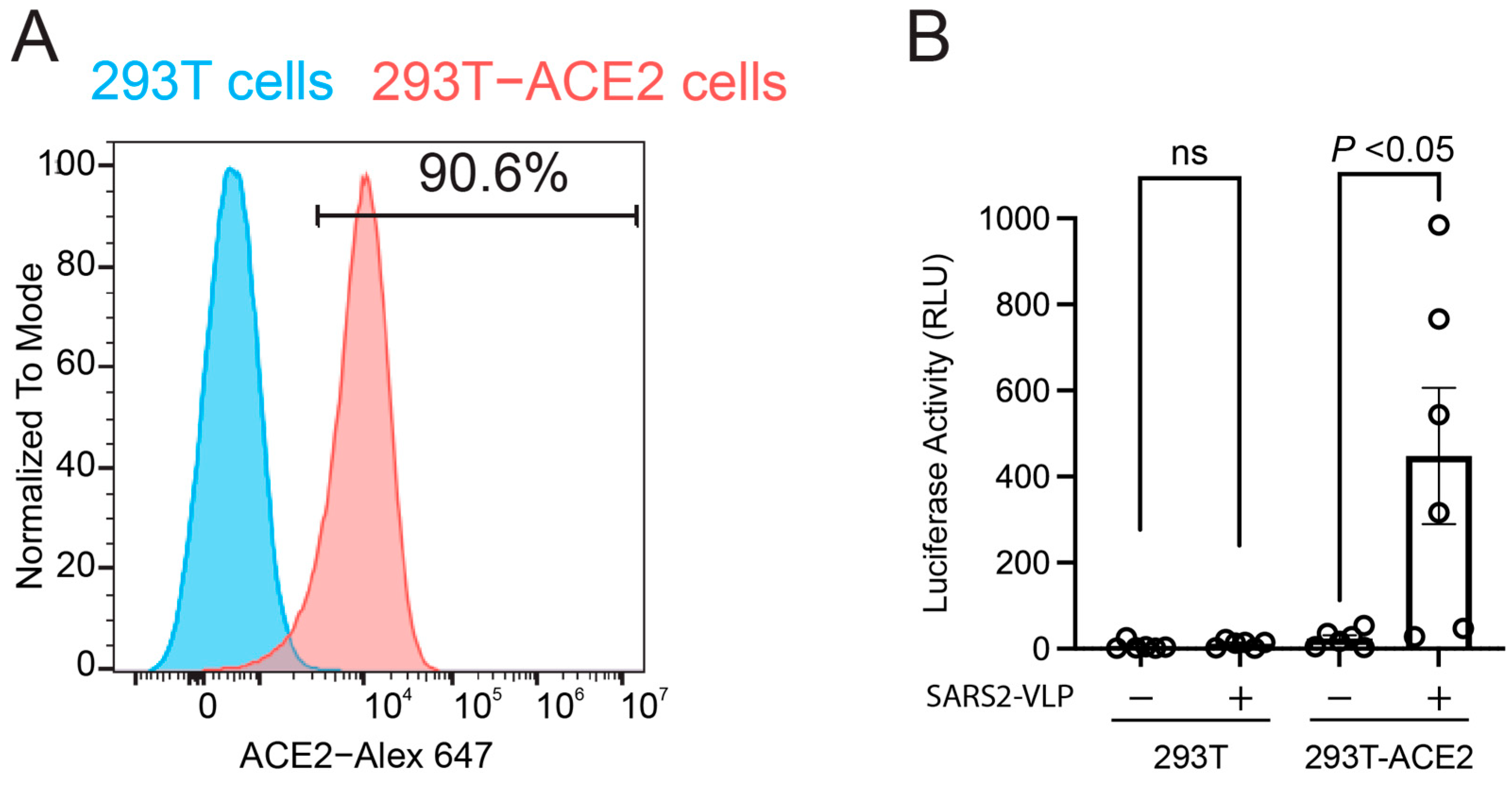
2.4. Cell Culture
2.5. Immunostaining
2.6. SARS-CoV-2 Viral-like Particle (VLP) Production
2.7. Single-Round Infection Assay
2.8. Statistical Analysis
3. Results
3.1. Molecular Docking Analysis of Angiotensin Peptides and Spike Protein Interactions
3.2. Impact of Angiotensin Peptides on SARS-CoV-2 Cell Entry Through ACE2 Receptor
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.J.; Dong, X.; Liu, G.H.; Gao, Y.D. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin. Rev. Allergy Immunol. 2023, 64, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Das Sarma, J. Murine-beta-coronavirus-induced neuropathogenesis sheds light on CNS pathobiology of SARS-CoV2. J. Neurovirol. 2021, 27, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yu, L.; Dong, J.; Liu, Y.; Zhang, L.; Liang, P.; Wang, L.; Chen, B.; Huang, L.; Song, C. Cellular poly(C) binding protein 2 interacts with porcine epidemic diarrhea virus papain-like protease 1 and supports viral replication. Vet. Microbiol. 2020, 247, 108793. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Sergi, C.; Kast, R.E.; Kanwar, B.A.; Bourbeau, J.; Oh, S.; Sohn, M.G.; Lee, C.J.; Coleman, M.D. Basic implications on three pathways associated with SARS-CoV-2. Biomed. J. 2025, 48, 100766. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, U.M.; Abokor, A.A.; Edwards, J.M.; Waigi, E.W.; Royfman, R.S.; Hasan, S.A.; Smedlund, K.B.; Hardy, A.M.G.; Chakravarti, R.; Koch, L.G. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol. Genom. 2021, 53, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Antony, P.; Vijayan, R. Role of SARS-CoV-2 and ACE2 variations in COVID-19. Biomed. J. 2021, 44, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Liu, Z.; Wang, X.; Liu, Q.; Xu, W.; Mao, Q.; Zhang, X.; Hao, A.; Xia, S.; Liu, Z.; et al. Early fusion intermediate of ACE2-using coronavirus spike acting as an antiviral target. Cell 2025, 188, 1297–1314.e24. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.; Gregor, A.; Cornbleet, M.A.; Allan, S.G.; McIntyre, M.A.; Grant, I.W.; Compton, G.K.; Leonard, R.C.; Smyth, J.F. cis-Platinum and vindesine in combination in the treatment of non-small cell lung cancer. Eur. J. Cancer Clin. Oncol. 1985, 21, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, T.; Zhou, Y. Mediators of SARS-CoV-2 entry are preferentially enriched in cardiomyocytes. Hereditas 2021, 158, 4. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, M.; Coperchini, F.; Ricci, G.; Denegri, M.; Croce, L.; Ngnitejeu, S.T.; Villani, L.; Magri, F.; Latrofa, F.; Chiovato, L. Detection of SARS-CoV-2 receptor ACE-2 mRNA in thyroid cells: A clue for COVID-19-related subacute thyroiditis. J. Endocrinol. Investig. 2021, 44, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Li, Z. Angiotensin-converting enzyme 2 (ACE2): SARS-CoV-2 receptor and RAS modulator. Acta Pharm. Sin. B 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef] [PubMed]
- Delaitre, C.; Boisbrun, M.; Lecat, S.; Dupuis, F. Targeting the Angiotensin II Type 1 Receptor in Cerebrovascular Diseases: Biased Signaling Raises New Hopes. Int. J. Mol. Sci. 2021, 22, 6738. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, J.; Ye, M.; Batlle, D. Plasma and Kidney Angiotensin Peptides: Importance of the Aminopeptidase A/Angiotensin III Axis. Am. J. Hypertens. 2015, 28, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Kemp, B.A.; Bell, J.F.; Rottkamp, D.M.; Howell, N.L.; Shao, W.; Navar, L.G.; Padia, S.H.; Carey, R.M. Intrarenal angiotensin III is the predominant agonist for proximal tubule angiotensin type 2 receptors. Hypertension 2012, 60, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Padia, S.H.; Kemp, B.A.; Howell, N.L.; Fournie-Zaluski, M.C.; Roques, B.P.; Carey, R.M. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-mediated natriuresis in rats. Hypertension 2008, 51, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Park, B.M.; Cha, S.A.; Lee, S.H.; Kim, S.H. Angiotensin IV protects cardiac reperfusion injury by inhibiting apoptosis and inflammation via AT4R in rats. Peptides 2016, 79, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Zulli, A.; Burrell, L.M.; Buxton, B.F.; Hare, D.L. ACE2 and AT4R are present in diseased human blood vessels. Eur. J. Histochem. 2008, 52, 39–44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gul, R.; Kim, U.H.; Alfadda, A.A. Renin-angiotensin system at the interface of COVID-19 infection. Eur. J. Pharmacol. 2021, 890, 173656. [Google Scholar] [CrossRef] [PubMed]
- Wigen, J.; Lofdahl, A.; Bjermer, L.; Elowsson-Rendin, L.; Westergren-Thorsson, G. Converging pathways in pulmonary fibrosis and COVID-19-The fibrotic link to disease severity. Respir. Med. X 2020, 2, 100023. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, A.; Spiller, L.; Wolke, C.; Lendeckel, U.; Weinert, S.; Hoffmann, J.; Bornfleth, P.; Kutschka, I.; Gardemann, A.; Isermann, B.; et al. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp. Biol. Med. 2017, 242, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.Y.; Zhang, Y.; Wu, Y.; Cannesson, M.; Cai, H. Therapeutic application of estrogen for COVID-19: Attenuation of SARS-CoV-2 spike protein and IL-6 stimulated, ACE2-dependent NOX2 activation, ROS production and MCP-1 upregulation in endothelial cells. Redox Biol. 2021, 46, 102099. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, Y.; Zhou, Z.; Liu, B.; Liu, Z.; Li, G. Relationship between Angiotensin II, Vascular Endothelial Growth Factor, and Arteriosclerosis Obliterans. Dis. Markers 2023, 2023, 1316821. [Google Scholar] [CrossRef] [PubMed]
- Vinh, A.; Widdop, R.E.; Chai, S.Y.; Gaspari, T.A. Angiotensin IV-evoked vasoprotection is conserved in advanced atheroma. Atherosclerosis 2008, 200, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Fatima, Z.; Sood, A.; Shukla, R.; Hanif, K. The Protective Effects of AT2R Agonist, CGP42112A, Against Angiotensin II-Induced Oxidative Stress and Inflammatory Response in Astrocytes: Role of AT2R/PP2A/NFkappaB/ROS Signaling. Neurotox. Res. 2021, 39, 1991–2006. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.M.D.; Du-Fay-de-Lavallaz, J.M.; Fugar, S.; Sarau, A.; Simmons, J.A.; Clark, B.; Sanghani, R.M.; Aggarwal, N.T.; Williams, K.A.; Doukky, R.; et al. Sex Differences in COVID-19 Hospitalization and Mortality. J. Women’s Health 2021, 30, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Chinn, J.; De Ferrante, M.; Kirby, K.A.; Hohmann, S.F.; Amin, A. Male gender is a predictor of higher mortality in hospitalized adults with COVID-19. PLoS ONE 2021, 16, e0254066. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, T.; Smilowitz, N.R.; Seda, B.; Xia, Y.; Hochman, J.; Berger, J.S. Sex Differences in Thrombosis and Mortality in Patients Hospitalized for COVID-19. Am. J. Cardiol. 2022, 170, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Gorenshtein, A.; Leibovitch, L.; Liba, T.; Stern, S.; Stern, Y. Gender Disparities in Neurological Symptoms of Long COVID: A Systematic Review and Meta-Analysis. Neuroepidemiology 2024, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.U.; Lyu, J.; Kim, K.D.; Chung, Y.C.; Yoon, G.Y.; Lee, S.; Hwang, I.; Shin, W.H.; Ko, J.; Lee, J.Y.; et al. SARS-CoV-2 Infection of Microglia Elicits Proinflammatory Activation and Apoptotic Cell Death. Microbiol. Spectr. 2022, 10, e01091-22. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.X.; Suzuki, Y.J. Angiotensin peptides enhance SARS-CoV-2 spike protein binding to its host cell receptors. bioRxiv 2024. bioRxiv:2024-12. [Google Scholar]
- Oliveira, K.X.; Bablu, F.E.; Gonzales, E.S.; Izumi, T.; Suzuki, Y.J. Naturally Occurring Angiotensin Peptides Enhance the SARS-CoV-2 Spike Protein Binding to Its Receptors. Int. J. Mol. Sci. 2025, 26, 6067. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhang, J.; Huang, W.; Zhang, Z.; Jia, X.; Wang, Z.; Shi, L.; Li, C.; Wolynes, P.G.; Zheng, S. DynamicBind: Predicting ligand-specific protein-ligand complex structure with a deep equivariant generative model. Nat. Commun. 2024, 15, 1071. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.M.; Taha, T.Y.; Tabata, T.; Chen, I.P.; Ciling, A.; Khalid, M.M.; Sreekumar, B.; Chen, P.Y.; Hayashi, J.M.; Soczek, K.M.; et al. Rapid assessment of SARS-CoV-2-evolved variants using virus-like particles. Science 2021, 374, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Maksimowski, N.A.; Scholey, J.W.; Williams, V.R.; Nephrotic Syndrome Study Network. Sex and kidney ACE2 expression in primary focal segmental glomerulosclerosis: A NEPTUNE study. PLoS ONE 2021, 16, e0252758. [Google Scholar] [CrossRef] [PubMed]
- Muthuraman, A.; Kaur, P. Renin-Angiotensin-Aldosterone System: A Current Drug Target for the Management of Neuropathic Pain. Curr. Drug Targets 2016, 17, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Scully, E.P.; Schumock, G.; Fu, M.; Massaccesi, G.; Muschelli, J.; Betz, J.; Klein, E.Y.; West, N.E.; Robinson, M.; Garibaldi, B.T.; et al. Sex and Gender Differences in Testing, Hospital Admission, Clinical Presentation, and Drivers of Severe Outcomes from COVID-19. Open Forum Infect. Dis. 2021, 8, ofab448. [Google Scholar] [CrossRef] [PubMed]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef] [PubMed]
- Sieurin, J.; Branden, G.; Magnusson, C.; Hergens, M.P.; Kosidou, K. A population-based cohort study of sex and risk of severe outcomes in COVID-19. Eur. J. Epidemiol. 2022, 37, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.W.; Krebs, L.T.; Stobb, J.W.; Harding, J.W. The angiotensin IV system: Functional implications. Front. Neuroendocr. 1995, 16, 23–52. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, J.; Moeller, I.; Jenkins, T.; Chai, S.Y.; Allen, A.M.; Ohishi, M.; Mendelsohn, F.A. Mapping tissue angiotensin-converting enzyme and angiotensin AT1, AT2 and AT4 receptors. J. Hypertens. 1998, 16 Pt 2, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.Y.; Bastias, M.A.; Clune, E.F.; Matsacos, D.J.; Mustafa, T.; Lee, J.H.; McDowall, S.G.; Paxinos, G.; Mendelsohn, F.A.; Albiston, A.L. Distribution of angiotensin IV binding sites (AT4 receptor) in the human forebrain, midbrain and pons as visualised by in vitro receptor autoradiography. J. Chem. Neuroanat. 2000, 20, 339–348. [Google Scholar] [CrossRef] [PubMed]
- de Gasparo, M.; Catt, K.J.; Inagami, T.; Wright, J.W.; Unger, T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 2000, 52, 415–472. [Google Scholar] [CrossRef] [PubMed]
- Gard, P.R. Cognitive-enhancing effects of angiotensin IV. BMC Neurosci. 2008, 9 (Suppl. 2), S15. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.W.; Harding, J.W. Contributions by the Brain Renin-Angiotensin System to Memory, Cognition, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 67, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, X.; Geng, D.; Mei, N.; Wu, P.Y.; Huang, C.C.; Jia, T.; Zhao, Y.; Wang, D.; Xiao, A.; et al. Cerebral Micro-Structural Changes in COVID-19 Patients—An MRI-based 3-month Follow-up Study. EClinicalMedicine 2020, 25, 100484. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wei, T.; Liu, X.; Liu, Y.; Song, W.; Que, X.; Xing, Y.; Wang, Z.; Tang, Y. Causal effects of COVID-19 on structural changes in specific brain regions: A Mendelian randomization study. BMC Med. 2023, 21, 261. [Google Scholar] [CrossRef] [PubMed]
- Martyniak, A.; Drozdz, D.; Tomasik, P.J. Classical and Alternative Pathways of the Renin-Angiotensin-Aldosterone System in Regulating Blood Pressure in Hypertension and Obese Adolescents. Biomedicines 2024, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, Y.; Mori, Y.; Tsutumi, Y.; Masaki, H.; Sakamoto, K.; Murasawa, S.; Maruyama, K.; Moriguchi, Y.; Tanaka, Y.; Iwasaka, T.; et al. Differential kinetics of circulating angiotensin IV and II after treatment with angiotensin II type 1 receptor antagonist and their plasma levels in patients with chronic renal failure. Clin. Nephrol. 1999, 51, 83–91. [Google Scholar] [PubMed]
- Gagliardi, S.; Hotchkin, T.; Hillmer, G.; Engelbride, M.; Diggs, A.; Tibebe, H.; Izumi, C.; Sullivan, C.; Cropp, C.; Lantz, O.; et al. Oxidative Stress in HIV-Associated Neurodegeneration: Mechanisms of Pathogenesis and Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 6724. [Google Scholar] [CrossRef]
- Bai, F.; Tomasoni, D.; Falcinella, C.; Barbanotti, D.; Castoldi, R.; Mule, G.; Augello, M.; Mondatore, D.; Allegrini, M.; Cona, A.; et al. Female gender is associated with long COVID syndrome: A prospective cohort study. Clin. Microbiol. Infect. 2022, 28, 611.e9–611.e16. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Schreinlechner, M.; Sappler, N.; Dolejsi, T.; Tilg, H.; Aulinger, B.A.; Weiss, G.; Bellmann-Weiler, R.; Adolf, C.; Wolf, D.; et al. Discontinuation versus continuation of renin-angiotensin-system inhibitors in COVID-19 (ACEI-COVID): A prospective, parallel group, randomised, controlled, open-label trial. Lancet Respir. Med. 2021, 9, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, M.; Winfred, S.B.; Meiyazhagan, G.; Venkatachalam, D.P. Mechanisms contributing to adverse outcomes of COVID-19 in obesity. Mol. Cell. Biochem. 2022, 477, 1155–1193. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.C.; Eldahshan, W.; Rutkowski, E. COVID-19-Related Stroke. Transl. Stroke Res. 2020, 11, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An evolving chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef] [PubMed]
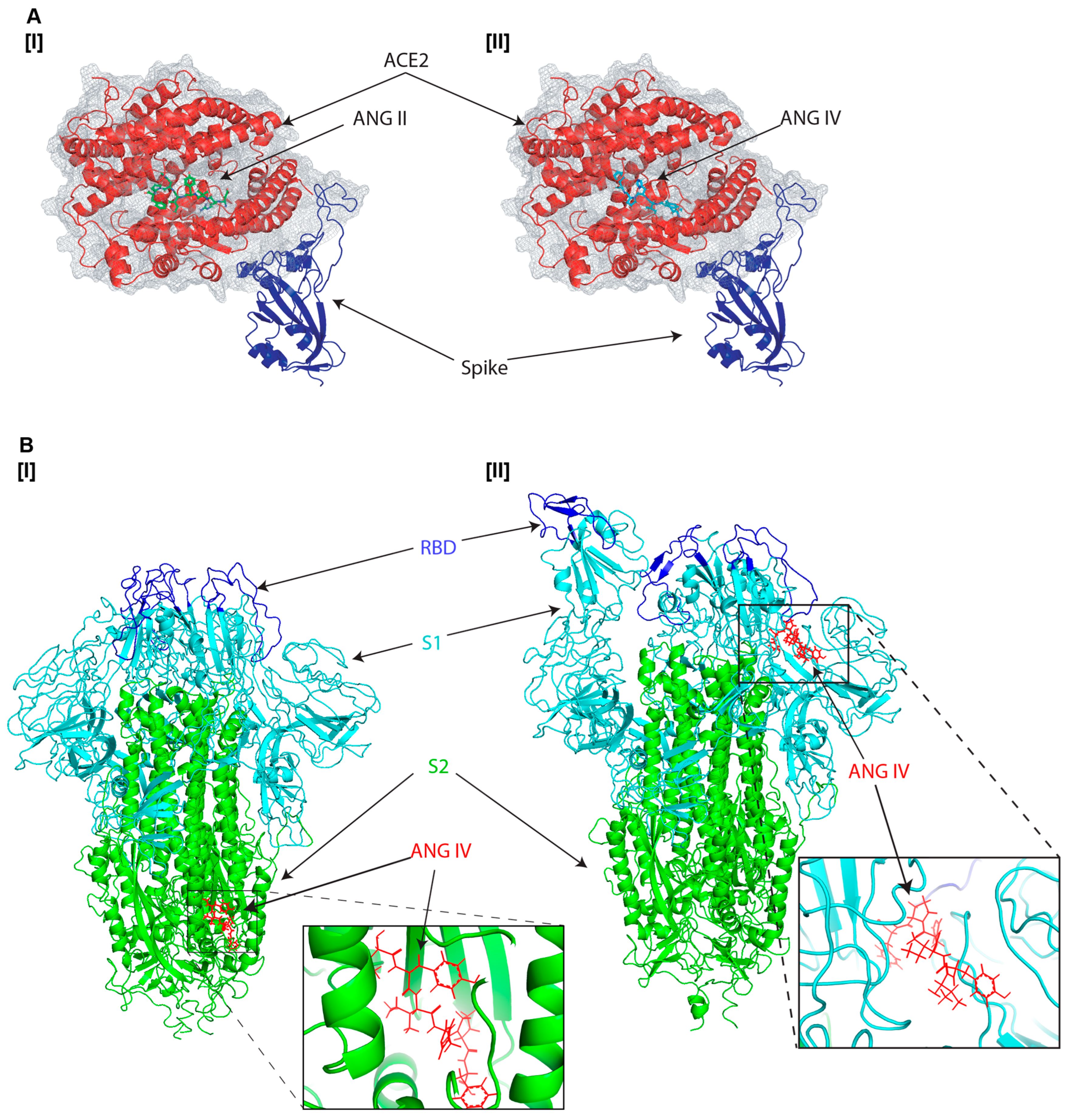

| Protein | Ligand | cLDDT | Binding Affinity |
|---|---|---|---|
| SARS-CoV-2 spike receptor-binding domain bound with ACE2 (PDB: 6M0J) | Angiotensin II | 0.5082692 | 5.285819 |
| SARS-CoV-2 spike receptor-binding domain bound with ACE2 (PDB: 6M0J) | Angiotensin IV | 0.6030191 | 4.9195795 |
| SARS-CoV-2 spike glycoprotein (closed state) (PDB: 6VXX) | Angiotensin II | 0.47392076 | 3.6205282 |
| SARS-CoV-2 spike ectodomain structure (open state) (PDB: 6VYB) | Angiotensin II | 0.34993827 | 6.679925 |
| Sample | Luc (RLU.) | Virus Exposure Time |
|---|---|---|
| No VLP | 123.46 | 24 h |
| 218.49 | 48 h | |
| SARS2-VLP | 2192.15 | 24 h |
| 1642.48 | 48 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagliardi, S.; Hotchkin, T.; Tibebe, H.; Hillmer, G.; Marquez, D.; Izumi, C.; Chang, J.; Diggs, A.; Ezaki, J.; Suzuki, Y.J.; et al. The Renin–Angiotensin System Modulates SARS-CoV-2 Entry via ACE2 Receptor. Viruses 2025, 17, 1014. https://doi.org/10.3390/v17071014
Gagliardi S, Hotchkin T, Tibebe H, Hillmer G, Marquez D, Izumi C, Chang J, Diggs A, Ezaki J, Suzuki YJ, et al. The Renin–Angiotensin System Modulates SARS-CoV-2 Entry via ACE2 Receptor. Viruses. 2025; 17(7):1014. https://doi.org/10.3390/v17071014
Chicago/Turabian StyleGagliardi, Sophia, Tristan Hotchkin, Hasset Tibebe, Grace Hillmer, Dacia Marquez, Coco Izumi, Jason Chang, Alexander Diggs, Jiro Ezaki, Yuichiro J. Suzuki, and et al. 2025. "The Renin–Angiotensin System Modulates SARS-CoV-2 Entry via ACE2 Receptor" Viruses 17, no. 7: 1014. https://doi.org/10.3390/v17071014
APA StyleGagliardi, S., Hotchkin, T., Tibebe, H., Hillmer, G., Marquez, D., Izumi, C., Chang, J., Diggs, A., Ezaki, J., Suzuki, Y. J., & Izumi, T. (2025). The Renin–Angiotensin System Modulates SARS-CoV-2 Entry via ACE2 Receptor. Viruses, 17(7), 1014. https://doi.org/10.3390/v17071014







