1. Introduction
Enteroviruses (EVs) are responsible for a diverse array of human diseases, including myocarditis, pericarditis, exanthems and enanthems, conjunctivitis, and meningitis; the majority of these conditions are mild and self-limiting. Nevertheless, certain enterovirus strains possess the capacity to induce severe and potentially fatal illnesses [
1]. EVs encompass enterovirus A (EV-A), B (EV-B), C (EV-C), and D (EV-D), with Echovirus type 18 (E18) serving as a prominent serotype within the EV-B category [
2].
Echovirus 18 (E18) was first identified in 1955 [
3], and, in recent years, there has been a multinational epidemic of this virus worldwide. Countries and regions such as the United States, Japan, India, and Germany have reported outbreaks of aseptic meningitis caused by E18, with most patients exhibiting symptoms consistent with meningitis [
4,
5,
6,
7]. In 2006, a total of 80 cases of E18 infection in young children were documented in southern Taiwan, with a case fatality rate of 4% among those diagnosed with meningitis [
8]. In 2007, a case of fatal encephalitis characterized by white matter lesions in infants, attributed to Echovirus 18, was reported in France. This observation underscored the potential role of enteroviral central nervous system (CNS) infections as a contributing factor to leukoencephalitis in infants [
9]. In 2015, an outbreak involving 61 pediatric cases of E18 infection presenting with aseptic encephalitis/meningitis was documented in Hebei Province, China. The primary clinical manifestations included headache, vomiting, fever, lethargy, and nausea. All patients recovered except for one individual who developed secondary epilepsy [
10]. Consequently, E18 has been identified as a significant etiological agent of aseptic meningitis among young children worldwide.
The advancement of vaccines and innovative therapeutics for enterovirus (EV) infections primarily focuses on the principal pathogens associated with hand, foot, and mouth disease (HFMD), notably EV71 and CVA16. Conventional vaccines, including the inactivated EV71 vaccine, represent the most thoroughly researched and developed options for HFMD prevention. Furthermore, recombinant HFMD vaccines utilizing recombinant DNA technology have been introduced; however, these are predominantly in the early or late phases of preclinical development [
11]. Animal models are essential for elucidating the mechanisms of enterovirus infections and for evaluating the efficacy of vaccines. For example, Elizabeth [
12] employed an interferon-deficient murine model to investigate the effectiveness of a trivalent vaccine composed of inactivated EV-A71, CVA16, and CVA6 during both active and passive immunity assessments.
The transmission of the majority of enteroviruses is believed to occur exclusively from human to human, indicating that humans serve as the primary natural hosts for these viruses. Nevertheless, under controlled experimental conditions, numerous enteroviruses have demonstrated the ability to infect mice, cotton rats, and various non-human primates. Many of these viral infection models utilize immunocompromised mice, such as neonatal or suckling mice and IFNAR knockout mice [
13,
14]. For instance, the establishment of a poliovirus oral infection system utilizing human poliovirus receptors enables the generation of transgenic mice that lack alpha/beta interferon receptors [
15]. Additionally, human SCARB2 transgenic mice serve as an infectious animal model for enterovirus 71 (EV71) [
16].
Currently, a multitude of clinical and epidemiological studies are concentrated on E18; however, there exists a significant gap in research regarding its pathology and the mechanisms that drive E18-induced meningeal inflammation. Progress in the development of novel drugs and vaccines targeting E18 has remained stagnant, making it imperative to establish an appropriate animal model for further investigation. Previously, Li J et al. simulated Echovirus 11 (E11) infection using 2-day-old IFNAR type I knockout (IFNAR -/-) mice, which exhibit susceptibility to enteroviruses. They identified symptoms that align with those observed clinically in human cases [
17]. A novel infection model employing 2-day-old IFNAR1 knockout mice has been developed to examine the pathogenicity of Echovirus 30 (E30) [
18].
Building upon this foundational work, our study involved the infection of Balb/c, C57BL/6J, and IFNAR1-KO mice through various methodologies to compare clinical manifestations, organ viral loads, and histopathological alterations among the infected subjects. Ultimately, we established an E18 infection animal model utilizing 2-day-old IFNAR1-KO mice exhibiting immunocompromised functions. This research aims to elucidate the mechanisms underlying E18 infection while facilitating future vaccine development initiatives alongside novel therapeutic strategies.
3. Discussion
Animal testing has played a pivotal role in the advancement of vaccines, antibiotics, and our fundamental comprehension of human disease mechanisms. Furthermore, animal models are essential for evaluating the efficacy and safety of a vaccine [
19]. Enterovirus 18 (E18) has emerged as one of the most significant pathogenic agents linked to aseptic meningitis in children in recent years. Currently, there are no specific therapeutics or vaccines available for E18 infection, nor has a suitable animal model for studying E18 virus infection been established.
To further elucidate the pathogenic mechanisms of E18 and its replication across diverse tissues and organs, it is imperative to investigate suitable animal models. In this study, several commonly used experimental mouse strains were initially identified for preliminary testing.
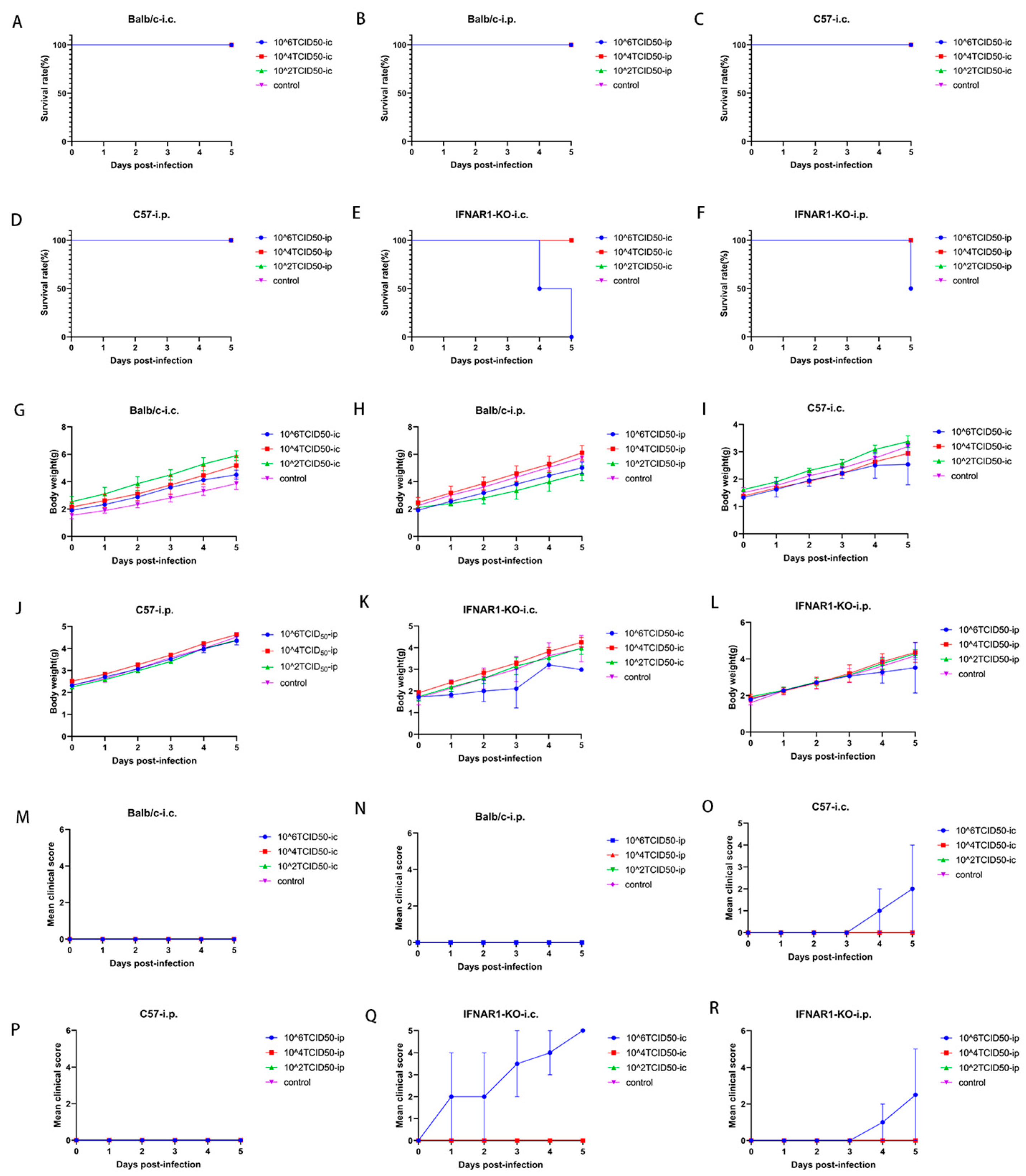
Balb/c neonatal mice were employed as experimental subjects and demonstrated pronounced pathological responses during enterovirus EV71 infection, characterized by skeletal muscle injury, weight loss, and a diminished survival rate [
20,
21]. Preliminary findings regarding E18 infection suggest that Balb/c mice exhibit resistance to the E18 virus, demonstrating no clinical alterations even after exposure to the highest viral concentrations. Typically, animal models utilized for studying Echovirus involve immunodeficient human transgenic mice that express the human homolog of the receptor while lacking expression of the interferon-alpha/beta receptor (IFNAR). For example, a mouse model for Echovirus type 1 (EV1) was established using transgenic mice expressing human integrin very late antigen 2 (VLA-2) [
22]. Furthermore, an in vivo model for Echovirus type 11 (E11) was developed using hFcRn-IFNAR mice, which are deficient in type I interferon (IFN) signaling and express the neonatal Fc receptor (hFcRn) [
23].
A further comparative analysis of C57 and IFNAR1-KO mice revealed that the infection symptoms, survival rates, and weight fluctuations in the animal models subjected to both injection methods for IFNAR1-KO mice were significantly more favorable than those observed in C57 mice. This finding underscores the critical role of type I interferon in the in vivo infection process of E18 virus. The absence of the type I interferon receptor markedly heightened the incidence of pathological manifestations following infection in these mice. Moreover, the intracranial administration of IFNAR1-KO exhibited superior outcomes compared to intraperitoneal injection, indicating that the blood–brain barrier may modulate viral infection and replication.
In C57 mice, hind limb paralysis, weight loss, and even mortality were observed only when the intracranial injection of the highest concentration gradient E18 was administered; under these conditions, viral titers were detectable in the tissue homogenate. Although no positive indicators of E11 infection have been identified in any tissues of wild-type (WT) C57BL/6J mice, this finding has also been corroborated in animal models infected with E30 [
17,
18]. In the current study, a subset of C57 mice exhibited hind limb paralysis following the intracranial administration of E18, with E18 virus identified in various tissue sites. This finding suggests that there are variations in the susceptibility of C57 mice to different serotypes of Echovirus strains.
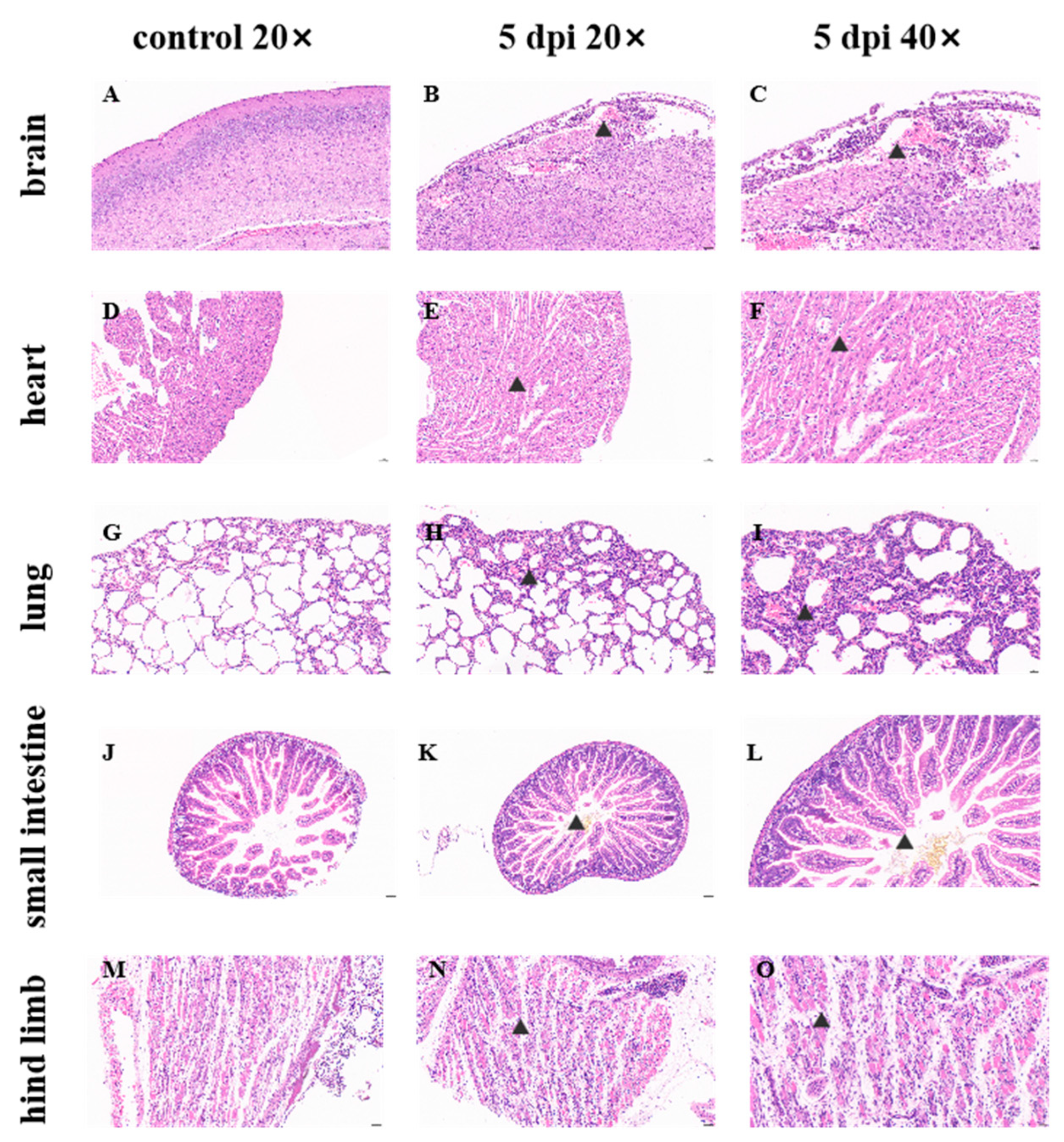
E18 was detected in all tissues and organs of both IFNAR1-KO and C57 mice, accompanied by corresponding pathological manifestations, indicating the extensive replication of the E18 virus in these murine models. No significant difference in viral load was observed between intracranial and intraperitoneal injections in brain tissue for both IFNAR1-KO and C57 mice, which may be attributed to the virus’s replication and transmission from the injection site to other tissues. The variation in E18 virus replication within the hearts of different mouse groups infected with E18 could be related to both the mouse strain type and injection method employed. Notably, intracranial injection yielded superior results for IFNAR1-KO mice compared to intraperitoneal injection; furthermore, when utilizing intracranial injection, IFNAR1-KO mice exhibited better outcomes than their C57 counterparts. There were no significant differences noted regarding E18 virus replication in lung tissue across various strains of mice or differing modes of injection. A comparative analysis of viral loads in the small intestine revealed that intracranial injections resulted in significantly higher viral loads for IFNAR1-KO mice relative to those receiving intraperitoneal injections. Additionally, the assessment of viral load within hind limb muscle indicated that C57 mice demonstrated a significantly greater viral load than IFNAR1-KO mice under conditions involving intraperitoneal administration.
Based on the aforementioned results, it can be concluded that variations in mouse strains and injection methodologies result in differing replication effects of E18 across various tissues. Notably, the viral load detected in numerous tissues, such as the heart and small intestine of IFNAR1-KO mice, exhibited significant discrepancies compared to other groups. While a high level of E18 virus was observed in the hind limb muscle following intraperitoneal injection in C57 mice, no individual signs of hind limb paralysis were noted; thus, the underlying reasons warrant further investigation. There was no statistically significant difference in viral load within muscle tissue between C57 mice and IFNAR1-KO mice exhibiting similar signs of hind limb paralysis. Although neurological symptoms were observed in mice, the comparable viral loads in brain tissues across groups suggest that these manifestations may involve indirect mechanisms (e.g., systemic inflammation), warranting further mechanistic investigation. Integrating findings from pathological sections across diverse tissue sites led to the conclusion that the impact of E18 virus on murine hind limb muscle is more pronounced than that on brain tissue, aligning with observations from infections caused by other Echoviruses such as E11 and E30 viruses in IFNAR-KO mice.
The clinical manifestations observed were in alignment with the viral load assessments and pathological analyses of brain tissue in the mouse model utilized in this study. Through a comprehensive evaluation of clinical signs, histopathological sections, and viral load quantifications across various tissues and organs, we established an E18 animal infection model employing 2-day-old IFNAR1-KO mice that were intracranially injected with 106 TCID50 of E18. This study elucidates the pathological characteristics of E18 for the first time within a murine model, thereby offering valuable insights and clues for future investigations into the mechanisms underlying pathological injury as well as the protective effects conferred by antibodies.
The differential susceptibility observed between C57BL/6J and IFNAR1-KO mice, particularly the strain-specific variations in clinical signs and viral replication, suggests that intrinsic differences in viral pathogenicity may drive these phenotypes. This is consistent with established mechanisms in related Echoviruses. Pathogenicity varies significantly between isolates of the same serotype. While the prototype E9 strain (Hill) isolated from a healthy child is non-pathogenic in newborn mice, a clinical isolate (E9/Barty) obtained from the cerebrospinal fluid (CSF) of a child with aseptic meningitis exhibits high virulence in newborn Swiss albino mice [
24]. Similarly, a clinical E30 isolate (A538) demonstrated lethality and susceptibility across multiple immunocompetent newborn mouse strains (ICR, BALB/c, KM, C57BL/6J) [
25], whereas another E30 clinical isolate (WZ16) showed no detectable viral replication in newborn C57BL/6Jmice [
18].
Specific genomic regions correlate with murine pathogenicity: comparative sequence analysis of the non-pathogenic E9/Hill strain and the virulent E9/Barty isolate identified a critical 310-aa segment including the RGD motif associated with murine virulence. Functional studies confirmed that mutating the RGD motif to RGE abolished infectivity in GMK cells. Furthermore, synthetic RGD peptides specifically inhibited the binding of the virulent E9/Barty strain but not the non-pathogenic E9/Hill strain, to GMK cells, establishing the RGD motif as a key determinant of E9 pathogenicity in this mode [
26].
Collectively, these findings strongly suggest that variability in pathogenicity among isolates of the same Echovirus serotype, including the observed differences across mouse strains, may be linked to discrete genomic variations. Consequently, to elucidate the mechanistic basis for the differential pathogenicity observed with our E18 isolate in various mouse strains, a critical next step involves comparative genomic sequence analysis. Specifically, sequencing the pathogenic E18 isolate used in this study alongside non-pathogenic E18 reference or clinical strains would be essential to identify potential virulence-associated mutations, potentially within functionally critical regions analogous to the RGD motif identified in E9.
This study has several limitations. First, while we observed significant differences between experimental and control groups at the highest gradient level, the current dose–response design did not demonstrate subsequent enhancement in virus culture titers. Second, the small sample size, though sufficient for preliminary findings, warrants cautious interpretation; future validation will employ larger cohorts (
n ≥ 5 per group) to ensure robustness. Additionally, the histopathological evaluation, while informative, lacked a standardized scoring system. Subsequent studies will adopt established criteria to improve objectivity and reproducibility. The mouse model selection also presents constraints: our study focused on three common strains for comparative analysis but did not include hFcRn-IFNAR-deficient mice, which have been shown to exhibit enhanced susceptibility to E11 infection [
23]. Finally, the pathogenesis underlying the positive clinical signs in C57BL/6J and IFNAR1-KO mice remains unresolved and merits targeted investigation.
Our identification of an E18 animal infection model utilizing IFNAR1-KO neonatal mice is consistent with findings from previous studies on E11 and E30 infections. This indicates that our model can facilitate horizontal comparisons across various types of Echovirus infections. The current model primarily simulates early systemic infection, while the mechanisms underlying human meningitis require further validation. Our future research will also focus on the molecular determinants of strain-specific pathogenesis in E18. It is crucial to directly integrate the role of the host inflammatory pathway in both C57 and IFNAR1-KO mice into our experimental design. Additionally, we intend to employ this model for drug efficacy assessments as well as vaccine evaluation research related to E18.
4. Method
4.1. Ethical Statement
Animal models were established utilizing specific pathogen-free (SPF) Balb/c, C57BL/6J, and IFNAR1-KO mice. All animal experiments were approved by the Laboratory Animal Ethics Committee of Southern Medical University (Approval No. D202310-1) and were conducted in accordance with relevant guidelines and regulations.
4.2. Mice, Cells, and Viruses
SPF Balb/c and C57BL/6J pregnant mice were procured from the Guangdong Experimental Animal Center. The immunocompromised IFNAR1 knockout (KO) mice used in this study were generated on a C57BL/6J genetic background (Catalog Number: T005534,GemPharmatech Co., Ltd., Nanjing, China). The C57BL/6J background was matched with wild-type control group (C57 mice) to minimize confounding factors in comparative analyses [
27]. This study utilizes CRISPR/Cas9 technology to edit the Ifnar1 gene, specifically targeting exons 2-6 of the IFNAR1-201 transcript (ENSMUST00000023689.10) as the knockout region, which encompasses a coding sequence of 718 base pairs. A PCR primer set was meticulously designed for validating gene expression in both wild-type and IFNAR1 knockout mice (F1: TACCCACCTGCCAAGGATTGA; R1: CAACTGAGCCATCTCTCCAGCAC; F2: CGTGGAGACTGAGCTGAGATGAAG; R2: CACTGTGTGTGTGCTGGAAATGACAG). All mice were housed in a life sciences laboratory animal facility within individually ventilated cages, lined with clean sawdust and supplemented with cotton for nesting purposes, thereby facilitating mating and subsequent pup production for further animal testing.
The E18 strain was isolated from a 2019 clinical case of E18 meningitis in an immunocompetent adult patient in Shenzhen. The virus used in experiments was at passage 3 (P3) after clinical isolation [
28]. Rhabdomyosarcoma cells (RD; ATCC) were cultured in DMEM medium (Pocell), supplemented with 10% fetal bovine serum (Procell) and 1% penicillin/streptomycin, at a temperature of 37 °C in a humidified atmosphere containing 5% CO
2. Upon achieving a cell density of 80–90%, the complete culture medium was replaced with one containing only 2% fetal bovine serum prior to viral infection, after which samples were collected following the observation of cytopathic effects (CPE). After undergoing three freeze–thaw cycles, all viruses present in the supernatant were filtered through a 0.22 μm filter and subsequently stored at −80 °C.
4.3. Animal Infection Experiments
Balb/c, C57BL/6J (C57), and IFNAR1-KO mice aged two days postnatal were subjected to infection with E18 using a concentration gradient of either 102, 104, or 106 TCID50 (50% tissue culture infectious dose). A total volume of 50 μL of the E18 suspension was administered via either intracranial or intraperitoneal routes. Control groups received an equivalent volume of uninfected medium while being housed separately from the infected subjects.
Weight trends, survival rates, and clinical disease scores were meticulously recorded on a daily basis until day 5 post-inoculation. The clinical disease grading system was delineated as follows: 0—healthy; 1—lethargy or inactivity; 2—emaciation or weakness in the hind limbs; 3—paralysis of one hind limb; 4—paralysis of both hind limbs; 5—dying or deceased.
On the fifth day following viral infection, anesthetized mice underwent aseptic extraction of their brains, hearts, lungs, intestines, and skeletal muscles from the contralateral hind limbs. Half of the harvested tissues were homogenized and stored at −80 °C for subsequent analyses, while the remaining half was fixed in a 4% paraformaldehyde solution for tissue preservation.
4.4. Viral Load Measurement
Tissues from the brain, hind limb muscles, heart, lung, and small intestine were collected at 5 days post-infection (5 dpi). The samples were rinsed with sterile PBS to remove excess blood, after which 500 μL of PBS containing 1% penicillin and streptomycin was added to each tissue sample. The supernatant was then homogenized using a frozen tissue grinder and subjected to three freeze–thaw cycles. Following centrifugation, the supernatant was carefully removed for viral load determination via qPCR probe method.
Viral RNA was extracted from 50 μL of the grinding supernatant, followed by quantitative analysis via reverse transcription quantitative PCR (RT-qPCR). A standard plasmid containing a 5′ untranslated region (UTR) sequence in E18 was synthesized, along with the requisite primers and probes for qPCR as detailed below (
Table 1).
4.5. Histopathological Analysis of E18 Infected Mice
Brain tissue, hind limb muscle, heart, lung, and small intestine from E18-infected mice were fixed in 4% paraformaldehyde for a duration of 24 h. The specimens were subsequently trimmed to achieve a standardized cross-section, conventionally processed, embedded in paraffin wax, and then sliced into sections with a thickness of 4 μm. These sections were mounted on slides treated with 3-aminopropyl triethoxysilane and stained using hematoxylin and eosin (HE). A microscopic examination was performed to elucidate the detailed histological characteristics of the tissue sections at various magnifications.
4.6. Statistical Analysis
Data were analyzed utilizing GraphPad Prism software (version 9.00; GraphPad, La Jolla, CA, USA) through unpaired two-tailed t-tests. Comparisons between the two groups were assessed using independent-sample t-tests. The results are presented as means ± standard deviations (SD). A p value of less than 0.05 was deemed to indicate a statistically significant difference between values.