The Effect on Mortality of Bacterial Co-Infections on Critically Ill Patients with Community-Acquired COVID-19 and Influenza Pneumonia: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
- PubMed: (ARDS OR “Acute Respiratory Distress Syndrome” OR “Respiratory failure” OR “Severe Pneumonia” OR SARS OR “Pneumonia” [MESH] “Severe acute respiratory syndrome” OR “Respiratory Distress Syndrome” [Mesh] OR “adult hyaline membrane disease” OR “postperfusion lung” OR “pump lung” OR “shock lung” OR “adult respiratory insufficiency syndrome” OR “SARS” OR MERS OR “middle east respiratory syndrome” OR Virus OR Viral OR “Virus Diseases” [MeSH]) AND (“Intensive Care Unit” OR ICU OR “Critical Care” OR “Intensive Care Units” [MeSH]) AND (Co-infection OR “Bacterial Co-Infection” OR “Co infection” OR “coinfection” OR superinfection OR “Coinfection” [MeSH]) AND (Mortality OR Death OR “Mortality” [MeSH])
- Scopus: (TITLE-ABS-KEY (ards OR “acute respiratory distress syndrome” OR “respiratory failure” OR “severe pneumonia” OR “severe acute respiratory syndrome” OR sars OR “respiratory distress syndrome” OR “pneumonia” OR “adult hyaline membrane disease” OR “postperfusion lung” OR “pump lung” OR “shock lung” OR “adult respiratory insufficiency syndrome” OR mers OR “middle east respiratory syndrome” OR virus OR viral OR “virus diseases”) OR AUTHKEY (ards OR “acute respiratory distress syndrome” OR “respiratory failure” OR “severe pneumonia” OR “severe acute respiratory syndrome” OR sars OR “respiratory distress syndrome” OR “pneumonia” OR “adult hyaline membrane disease” OR “postperfusion lung” OR “pump lung” OR “shock lung” OR “adult respiratory insufficiency syndrome” OR mers OR “middle east respiratory syndrome” OR virus OR viral OR “virus diseases”)) AND (TITLE-ABS-KEY (“intensive care unit” OR icu OR “critical care” OR “intensive care units”) OR AUTHKEY (“intensive care unit” OR icu OR “critical care” OR “intensive care units”)) AND (TITLE-ABS-KEY (“co-infection” OR “bacterial co-infection” OR “co infection” OR coinfection OR superinfection) OR AUTHKEY (“co-infection” OR “bacterial co-infection” OR “co infection” OR coinfection OR superinfection)) AND (TITLE-ABS-KEY (mortality OR death OR fatality) OR AUTHKEY (mortality OR death OR fatality))
- Web of Science: TS = (ARDS OR “acute respiratory distress syndrome” OR “respiratory failure” OR “severe pneumonia” OR “severe acute respiratory syndrome” OR SARS OR “respiratory distress syndrome” OR “pneumonia” OR “adult hyaline membrane disease” OR “postperfusion lung” OR “pump lung” OR “shock lung” OR “adult respiratory insufficiency syndrome” OR MERS OR “middle east respiratory syndrome” OR virus OR viral OR “virus diseases”) AND TS = (“intensive care unit” OR ICU OR “critical care” OR “intensive care units”) AND TS = (“co-infection” OR “bacterial co-infection” OR “co infection” OR coinfection OR superinfection) AND TS = (mortality OR death OR fatality)
- Cochrane: #1 ARDS: ti,ab,kw, #2 “acute respiratory distress syndrome”: ti,ab,kw, #3 “respiratory failure”: ti,ab,kw, #4 “severe pneumonia”: ti,ab,kw, #5 SARS: ti,ab,kw, #6 “severe acute respiratory syndrome”: ti,ab,kw, #7 “respiratory distress syndrome”: ti,ab,kw, #8 pneumonia: ti,ab,kw, #9 “adult hyaline membrane disease”: ti,ab,kw, #10 “postperfusion lung”: ti,ab,kw, #11 “pump lung”: ti,ab,kw, #12 “shock lung”: ti,ab,kw, #13 “adult respiratory insufficiency syndrome”: ti,ab,kw, #14 MERS: ti,ab,kw, #15 “middle east respiratory syndrome”: ti,ab,kw, #16 virus: ti,ab,kw, #17 viral: ti,ab,kw, #18 “virus diseases”: ti,ab,kw, #19 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18), #20 “intensive care unit”: ti,ab,kw, #21 ICU: ti,ab,kw, #22 “critical care”: ti,ab,kw, #23 “intensive care units”: ti,ab,kw, #24 (#20 OR #21 OR #22 OR #23), #25 “co-infection”: ti,ab,kw, #26 “bacterial co-infection”: ti,ab,kw, #27 “co infection”: ti,ab,kw, #28 coinfection:ti,ab,kw, #29 superinfection: ti,ab,kw, #30 (#25 OR #26 OR #27 OR #28 OR #29), #31 mortality: ti,ab,kw, #32 death:ti,ab,kw, #33 fatality: ti,ab,kw, #34 (#31 OR #32 OR #33), #35 (#19 AND #24 AND #30 AND #34).
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias
3.4. Results of Individual Studies
4. Discussion
4.1. Limitations
4.2. Implications
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrer, M.; Travierso, C.; Cilloniz, C.; Gabarrus, A.; Ranzani, O.T.; Polverino, E.; Liapikou, A.; Blasi, F.; Torres, A. Severe Community-Acquired Pneumonia: Characteristics and Prognostic Factors in Ventilated and Non-Ventilated Patients. PLoS ONE 2018, 13, e0191721. [Google Scholar] [CrossRef] [PubMed]
- Niederman, M.S.; Torres, A. Severe Community-Acquired Pneumonia. Eur. Respir. Rev. 2022, 31, 220123. [Google Scholar] [CrossRef] [PubMed]
- Martín-Loeches, I.; Sanchez-Corral, A.; Diaz, E.; Granada, R.M.; Zaragoza, R.; Villavicencio, C.; Albaya, A.; Cerdá, E.; Catalán, R.M.; Luque, P.; et al. Community-Acquired Respiratory Coinfection in Critically Ill Patients With Pandemic 2009 Influenza A(H1N1) Virus. Chest 2011, 139, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W.; Rubinson, L.; Uyeki, T.M.; Vaughn, F.L.; John, B.B.; Miller, R.R.; Higgs, E.; Randolph, A.G.; Smoot, B.E.; Thompson, B.T. Critical Illness from 2009 Pandemic Influenza A Virus and Bacterial Coinfection in the United States. Crit Care Med. 2012, 40, 1487–1498. [Google Scholar] [CrossRef]
- Santos, A.P.; Gonçalves, L.C.; Oliveira, A.C.C.; Queiroz, P.H.P.; Ito, C.R.M.; Santos, M.O.; Carneiro, L.C. Bacterial Co-Infection in Patients with COVID-19 Hospitalized (ICU and Not ICU): Review and Meta-Analysis. Antibiotics 2022, 11, 894. [Google Scholar] [CrossRef]
- Arranz-Herrero, J.; Presa, J.; Rius-Rocabert, S.; Utrero-Rico, A.; Arranz-Arija, J.Á.; Lalueza, A.; Escribese, M.M.; Ochando, J.; Soriano, V.; Nistal-Villan, E. Determinants of Poor Clinical Outcome in Patients with Influenza Pneumonia: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2023, 131, 173–179. [Google Scholar] [CrossRef]
- Qiao, M.; Moyes, G.; Zhu, F.; Li, Y.; Wang, X. The Prevalence of Influenza Bacterial Co-Infection and Its Role in Disease Severity: A Systematic Review and Meta-Analysis. J. Glob. Health 2023, 13, 04063. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-Infections in People with COVID-19: A Systematic Review and Meta-Analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Alhumaid, S.; Al Mutair, A.; Al Alawi, Z.; Alshawi, A.M.; Alomran, S.A.; Almuhanna, M.S.; Almuslim, A.A.; Bu Shafia, A.H.; Alotaibi, A.M.; Ahmed, G.Y.; et al. Coinfections with Bacteria, Fungi, and Respiratory Viruses in Patients with SARS-CoV-2: A Systematic Review and Meta-Analysis. Pathogens 2021, 10, 809. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial Co-Infection and Secondary Infection in Patients with COVID-19: A Living Rapid Review and Meta-Analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Klein, E.Y.; Monteforte, B.; Gupta, A.; Jiang, W.; May, L.; Hsieh, Y.; Dugas, A. The Frequency of Influenza and Bacterial Coinfection: A Systematic Review and Meta-analysis. Influenza Other Respir. Viruses 2016, 10, 394–403. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Schwarzer, G. Meta: An R Package for Meta-Analysis. R News 2007, 7, 40–45. [Google Scholar]
- Zelus, C.S.; Blaha, M.A.; Samson, K.K.; Kalil, A.C.; Van Schooneveld, T.C.; Marcelin, J.R.; Cawcutt, K.A. Lower Respiratory Tract Coinfection in the ICU: Prevalence and Clinical Significance of Coinfection Detected via Microbiological Analysis of Bronchoalveolar Lavage Fluid With a Comparison of Invasive Methodologies. Crit. Care Explor. 2022, 4, e0708. [Google Scholar] [CrossRef] [PubMed]
- Voiriot, G.; Visseaux, B.; Cohen, J.; Nguyen, L.B.L.; Neuville, M.; Morbieu, C.; Burdet, C.; Radjou, A.; Lescure, F.-X.; Smonig, R.; et al. Viral-Bacterial Coinfection Affects the Presentation and Alters the Prognosis of Severe Community-Acquired Pneumonia. Crit. Care 2016, 20, 375. [Google Scholar] [CrossRef] [PubMed]
- Verdier, V.; Lilienthal, F.; Desvergez, A.; Gazaille, V.; Winer, A.; Paganin, F. Severe Forms of Influenza Infections Admitted in Intensive Care Units: Analysis of Mortality Factors. Influenza Other Respir. Viruses 2023, 17, e13168. [Google Scholar] [CrossRef]
- Kim, T.; Huh, J.W.; Hong, S.-B.; Jung, J.; Kim, M.J.; Chong, Y.P.; Sung, H.; Doh, K.H.; Kim, S.-H.; Lee, S.-O.; et al. Epidemiology and Characteristics of Respiratory Syncytial Virus Pneumonia in Critically Ill Adults. Open Forum Infect. Dis. 2023, 10, ofad131. [Google Scholar] [CrossRef]
- Karaca, B.; Aksun, M.; Karahan, N.A.; Girgin, S.; Ormen, B.; Tuzen, A.S.; Demirdal, T.; Sencan, A. Are Bacterial Coinfections Really Rare in COVID-19 Intensive Care Units? Eur. J. Med. Res. 2023, 28, 43. [Google Scholar] [CrossRef]
- Cohen, R.; Babushkin, F.; Finn, T.; Geller, K.; Alexander, H.; Datnow, C.; Uda, M.; Shapiro, M.; Paikin, S.; Lellouche, J. High Rates of Bacterial Pulmonary Co-Infections and Superinfections Identified by Multiplex PCR among Critically Ill COVID-19 Patients. Microorganisms 2021, 9, 2483. [Google Scholar] [CrossRef]
- Alqahtani, A.; Alamer, E.; Mir, M.; Alasmari, A.; Alshahrani, M.M.; Asiri, M.; Ahmad, I.; Alhazmi, A.; Algaissi, A. Bacterial Coinfections Increase Mortality of Severely Ill COVID-19 Patients in Saudi Arabia. Int. J. Environ. Res. Public Health 2022, 19, 2424. [Google Scholar] [CrossRef]
- Alhoufie, S.T.; Mumena, W.A.; Alsharif, N.; Makhdoom, H.M.; Almutawif, Y.A.; Alfarouk, K.O.; Alharbi, M.Z.; Aljabri, K.; Aljifri, A. Epidemiological Characteristics and Outcomes Predictors for Intensive Care Unit COVID-19 Patients in Al-Madinah, Saudi Arabia. Retrospective Cohort Study. Infect. Drug Resist. 2023, 16, 5573–5586. [Google Scholar] [CrossRef] [PubMed]
- Siow, W.T.; Koay, E.S.-C.; Lee, C.K.; Lee, H.K.; Ong, V.; Ngerng, W.J.; Lim, H.F.; Tan, A.; Tang, J.W.-T.; Phua, J. The Use of Polymerase Chain Reaction Amplification for the Detection of Viruses and Bacteria in Severe Community-Acquired Pneumonia. Respiration 2016, 92, 286–294. [Google Scholar] [CrossRef]
- Roger, C.; Collange, O.; Mezzarobba, M.; Abou-Arab, O.; Teule, L.; Garnier, M.; Hoffmann, C.; Muller, L.; Lefrant, J.-Y.; Guinot, P.G.; et al. French Multicentre Observational Study on SARS-CoV-2 Infections Intensive Care Initial Management: The FRENCH CORONA Study. Anaesth. Crit. Care Pain Med. 2021, 40, 100931. [Google Scholar] [CrossRef]
- Raymond, M.; Martin, M.; Lamouche-Wilquin, P.; Blonz, G.; Decamps, P.; Agbakou, M.; Desmedt, L.; Reignier, J.; Lascarrou, J.-B.; Canet, E. Clinical Features and Outcome of Influenza Pneumonia in Critically-Ill Immunocompromised Patients. Medicine 2022, 101, e32245. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; May, A.; Tan, L.; Hughes, H.; Jones, J.P.; Harrison, W.; Bradburn, S.; Tyrrel, S.; Muthuswamy, B.; Berry, N.; et al. Comparative Incidence of Early and Late Bloodstream and Respiratory Tract Co-Infection in Patients Admitted to ICU with COVID-19 Pneumonia versus Influenza A or B Pneumonia versus No Viral Pneumonia: Wales Multicentre ICU Cohort Study. Crit. Care 2022, 26, 158. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-J.; Zhou, C.-C.; Huang, F.; Shen, F.-M.; Li, Y.-C. Clinical Features of Omicron SARS-CoV-2 Variants Infection Associated with Co-Infection and ICU-Acquired Infection in ICU Patients. Front. Public Health 2024, 11, 1320340. [Google Scholar] [CrossRef]
- Fartoukh, M.; Nseir, S.; Mégarbane, B.; Cohen, Y.; Lafarge, A.; Contou, D.; Thille, A.W.; Galerneau, L.-M.; Reizine, F.; Cour, M.; et al. Respiratory Multiplex PCR and Procalcitonin to Reduce Antibiotic Exposure in Severe SARS-CoV-2 Pneumonia: A Multicentre Randomized Controlled Trial. Clin. Microbiol. Infect. 2023, 29, 734–743. [Google Scholar] [CrossRef]
- Briguglio, M.; Crespi, T.; Pino, F.; Mazzocchi, M.; Porta, M.; De Vecchi, E.; Banfi, G.; Perazzo, P. Clinical Characteristics of Severe COVID-19 Patients Admitted to an Intensive Care Unit in Lombardy During the Italian Pandemic. Front. Med. 2021, 8, 582896. [Google Scholar] [CrossRef]
- Delhommeau, G.; Buetti, N.; Neuville, M.; Siami, S.; Cohen, Y.; Laurent, V.; Mourvillier, B.; Reignier, J.; Goldgran-Toledano, D.; Schwebel, C.; et al. Bacterial Pulmonary Co-Infections on ICU Admission: Comparison in Patients with SARS-CoV-2 and Influenza Acute Respiratory Failure: A Multicentre Cohort Study. Biomedicines 2022, 10, 2646. [Google Scholar] [CrossRef]
- Silva, D.L.; Lima, C.M.; Magalhães, V.C.R.; Baltazar, L.M.; Peres, N.T.A.; Caligiorne, R.B.; Moura, A.S.; Fereguetti, T.; Martins, J.C.; Rabelo, L.F.; et al. Fungal and Bacterial Coinfections Increase Mortality of Severely Ill COVID-19 Patients. J. Hosp. Infect. 2021, 113, 145–154. [Google Scholar] [CrossRef]
- Shih, E.; Michael DiMaio, J.; Squiers, J.J.; Banwait, J.K.; Kussman, H.M.; Meyers, D.P.; Meidan, T.G.; Sheasby, J.; George, T.J. Bloodstream and Respiratory Coinfections in Patients with COVID-19 on ECMO. J. Card. Surg. 2022, 37, 3609–3618. [Google Scholar] [CrossRef] [PubMed]
- López-Herrero, R.; Sánchez-de Prada, L.; Tamayo-Velasco, A.; Lorenzo-López, M.; Gómez-Pesquera, E.; Sánchez-Quirós, B.; De La Varga-Martínez, O.; Gómez-Sánchez, E.; Resino, S.; Tamayo, E.; et al. Epidemiology of Bacterial Co-Infections and Risk Factors in COVID-19-Hospitalized Patients in Spain: A Nationwide Study. Eur. J. Public Health 2023, 33, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, V.; Lawrence, H.; Lansbury, L.E.; Webb, K.; Safavi, S.; Zainuddin, N.I.; Huq, T.; Eggleston, C.; Ellis, J.; Thakker, C.; et al. Co-Infection in Critically Ill Patients with COVID-19: An Observational Cohort Study from England: Read the Story behind the Paper on the Microbe Post Here. J. Med. Microbiol. 2021, 70, 001350. [Google Scholar] [CrossRef] [PubMed]
- Aldali, J.A.; Aldali, H.J.; Aljohani, R.; Algahtani, M.; Meo, S.A.; Alharbi, S.; Al-Afghani, H.; Aldabaseh, L.N.; Al Rubai, E.H.; Fallata, A.; et al. Implications of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infected Hospitalised Patients with Co-Infections and Clinical Outcomes. Microorganisms 2023, 11, 1921. [Google Scholar] [CrossRef]
- Santus, P.; Danzo, F.; Signorello, J.C.; Rizzo, A.; Gori, A.; Antinori, S.; Gismondo, M.R.; Brambilla, A.M.; Contoli, M.; Rizzardini, G.; et al. Burden and Risk Factors for Coinfections in Patients with a Viral Respiratory Tract Infection. Pathogens 2024, 13, 993. [Google Scholar] [CrossRef]
- Rozencwajg, S.; Bréchot, N.; Schmidt, M.; Hékimian, G.; Lebreton, G.; Besset, S.; Franchineau, G.; Nieszkowska, A.; Leprince, P.; Combes, A.; et al. Co-Infection with Influenza-Associated Acute Respiratory Distress Syndrome Requiring Extracorporeal Membrane Oxygenation. Int. J. Antimicrob. Agents 2018, 51, 427–433. [Google Scholar] [CrossRef]
- Marcoux, D.; Etienne, I.; Van Muylem, A.; Bogossian, E.G.; Yin, N.; Taccone, F.S.; Hites, M. A Retrospective, Monocentric Study Comparing Co and Secondary Infections in Critically Ill COVID-19 and Influenza Patients. Antibiotics 2022, 11, 704. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yang, K.S.; Chung, Y.; Lee, K.-B.; Suh, J.W.; Kim, S.B.; Sohn, J.W.; Yoon, Y.K. Epidemiologic Characteristics and Clinical Significance of Respiratory Viral Infections Among Adult Patients Admitted to the Intensive Care Unit. Front. Med. 2022, 9, 829624. [Google Scholar] [CrossRef]
- Howard-Jones, A.R.; Huang, S.; Orde, S.R.; Branley, J.M. Risk Factors for Mortality in Severe COVID-19: Exploring the Interplay of Immunomodulatory Therapy and Coinfection. Anaesth. Intensive Care 2024, 52, 52–63. [Google Scholar] [CrossRef]
- Cillóniz, C.; Ewig, S.; Ferrer, M.; Polverino, E.; Gabarrús, A.; Puig De La Bellacasa, J.; Mensa, J.; Torres, A. Community-Acquired Polymicrobial Pneumonia in the Intensive Care Unit: Aetiology and Prognosis. Crit. Care 2011, 15, R209. [Google Scholar] [CrossRef]
- For the Efraim Investigators and the Nine-I Study Group; Martin-Loeches, I.; Lemiale, V.; Geoghegan, P.; McMahon, M.A.; Pickkers, P.; Soares, M.; Perner, A.; Meyhoff, T.S.; Bukan, R.B.; et al. Influenza and Associated Co-Infections in Critically Ill Immunosuppressed Patients. Crit. Care 2019, 23, 152. [Google Scholar] [CrossRef] [PubMed]
- Nebreda-Mayoral, T.; Miguel-Gómez, M.A.; March-Rosselló, G.A.; Puente-Fuertes, L.; Cantón-Benito, E.; Martínez-García, A.M.; Muñoz-Martín, A.B.; Orduña-Domingo, A. Infección bacteriana/fúngica en pacientes con COVID-19 ingresados en un hospital de tercer nivel de Castilla y León, España. Enferm. Infecc. Microbiol. Clín. 2022, 40, 158–165. [Google Scholar] [CrossRef]
- Schoettler, J.J.; Sandrio, S.; Boesing, C.; Bauer, L.; Miethke, T.; Thiel, M.; Krebs, J. Bacterial Co- or Superinfection in Patients Treated in Intensive Care Unit with COVID-19- and Influenza-Associated Pneumonia. Pathogens 2023, 12, 927. [Google Scholar] [CrossRef] [PubMed]
- Aissaoui, Y.; Ennassimi, Y.; Myatt, I.; El Bouhiaoui, M.; Nabil, M.; Bahi, M.; Arsalane, L.; Miloudi, M.; Belhadj, A. What Happened during COVID-19 in African ICUs? An Observational Study of Pulmonary Co-Infections, Superinfections, and Mortality in Morocco. PLoS ONE 2022, 17, e0278175. [Google Scholar] [CrossRef]
- Aziza, E.; Slemko, J.; Zapernick, L.; Smith, S.W.; Lee, N.; Sligl, W.I. Outcomes among Critically Ill Adults with Influenza Infection. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2021, 6, 269–277. [Google Scholar] [CrossRef]
- Lee, W.-C.; Ho, M.-C.; Leu, S.-W.; Chang, C.-C.; Lin, C.-K.; Lin, C.-M.; Fang, Y.-H.; Huang, S.-Y.; Lin, Y.-C.; Chuang, M.-C.; et al. The Impacts of Bacterial Co-Infections and Secondary Bacterial Infections on Patients with Severe Influenza Pneumonitis Admitted to the Intensive Care Units. J. Crit. Care 2022, 72, 154164. [Google Scholar] [CrossRef]
- Rouzé, A.; Martin-Loeches, I.; Povoa, P.; Metzelard, M.; Du Cheyron, D.; Lambiotte, F.; Tamion, F.; Labruyere, M.; Boulle Geronimi, C.; Nieszkowska, A.; et al. Early Bacterial Identification among Intubated Patients with COVID-19 or Influenza Pneumonia: A European Multicenter Comparative Clinical Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 546–556. [Google Scholar] [CrossRef]
- Bergmann, F.; Gabler, C.; Nussbaumer-Pröll, A.; Wölfl-Duchek, M.; Blaschke, A.; Radtke, C.; Zeitlinger, M.; Jorda, A. Early Bacterial Coinfections in Patients Admitted to the ICU With COVID-19 or Influenza: A Retrospective Cohort Study. Crit. Care Explor. 2023, 5, e0895. [Google Scholar] [CrossRef] [PubMed]
- Chu, R.B.H.; Zhao, S.; Zhang, J.Z.; Chan, K.C.K.; Ng, P.Y.; Chan, C.; Fong, K.M.; Au, S.Y.; Yeung, A.W.T.; Chan, J.K.H.; et al. Comparison of COVID-19 with Influenza A in the ICU: A Territory-Wide, Retrospective, Propensity Matched Cohort on Mortality and Length of Stay. BMJ Open 2023, 13, e067101. [Google Scholar] [CrossRef]
- Doubravská, L.; Htoutou Sedláková, M.; Fišerová, K.; Klementová, O.; Turek, R.; Langová, K.; Kolář, M. Bacterial Community- and Hospital-Acquired Pneumonia in Patients with Critical COVID-19—A Prospective Monocentric Cohort Study. Antibiotics 2024, 13, 192. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Schultz, M.J.; Vincent, J.-L.; Alvarez-Lerma, F.; Bos, L.D.; Solé-Violán, J.; Torres, A.; Rodriguez, A. Increased Incidence of Co-Infection in Critically Ill Patients with Influenza. Intensive Care Med. 2017, 43, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Estenssoro, E.; Ríos, F.G.; Apezteguía, C.; Reina, R.; Neira, J.; Ceraso, D.H.; Orlandi, C.; Valentini, R.; Tiribelli, N.; Brizuela, M.; et al. Pandemic 2009 Influenza A in Argentina: A Study of 337 Patients on Mechanical Ventilation. Am. J. Respir. Crit. Care Med. 2010, 182, 41–48. [Google Scholar] [CrossRef]
- Goncalves Mendes Neto, A.; Lo, K.B.; Wattoo, A.; Salacup, G.; Pelayo, J.; DeJoy, R.; Bhargav, R.; Gul, F.; Peterson, E.; Albano, J.; et al. Bacterial Infections and Patterns of Antibiotic Use in Patients with COVID-19. J. Med. Virol. 2021, 93, 1489–1495. [Google Scholar] [CrossRef]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; De Silva, T.I.; et al. Co-Infections, Secondary Infections, and Antimicrobial Use in Patients Hospitalised with COVID-19 during the First Pandemic Wave from the ISARIC WHO CCP-UK Study: A Multicentre, Prospective Cohort Study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef]
- Patton, M.J.; Orihuela, C.J.; Harrod, K.S.; Bhuiyan, M.A.N.; Dominic, P.; Kevil, C.G.; Fort, D.; Liu, V.X.; Farhat, M.; Koff, J.L.; et al. COVID-19 Bacteremic Co-Infection Is a Major Risk Factor for Mortality, ICU Admission, and Mechanical Ventilation. Crit. Care 2023, 27, 34. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, P.; Johansson, N.; Ternhag, A.; Abdel-Halim, L.; Hedlund, J.; Nauclér, P. Bacterial Co-Infections in Community-Acquired Pneumonia Caused by SARS-CoV-2, Influenza Virus and Respiratory Syncytial Virus. BMC Infect. Dis. 2022, 22, 108. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of Co-Infections and Superinfections in Hospitalized Patients with COVID-19: A Retrospective Cohort Study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Shafran, N.; Shafran, I.; Ben-Zvi, H.; Sofer, S.; Sheena, L.; Krause, I.; Shlomai, A.; Goldberg, E.; Sklan, E.H. Secondary Bacterial Infection in COVID-19 Patients Is a Stronger Predictor for Death Compared to Influenza Patients. Sci. Rep. 2021, 11, 12703. [Google Scholar] [CrossRef]
- Beumer, M.C.; Koch, R.M.; Van Beuningen, D.; OudeLashof, A.M.; Van De Veerdonk, F.L.; Kolwijck, E.; Van Der Hoeven, J.G.; Bergmans, D.C.; Hoedemaekers, C.W.E. Influenza Virus and Factors That Are Associated with ICU Admission, Pulmonary Co-Infections and ICU Mortality. J. Crit. Care 2019, 50, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bartley, P.S.; Deshpande, A.; Yu, P.-C.; Klompas, M.; Haessler, S.D.; Imrey, P.B.; Zilberberg, M.D.; Rothberg, M.B. Bacterial Coinfection in Influenza Pneumonia: Rates, Pathogens, and Outcomes. Infect. Control Hosp. Epidemiol. 2022, 43, 212–217. [Google Scholar] [CrossRef]
- Chen, Z.; Zhan, Q.; Huang, L.; Wang, C. Coinfection and Superinfection in ICU Critically Ill Patients with Severe COVID-19 Pneumonia and Influenza Pneumonia: Are the Pictures Different? Front. Public Health 2023, 11, 1195048. [Google Scholar] [CrossRef] [PubMed]
- Contou, D.; Claudinon, A.; Pajot, O.; Micaëlo, M.; Longuet Flandre, P.; Dubert, M.; Cally, R.; Logre, E.; Fraissé, M.; Mentec, H.; et al. Bacterial and Viral Co-Infections in Patients with Severe SARS-CoV-2 Pneumonia Admitted to a French ICU. Ann. Intensive Care 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Self, W.H.; Wunderink, R.G.; Williams, D.J.; Zhu, Y.; Anderson, E.J.; Balk, R.A.; Fakhran, S.S.; Chappell, J.D.; Casimir, G.; Courtney, D.M.; et al. Staphylococcus aureus Community-Acquired Pneumonia: Prevalence, Clinical Characteristics, and Outcomes. Clin. Infect. Dis. 2016, 63, 300–309. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.J.; Myong, J.-P.; Lee, Y.-H.; Kim, B.Y.; Hwang, A.; Kim, G.O.; Jeong, S.H.; Yoon, H.K.; An, T.J.; et al. Prior Pneumococcal Vaccination Improves In-Hospital Mortality among Elderly Population Hospitalized Due to Community-Acquired Pneumonia. BMC Pulm. Med. 2024, 24, 168. [Google Scholar] [CrossRef] [PubMed]


| Study | Country | Setting | Design | Study Period | Sample Size | Invasive Ventilation | Population |
|---|---|---|---|---|---|---|---|
| <48 h from Hospital Admission | |||||||
| Aissaoui et al. (2022) [44] | Morocco | ICU (single center) | Retrospective cohort | 2020–2021 | 155 | 42% | COVID-19 |
| Doubravská et al. (2024) [50] | Czech Republic | ICU (single center) | Prospective cohort | 2020–2022 | 171 | 69% | COVID-19 |
| Lee et al. (2022) [46] | Taiwan | ICU (multicenter) | Retrospective cohort | 2014–2018 | 117 | 63.3% | Influenza |
| Martin-Loeches et al. (2017) [51] | Spain | ICU (multicenter) | Prospective cohort | 2009–2015 | 2901 | 53% | Influenza |
| Schoettler et al. (2023) [43] | Germany | ICU (single-center) | Retrospective cohort | 2009–2022 | 190 | 78.1% | COVID-19, Influenza |
| <48 h from ICU Admission | |||||||
| Chu et al. (2023) [49] | Hong Kong | ICU (multicenter) | Retrospective cohort | 2015–2021 | 746 | COVID-19: 43.4% Influenza: 53.1% | COVID-19, Influenza |
| Aziza et al. (2021) [45] | Canada | ICU (single center) | Retrospective cohort | 2014–2019 | 130 | 83% | Influenza |
| Rouzé et al. * (2021) [47] | Europe | ICU (multicenter) | Retrospective cohort | 2016–2020 | 1050 | 100% | COVID-19, Influenza |
| Bergmann et al. (2023) [48] | Germany | ICU (single center) | Retrospective cohort | 2015–2022 | 328 | No data | COVID-19, Influenza |
| Estenssoro et al. (2010) [52] | Argentina | ICU (multicenter) | Prospective cohort | 2009 | 337 | 81% | Influenza |
| Study | S1: | S2: | S3: | S4: | C1: | O1: | O2: | O3: | Total |
|---|---|---|---|---|---|---|---|---|---|
| Aissaoui et al. [44] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9/9 |
| Aziza et al. [45] | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 9/9 |
| Lee et al. [46] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9/9 |
| Martin-Loeches et al. [51] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9/9 |
| Bergmann et al. [48] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9/9 |
| Chu et al. [49] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9/9 |
| Doubravská et al. [50] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7/9 |
| Rouzé et al. [47] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9/9 |
| Estenssoro et al. [52] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9/9 |
| Schoettler et al. [43] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9/9 |
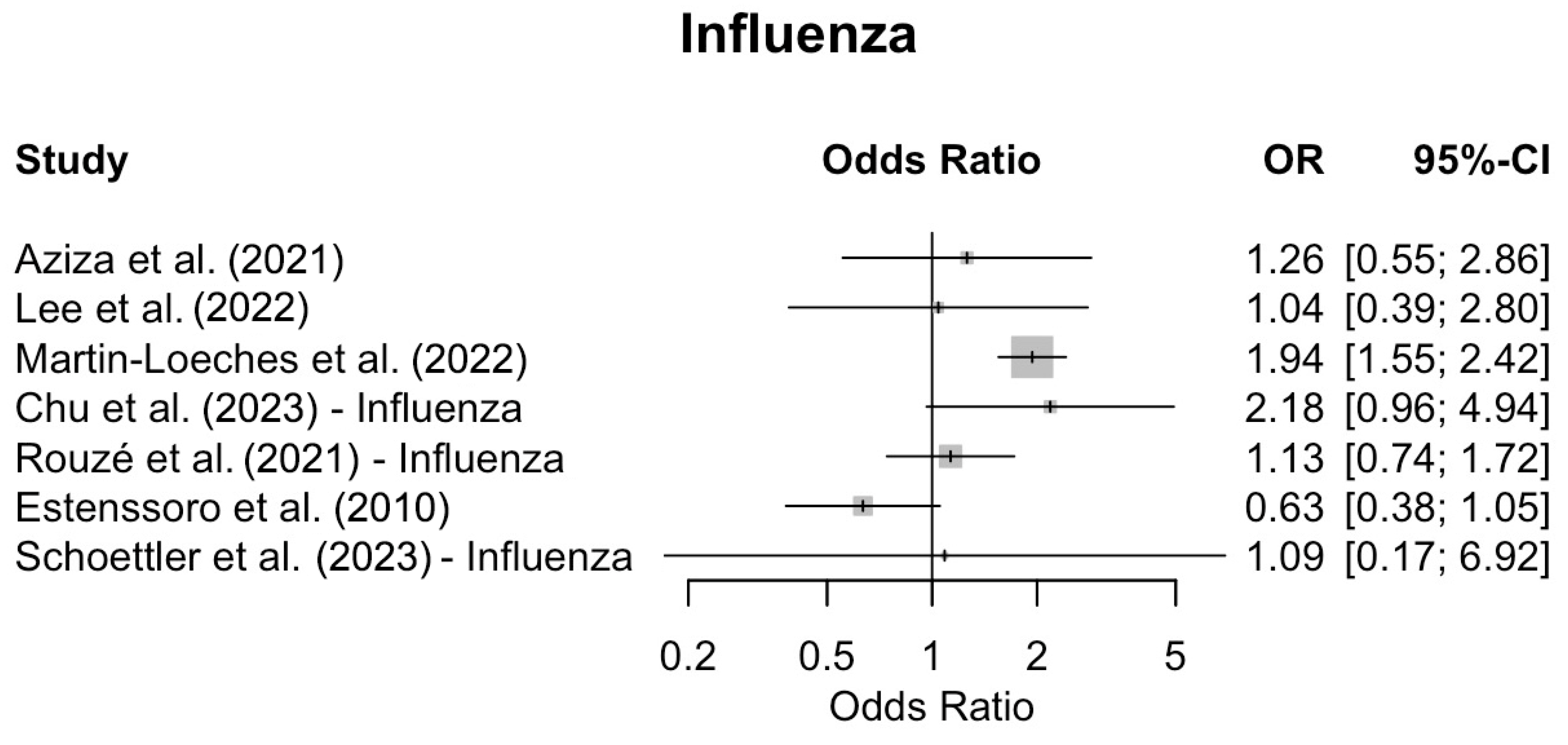
| Study | Mortality | Co-Infections | Co-Infected Mortality (n/N) | Non-Infected Mortality (n/N) | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|---|---|---|---|
| <48 h from Hospital Admission | |||||||
| Aissaoui et al. [44] | Hospital | COVID-19: 6/155 | COVID-19: 5/6 | COVID-19: 54/149 | 8.8 | (1.001–77.26) | 0.030 |
| Doubravská et al. [50] | 28-d | COVID-19: 46/171 | COVID-19: 27/46 | COVID-19: 49/125 | 2.2 | 1.11–4.39 | 0.025 |
| Lee et al. [46] | ICU | Influenza: 32/117 | Influenza: 7/32 | Influenza: 18/85 | 1.04 | (0.39–2.8) | 1 |
| Martin-Loeches et al. [51] | ICU | Influenza:451/2901 | Influenza: 147/451 | Influenza: 446/2233 | 1.94 | (1.55–2.42) | <0.001 |
| Schoettler et al. [43] | Hospital | COVID-19: 15/114 Influenza: 5/76 | COVID-19: 6/15 Influenza: 2/5 | COVID-19: 34/99 Influenza: 27/71 | 1.27 1.09 | 0.42–3.88 0.17–6.92 | 0.67 0.93 |
| <48 h from ICU admission | |||||||
| Chu et al. [49] | Hospital | COVID-19: 71/373 Influenza: 80/373 | COVID-19: 21/71 Influenza: 10/80 | COVID-19: 43/302 Influenza: 18/293 | 2.53 2.18 | 1.38–4.62 0.96–4.94 | 0.004 0.089 |
| Aziza et al. [45] | 30-d | Influenza: 55/130 | Influenza: 14/55 | Influenza: 16/75 | 1.26 | (0.55–2.86) | 0.674 |
| Rouzé et al. [47] | 28-d | COVID-19: 55/568 Influenza: 162/482 | COVID-19: 24/55 Influenza: 47/162 | COVID-19: 142/513 Influenza: 85/320 | 2.02 1.13 | 1.15–3.57 0.74–1.72 | 0.018 0.589 |
| Bergmann et al. [48] | 30-d | COVID-19: 68/289 Influenza: 8/39 | COVID-19: 21/68 Influenza: no data | COVID-19: 40/221 Influenza: no data | 2.02 No data | (1.09–3.75) No data | 0.027 >0.05 |
| Estenssoro et al. [52] | Hospital | Influenza: 80/337 | Influenza: 40/80 | Influenza: 150/245 | 0.63 | 0.38–1.05 | 0.089 |
| Genus | COVID-19 (N = 613) | COVID-19 <48 h ICU (N = 499) | COVID-19 <48 h Hospital (N = 114) | Influenza (N = 789) | Influenza <48 h ICU (N = 193) | Influenza <48 h Hospital (N = 596) |
|---|---|---|---|---|---|---|
| Acinetobacter spp. | 2 (0.3%) | 2 (0.4%) | 0 (0%) | 18 (2.3%) | 2 (1.0%) | 16 (2.7%) |
| Bacteroides spp. | 0 (0%) | – | – | 1 (0.1%) | 0 (0%) | 1 (0.2%) |
| Burkholderia spp. | 0 (0%) | – | – | 1 (0.1%) | 1(0.5%) | – |
| Chlamydophila spp. | 0 (0%) | – | – | 3 (0.4%) | 0 (0%) | 3 (0.5%) |
| Citrobacter spp. | 3 (0.5%) | 1 (0.2%) | 2 (1.8%) | 0 (0%) | 0 (0%) | – |
| Enterobacter spp. | 11 (1.8%) | 8 (1.6%) | 3 (2.6%) | 6 (0.8%) | 3 (1.6%) | 5 (0.8%) |
| Enterococcus spp. | 14 (2.3%) | 13 (2.6%) | 1 (0.9%) | 2 (0.3%) | 2 (1.0%) | – |
| Escherichia coli | 12 (2.0%) | 10 (2.0%) | 2 (1.8%) | 22 (2.8%) | 8 (4.1%) | 14 (2.3%) |
| Haemophilus influenzae | 12 (2.0%) | 11 (2.2%) | 1 (0.9%) | 36 (4.6%) | 19 (9.8%) | 17 (2.9%) |
| Klebsiella spp. | 25 (4.1%) | 19 (3.8%) | 6 (5.3%) | 31 (3.9%) | 4 (2.1%) | 27 (4.5%) |
| Legionella spp. | 0 (0%) | – | – | 5 (0.6%) | 0 (0%) | 5 (0.8%) |
| Moraxella catarrhalis | 4 (0.7%) | 4 (0.8%) | – | 1 (0.1%) | 1 (0.5%) | – |
| Morganella spp. | 1 (0.2%) | 0 (0.0%) | 1 (0.9%) | 5 (0.6%) | 4 (2.1%) | 1 (0.2%) |
| Mycobacterium spp. | 0 (0%) | – | – | 3 (0.4%) | 0 (0%) | 3 (0.5%) |
| Mycoplasma spp. | 0 (0%) | – | – | 4 (0.5%) | 0 (0%) | 4 (0.7%) |
| Nocardia spp. | 0 (0%) | – | – | 1 (0.1%) | 0 (0%) | 1 (0.2%) |
| Pneumocystis spp. | 0 (0%) | – | – | 4 (0.5%) | 0 (0%) | 4 (0.7%) |
| Proteus spp. | 9 (1.5%) | 5 (1.0%) | 4 (3.5%) | 0 (0.0%) | – | – |
| Pseudomonas aeruginosa | 15 (2.4%) | 13 (2.6%) | 2 (1.8%) | 59 (7.5%) | 0 (0%) | 59 (9.9%) |
| Serratia spp. | 8 (1.3%) | 6 (1.2%) | 2 (1.8%) | 6 (0.8%) | 1 (0.5%) | 5 (0.8%) |
| Shewanella spp. | 0 (0%) | – | – | 1 (0.1%) | 0 (0%) | 1 (0.2%) |
| Staphylococcus aureus | 66 (10.8%) | 50 (10.0%) | 16(14.0%) | 120 (15.2%) | 53 (27.5%) | 67 (11.2%) |
| Staphylococcus spp. | 1 (0.2%) | 1 (0.2%) | – | 6 (0.8%) | 2 (1.0%) | 4 (0.7%) |
| Stenotrophomonas spp. | 4 (0.7%) | 2 (0.4%) | 2 (1.8%) | 5 (0.6%) | 1 (0.5%) | 4 (0.7%) |
| Streptococcus spp. | 33 (5.4%) | 32 (6.4%) | 1 (0.9%) | 313 (39.7%) | 63 (32.6%) | 250 (41.9%) |
| COVID-19 + Influenza | Co-Infections | |||
|---|---|---|---|---|
| Sample | Method | Sample | Method | |
| Aissaoui et al. (2022) [44] | Nasopharyngeal Swab—awake, Lower Respiratory Tract—intubated | RT-PCR | Blood, Lower Respiratory tract | Cultures |
| Aziza et al. (2021) [45] | Upper/lower respiratory track | PCR | Respiratory/blood | Cultures |
| Lee et al. (2022) [46] | Nasopharyngeal/pharyngeal swab | PCR | Respiratory/blood | Cultures |
| Martin-Loeches et al. (2017) [51] | Nasopharyngeal swab | PCR | Endotracheal aspirates/blood/pleural fluid | Cultures |
| Bergmann et al. (2023) [48] | No data | PCR | Sputum/endotracheal aspirates/BAL/blood; Urine | Cultures; Antigen test for S. pneumoniae & L. pneumophila |
| Chu et al. (2023) [49] | No data | PCR | Sputum/tracheal aspirate/BAL/Blood/pleural | Cultures |
| Doubravská et al. (2024) [50] | Nasopharyngeal/endotracheal | PCR | Blood/lower respiratory tract; Urine | Cultures, PCR for bacterial nucleic acid, serological methods for mycoplasma and chlamydophila; Antigen test for S. pneumoniae & L. pneumophila |
| Rouzé et al. (2021) [47] | Nasopharyngeal/respiratory secretions | PCR | Endotracheal aspirates/Blood; Urine | Cultures; Antigen test for S. pneumoniae & L. pneumophila |
| Estenssoro et al. (2010) [52] | Respiratory specimens | RT-PCR | Respiratory specimens | No data |
| Schoettler et al. (2023) [43] | Nasal/Throat swab/tracheal aspirate/Bal | PCR | Respiratory sample | No data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menis, A.A.; Gerovasileiou, E.; Mantzarlis, K.; Manoulakas, E.; Deskata, K.; Vazgiourakis, V.; Makris, D.; Dimopoulos, G. The Effect on Mortality of Bacterial Co-Infections on Critically Ill Patients with Community-Acquired COVID-19 and Influenza Pneumonia: A Systematic Review. Viruses 2025, 17, 851. https://doi.org/10.3390/v17060851
Menis AA, Gerovasileiou E, Mantzarlis K, Manoulakas E, Deskata K, Vazgiourakis V, Makris D, Dimopoulos G. The Effect on Mortality of Bacterial Co-Infections on Critically Ill Patients with Community-Acquired COVID-19 and Influenza Pneumonia: A Systematic Review. Viruses. 2025; 17(6):851. https://doi.org/10.3390/v17060851
Chicago/Turabian StyleMenis, Apostolos A., Efrosyni Gerovasileiou, Konstantinos Mantzarlis, Efstratios Manoulakas, Konstantina Deskata, Vasileios Vazgiourakis, Demosthenes Makris, and George Dimopoulos. 2025. "The Effect on Mortality of Bacterial Co-Infections on Critically Ill Patients with Community-Acquired COVID-19 and Influenza Pneumonia: A Systematic Review" Viruses 17, no. 6: 851. https://doi.org/10.3390/v17060851
APA StyleMenis, A. A., Gerovasileiou, E., Mantzarlis, K., Manoulakas, E., Deskata, K., Vazgiourakis, V., Makris, D., & Dimopoulos, G. (2025). The Effect on Mortality of Bacterial Co-Infections on Critically Ill Patients with Community-Acquired COVID-19 and Influenza Pneumonia: A Systematic Review. Viruses, 17(6), 851. https://doi.org/10.3390/v17060851







