Abstract
In agreement with WHO recommendations, the Emilia-Romagna Region, Italy, implemented a population-wide HCV screening program for the treatment of the large asymptomatic infected population. From January 2022, the free-of-charge screening targeted all residents born between 1969 and 1989, prison inmates, and injection drug users. Participants were recruited using phone messages, electronic health record notifications, public advertisement, and direct contact with general practitioners. A single blood sample was collected for anti-HCV IgG testing and, if positive, for reflex HCV–RNA testing. Infected subjects were offered an evidence-based therapeutic pathway. By June 2024, 72.8% of high-risk subjects (n = 19,732), and 36.9% of the general population (n = 488,065) had been screened. A total of 1032 individuals were positive based on the HCV–RNA test, and the detection rate widely differed between the high-risk and the general population (23.8‰ vs. 1.2‰, respectively). Of the infected individuals, 88.1% were seen by a specialist physician, and 74.3% (n = 767) started antiviral therapy. Thanks to multiple recruitment approaches, over one third of the general population participated in HCV screening. The program performance was substantially greater among high-risk individuals compared to the general population. To achieve WHO targets, policymakers might consider expanding the screening to other high-risk subgroups and/or adapting birth cohorts.
Keywords:
Hepatitis C Virus; screening; HCV prevalence; prison inmates; drug addiction; 1969–1989; Italy 1. Introduction
Hepatitis C Virus (HCV) infection is one of the most prevalent blood-borne disease globally and is a major cause of chronic hepatitis [1,2]. More than 70 million patients are estimated to live with chronic HCV infection world-wide, and approximately 350,000 related deaths occur annually [2,3,4]. If not treated, chronic hepatitis progresses to liver cirrhosis and hepatocellular carcinoma within 20 years in about 10% and 2% of infected patients, respectively [5]. The advent of direct-acting antiviral agents (DAAs) in 2014 drastically improved HCV treatment by offering a simplified protocol and sustained viral response in 95% of patients [6,7]. To address this burden, the World Health Organization (WHO) developed strategies in 2016 to eliminate HCV as a public health threat by 2030, setting the target of reducing its global mortality by 65% [8]. To achieve this goal, countries were encouraged to develop screening programs to increase testing and treatment uptake [1], in order to identify the large population with asymptomatic HCV [7].
Italy has the highest estimated seroprevalence of HCV in Europe and, in line with WHO objectives, has allocated over EUR 70 million to develop free regional screening programs [9,10]. In January 2022, the Emilia-Romagna Region, located in northern Italy, started a population-wide screening program to identify HCV-positive adults and promote treatment [11]. We present the results of the first two years of the screening program, in terms of screening adherence, detection rates, and treatment uptake.
2. Materials and Methods
We analyzed several measures of effectiveness of the HCV screening program “C devi pensare” in the Emilia-Romagna Region, from its beginning to June 2024 [12]. The program targeted all residents (or those temporarily living in the region) born between 1969 and 1989, plus prison inmates and individuals with drug addiction, regardless of their official residency or age, although data were only collected for individuals aged 18 years or over). The rationale for limiting invitations to the 1969–1989 birth cohorts lies in the estimated highest prevalence of HCV infection and the proportion of immigrants [12,13], and in the prospective advantage conferred by DAA treatment in this age group [14]. The subjects from the eligible birth cohorts were recruited via automated short message service (SMS) and notifications on their electronic health record (EHR), and individuals under institutional care (prison inmates and individuals with drug addiction disorders) were actively recruited by their healthcare providers. The people who injected drugs were identified among the users of outpatient services at National Health Service addiction recovery clinics [15]. Also, general practitioners within the National Health Service were asked to invite their patients to participate, informing them about the purpose and procedures and the therapeutic pathway in the case of a positive result. Finally, screening was offered to eligible individuals when scheduling blood tests at public healthcare facilities. The program also employed various communication strategies to encourage participation, including billboard advertisements in public spaces, a social media campaign, and the distribution of multilingual flyers to reach immigrant communities [11].
All public hospitals and outpatient blood collection facilities located in Emilia-Romagna were required to participate. After written informed consent was obtained, a single venous blood draw was collected, stored, and sent for HCV testing at a single Microbiology laboratory, designated by each Local Health Unit (LHU). No prescription from the primary care physician was requested, and the exam was free of charge. Each blood sample underwent a two-step reflex testing pathway: it was screened for anti-HCV IgG antibodies by enzyme-linked immunosorbent assay (ELISA); if reactive, it was further evaluated for HCV-RNA detection using real-time polymerase chain reaction (RT-PCR) technology.
The subjects who also tested positive in the second test were diagnosed with a confirmed active infection and promptly contacted by a specialist physician, who explained the therapeutic pathway and proposed the start of therapy according to the Italian national guidelines [16]. The physicians were specialists in Infectious Diseases or Gastroenterology who worked in the reference center of each LHU. The test results were uploaded to web-based platforms and sent to LHU data centers. After referral to an healthcare center for further care, the screening data were anonymized in compliance with European General Data Protection Regulation (GDPR) policies [17]. Only aggregated data were then shared with the Emilia-Romagna Region Welfare Department for the current analyses. As the study was conducted using only aggregated, already collected data, tailored to an evaluation of efficacy explicitly requested by the Italian Minister of Health, the Emilia-Romagna Region approved the analysis protocol internally and did not require a formal evaluation from the Regional Ethics Committee.
The outcomes of the study were the following:
- - Anti-HCV IgG Screening Adherence—Proportion of individuals who underwent the first-level test among all the invited subjects;
- - HCV-RNA Screening Adherence—Proportion of individuals who underwent the second-level test among the subjects who tested positive in the first-level test (anti-HCV IgG);
- - Detection Rate—Proportion of individuals who tested positive in the confirmatory HCV-RNA test among the subjects who underwent the first-level test (anti-HCV IgG test);
- - Positive Predictive Value (PPV) of the Anti-HCV IgG Screening Test—Proportion of individuals who tested positive in the confirmatory HCV-RNA test among the subjects that tested positive in the first-level test (conventionally calculated for screening programs to assess the performance of first-level tests in correctly identifying, out of an asymptomatic population, the individuals at high risk of having the disease [18,19,20]);
- - Outpatient Visit Adherence—Proportion of individuals who were visited at least once by a specialist physician among the subjects that tested positive in the confirmatory HCV-RNA test;
- - Treatment Uptake—Proportion of individuals who started antiviral therapy among the subjects who tested positive in the confirmatory HCV-RNA test.
Descriptive statistics were used to summarize the main characteristics of the sample and compute the primary outcomes. For HCV infection detection rate, the specialist visit adherence rate, and the antiviral therapy uptake, 95% confidence intervals (CIs) were computed using Stata, version 13.0 (Stata Corp., College Station, TX, USA, 2013).
3. Results
Overall, 1,364,341 individuals were eligible for the HCV screening program from January 2022 to June 2024: 1,330,562 were residents born between 1969 and 1989; 24,678 were individuals with drug addiction disorders, and 9101 were prison inmates (Table 1). Almost all the subjects of the birth cohort (99.4%), and approximately 80% of the individuals in institutional care were invited to participate.

Table 1.
Results of the HCV infection screening program in the Emilia-Romagna Region, overall and by population subgroup (data as of 30 June 2024).
The adherence to the first-level test largely varied across subgroups: the majority of the invited prison inmates and addiction service users participated to the screening (93.8% and 64.7%, respectively), while the overall uptake in residents in the birth cohort was 36.9%.
The total number of individuals who tested positive in the first-level test (anti-HCV IgG) was 5858, and the positivity rate also differed widely by population group, being much higher among the institutionalized individuals (approximately 100 × 1000 participants) than the residents (8.0 × 1000).
The vast majority of the positive subjects agreed to participate in the second-level screening (approximately 90%; n = 5295). Of them, 1032 (19.5%) also tested positive in the second-level, confirmatory HCV-RNA test and were considered infected, corresponding to an overall detection rate of 2.0 × 1000 participants (95% CI: 1.9–2.2). It is important to note that the incidence of infection varied widely across groups, being much higher among the institutionalized subjects (>20 × 1000 participants; p < 0.001).
With regard to program performance (Figure 1 and Table 1), the overall positive predictive value of the first-level test again differed largely by population, with lower values observed among the residents (15.5%) than the other subgroups (28.3%). In contrast, although a high adherence to the treatment program was observed in all groups, the residents showed a higher participation in both the outpatient visit and drug therapy (95.9% and 80.4%, respectively), especially when compared to the addiction service users (75.8% and 63.9%, respectively).
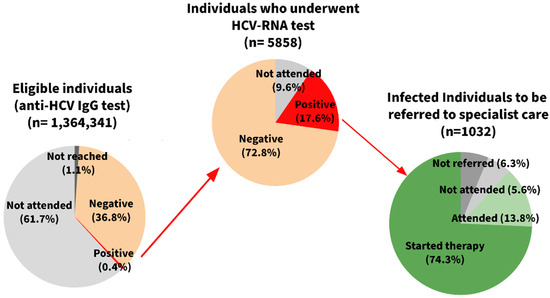
Figure 1.
Pathway of the Hepatitis C Virus (HCV) infection screening program in the Emilia-Romagna Region (data as of 30 June 2024).
4. Discussion
The Emilia-Romagna Region carried out the first Western European study reporting the results of HCV screening in both the general population (birth cohorts 1969–1989) and underserved subgroups, such as prison inmates and individuals with drug addiction disorders. The main findings from the first two years of this population-wide campaign are the following: (a) it was possible to identify a total of 1032 HCV chronically infected subjects, 88.1% of whom were enrolled in the designated clinical pathways; (b) the overall screening performance (screening adherence, detection rate, and positive predictive value) was substantially greater among the institutionalized subjects (prison inmates and persons with drug addiction disorders) than in the general population, highlighting the need to enroll these hard-to-reach segments of population.
The overall adherence to HCV screening in the general population was approximately 40%. On one hand, a few similar programs in other countries have reached higher levels [21]. On the other hand, many programs have shown worse results, and the only other screening (against colorectal cancer) targeting the general population of both sexes in the same region showed a very similar 40% adherence, although it was activated two decades prior [22,23]. The fact that the residents had already used screening services for a long time might have contributed to the relatively high adherence to a newly established screening system against an infectious disease. Indeed, the Emilia-Romagna region started in the 1990s to provide free, organized screening programs for colon, breast, and cervical cancers [24,25].
Thus far, two large population studies have reported on the effectiveness of DAAs in chronically HCV-infected patients: a sharp decrease in all-cause mortality and hepatocellular carcinoma incidence was observed in a French cohort study, while a decline in liver-related mortality and decompensated cirrhosis diagnoses, together with a plateau in the previously increasing rates of hepatocellular carcinoma and all-cause mortality, were documented in an Australian pre-post evaluation [26,27]. In line with these findings, in this program, the early identification of more than a thousand HCV-infected subjects, which lead to the subsequent treatment of more than 700 patients, represented a step forward toward the WHO’s 2030 HCV elimination targets [8]. To fully achieve these targets, however, several authors suggested extending the screening program to an older population [28,29,30,31,32]. Clearly, such an extension should be informed by an in-depth cost–benefit analysis, which requires data on HCV prevalence, diagnostic test accuracy, DAA therapy effectiveness, and cost [14,33,34]. This information is not available yet, as this is the first interim report, and a comprehensive evaluation will be performed five years after the start of the screening.
The HCV-RNA detection rate recorded in the general population of the Emilia-Romagna was comparable to the one recently reported from the 1969–1989 birth cohort in the neighboring Lombardy region (1.0‰) [10]. Similarly, two Italian screenings in the same birth cohorts recently reported detection rates of 0.5‰ in a COVID-19 vaccination setting and 0.7‰ among hospitalized patients [35,36]. In line with a recent modeling study by the Polaris Observatory [32], these findings suggest that the Italian HCV chronic infection prevalence is similar to rates in other Western European countries (3.0‰) and significantly lower than the values typically reported in Central and Eastern European nations (8.0‰ and 29.0‰, respectively). Indeed, in 2016, Italy was thought to have the highest HCV seroprevalence in the EU [9]. In contrast, this study showed an anti-HCV IgG detection rate comparable to other European countries (5.4‰ to 15.0‰, among the general population) [37]. This considerably lower seroprevalence may be explained by three main factors: (a) the fact that previous estimates relied on studies published nearly twenty years ago that included populations from Southern Italy, where the prevalence is higher; (b) the cumulative effect of time (aging, all-cause and liver-related deaths, and declining incident infections); and (c) the growing use of effective treatments [38,39,40]. When the results of additional government funded region-wide screening programs are available, it will be possible to more precisely update the Italian HCV national burden estimates.
As mentioned, the individuals under institutional care exhibited much higher HCV-RNA detection rates than the general population, in agreement with the well-established evidence regarding HCV prevalence among high-risk populations and transmission patterns (e.g., injection drug use) [41,42]. In settings characterized by high financial constraints, this finding confirms the importance of implementing targeted policies and defined micro-elimination strategies to identify and cure HCV-infected individuals among high-risk populations with a greater cost-effective approach [43]. The adherence rate to the first-level test was also higher among the underserved subjects compared to the general population, suggesting that the tailored recruitment strategies had a positive impact in engaging such hard-to-reach subgroups [41,42,44,45,46]. Indeed, while the adherence rate to the first-level test in the general population was not substantially higher than those reported in other HCV screening studies [10,21], the screening uptake among underserved subgroups was higher compared to most of the recent literature [47,48,49,50,51,52], probably because many of these individuals were already being screened in the context of their care pathways. Overall, given the above findings, HCV screening could be effectively extended to additional subgroups that are known to have a higher prevalence of HCV chronic infection compared to the general population (e.g., patients undergoing hemodialysis, sex workers, and men who have sex with men—MSM) [47]. Indeed, MSM were not targeted by the Emilia-Romagna screening program, although an HCV chronic infection prevalence of 3.8‰, estimated by a recent meta-analysis, suggests that the early diagnosis of HCV infection could prevent substantial morbidity in this group [53].
Consistent with several previous studies [36,54,55,56], reporting rates of true-positive subjects ranging from 18% and 36% after the first-level test; in this sample, we found that approximately one out of five subjects with a positive anti-HCV IgG test actually tested positive in the HCV-RNA test. As suggested by Beltrami et al. [57], this could be partially explained by the absence of exclusion criteria in the screening program, leading to the inclusion of individuals already treated with DAAs or on regular follow-up for known HCV infection. Future research is needed to estimate the impact of potential exclusion criteria on the PPV of the first-level test, which would allow us to refine the screening protocol and reduce the false-positive rate.
Despite employing a centralized, validated one-step method to perform two laboratory tests together, from a single blood draw, approximately 10% of the samples could not be assessed for the confirmatory test (HCV-RNA detection). It is worth noting that this loss of data was not due to laboratory, management, or informatics deficiencies but was caused by directives issued by the Data Protection Authority of one LHU. In this unit, at the beginning of the program, the second-level test was not allowed due to concerns regarding informed consent. The format of the informed consent was rapidly revised, and the limitation was resolved within the first 15 days of the program, but we could not process the samples collected before the change.
In this program, almost nine out of ten HCV-infected individuals began the specialized care pathway provided by the program. This robust linkage-to-care result represents an important accomplishment, given the heterogenous performances recorded in the literature [42,45,58,59]. However, for once, this indicator of screening performance was poorer among the individuals in institutional care compared to the general population, suggesting the existence of potential barriers in these subgroups, such as the high turnover rate and frequent transfers among prisoners, and socio-economic vulnerabilities among subjects with drug abuse disorders [42,45,46]. A recent systematic review suggests potential strategies to enhance the drug abuser linkage-to-care result, including individual navigation and education regarding the tolerability of DAA therapy [41].
The strengths of this study include the involvement of all the residents of the Emilia-Romagna Region (born between 1969 and 1989), prison inmates, and individuals with drug addiction disorders, with a tailored pathway for these high-risk, hard-to-reach subgroups and the use of a reflex test, requiring a single blood draw, processed with a validated two-step technology. However, this study also has limitations that must be considered when interpreting the results. First, as the study design was based on aggregated data, because of strict privacy requirements, the demographic and clinical characteristics of the subjects who participated in the program could not be analyzed. Second, as the individuals who tested negative did not undergo the confirmatory test, it was not possible to assess the specificity and sensitivity of the first-level test. Third, given that the screening was directed towards the entire population, some of the positive subjects already knew that they were infected. This helped in producing more reliable estimates of the infection prevalence but may have reduced the benefit to the population, as some of the individuals were already on therapy and the screening was only useful as a monitoring tool. Finally, as mentioned, we could not evaluate the effectiveness of the therapy and the costs of the program at this stage.
In conclusion, the HCV screening program in the Emilia-Romagna Region, conducted from January 2022 to June 2024, targeting both the general population (1969–89 birth cohort) and individuals under institutional care, identified 1032 new chronic infections, the vast majority of which have been taken care of by a specialist physician. Thanks to multiple recruitment approaches, more than one third of the general population participated to the program. The screening adherence, detection rate, and positive predictive value were substantially greater among prison inmates and subjects with drug addiction disorders compared to the general population, thanks to tailored strategies engaging these hard-to-reach subgroups, highlighting the value of these approaches. Policymakers might consider expanding screening to other high-risk subgroups and adapting the birth cohorts targeted as new data emerge to achieve the HCV elimination targets recommended by the WHO. As the program remains active, future analyses will focus on the long-term cost–benefit ratio of the program.
Author Contributions
Conceptualization, G.I., M.F., G.M. (Giovanna Mattei), G.M. (Giulio Matteo), G.D. and L.M.; methodology, E.R.D.G., A.B., C.A.M., M.E.F. and L.M.; software, G.I., A.B. and L.M.; validation, M.E.F. and L.M.; formal analysis, A.B. and C.A.M.; investigation, G.I., M.F. and E.R.D.G.; resources, G.M. (Giovanna Mattei), G.M. (Giulio Matteo) and G.D.; data curation, G.I., G.M. (Giovanna Mattei), G.M. (Giulio Matteo) and G.D.; writing—original draft preparation, G.I., M.F., A.B. and L.M.; writing—review and editing, C.A.M., M.E.F. and L.M.; visualization, E.R.D.G., M.F. and A.B.; supervision, C.A.M., M.E.F. and L.M.; project administration, G.M. (Giovanna Mattei), G.M. (Giulio Matteo), G.D. and Regional HCV Working Group; funding acquisition, G.M. (Giovanna Mattei), G.M. (Giulio Matteo) and G.D. All authors have read and agreed to the published version of the manuscript.
Funding
The project was funded by the Italian Ministers of Health and Economy, Law Decree 14 May 2021 [Implementation of national screening for HCV elimination], Gazzetta Ufficiale Serie Generale n.162, 8 July 2021 (21A04075).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the internal board of the Emilia-Romagna Region; it did not request a formal evaluation by the Regional Ethics Committee, as the screening program and its efficacy evaluation were explicitly requested by the Italian Minister of Health and the analyses were conducted using only aggregated, secondary data.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study and the exclusive use of aggregated secondary data.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Regional HCV Working Group (alphabetic order): Marco Battini (Emilia-Romagna Region), Cinzia Campari (AUSL Reggio Emilia), Margherita De Lillo (AUSL Imola), Elisabetta Fioretti (AUSL Modena), Francesco Foschi (AUSL Romagna), Zaynalabedin Kahfian (AUSL Modena), Vittorio Laviola (Emilia-Romagna Region), Francesca Mezzetti (AUSL Bologna), Caterina Palmonari (AUSL Ferrara), Monica Pini (AUSL Parma), Alessandra Rampini (AUSL Piacenza), Vittorio Sambri (AUSL Romagna), and Alessio Saponaro (Emilia-Romagna Region).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Guidelines for the Screening, Care and Treatment of Persons with Hepatitis C Infection; World Health Organization: Geneva, Switzerland, 2014. Available online: https://iris.who.int/bitstream/handle/10665/111747/9789241548755_eng.pdf (accessed on 13 December 2024).
- Jefferies, M.; Rauff, B.; Rashid, H.; Lam, T.; Rafiq, S. Update on global epidemiology of viral hepatitis and preventive strategies. World J. Clin. Cases 2018, 6, 589–599. [Google Scholar] [CrossRef]
- Page, K.; Melia Michael, T.; Veenhuis Rebecca, T.; Winter, M.; Rousseau Kimberly, E.; Massaccesi, G.; Osburn William, O.; Forman, M.; Thomas, E.; Thornton, K.; et al. Randomized Trial of a Vaccine Regimen to Prevent Chronic HCV Infection. New Engl. J. Med. 2021, 384, 541–549. [Google Scholar] [CrossRef]
- CDA Foundation. Polaris Observatory Database. 2024. Available online: https://cdafound.org/polaris-countries-database/ (accessed on 4 December 2024).
- Mah’moud, M.A. Current Management of Hepatitis C Virus Infection. N. Carol. Med. J. 2016, 77, 188–193. [Google Scholar] [CrossRef]
- Tamborini Permunian, E.; Gervaso, L.; Gerdes, V.; Moja, L.; Guasti, L.; Squizzato, A. Direct-acting antiviral drugs for chronic Hepatitis C and risk of major vascular events: A systematic review. Intern. Emerg. Med. 2018, 13, 775–790. [Google Scholar] [CrossRef]
- Bakhai, S.; Nallapeta, N.; El-Atoum, M.; Arya, T.; Reynolds, J.L. Improving Hepatitis C screening and diagnosis in patients born between 1945 and 1965 in a safety-net primary care clinic. BMJ Open Qual. 2019, 8, e000577. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021. Towards Ending Viral Hepatitis; World Health Organization: Geneva, Switzerland, 2016. Available online: https://iris.who.int/handle/10665/246177 (accessed on 4 December 2024).
- European Centres for Disease Prevention and Control. Systematic Review on Hepatitis B and C Prevalence in the EU/EEA; ECDC: Stockholm, Sweden, 2016. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/systematic-review-hepatitis-B-C-prevalence.pdf (accessed on 4 December 2024).
- D’Ambrosio, R.; Piccinelli, S.; Beccalli, B.; Spinetti, A.; Puoti, M.; Fagiuoli, S.; Magni, C.F.; Vavassori, A.; Sacchi, P.; Castaldi, S.; et al. A territory-wide opportunistic, hospital-based HCV screening in the general population from northern Italy: The 1969–1989 birth-cohort. Liver Int. 2023, 43, 2645–2656. [Google Scholar] [CrossRef]
- Emilia-Romagna. Screening Epatite C. 2023. Available online: https://salute.regione.emilia-romagna.it/screeningepatitec (accessed on 9 January 2025).
- Kondili, L.A.; Robbins, S.; Blach, S.; Gamkrelidze, I.; Zignego, A.L.; Brunetto, M.R.; Raimondo, G.; Taliani, G.; Iannone, A.; Russo, F.P.; et al. Forecasting Hepatitis C liver disease burden on real-life data. Does the hidden iceberg matter to reach the elimination goals? Liver Int. 2018, 38, 2190–2198. [Google Scholar] [CrossRef]
- Foreign Citizens in Italy-2023. Tuttitalia.it. 2024. Available online: https://www.tuttitalia.it/statistiche/cittadini-stranieri-2023/ (accessed on 4 June 2025).
- Kondili, L.A.; Gamkrelidze, I.; Blach, S.; Marcellusi, A.; Galli, M.; Petta, S.; Puoti, M.; Vella, S.; Razavi, H.; Craxi, A.; et al. Optimization of Hepatitis C Virus screening strategies by birth cohort in Italy. Liver Int. 2020, 40, 1545–1555. [Google Scholar] [CrossRef]
- Granozzi, B.; Guardigni, V.; Badia, L.; Rosselli Del Turco, E.; Zuppiroli, A.; Tazza, B.; Malosso, P.; Pieralli, S.; Viale, P.; Verucchi, G. Out-of-Hospital Treatment of Hepatitis C Increases Retention in Care among People Who Inject Drugs and Homeless Persons: An Observational Study. J. Clin. Med. 2021, 10, 4955. [Google Scholar] [CrossRef]
- Craxì, A.; Perno, C.F.; Viganò, M.; Ceccherini-Silberstein, F.; Petta, S. From current status to optimization of HCV treatment: Recommendations from an expert panel. Dig. Liver Dis. 2016, 48, 995–1005. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons with Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95/46/EC (General Data Protection Regulation); European Union: Brussels, Belgium, 2016. Available online: http://data.europa.eu/eli/reg/2016/679/oj (accessed on 4 June 2025).
- Italian Ministry of Health; National Screening Observatory. Recommendations for the Planning and Implementation of Population Screenings for the Prevention of Breast, Colon, and Cervical Cancers; Italian Ministry of Health: Rome, Italy, 2006. Available online: https://www.osservatorionazionalescreening.it/sites/default/files/allegati/screening_.pdf (accessed on 4 June 2025).
- Zorzi, M.; Hassan, C.; Capodaglio, G.; Fedato, C.; Montaguti, A.; Turrin, A.; Rosano, A.; Monetti, D.; Stocco, C.; Baracco, S.; et al. Long-term performance of colorectal cancerscreening programmes based on the faecal immunochemical test. Gut 2018, 67, 2124–2130. [Google Scholar] [CrossRef]
- Moshina, N.; Ursin, G.; Roman, M.; Sebuødegård, S.; Hofvind, S. Positive predictive values by mammographic density and screening mode in the Norwegian Breast Cancer Screening Program. Eur. J. Radiol. 2016, 85, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.A.; Kiener, T.; Johnson, H.A.; Li, K.H.; Limburg, P.J.; Fendrick, A.M.; Kisiel, J.B.; Ebner, D.W. Adherence to recommended blood-based screening tests for cancer and chronic diseases: A systematic literature review. Prev. Med. 2025, 191, 108213. [Google Scholar] [CrossRef]
- Zorzi, M. 2023 Survey: Extension and Uptake. 2024. Available online: https://www.giscor.it/wp-content/uploads/2024/04/1_ZORZI_giscor_2024.pdf (accessed on 4 June 2025).
- Giorgi Rossi, P.; Vicentini, M.; Sacchettini, C.; Di Felice, E.; Caroli, S.; Ferrari, F.; Mangone, L.; Pezzarossi, A.; Roncaglia, F.; Campari, C.; et al. Impact of Screening Program on Incidence of Colorectal Cancer: A Cohort Study in Italy. Am. J. Gastroenterol. 2015, 110, 1359–1366. [Google Scholar] [CrossRef]
- Italian Government. DPCM January 12, 2017—Definition and Update fo the Essential Healthcare Levels as to Art. 1 of the Legislative Decree December 30, 1992, n. 502. 2017. Available online: https://www.gazzettaufficiale.it/eli/id/2017/03/18/17A02015/sg (accessed on 4 June 2025).
- Giorgi Rossi, P.; Caroli, S.; Mancini, S.; de’ Bianchi, P.S.; Finarelli, A.C.; Naldoni, C.; Bucchi, L.; Falcini, F. Screening history of cervical cancers in Emilia-Romagna, Italy: Defining priorities to improve cervical cancer screening. Eur. J. Cancer Prev. 2015, 24, 128–134. [Google Scholar] [CrossRef]
- Carrat, F.; Fontaine, H.; Dorival, C.; Simony, M.; Diallo, A.; Hezode, C.; De Ledinghen, V.; Larrey, D.; Haour, G.; Bronowicki, J.P.; et al. Clinical outcomes in patients with chronic Hepatitis C after direct-acting antiviral treatment: A prospective cohort study. Lancet 2019, 393, 1453–1464. [Google Scholar] [CrossRef]
- Alavi, M.; Law, M.G.; Valerio, H.; Grebely, J.; Amin, J.; Hajarizadeh, B.; Selvey, C.; George, J.; Dore, G.J. Declining Hepatitis C Virus-related liver disease burden in the direct-acting antiviral therapy era in New South Wales, Australia. J. Hepatol. 2019, 71, 281–288. [Google Scholar] [CrossRef]
- Deuffic-Burban, S.; Huneau, A.; Verleene, A.; Brouard, C.; Pillonel, J.; Le Strat, Y.; Cossais, S.; Roudot-Thoraval, F.; Canva, V.; Mathurin, P.; et al. Assessing the cost-effectiveness of Hepatitis C screening strategies in France. J. Hepatol. 2018, 69, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, L.; La Mantia, C.; Di Marco, V. Hepatitis C: Standard of Treatment and What to Do for Global Elimination. Viruses 2022, 14, 505. [Google Scholar] [CrossRef]
- Zhou, H.; Yan, M.; Che, D.; Wu, B. Universal screening for HCV infection in China: An effectiveness and cost-effectiveness analysis. JHEP Rep. 2024, 6, 101000. [Google Scholar] [CrossRef]
- Kondili, L.A.; Blach, S.; Razavi, H.; Craxì, A. Tailored screening and dedicated funding for direct acting antiviral drugs: How to keep Italy on the road to Hepatitis C Virus elimination? Ann. Ist. Super. Sanita 2020, 56, 325–329. [Google Scholar] [CrossRef]
- Polaris Observatory HCV Collaborators. Global change in Hepatitis C Virus prevalence and cascade of care between 2015 and 2020: A modelling study. Lancet Gastroenterol. Hepatol. 2022, 7, 396–415. [Google Scholar] [CrossRef]
- Marcellusi, A.; Simonelli, C.; Mennini, F.S.; Kondili, L.A.; on behalf of PITER Collaborating Group. Economic Consequences of Anti-HCV Treatment of Patients Diagnosed Through Screening in Italy: A Prospective Modelling Analysis. Appl. Health Econ. Health Policy 2022, 20, 133–143. [Google Scholar] [CrossRef]
- Ledesma, F.; Buti, M.; Domínguez-Hernández, R.; Casado, M.A.; Esteban, R. Is the universal population Hepatitis C Virus screening a cost-effective strategy? A systematic review of the economic evidence. Rev. Esp. Quimioter. 2020, 33, 240–248. [Google Scholar] [CrossRef]
- D’Ambrosio, R.; Rizzardini, G.; Puoti, M.; Fagiuoli, S.; Anolli, M.P.; Gabiati, C.; D’Amico, F.; Pasulo, L.; Restelli, U.; Colombo, M.; et al. Implementation of HCV screening in the 1969-1989 birth-cohort undergoing COVID-19 vaccination. Liver Int. 2022, 42, 1012–1016. [Google Scholar] [CrossRef]
- Viganò, M.; Cerini, F.; Ridolfo, S.; Rumi, M.G. Hepatitis C Virus screening in the 1969–1989 birth cohort: Is not enough! Liver Int. 2022, 42, 1915. [Google Scholar] [CrossRef]
- Han, R.; Zhou, J.; François, C.; Toumi, M. Prevalence of Hepatitis C infection among the general population and high-risk groups in the EU/EEA: A systematic review update. BMC Infect. Dis. 2019, 19, 655. [Google Scholar] [CrossRef]
- Guadagnino, V.; Stroffolini, T.; Rapicetta, M.; Costantino, A.; Kondili, L.A.; Menniti-Ippolito, F.; Caroleo, B.; Costa, C.; Griffo, G.; Loiacono, L.; et al. Prevalence, risk factors, and genotype distribution of Hepatitis C Virus infection in the general population: A community-based survey in southern Italy. Hepatology 1997, 26, 1006–1011. [Google Scholar] [CrossRef]
- Stroffolini, T.; Stroffolini, G. Prevalence and Modes of Transmission of Hepatitis C Virus Infection: A Historical Worldwide Review. Viruses 2024, 16, 1115. [Google Scholar] [CrossRef]
- Andriulli, A.; Stroffolini, T.; Mariano, A.; Valvano, M.R.; Grattagliano, I.; Ippolito, A.M.; Grossi, A.; Brancaccio, G.; Coco, C.; Russello, M.; et al. Declining prevalence and increasing awareness of HCV infection in Italy: A population-based survey in five metropolitan areas. Eur. J. Intern. Med. 2018, 53, 79–84. [Google Scholar] [CrossRef]
- Cunningham, E.B.; Wheeler, A.; Hajarizadeh, B.; French, C.E.; Roche, R.; Marshall, A.D.; Fontaine, G.; Conway, A.; Bajis, S.; Valencia, B.M.; et al. Interventions to enhance testing and linkage to treatment for Hepatitis C infection for people who inject drugs: A systematic review and meta-analysis. Int. J. Drug Policy 2023, 111, 103917. [Google Scholar] [CrossRef]
- Kronfli, N.; Linthwaite, B.; Kouyoumdjian, F.; Klein, M.B.; Lebouché, B.; Sebastiani, G.; Cox, J. Interventions to increase testing, linkage to care and treatment of Hepatitis C Virus (HCV) infection among people in prisons: A systematic review. Int. J. Drug Policy 2018, 57, 95–103. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Safreed-Harmon, K.; Thursz, M.R.; Dillon, J.F.; El-Sayed, M.H.; Elsharkawy, A.M.; Hatzakis, A.; Jadoul, M.; Prestileo, T.; Razavi, H.; et al. The Micro-Elimination Approach to Eliminating Hepatitis C: Strategic and Operational Considerations. Semin. Liver Dis. 2018, 38, 181–192. [Google Scholar] [CrossRef]
- Pavarin, R.M.; Badia, L.; Nisi, S.; Iormetti, C.; Caputo, F. HCV testing and linkage to care for Hepatitis C Virus infection for marginalized drug user populations attending a harm reduction service in Bologna, Italy. Infez. Med. 2024, 32, 373–380. [Google Scholar] [CrossRef]
- Barror, S.; Avramovic, G.; Oprea, C.; Surey, J.; Story, A.; Macías, J.; Cullen, W.; Crowley, D.; Horan, A.; Naughton, A.M.; et al. HepCare Europe: A service innovation project. HepCheck: Enhancing HCV identification and linkage to care for vulnerable populations through intensified outreach screening. A prospective multisite feasibility study. J. Antimicrob. Chemother. 2019, 74 (Suppl. S3), v39–v46. [Google Scholar] [CrossRef]
- Flisiak, R.; Zarębska-Michaluk, D.; Ciupkeviciene, E.; Drazilova, S.; Frankova, S.; Grgurevic, I.; Hunyady, B.; Jarcuska, P.; Kupčinskas, L.; Makara, M.; et al. HCV Elimination in Central Europe with Particular Emphasis on Microelimination in Prisons. Viruses 2022, 14, 482. [Google Scholar] [CrossRef]
- Huang, C.-F.; Chen, G.-J.; Hung, C.-C.; Yu, M.-L. HCV Microelimination for High-risk Special Populations. J. Infect. Dis. 2023, 228, S168–S179. [Google Scholar] [CrossRef]
- Lopes, S.S.; Pericot-Valverde, I.; Lum, P.J.; Taylor, L.E.; Mehta, S.H.; Tsui, J.I.; Feinberg, J.; Kim, A.Y.; Norton, B.L.; Page, K.; et al. Overreporting of adherence to Hepatitis C direct-acting antiviral therapy and sustained virologic response among people who inject drugs in the HERO study. BMC Infect. Dis. 2024, 24, 251. [Google Scholar] [CrossRef]
- Werling, K.; Hunyady, B.; Makara, M.; Nemesi, K.; Horváth, G.; Schneider, F.; Enyedi, J.; Müller, Z.; Lesch, M.; Péterfi, Z.; et al. Hepatitis C Screening and Treatment Program in Hungarian Prisons in the Era of Direct Acting Antiviral Agents. Viruses 2022, 14, 308. [Google Scholar] [CrossRef]
- Cambianica, A.; Marchese, V.; Pennati, F.; Faustinelli, A.; Migliorati, M.; Roda, F.; Spinetti, A.; Zaltron, S.; Fiorentini, S.; Caruso, A.; et al. Chronic Hepatitis C Cascade of Care in Prisoners-Is There Still Some Work to Do? Analysis of Two Large Penitentiaries in Northern Italy. Int. J. Environ. Res. Public Health 2024, 21, 104. [Google Scholar] [CrossRef]
- Fernandes, N.D.; Banik, S.; Abughali, N.; Sthapit, B.; Abdullah, N.; Fragassi, P. Hepatitis C Virus Screening Among Adolescents Attending a Drug Rehabilitation Center. J. Pediatric Infect. Dis. Soc. 2020, 9, 437–441. [Google Scholar] [CrossRef]
- Izzo, C.; Masarone, M.; Torre, P.; Melara, G.; De Matteis, G.; De Luna, A.; Pagano, A.M.; Persico, M. Solving the Gap Between HCV Detection and Treatment in Prison HCVRNA Testing and Treatment in a Cohort of Newly Arrived Convicts in Southern Italy. Rev. Recent. Clin. Trials 2021, 16, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Traeger, M.W.; Harney, B.L.; Sacks-Davis, R.; van Santen, D.K.; Cornelisse, V.J.; Wright, E.J.; Hellard, M.E.; Doyle, J.S.; Stoové, M.A. Incidence and Prevalence of Hepatitis C Virus Among HIV-Negative Gay and Bisexual Men Using HIV Pre-exposure Prophylaxis (PrEP): A Systematic Review and Meta-analysis. Open Forum Infect. Dis. 2023, 10, ofad401. [Google Scholar] [CrossRef]
- Dettori, S.; Russo, C.; Mora, S.; Giacomini, M.; Taramasso, L.; Dentone, C.; Vena, A.; Bassetti, M.; Di Biagio, A. Prevalence of Viral Hepatitis in Unselected, Consecutively Enrolled Patients Hospitalised for SARS-CoV-2. J. Community Health 2022, 47, 800–805. [Google Scholar] [CrossRef]
- Rosato, V.; Kondili, L.A.; Nevola, R.; Perillo, P.; Mastrocinque, D.; Aghemo, A.; Claar, E. Elimination of Hepatitis C in Southern Italy: A Model of HCV Screening and Linkage to Care among Hospitalized Patients at Different Hospital Divisions. Viruses 2022, 14, 1096. [Google Scholar] [CrossRef] [PubMed]
- Torre, P.; Annunziata, M.; Sciorio, R.; Coppola, C.; Masarone, M.; Persico, M. Hepatitis C screening during SARS-CoV-2 testing or vaccination. Experience in an area of southern Italy in the province of Salerno. Liver Int. 2022, 42, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, M.; Pagani, G.; Conti, F.; Pezzati, L.; Casalini, G.; Rondanin, R.; Prina, A.; Zagari, A.; Galli, M.; Giacomelli, A. HCV point of care screening in people tested for SARS-CoV-2 in a social-housing neighbourhood of Milan, Italy. Liver Int. 2023, 43, 251–255. [Google Scholar] [CrossRef]
- Petroff, D.; Bätz, O.; Jedrysiak, K.; Lüllau, A.; Kramer, J.; Möller, H.; Heyne, R.; Jäger, B.; Berg, T.; Wiegand, J. From Screening to Therapy: Anti-HCV Screening and Linkage to Care in a Network of General Practitioners and a Private Gastroenterology Practice. Pathogens 2021, 10, 1570. [Google Scholar] [CrossRef]
- Yousafzai, M.T.; Bajis, S.; Alavi, M.; Grebely, J.; Dore, G.J.; Hajarizadeh, B. Global cascade of care for chronic Hepatitis C Virus infection: A systematic review and meta-analysis. J. Viral Hepat. 2021, 28, 1340–1354. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).