Gene Expression Modulation in Bovine Endometrial Cells Infected with Gammaherpesvirus Type 4 and Exposed to Lipopolysaccharide in the Presence of Platelet-Rich Plasma
Abstract
1. Introduction
2. Materials and Methods
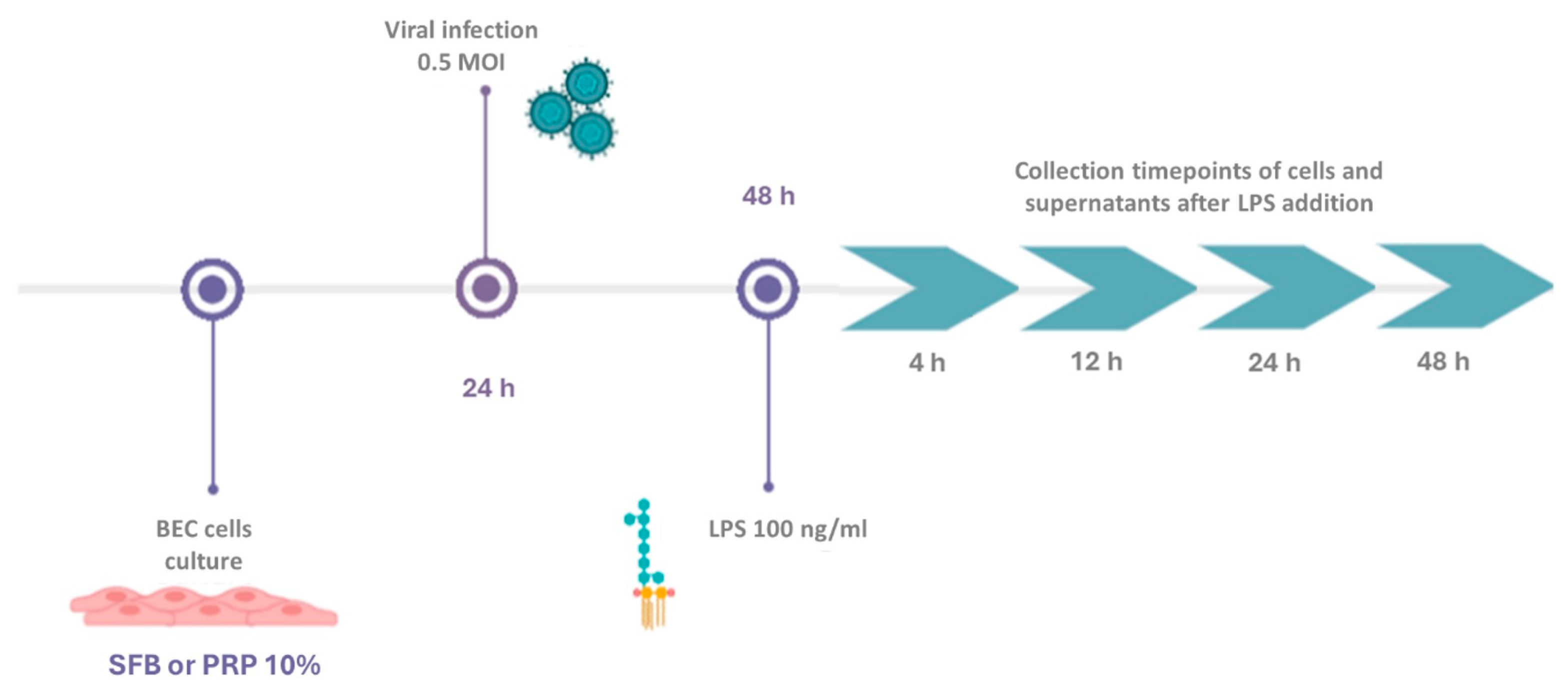
2.1. Culture of Bovine Endometrial Cells (BECs)
2.2. Viral Infection
2.3. Preparation of Platelet-Rich Plasma (PRP)
2.4. Viral Infection and PRP Treatment
2.5. RT‒qPCR
2.6. Statistical Analysis
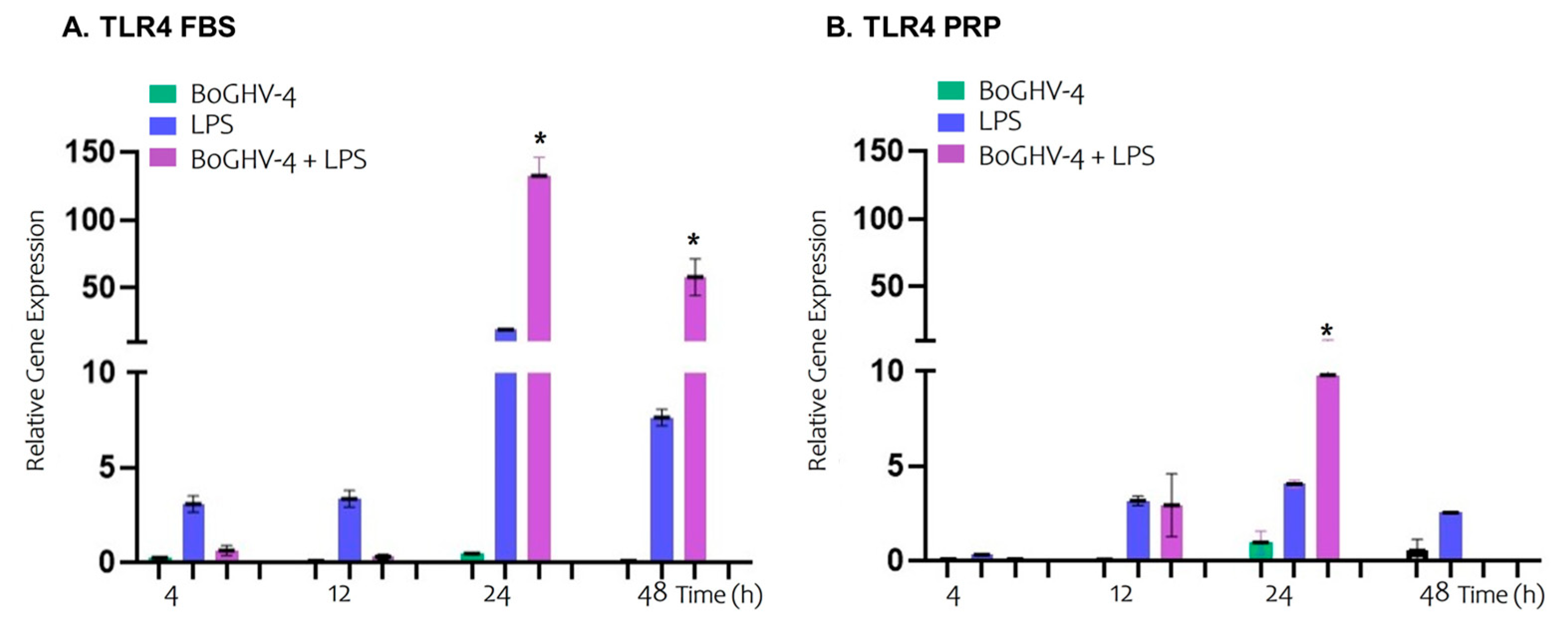
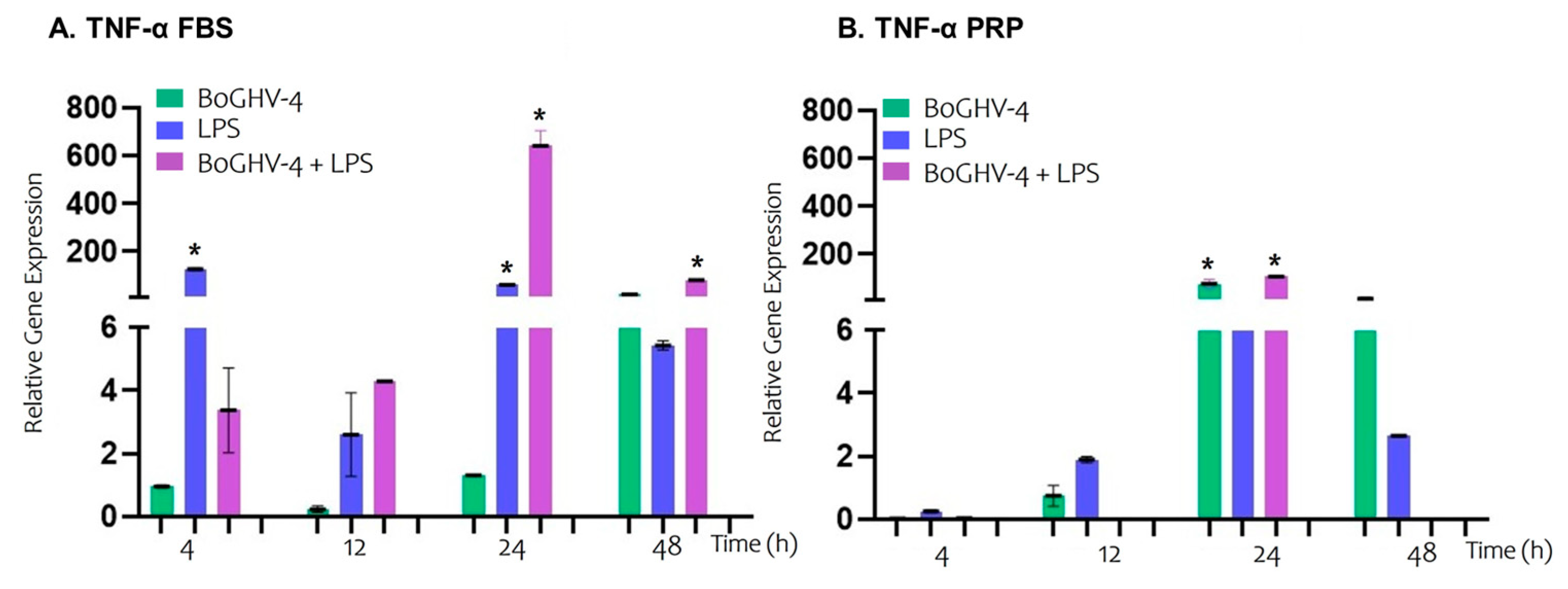
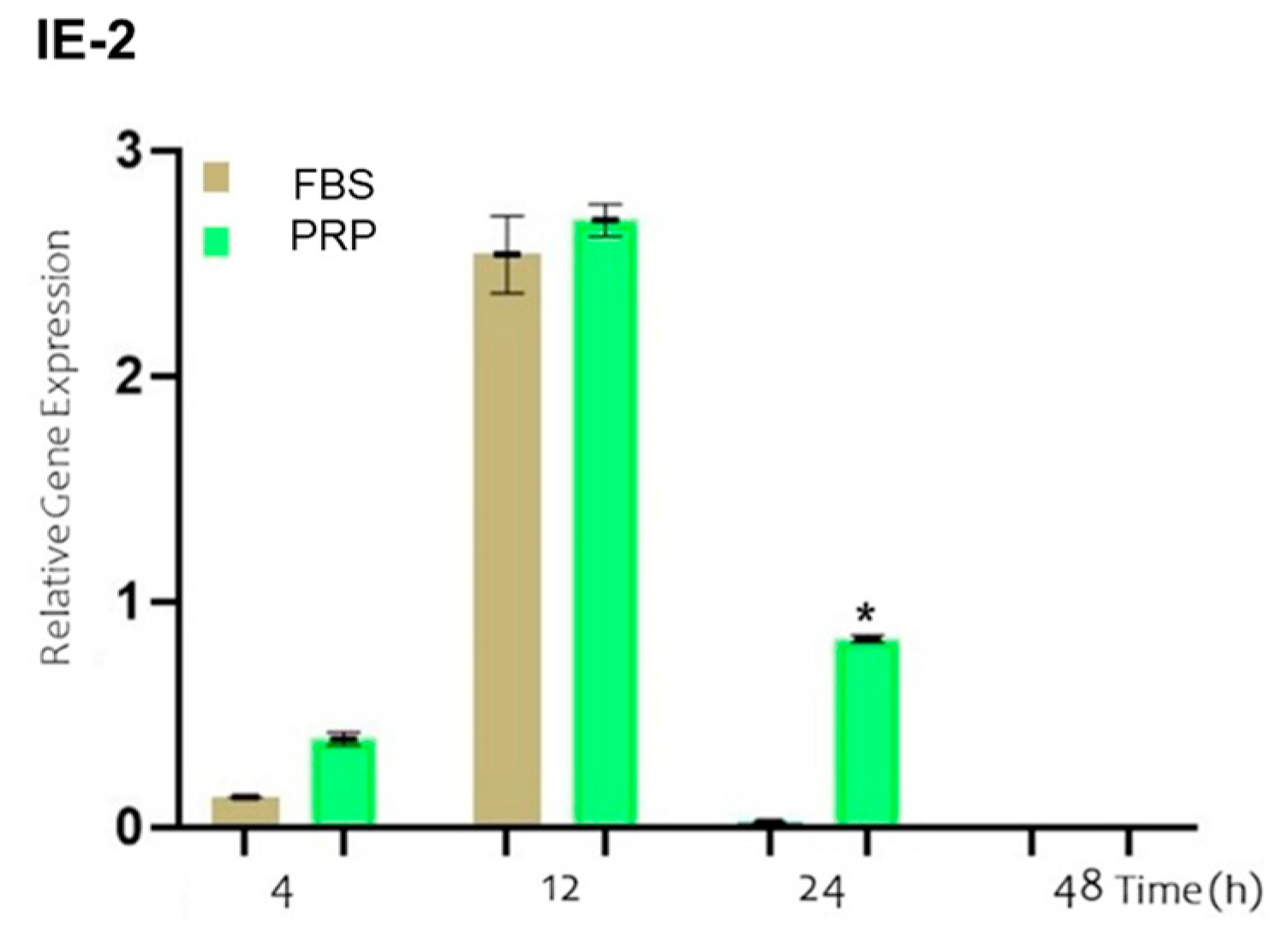
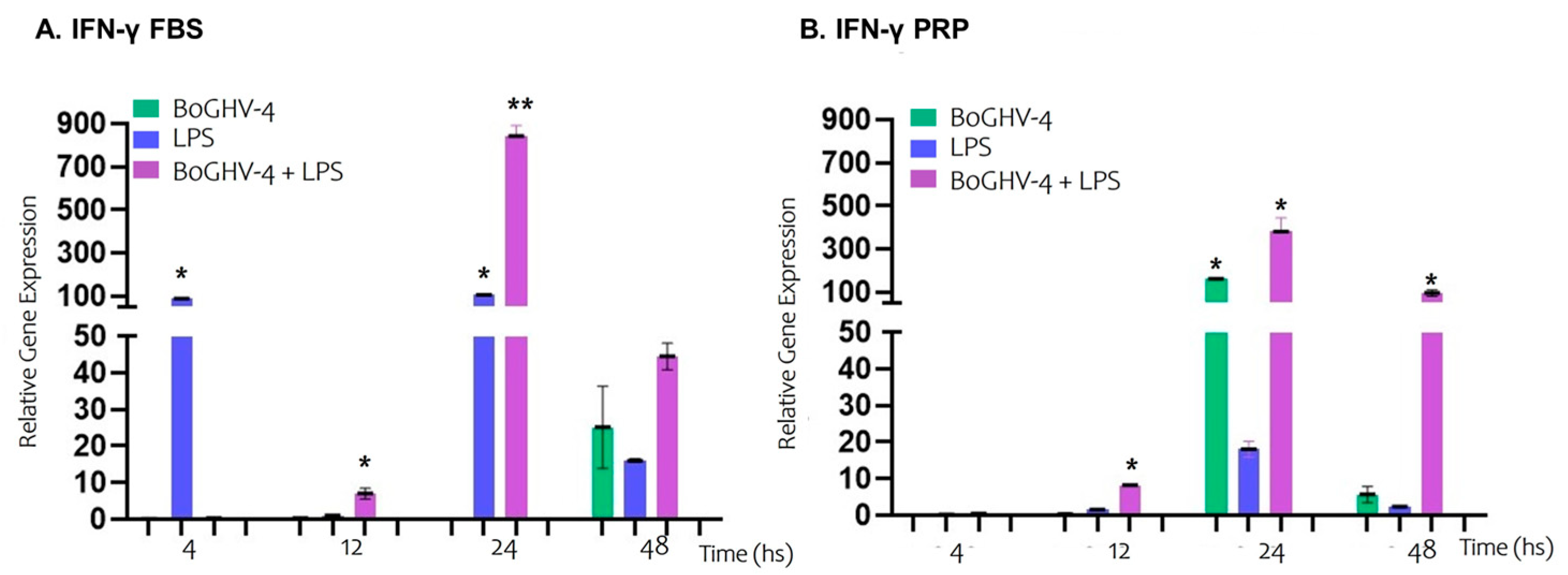
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BoGHV-4 | Bovine Gammaherpesvirus Type 4 |
| PRP | Platelet-rich plasma |
| LPS | Lipopolysaccharide |
| TLR4 | Toll-like receptor 4 |
| IE-2 | Viral immediate–early gene |
| TNF-α | Tumor necrosis factor-alpha |
| IL-8 | Interleukin-8 |
| IFN-γ | Interferon-gamma |
| BEC | Bovine endometrial cells |
| FBS | Fetal bovine serum |
| BoAHV-1 | Bovine Alphaherpesvirus 1 |
| BVDV | Bovine Viral Diarrhea virus |
| MDBK | Madin–Darby Bovine Kidney |
| hpi | hours post-infection |
| PPP | Platelet-poor plasma |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| Ct | threshold cycle |
References
- LeBlanc, S.J.; Duffield, T.F.; Leslie, K.E.; Bateman, K.G.; Keefe, G.P.; Walton, J.S.; Johnson, W.H. The effect of treatment of clinical endometritis on reproductive performance in dairy cows. J. Dairy Sci. 2002, 85, 2237–2249. [Google Scholar] [CrossRef] [PubMed]
- Johnston-MacAnanny, E.B.; Hartnett, J.; Engmann, L.L.; Nulsen, J.C.; Sanders, M.M.; Benadiva, C.A. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil. Steril. 2010, 93, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Plöntzke, J.; Madoz, L.V.; De la Sota, R.L.; Drillich, M.; Heuwieser, W. Subclinical endometritis and its impact on reproductive performance in grazing dairy cattle in Argentina. Anim. Reprod. Sci. 2010, 122, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Giuliodori, M.J.; Magnasco, R.P.; Becu-Villalobos, D.; Lacau-Mengido, I.M.; Risco, C.A.; de la Sota, R.L. Clinical endometritis in an Argentinean herd of dairy cows: Risk factors and reproductive efficiency. J. Dairy Sci. 2013, 96, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Novikov, A.; Breton, A.; Caroff, M. Micromethods for isolation and structural characterization of lipid A, and polysaccharide regions of bacterial lipopolysaccharides. In Microbial Toxins; Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1600. [Google Scholar]
- Williams, E.J.; Fischer, D.; Noakes, D.; England, G.; Rycroft, A.; Dobson, H.; Sheldon, I. The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology 2007, 68, 549–559. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Roberts, M.H. Toll-like receptor 4 mediates the response of epithelial and stromal cells to lipopolysaccharide in the endometrium. PLoS ONE 2010, 5, e12906. [Google Scholar] [CrossRef]
- Davison, A.J.; Eberle, R.; Ehlers, B.; Hayward, G.S.; McGeoch, D.J.; Minson, A.C.; Pellett, P.E.; Roizman, B.; Studdert, M.J.; Thiry, E. The order Herpesvirales. Arch. Virol. 2009, 154, 171–177. [Google Scholar] [CrossRef]
- Donofrio, G.; Cavirani, S.; Taddei, S.; Flammini, C.F. Activation of bovine herpesvirus 4 lytic replication in a nonpermissive cell line by overexpression of BoHV-4 immediate early (IE) 2 gene. J. Virol. Methods 2004, 116, 203–207. [Google Scholar] [CrossRef]
- Egyed, L. Replication of bovine herpesvirus type 4 in human cells in vitro. J. Clin. Microbiol. 1998, 36, 2109–2111. [Google Scholar] [CrossRef]
- Gillet, L.; Dewals, B.; Farnir, F.; De Level, L.; Vanderplasschen, A. Bovine herpesvirus 4 induces apoptosis of human carcinoma cell lines in vitro and in vivo. Cancer Res. 2005, 65, 9463–9472. [Google Scholar] [CrossRef]
- Donofrio, G.; Herath, S.; Sartori, C.; Cavirani, S.; Flammini, C.F.; Sheldon, I.M. Bovine herpesvirus 4 is tropic for bovine endometrial cells and modulates endocrine function. Reproduction 2007, 134, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Donofrio, G.; Ravanetti, L.; Cavirani, S.; Herath, S.; Capocefalo, A.; Sheldon, I.M. Bacterial infection of endometrial stromal cells influences bovine herpesvirus 4 immediate early gene activation: A new insight into bacterial and viral interaction for uterine disease. Reproduction 2008, 136, 361–366. [Google Scholar] [CrossRef]
- Bilge-Dagalp, S.; Gungor, E.; Demir, A.B.; Pınar Muz, D.; Yılmaz, V.; Oguzoglu, T.Ç.; Ataseven, V.S.; Alkan, F. The investigation of the presence of bovine herpesvirus type 4 (BoHV-4) in cows with metritis in a dairy herd. Ank. Univ. Vet. Fak. Derg. 2010, 57, 87–91. [Google Scholar] [CrossRef]
- Daǧalp, S.B.; Gungor, E.; Demir, A.; Pinar-Muz, D.; Yilmaz, V.; Oğuzoğlu, T.; Ataseven, V.; Alkan, F. The investigation of the herpesviruses (BoHV-1 and BoHV-4) on the occurrence of the reproductive disorders in dairy cattle herds, Turkey. Rev. Med. Vet. 2012, 163, 206–211. [Google Scholar]
- Donofrio, G.; Flammini, C.F.; Scatozza, F.; Cavirani, S. Detection of bovine herpesvirus 4 (BoHV-4) DNA in the cell fraction of milk of dairy cattle with history of BoHV-4 infection. J. Clin. Microbiol. 2000, 38, 4668–4671. [Google Scholar] [CrossRef]
- Izumi, Y.; Tsuduku, S.; Murakami, K.; Tsuboi, T.; Konishi, M.; Haritani, M.; Kamiyoshi, T.; Kimura, K.; Sentsui, H. Characterization of bovine herpesvirus type 4 isolated from cattle with mastitis and subclinical infection by the virus among cattle. J. Vet. Med. Sci. 2006, 68, 189–193. [Google Scholar] [CrossRef]
- Miyano, H.; Haritani, M.; Sentsui, H.; Tsuboi, T.; Tanimura, N.; Kimura, K.M.; Kobayashi, M.; Obara, N.; Akimoto, Y. Mammary lesions associated with bovine herpesvirus type 4 in a cow with clinical mastitis. J. Vet. Med. Sci. 2004, 66, 457–460. [Google Scholar] [CrossRef]
- Nikolin, V.M.; Donofrio, G.; Miloševic, B.; Taddei, S.; Radosavljevic, V.; Milicevic, V. First Serbian isolates of bovine herpesvirus 4 (BoHV-4) from a herd with a history of postpartum metritis. New Microbiol. 2007, 30, 53–57. [Google Scholar] [PubMed]
- Verna, A.E.; Manrique, J.; Pérez, S.; Leunda, M.; Pereyra, S.; Jones, L.; Odeón, A. Genomic analysis of bovine herpesvirus type 4 (BoHV-4) from Argentina: High genetic variability and novel phylogenetic groups. Vet. Microbiol. 2012, 160, 1–8. [Google Scholar] [CrossRef]
- Romeo, F.; Spetter, M.J.; Moran, P.; Pereyra, S.; Odeon, A.; Perez, S.E.; Verna, A.E. Analysis of the transcripts encoding for antigenic proteins of bovine gammaherpesvirus 4. J. Vet. Sci. 2020, 21, e5. [Google Scholar] [CrossRef]
- Romeo, F.; Louge-Uriarte, E.; Gonzalez-Altamiranda, E.; Delgado, S.; Pereyra, S.; Morán, P.; Odeón, A.; Pérez, S.; Verna, A.E. Gene expression and in vitro replication of bovine gammaherpesvirus type 4. Arch. Virol. 2021, 166, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, J.; Guillaume, J.; Boulanger, D.; Thiry, E.; Bublot, M.; Pastoret, P.P. Neutralization of bovine herpesvirus type 4 by pairs of monoclonal antibodies raised against two glycoproteins and identification of antigenic determinants involved in neutralization. J. Gen. Virol. 1990, 71, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.; Meade, K.G.; Herath, S.; Eckersall, P.D.; Gonzalez, D.; O White, J.; Conlan, R.S.; O'Farrelly, C.; Sheldon, I.M. Toll-like receptor and antimicrobial peptide expression in the bovine endometrium. Reprod. Biol. Endocrinol. 2008, 6, 53. [Google Scholar] [CrossRef]
- Herath, S.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Use of the cow as a large animal model of uterine infection and immunity. J. Reprod. Immunol. 2006, 69, 13–22. [Google Scholar] [CrossRef]
- Franceschi, V.; Capocefalo, A.; Ravanetti, L.; Vanderplasschen, A.; Gillet, L.; Cavirani, S.; van Santen, V.L.; Donofrio, G. Bovine herpesvirus 4 immediate early 2 (Rta) gene is an essential gene and is duplicated in bovine herpesvirus 4 isolate U. Vet. Microbiol. 2011, 148, 219–231. [Google Scholar] [CrossRef]
- Donofrio, G.; Capocefalo, A.; Franceschi, V.; Price, S.; Cavirani, S.; Sheldon, I.M. The chemokine IL-8 is upregulated in bovine endometrial stromal cells by the BoHV-4 IE-2 gene product, ORF50/Rta: A step ahead toward a mechanism for BoHV-4 induced endometritis. Biol. Reprod. 2010, 83, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Jacca, S.; Franceschi, V.; Agosti, M.; Cavirani, S.; Mistretta, F.; Donofrio, G. Interferon gamma-mediated BoHV-4 replication restriction in bovine endometrial stromal cells is host IDO1 gene expression independent and BoHV-4 IE-2 gene expression dependent. Biol. Reprod. 2014, 91, 112. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.J. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef]
- Shams-Esfandabadi, N.; Shirazi, A.; Ghasemzadeh-Nava, H. Pregnancy rate following postinsemination intrauterine treatment of endometritis in dairy cattle. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2004, 51, 155–156. [Google Scholar] [CrossRef]
- Heuwieser, W.; Tenhagen, B.A.; Tischer, M.; Lühr, J.; Blum, H. Effect of three programmes for the treatment of endometritis on the reproductive performance of a dairy herd. Vet. Rec. 2000, 146, 338–341. [Google Scholar] [CrossRef]
- Knutti, B.; Küpfer, U.; Busato, A. Reproductive Efficiency of Cows with Endometritis after Treatment with Intrauterine Infusions or Prostaglandin Injections, or No Treatment. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2000, 47, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-rich plasma: New performance understandings and therapeutic considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant. Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef]
- Leslie, M. Beyond clotting: The powers of platelets. Science 2010, 328, 562–564. [Google Scholar] [CrossRef]
- Sánchez-González, D.J.; Méndez-Bolaina, E.; Trejo-Bahena, N.I. Platelet-rich plasma peptides: Key for regeneration. Int. J. Pept. 2012, 2012, 532519. [Google Scholar] [CrossRef] [PubMed]
- Iacopetti, I.; Patruno, M.; Melotti, L.; Martinello, T.; Bedin, S.; Badon, T.; Righetto, E.M.; Perazzi, A. Autologous platelet-rich plasma enhances the healing of large cutaneous wounds in dogs. Front. Vet. Sci. 2020, 7, 575449. [Google Scholar] [CrossRef]
- Romeo, F.; Uriarte, E.L.; Delgado, S.; González-Altamiranda, E.; Pereyra, S.; Morán, P.; Odeón, A.; Pérez, S.; Verna, A. Effect of bovine viral diarrhea virus on subsequent infectivity of bovine gammaherpesvirus 4 in endometrial cells in primary culture: An in vitro model of viral coinfection. J. Virol. Methods 2021, 291, 114097. [Google Scholar] [CrossRef]
- Florencia, R.; Julieta, M.; Sandra, P.; Enrique, L.U.; Maia, M.; German, C.; Leunda, M.R.; Erika, G.A.; Susana, P.; Maximiliano, S.; et al. Characterization of the first bovine gammaherpesvirus 4 strain isolated from an aborted bovine fetus in Argentina. Arch. Virol. 2020, 165, 719–723. [Google Scholar] [CrossRef]
- Spetter, M.J.; Uriarte, E.L.L.; Armendano, J.I.; Álvarez, I.; Norero, N.S.; Storani, L.; Pereyra, S.B.; Verna, A.E.; Odeón, A.C.; Altamiranda, E.A.G. Frequency of bovine viral diarrhea virus (BVDV) in Argentinean bovine herds and comparison of diagnostic tests for BVDV detection in bovine serum samples: A preliminary study. Braz. J. Microbiol. 2021, 52, 467–475. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Palagiano, P.; Graziano, L.; Scarabello, W.; Berni, P.; Andreoli, V.; Grolli, S. Platelet-Rich Plasma Treatment Supported by Ultrasound Detection of Septa in Recurrent Canine Aural Hematoma: A Case Series. Animals 2023, 13, 2456. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Williams, E.J.; Miller, A.N.A.; Nash, D.M.; Herath, S. Uterine diseases in cattle after parturition. Vet. J. 2008, 176, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J.; Herath, S.; England, G.C.W.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Effect of Escherichia coli infection of the bovine uterus from the whole animal to the cell. Animal 2008, 2, 1153–1157. [Google Scholar] [CrossRef]
- Chanrot, M.; Blomqvist, G.; Guo, Y.; Ullman, K.; Juremalm, M.; Bage, R.; Donofrio, G.; Valarcher, J.-F.; Humblot, P. Bovine herpes virus type 4 alters TNF-α and IL-8 profiles and impairs the survival of bovine endometrial epithelial cells. Reprod. Biol. 2017, 17, 225–232. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, B.; Mao, W.; Gao, R.; Feng, S.; Qian, Y.; Wu, J.; Zhang, S.; Gao, L.; Fu, C.; et al. PGE2 downregulates LPS-induced inflammatory responses via the TLR4-NF-κB signalling pathway in bovine endometrial epithelial cells. Prostaglandins Leukot. Essent. Fat. Acids 2018, 129, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Noakes, D.E.; Rycroft, A.N.; Pfeiffer, D.U.; Dobson, H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction 2002, 123, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Dobson, H. Postpartum uterine health in cattle. Anim. Reprod. Sci. 2004, 82–83, 295–306. [Google Scholar] [CrossRef]
- Verna, A.; Leunda, M.; Louge Iriarte, E.; Lomonaco, M.; Pereyra, S.; Odeón, A. Primera evidencia virológica de herpesvirus bovino tipo 4 (BoHV-4) en Argentina. Rev. Argent. Microbiol. 2008, 40 (Suppl. 1), 54–55. [Google Scholar]
- Donofrio, G.; Cavirani, S.; Van Santen, V.; Flammini, C.F. Potential secondary pathogenic role for bovine herpesvirus 4. J. Clin. Microbiol. 2005, 43, 3421–3426. [Google Scholar] [CrossRef]
- Tebaldi, G.; Jacca, S.; Montanini, B.; Capra, E.; Rosamilia, A.; Sala, A.; Stella, A.; Castiglioni, B.; Ottonello, S.; Donofrio, G. Virus-mediated metalloproteinase 1 induction revealed by transcriptome profiling of bovine herpesvirus 4-infected bovine endometrial stromal cells. Biol. Reprod. 2016, 95, 12. [Google Scholar] [CrossRef][Green Version]
- LeBlanc, S.J.; Duffield, T.; Leslie, K.; Bateman, K.; Keefe, G.; Walton, J.; Johnson, W. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J. Dairy. Sci. 2002, 85, 2223–2236. [Google Scholar] [CrossRef] [PubMed]
- Runciman, D.J.; Anderson, G.A.; Malmo, J. Comparison of two methods of detecting purulent vaginal discharge in postpartum dairy cows and effect of intrauterine cephapirin on reproductive performance. Aust. Vet. J. 2009, 87, 369–378. [Google Scholar] [CrossRef]
- Xiong, T.; Zheng, X.; Zhang, K.; Wu, H.; Dong, Y.; Zhou, F.; Cheng, B.; Li, L.; Xu, W.; Su, J.; et al. Ganluyin ameliorates DSS-induced ulcerative colitis by inhibiting the enteric-origin LPS/TLR4/NF-κB pathway. J. Ethnopharmacol. 2022, 289, 115001. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, B.; Feng, X.; Liu, Z.; Liang, D.; Li, F.; Li, D.; Cao, Y.; Feng, S.; Zhang, X.; et al. Lipopolysaccharide increases Toll-like receptor 4 and downstream Toll-like receptor signalling molecules expression in bovine endometrial epithelial cells. Vet. Immunol. Immunopathol. 2013, 151, 20–27. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Wen, X.; Hao, C.; Ma, J.; Yan, L. Platelet rich plasma alleviates endometritis induced by lipopolysaccharide in mice by inhibiting TLR4/NF-κB signalling pathway. Am. J. Reprod. Immunol. 2024, 91, e13833. [Google Scholar] [CrossRef] [PubMed]
- Herath, S.; Fischer, D.P.; Werling, D.; Williams, E.J.; Lilly, S.T.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Expression and function of toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology 2006, 147, 562–570. [Google Scholar] [CrossRef]
- Donofrio, G.; Franceschi, V.; Capocefalo, A.; Cavirani, S.; Sheldon, I.M. Isolation and characterization of bovine herpesvirus 4 (BoHV-4) from a cow affected by post partum metritis and cloning of the genome as a bacterial artificial chromosome. Reprod. Biol. Endocrinol. 2009, 7, 83. [Google Scholar] [CrossRef]
- Jacca, S.; Franceschi, V.; Colagiorgi, A.; Sheldon, M.; Donofrio, G. Bovine endometrial stromal cells support tumor necrosis factor alpha-induced bovine herpesvirus type 4 enhanced replication. Biol. Reprod. 2013, 88, 135. [Google Scholar] [CrossRef]

| Primer Sequences | ||
|---|---|---|
| Name | Type | Sequences |
| GAPDH | FWD | 5′-TTC TGG CAA AGT GGA CAT CGT-3′ |
| GAPDH | REV | 5′-CCT GAC TGT GCC GTT GAA CTT-3′ |
| IL-8 | FWD | 5′-CCT CTT GTT CAA TAT GAC TTC CA-3′ |
| IL-8 | REV | 5′-GGC CCA CTC TCA ATA ACT CTC-3′ |
| TNF-α | FWD | 5′-CCA CGT TGT AGC CGA CAT CA-3′ |
| TNF-α | REV | 5′-CTG GTT GTC TTC CAG CTT CAC A-3′ |
| IE-2 | FWD | 5′-ACA AAC ACA CAG ACC AGT CA-3′ |
| IE-2 | REV | 5′-GTT TCA CAA CAG ATT GAG CA-3′ |
| TLR4 | FWD | 5′-TGC CTT CAC TAC AGG GAC TTT-3′ |
| TLR4 | REV | 5′-AGT GTC GCT GTT GAA GTC-3′ |
| IFN-γ | FWD | 5′-CAG CTC TGA GAA ACT GGA GGA CTT-3′ |
| IFN-γ | REV | 5′-GTG GCT GGA GTG GTT ATT AG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, S.; Álvarez, I.; Andreoli, V.; Delgado, S.; Perez, S.; Pereyra, S.; Romeo, F.; Grolli, S.; Verna, A.E. Gene Expression Modulation in Bovine Endometrial Cells Infected with Gammaherpesvirus Type 4 and Exposed to Lipopolysaccharide in the Presence of Platelet-Rich Plasma. Viruses 2025, 17, 744. https://doi.org/10.3390/v17060744
López S, Álvarez I, Andreoli V, Delgado S, Perez S, Pereyra S, Romeo F, Grolli S, Verna AE. Gene Expression Modulation in Bovine Endometrial Cells Infected with Gammaherpesvirus Type 4 and Exposed to Lipopolysaccharide in the Presence of Platelet-Rich Plasma. Viruses. 2025; 17(6):744. https://doi.org/10.3390/v17060744
Chicago/Turabian StyleLópez, Sofía, Ignacio Álvarez, V. Andreoli, S. Delgado, S. Perez, S. Pereyra, F. Romeo, S. Grolli, and Andrea Elizabeth Verna. 2025. "Gene Expression Modulation in Bovine Endometrial Cells Infected with Gammaherpesvirus Type 4 and Exposed to Lipopolysaccharide in the Presence of Platelet-Rich Plasma" Viruses 17, no. 6: 744. https://doi.org/10.3390/v17060744
APA StyleLópez, S., Álvarez, I., Andreoli, V., Delgado, S., Perez, S., Pereyra, S., Romeo, F., Grolli, S., & Verna, A. E. (2025). Gene Expression Modulation in Bovine Endometrial Cells Infected with Gammaherpesvirus Type 4 and Exposed to Lipopolysaccharide in the Presence of Platelet-Rich Plasma. Viruses, 17(6), 744. https://doi.org/10.3390/v17060744







