The COVID-19 Infection Did Not Aggravate the Mortality of Long-Term Care Facility Residents Under Strict Infection Control and with Immediate Anti-Viral Treatment: Real-World Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Prevention and Diagnostic Protocols
2.2. Treatment Protocols
2.3. Comparison of Demographic Parameters
2.4. Comparison of Symptoms
2.5. Survival Time Comparison
2.6. Statistical Analysis
3. Results
3.1. Symptom Profile and Acute Outcomes
3.2. Survival Analysis: Infected vs. Uninfected
3.2.1. Stratified Survival Analysis by Age
3.2.2. Stratified Analysis by Sex
3.2.3. Stratified Survival Analysis by Survival Status and CCI
3.3. Comparison Between Periods
4. Discussion
5. Conclusions
6. Limitation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LTCF | Long-term care facility |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| COVID-19 | Coronavirus disease-2019 |
| VOC | Variant of concern |
| C.I. | Confidence Interval |
| PCR | Polymerase chain reaction |
| NEAR | Nicking enzyme amplification reaction |
References
- Worldometer. Statistics. Available online: https://www.worldometers.info/coronavirus/ (accessed on 15 February 2025).
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://data.who.int/dashboards/covid19/data/ (accessed on 15 February 2025).
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Consortium, C.-G.U.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Tzou, P.L.; Nouhin, J.; Gupta, R.K.; de Oliveira, T.; Kosakovsky Pond, S.L.; Fera, D.; Shafer, R.W. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 2021, 22, 757–773. [Google Scholar] [CrossRef]
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E.; Ledford, H. How bad is Omicron? What scientists know so far. Nature 2021, 600, 197–199. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradnik, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.E.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022, 185, 467–484.e15. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef]
- Singhal, T. The Emergence of Omicron: Challenging Times Are Here Again! Indian J. Pediatr. 2022, 89, 490–496. [Google Scholar] [CrossRef]
- Danielle-Iuliano, A.; Brunkard, J.M.; Boehmer, T.K.; Peterson, E.; Adjei, S.; Binder, A.M.; Cobb, S.; Graff, P.; Hidalgo, P.; Panaggio, M.J.; et al. Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods—United States, December 2020–January 2022. In Morbidity and Mortality Report; CDC: Atlanta, GA, USA, 2022. Available online: https://www.cdc.gov/mmwr/volumes/71/wr/mm7104e4.htm/ (accessed on 15 February 2025).
- Ahmad, S.J.; Degiannis, J.R.; Borucki, J.; Pouwels, S.; Rawaf, D.L.; Lala, A.; Whiteley, G.S.; Head, M.; Simpson, A.; Archid, R.; et al. Fatality Rates After Infection With the Omicron Variant (B.1.1.529): How Deadly has it been? A Systematic Review and Meta-Analysis. J. Acute Med. 2024, 14, 51–60. [Google Scholar]
- Burton, J.K.; Reid, M.; Gribben, C.; Caldwell, D.; Clark, D.N.; Hanlon, P.; Quinn, T.J.; Fischbacher, C.; Knight, P.; Guthrie, B.; et al. Impact of COVID-19 on care-home mortality and life expectancy in Scotland. Age Ageing 2021, 50, 1029–1037. [Google Scholar] [CrossRef]
- Parra, P.N.B.; Atanasov, V.; Whittle, J.; Meurer, J.; Luo, Q.E.; Zhang, R.; Black, B. The Effect of the COVID-19 Pandemic on the Elderly: Population Fatality Rates, COVID Mortality Percentage, and Life Expectancy Loss. Elder Law J. 2022, 30, 33–80. [Google Scholar] [PubMed]
- Goldstein, J.R.; Lee, R.D. Demographic perspectives on the mortality of COVID-19 and other epidemics. Proc. Natl. Acad. Sci. USA 2020, 117, 22035–22041. [Google Scholar] [CrossRef]
- Ma, B.H.; Yip, T.C.; Lui, G.C.; Lai, M.S.; Hui, E.; Wong, V.W.; Tse, Y.K.; Chan, H.L.; Hui, D.S.; Kwok, T.C.; et al. Clinical Outcomes Following Treatment for COVID-19 with Nirmatrelvir/Ritonavir and Molnupiravir Among Patients Living in Nursing Homes. JAMA Netw. 2023, 6, e2310887. [Google Scholar] [CrossRef]
- CDC. COVID-19 Treatment Clinical Care for Outpatients. Available online: https://www.cdc.gov/covid/hcp/clinical-care/outpatient-treatment.html/ (accessed on 15 February 2025).
- Shimizu, H.; Kawase, J.; Hayashi, M.; Imaizumi, K.; Ito, Y.; Okazawa, M. COVID-19 symptom-onset to diagnosis and diagnosis to treatment intervals are significant predictors of disease progression and hospitalization in high-risk patients: A real world analysis. Respir. Investig. 2023, 61, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Jian, W.H.; Liang, Z.Y.; Guan, W.J.; Liang, W.H.; Chen, R.C.; Tang, C.L.; Wang, T.; Liang, H.R.; Li, Y.M.; et al. Earlier diagnosis improves COVID-19 prognosis: A nationwide retrospective cohort analysis. Ann. Transl. Med. 2021, 9, 941. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Gong, T.; Wang, G.; Wang, J.; Guo, X.; Cai, E.; Li, S.; Li, X.; Yu, Y.; Lin, L.; et al. Timely Diagnosis and Treatment Shortens the Time to Resolution of Coronavirus Disease (COVID-19) Pneumonia and Lowers the Highest and Last CT Scores From Sequential Chest CT. Am. J. Roentgenol. 2020, 215, 367–373. [Google Scholar] [CrossRef]
- Nakazawa, E.; Ino, H.; Akabayashi, A. Chronology of COVID-19 Cases on the Diamond Princess Cruise Ship and Ethical Considerations: A Report From Japan. Disaster Med. Public Health Prep. 2020, 14, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T.; Inokuchi, H.; Yasunaga, H. Services in public long-term care insurance in Japan. Ann. Clin. Epidemiol. 2024, 6, 1–4. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Schultz, L.R.; Peterson, E.L.; Breslau, N. Graphing survival curve estimates for time-dependent covariates. Int. J. Methods Psychiatr. Res. 2002, 11, 68–74. [Google Scholar] [CrossRef]
- Zheng, Q.; Otahal, P.; Cox, I.A.; de Graaff, B.; Campbell, J.A.; Ahmad, H.; Walters, E.H.; Palmer, A.J. The influence of immortal time bias in observational studies examining associations of antifibrotic therapy with survival in idiopathic pulmonary fibrosis: A simulation study. Front. Med. 2023, 10, 1157706. [Google Scholar] [CrossRef]
- Suissa, S.; Dell’Aniello, S. Time-related biases in pharmacoepidemiology. Pharmacoepidemiol. Drug Saf. 2020, 29, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Hashan, M.R.; Smoll, N.; King, C.; Ockenden-Muldoon, H.; Walker, J.; Wattiaux, A.; Graham, J.; Booy, R.; Khandaker, G. Epidemiology and clinical features of COVID-19 outbreaks in aged care facilities: A systematic review and meta-analysis. Eclinical Med. 2021, 33, 100771. [Google Scholar] [CrossRef] [PubMed]
- Duong, B.V.; Larpruenrudee, P.; Fang, T.; Hossain, S.I.; Saha, S.C.; Gu, Y.; Islam, M.S. Is the SARS CoV-2 Omicron Variant Deadlier and More Transmissible Than Delta Variant? Int. J. Environ. Res. Public Health 2022, 19, 4586. [Google Scholar] [CrossRef]
- Severity of Disease Associated with Omicron Variant as Compared with Delta Variant in hospitalized Patients with Suspected or Confirmed SARS-CoV-2 Infection. Available online: https://www.who.int/publications/i/item/9789240051829?utm_source=chatgpt.com/ (accessed on 15 February 2025).
- Guillermo, V.; Sanchez, G.V.; Biedron, C.; Fink, L.R.; Hatfield, K.M.; Polistico, J.M.F.; Meyer, M.P.; Noe, R.S.; Copen, C.E.; Lyons, A.K.; et al. Initial and Repeated Point Prevalence Surveys to Inform SARS-CoV-2 Infection Prevention in 26 Skilled Nursing Facilities—Detroit, Michigan, March–May 2020. In Morbidity and Mortality Weekly Report (MMWR); Center for Disease Control and Prevention: Atlanta, GA, USA, 2020; pp. 882–886. [Google Scholar]
- Madewell, Z.J.; Yang, Y.; Longini, I.M.; Jr Halloran, M.E.; Vespignani, A.; Dean, N.E. Rapid review and meta-analysis of serial intervals for SARS-CoV-2 Delta and Omicron variants. BMC Infect. Dis. 2023, 23, 429. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Y.; Kummer, A.G.; Zhao, Y.; Hu, Z.; Wang, Y.; Liu, H.; Ajelli, M.; Yu, H. Assessing changes in incubation period, serial interval, and generation time of SARS-CoV-2 variants of concern: A systematic review and meta-analysis. BMC Med. 2023, 21, 374. [Google Scholar] [CrossRef] [PubMed]
- Oksuzyan, A.; Juel, K.; Vaupel, J.W.; Christensen, K. Men: Good health and high mortality. Sex differences in health and aging. Aging Clin. Exp. Res. 2008, 20, 91–102. [Google Scholar] [CrossRef]
- Crimmins, E.M.; Shim, H.; Zhang, Y.S.; Kim, J.K. Differences between Men and Women in Mortality and the Health Dimensions of the Morbidity Process. Clin. Chem. 2019, 65, 135–145. [Google Scholar] [CrossRef]
- Luy, M.; Gast, K. Do women live longer or do men die earlier? Reflections on the causes of sex differences in life expectancy. Gerontology 2014, 60, 143–153. [Google Scholar] [CrossRef]
- Pijls, B.G.; Jolani, S.; Atherley, A.; Derckx, R.T.; Dijkstra, J.I.R.; Franssen, G.H.L.; Hendriks, S.; Richters, A.; Venemans-Jellema, A.; Zalpuri, S.; et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: A meta-analysis of 59 studies. BMJ Open 2021, 11, e044640. [Google Scholar] [CrossRef]
- Yamaguchi, D.; Chimed-Ochir, O.; Yumiya, Y.; Kishita, E.; Akita, T.; Tanaka, J.; Kubo, T. Potential Risk Factors to COVID-19 Severity: Comparison of SARS-CoV-2 Delta- and Omicron-Dominant Periods. Int. J. Environ. Res. Public Health 2024, 21, 322. [Google Scholar] [CrossRef]
- Fan, Y.; Li, X.; Zhang, L.; Wan, S.; Zhang, L.; Zhou, F. SARS-CoV-2 Omicron variant: Recent progress and future perspectives. Signal Transduct. Target. Ther. 2022, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, L.E.; Ngeh, S.; Cadby, G.; Hutcheon, K.; Effle, P.V. SARS-CoV-2 Vaccine Effectiveness against Omicron Variant in Infection-Naive Population, Australia, 2022; Center for Disease Control and Prevention: Atlanta, GA, USA, 2023. Available online: https://www.cdc.gov/mmwr/volumes/73/wr/mm7312a5.htm/ (accessed on 15 February 2025).
- Chidambaram, P.; Rudowitz, R.; Neuman, T. Key Questions About Nursing Home Cases, Deaths, and Vaccinations as Omicron Spreads in the United States, in Coronavirus (COVID-19). 2022. Available online: https://www.kff.org/coronavirus-covid-19/issue-brief/key-questions-about-nursing-home-cases-deaths-and-vaccinations-as-omicron-spreads-in-the-united-states/?utm_source=chatgpt.com/ (accessed on 15 February 2025).
- Link-Gelles, R.; Rowley, E.A.K.; DeSilva, M.B.; Dascomb, K.; Irving, S.A.; Klein, N.P.; Grannis, S.J.; Ong, T.C.; Weber, Z.A.; Fleming-Dutra, K.E.; et al. Interim Effectiveness of Updated 2023–2024 (Monovalent XBB.1.5) COVID-19 Vaccines Against COVID-19–Associated Hospitalization Among Adults Aged ≥18 Years with Immunocompromising Conditions—VISION Network, September 2023–February 2024. In Morbidity and Mortality Report; Center for Disease Control and Prevention: Atlanta, GA, USA, 2024; pp. 271–276. [Google Scholar]
- Jones, R.P.; Ponomarenko, A. COVID-19-Related Age Profiles for SARS-CoV-2 Variants in England and Wales and States of the USA (2020 to 2022): Impact on All-Cause Mortality. Infect. Dis. Rep. 2023, 15, 600–634. [Google Scholar] [CrossRef]
- Chen, L.K.; Peng, L.N.; Lin, M.H.; Lai, H.Y.; Hwang, S.J.; Lan, C.F. Predicting mortality of older residents in long-term care facilities: Comorbidity or care problems? J. Am. Med. Dir. Assoc. 2010, 11, 567–571. [Google Scholar] [CrossRef]
- Checovich, M.M.; Barlow, S.; Shult, P.; Reisdorf, E.; Temte, J.L. Evaluation of Viruses Associated With Acute Respiratory Infections in Long-Term Care Facilities Using a Novel Method: Wisconsin, 2016–2019. J. Am. Med. Dir. Assoc. 2020, 21, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Influenza 2019/20 season, Japan. In Infectious Agents Surveillance Report; National Institute of Infectious Diseases: Tokyo, Japan, 2020; pp. 191–193.
- Otsuka, M.; Kasamatsu, A.; Arima, Y.; Takahashi, T.; Arashiro, T.; Komase, K.; Shimbashi, R.; Tsuchihashi, Y.; Kobayashi, Y.; Takahara, O.; et al. Respiratory syncytial virus infection notification trends and interpretation of the reported case data, 2018–2021, Japan. Jpn. J. Infect. Dis. 2025, 78, 51–54. [Google Scholar] [CrossRef]
- Sakamoto, H.; Ishikane, M.; Ueda, P. Seasonal Influenza Activity During the SARS-CoV-2 Outbreak in Japan. JAMA 2020, 323, 1969–1971. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, M.; Tsuzuki, S.; Nakamoto, T.; Iwamoto, N. Resurgence of Respiratory Syncytial Virus Infections during COVID-19 Pandemic, Tokyo, Japan. Emerg. Infect. Dis. 2021, 27, 2969–2970. [Google Scholar] [CrossRef]
- Fukuda, Y.; Togashi, A.; Hirakawa, S.; Yamamoto, M.; Fukumura, S.; Nawa, T.; Honjo, S.; Kunizaki, J.; Nishino, K.; Tanaka, T.; et al. Resurgence of human metapneumovirus infection and influenza after three seasons of inactivity in the post-COVID-19 era in Hokkaido, Japan, 2022–2023. J. Med. Virol. 2023, 95, e29299. [Google Scholar] [CrossRef]

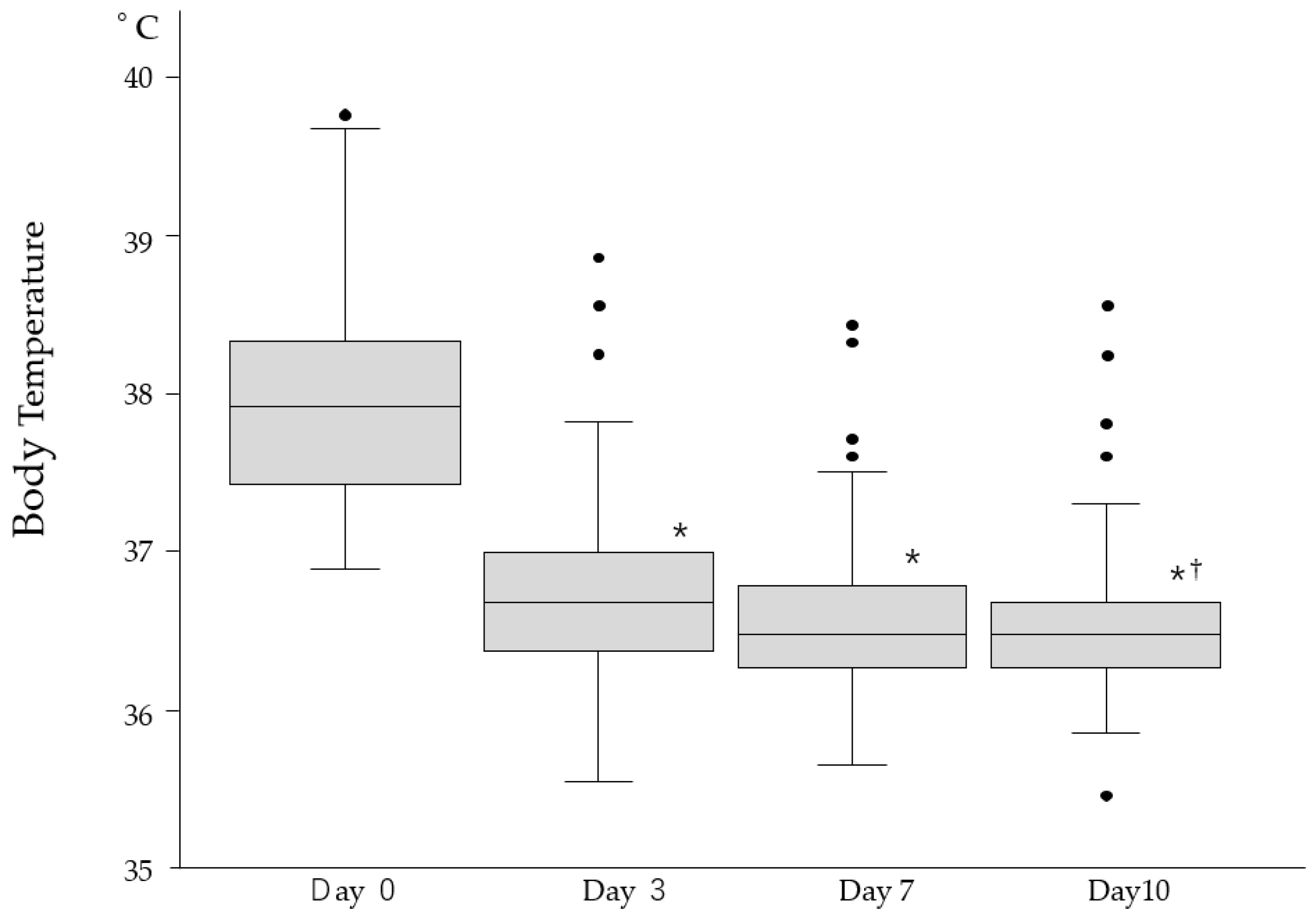
| Symptoms | Day 0 (n = 244) | Day 3 (n = 244) | Day 7 (n = 244) | Day 10 (n = 244) |
|---|---|---|---|---|
| High body temperature (%) | 108 (44.3) | 41 (16.8) * | 24 (9.8) * | 14 (5.7) * |
| Upper airway symptoms (%) | 25 (10.2) | 22 (9.0) * | 13 (5.3) * | 1 (0.4) * |
| Respiratory symptoms (%) | 41 (16.8) | 56 (23.0) | 34 (13.9) | 19 (7.8) † |
| Neurological symptoms (%) | 10 (4.1) | 2 (0.8) | 2 (0.8) | 0 (0.0) * |
| General fatigue (%) | 1 (0.4) | 4 (1.6) | 5 (2.1) | 6 (2.5) |
| Abdominal symptoms (%) | 3 (1.2) | 3 (1.2) | 4 (1.6) | 3 (1.2) |
| Any symptoms of above (%) | 141 (59.0) | 99(41.0) | 65 (26.6) | 34 (13.9) * |
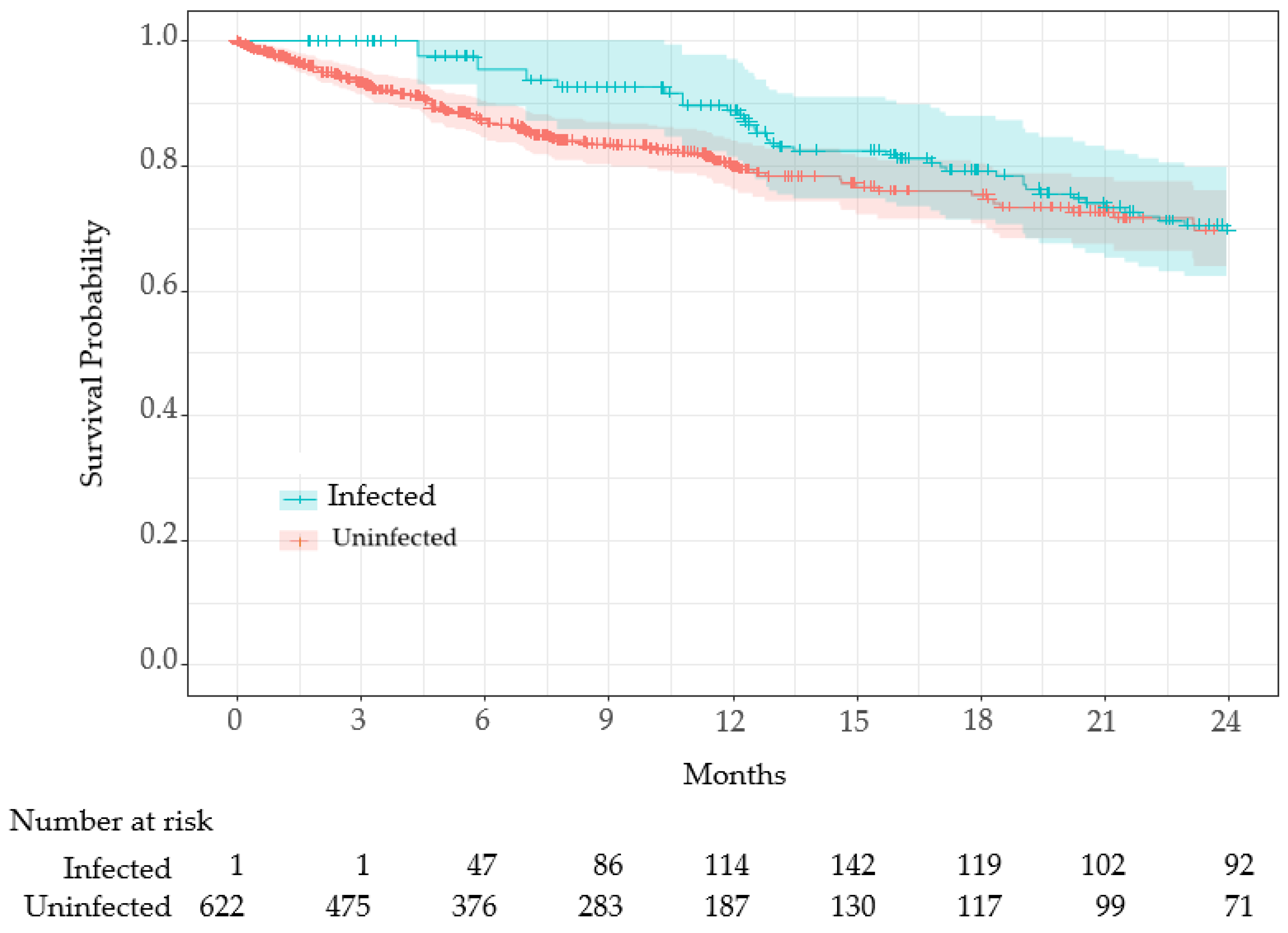
| Characteristics | Uninfected (n = 392) 1 | Infected (n = 231) 1 | p-Value 2 |
|---|---|---|---|
| Number of Deaths | 102 (26%) | 37 (16%) | 0.004 |
| Age (years) | 89 (7) | 88 (6) | 0.45 |
| Sex (% of males) | 81 (21%) | 19 (8.2%) | <0.001 |
| Degree of care | 0.78 | ||
| Low | 37 (9.4%) | 25 (11%) | |
| Moderate | 187 (48%) | 112 (48%) | |
| High | 168 (43%) | 94 (41%) | |
| Charlson comorbidity index | 1.24 (0.96) | 1.27 (0.94) | 0.77 |
| Number of vaccinations | <0.001 | ||
| 0 | 145 (37%) | 50 (22%) | |
| 1 | 6 (1.5%) | 1 (0.4%) | |
| 2 | 240 (61%) | 177 (77%) | |
| 3 | 1 (0.3%) | 3 (1.3%) |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Term | Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value |
| COVID-19 infection | 1.43 | 0.95–2.15 | 0.08 | 1.36 | 0.91–2.04 | 0.14 |
| Age | 1.07 | 1.04–1.11 | <0.001 | |||
| Sex | ||||||
| Female | 1.00 | (Ref) | ||||
| Male | 3.25 | 2.19–4.83 | <0.001 | |||
| Degree of Care | ||||||
| Low | 1.00 | (Ref) | ||||
| Moderate | 1.82 | 0.73–4.56 | 0.20 | |||
| High | 3.66 | 1.50–8.95 | 0.005 | |||
| Charlson comorbidity index | 1.10 | 0.93–1.29 | 0.26 | |||
| Number of Vaccinations | 0.83 | 0.68–1.03 | 0.09 | |||
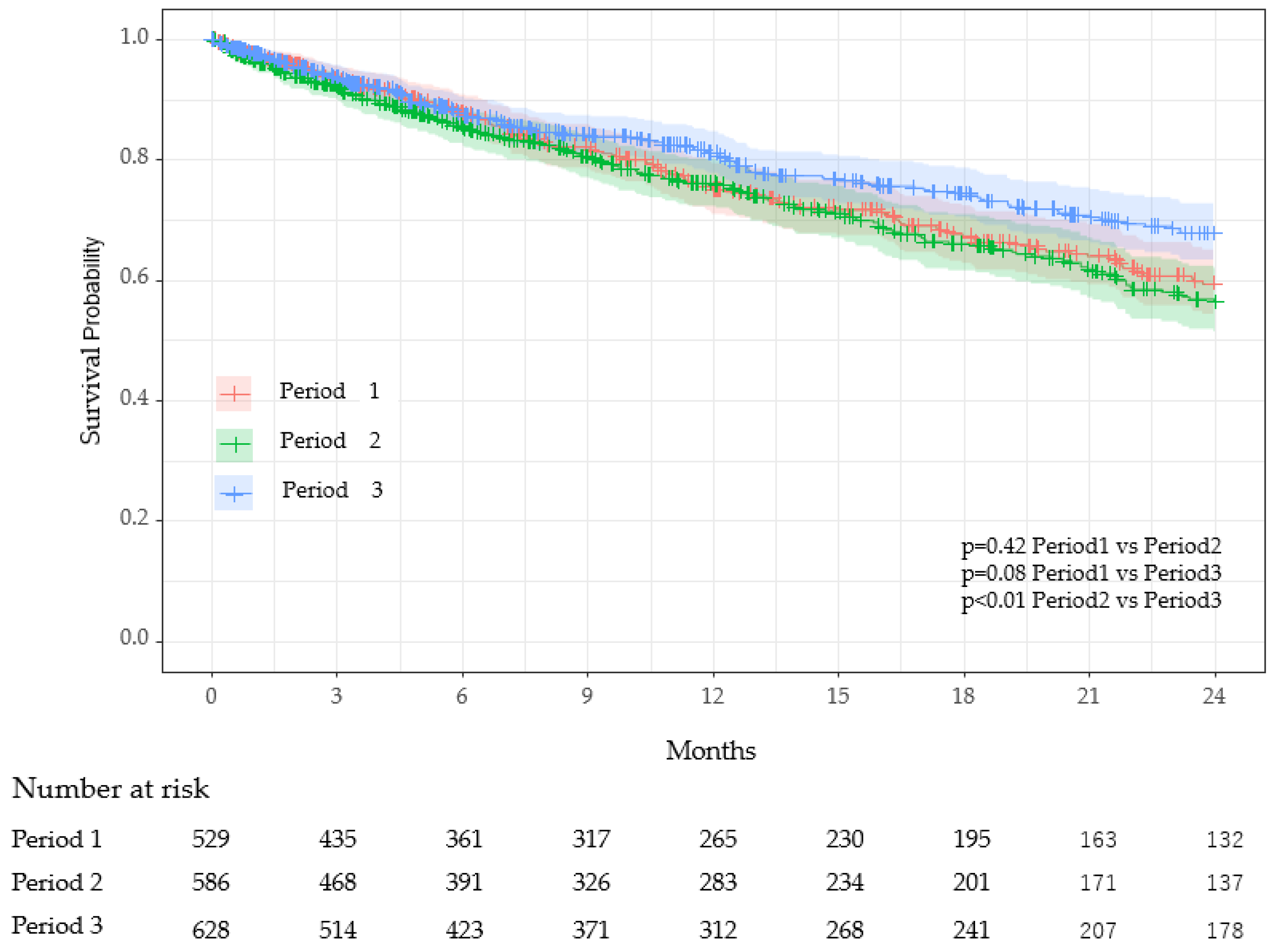
| Characteristics | Period 1 (n = 529) 1 | Period 2 (n = 589) 1 | Period 3 (n = 630) 1 |
|---|---|---|---|
| Number of Infections | |||
| 0 | 529 | 589 | 399 |
| 1 | 0 | 0 | 210 |
| 2 | 0 | 0 | 20 |
| 3 | 0 | 0 | 1 |
| Age (years) | 88 (7) | 89 (6) | 89 (6) |
| Sex (% of male) | 85 (16%) | 92 (16%) | 103 (16%) |
| Person–year | 1.07 (0.73) | 1.01 (0.74) | 1.07 (0.74) |
| Number of Deaths | 149 (28%) | 172 (29%) | 139 (22%) * |
| Degree of care | |||
| Low | 63 (12%) | 78 (13%) | 63 (10%) |
| Moderate | 236 (45%) | 270 (46%) | 302 (48%) |
| High | 230 (43%) | 241 (41%) | 265 (42%) |
| Charlson comorbidity index | 1.60 (1.19) | 1.37 (1.04) † | 1.36 (1.05) †§ |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Term | Hazard Ratio | 95% CI. | p-Value 1 | Hazard Ratio | 95% C.I. | p-Value 1 |
| Period 1 vs. Period 2 | 1.09 | 0.88–1.36 | 0.42 | 1.09 | 0.88–1.36 | 0.42 |
| Period 1 vs. Period 3 | 0.78 | 0.62–0.99 | 0.09 | 0.80 | 0.63–1.01 | 0.14 |
| Period 2 vs. Period 3 | 0.72 | 0.57–0.90 | 0.01 | 0.73 | 0.58–0.92 | 0.02 |
| Age | 1.05 | 1.04–1.07 | <0.001 | |||
| Sex | 2.47 | 1.94–3.16 | <0.001 | |||
| Female | 1.00 | (Ref) | ||||
| Male | 2.47 | 1.94–3.16 | <0.001 | |||
| Degree of Care | ||||||
| Low | 1.00 | (Ref) | ||||
| Moderate | 1.56 | 1.03–2.34 | 0.035 | |||
| High | 2.99 | 2.01–4.45 | <0.001 | |||
| CCI | 1.07 | 1.00–1.54 | 0.065 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, H.; Kawase, J.; Higashi, Y.; Nabeno, H.; Hayashi, M.; Imaizumi, K.; Ito, Y.; Matsunaga, M.; Okazawa, M. The COVID-19 Infection Did Not Aggravate the Mortality of Long-Term Care Facility Residents Under Strict Infection Control and with Immediate Anti-Viral Treatment: Real-World Analysis. Viruses 2025, 17, 625. https://doi.org/10.3390/v17050625
Shimizu H, Kawase J, Higashi Y, Nabeno H, Hayashi M, Imaizumi K, Ito Y, Matsunaga M, Okazawa M. The COVID-19 Infection Did Not Aggravate the Mortality of Long-Term Care Facility Residents Under Strict Infection Control and with Immediate Anti-Viral Treatment: Real-World Analysis. Viruses. 2025; 17(5):625. https://doi.org/10.3390/v17050625
Chicago/Turabian StyleShimizu, Hideyasu, Jin Kawase, Yuko Higashi, Hiroyuki Nabeno, Masamichi Hayashi, Kazuyoshi Imaizumi, Yuji Ito, Masaaki Matsunaga, and Mitsushi Okazawa. 2025. "The COVID-19 Infection Did Not Aggravate the Mortality of Long-Term Care Facility Residents Under Strict Infection Control and with Immediate Anti-Viral Treatment: Real-World Analysis" Viruses 17, no. 5: 625. https://doi.org/10.3390/v17050625
APA StyleShimizu, H., Kawase, J., Higashi, Y., Nabeno, H., Hayashi, M., Imaizumi, K., Ito, Y., Matsunaga, M., & Okazawa, M. (2025). The COVID-19 Infection Did Not Aggravate the Mortality of Long-Term Care Facility Residents Under Strict Infection Control and with Immediate Anti-Viral Treatment: Real-World Analysis. Viruses, 17(5), 625. https://doi.org/10.3390/v17050625






