Expression Profile of Human Cytomegalovirus UL111A cmvIL-10 and LAcmvIL-10 Transcripts in Primary Cells and Cells from Renal Transplant Recipients
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Cell Infections
2.3. Samples from Kidney Transplant Recipients
2.4. Serology
2.5. Monitoring of Viral Load in Plasma Samples
2.6. Clinical Evaluation and Antiviral Therapy
2.7. Peripheral Blood Mononuclear Cells (PBMCs) Isolation
2.8. Acid Nucleic Extraction and cDNA Synthesis
2.9. Primer Design and RT-qPCR Optimization
2.10. Standard Curve for Absolute Quantification UL111A Transcripts in PBMC
2.11. Quantitative Reverse Transcription PCR (RT-qPCR) Analysis
2.12. Statistical Analysis
3. Results
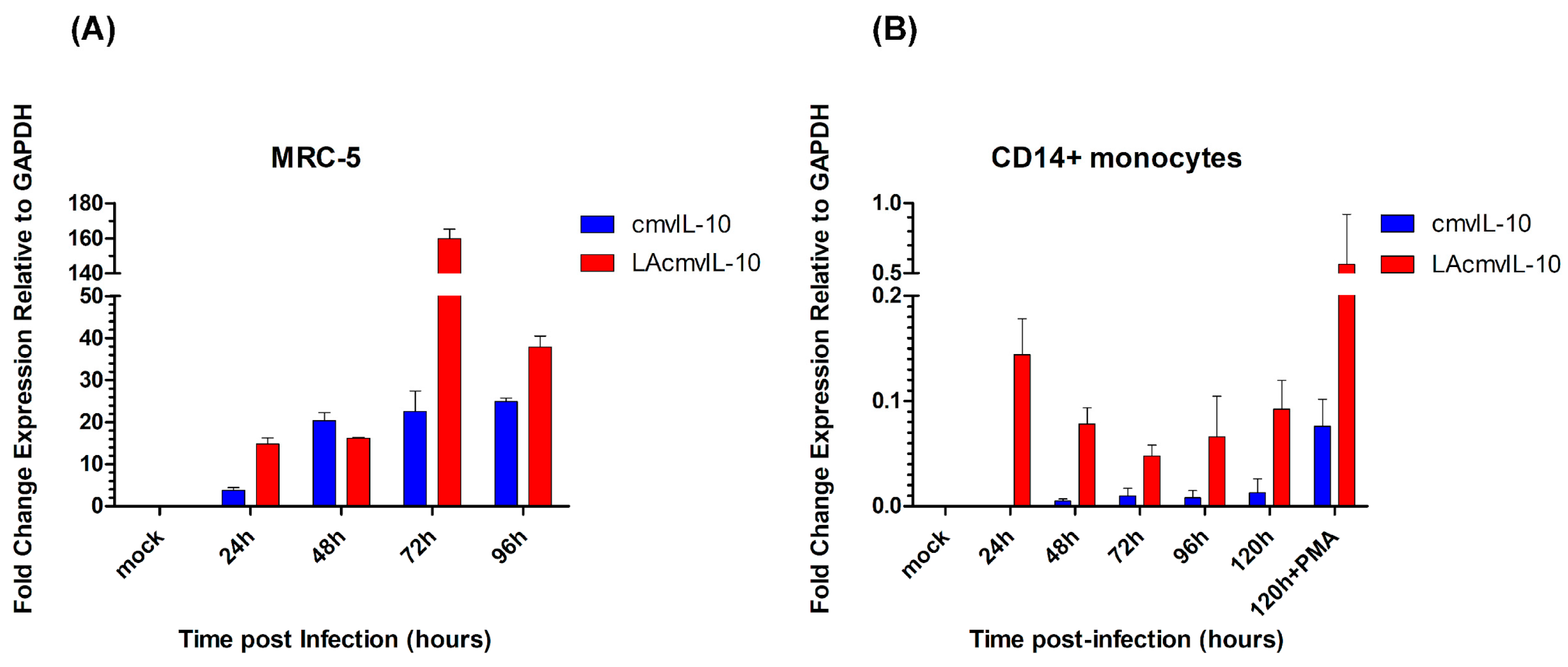
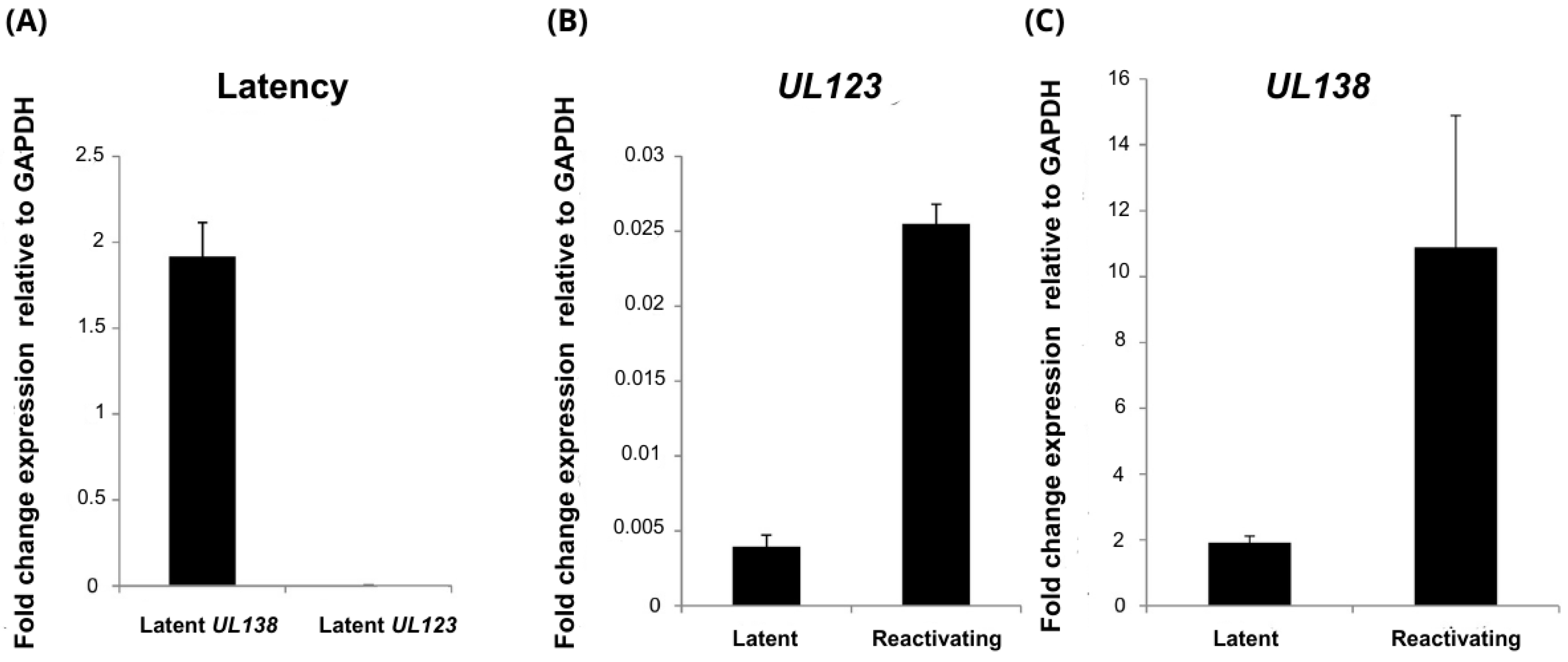
3.1. Relative Levels of UL111A Transcripts cmvIL-10 and LAcmvIL-10 in Productive and Latently Infected Cells
3.2. Patient History and Clinical Data of Transplant Recipients
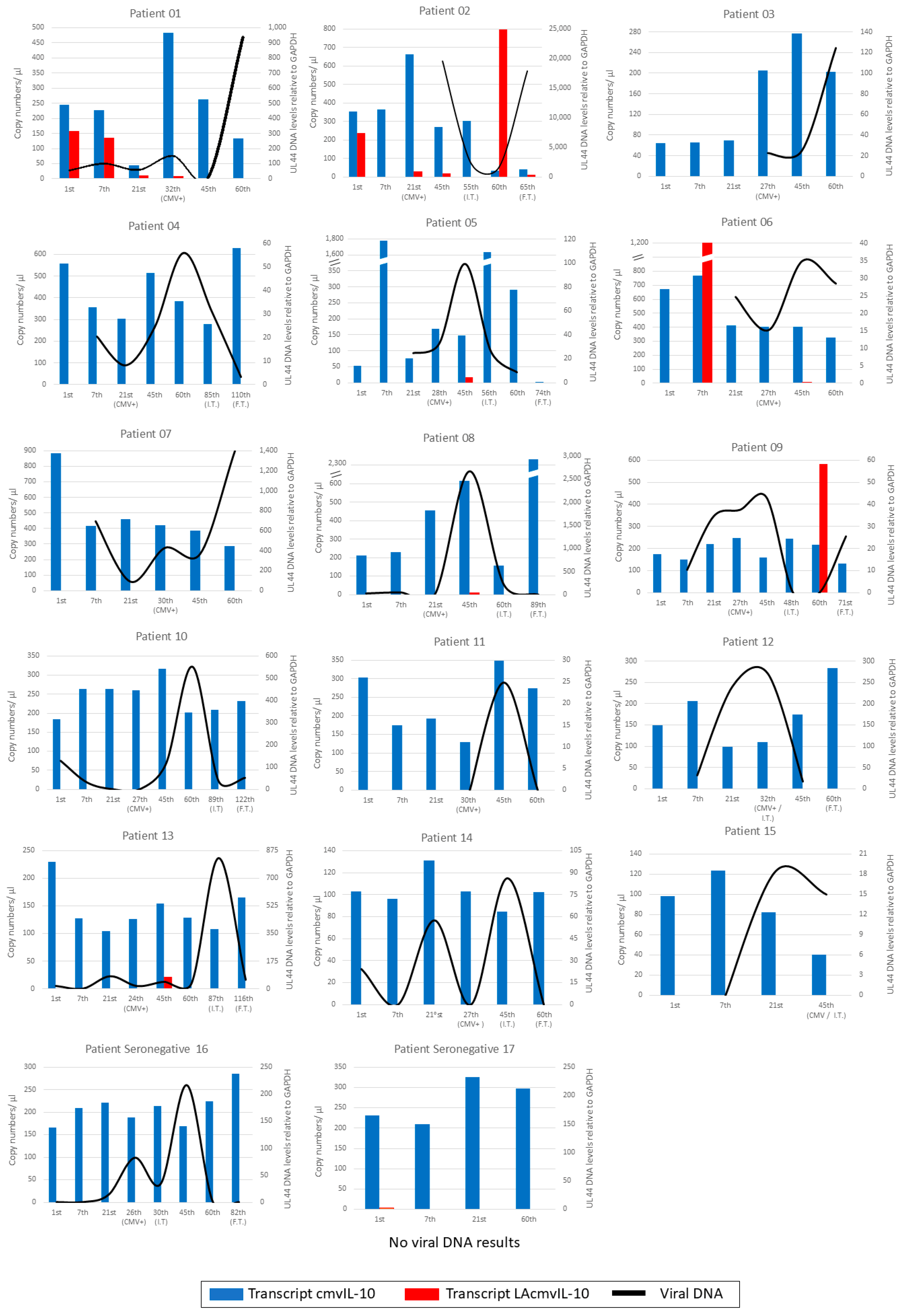
3.3. Monitoring Intracellular and Extracellular Viral DNA in Patient Samples
3.4. Kinetics of UL111A Transcripts in PBMCs of Transplant Recipients
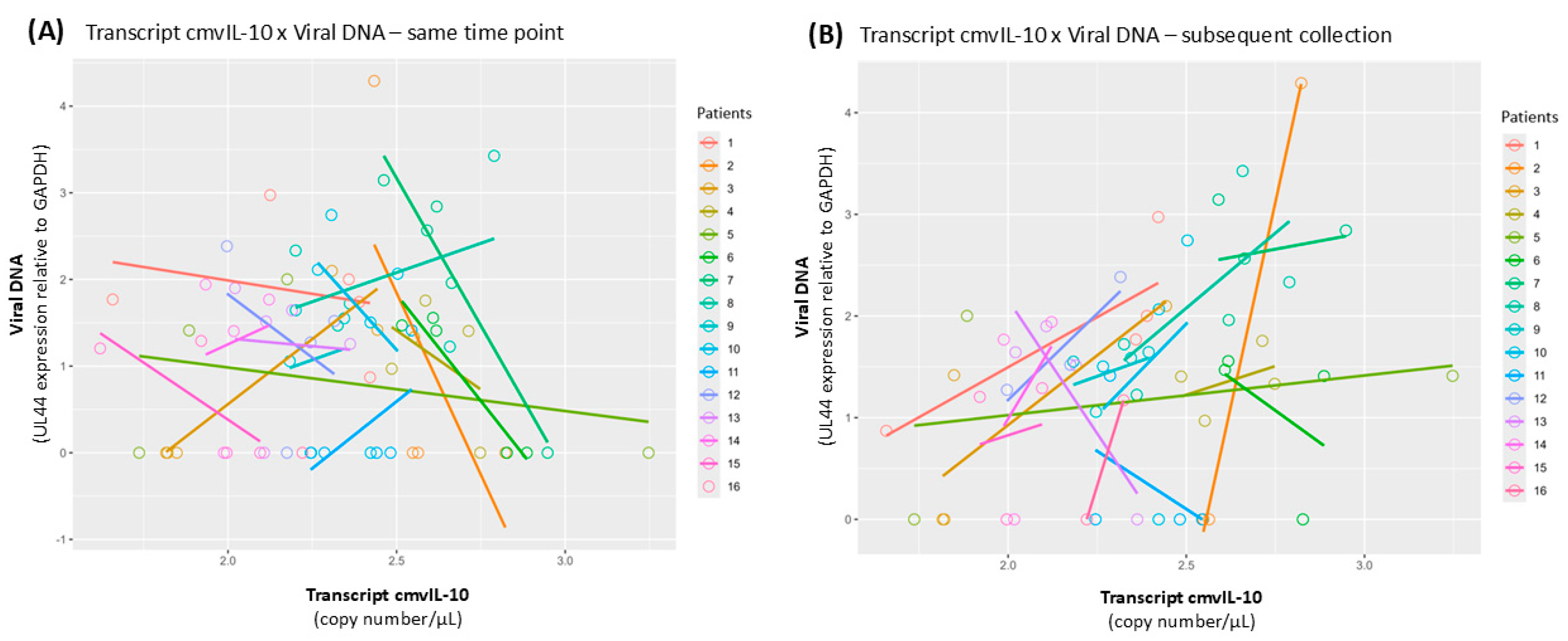
3.5. Covariance Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| cIL-10 | cellular interleukin-10 |
| cmvIL-10 | Cytomegalovirus IL-10 (isoform A) |
| Ct | Cycle threshold |
| LAcmvIL-10 | Latency-associated cytomegalovirus IL-10 (isoform B) |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| HCMV | Human cytomegalovirus (Cytomegalovirus humanbeta5) |
| hpi | Hours post-infection |
| IE | Immediate-early |
| MOI | Multiplicity of infection |
| NTC | No-template control |
| PBMC | Peripheral blood mononuclear cells |
| PMA | Phorbolmyristate acetate |
References
- Griffiths, P.; Baraniak, I.; Reeves, M. The Pathogenesis of Human Cytomegalovirus. J. Pathol. 2015, 235, 288–297. [Google Scholar] [CrossRef]
- Chan, S.T.; Logan, A.C. The Clinical Impact of Cytomegalovirus Infection Following Allogeneic Hematopoietic Cell Transplantation: Why the Quest for Meaningful Prophylaxis Still Matters. Blood Rev. 2017, 31, 173–183. [Google Scholar] [CrossRef]
- Malinis, M.; Boucher, H.W. Screening of Donor and Candidate Prior to Solid Organ Transplantation—Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13548. [Google Scholar] [CrossRef] [PubMed]
- Cukuranovic, J.; Ugrenovic, S.; Jovanovic, I.; Visnjic, M.; Stefanovic, V. Viral Infection in Renal Transplant Recipients. Sci. World J. 2012, 2012, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Collins-McMillen, D.; Buehler, J.; Peppenelli, M.; Goodrum, F. Molecular Determinants and the Regulation of Human Cytomegalovirus Latency and Reactivation. Viruses 2018, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Söderberg-Nauclér, C.; Fish, K.N.; Nelson, J.A. Reactivation of Latent Human Cytomegalovirus by Allogeneic Stimulation of Blood Cells from Healthy Donors. Cell 1997, 91, 119–126. [Google Scholar] [CrossRef]
- Taylor-Wiedeman, J.; Sissons, P.; Sinclair, J. Induction of Endogenous Human Cytomegalovirus Gene Expression after Differentiation of Monocytes from Healthy Carriers. J. Virol. 1994, 68, 1597–1604. [Google Scholar]
- Reeves, M.B.; Compton, T. Inhibition of Inflammatory Interleukin-6 Activity via Extracellular Signal-Regulated Kinase–Mitogen-Activated Protein Kinase Signaling Antagonizes Human Cytomegalovirus Reactivation from Dendritic Cells. J. Virol. 2011, 85, 12750–12758. [Google Scholar] [CrossRef]
- Sinclair, J.; Poole, E. Human Cytomegalovirus Latency and Reactivation in and beyond the Myeloid Lineage. Future Virol. 2014, 9, 557–563. [Google Scholar]
- Shnayder, M.; Nachshon, A.; Krishna, B.; Poole, E.; Boshkov, A.; Binyamin, A.; Maza, I.; Sinclair, J.; Schwartz, M.; Stern-Ginossar, N. Defining the Transcriptional Landscape during Cytomegalovirus Latency with Single-Cell RNA Sequencing. MBio 2018, 9, e00013-18. [Google Scholar] [CrossRef]
- Wills, M.R.; Poole, E.; Lau, B.; Krishna, B.; Sinclair, J.H. The Immunology of Human Cytomegalovirus Latency: Could Latent Infection Be Cleared by Novel Immunotherapeutic Strategies? Cell. Mol. Immunol. 2015, 12, 128–138. [Google Scholar]
- Mason, G.M.; Poole, E.; Sissons, J.G.P.; Wills, M.R.; Sinclair, J.H. Human Cytomegalovirus Latency Alters the Cellular Secretome, Inducing Cluster of Differentiation (CD)4+ T-Cell Migration and Suppression of Effector Function. Proc. Natl. Acad. Sci. USA. 2012, 109, 14538–14543. [Google Scholar] [CrossRef] [PubMed]
- Avdic, S.; Cao, J.Z.; Cheung, A.K.L.; Abendroth, A.; Slobedman, B. Viral Interleukin-10 Expressed by Human Cytomegalovirus during the Latent Phase of Infection Modulates Latently Infected Myeloid Cell Differentiation. J. Virol. 2011, 85, 7465–7471. [Google Scholar] [CrossRef] [PubMed]
- Poole, E.; Avdic, S.; Hodkinson, J.; Jackson, S.; Wills, M.; Slobedman, B.; Sinclair, J. Latency-Associated Viral Interleukin-10 (IL-10) Encoded by Human Cytomegalovirus Modulates Cellular IL-10 and CCL8 Secretion during Latent Infection through Changes in the Cellular MicroRNA Hsa-MiR-92a. J. Virol. 2014, 88, 13947–13955. [Google Scholar] [CrossRef]
- Avdic, S.; McSharry, B.P.; Steain, M.; Poole, E.; Sinclair, J.; Abendroth, A.; Slobedman, B. Human Cytomegalovirus-Encoded Human Interleukin-10 (IL-10) Homolog Amplifies Its Immunomodulatory Potential by Upregulating Human IL-10 in Monocytes. J. Virol. 2016, 90, 3819–3827. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.; Reeves, M. Pathogenesis of Human Cytomegalovirus in the Immunocompromised Host. Nat. Rev. Microbiol. 2021, 19, 759–773. [Google Scholar] [CrossRef]
- Kotenko, S.V.; Saccani, S.; Izotova, L.S.; Mirochnitchenko, O.V.; Pestka, S. Human Cytomegalovirus Harbors Its Own Unique IL-10 Homolog (CmvIL-10). Proc. Natl. Acad. Sci. USA 2000, 97, 1695–1700. [Google Scholar] [CrossRef]
- Chang, W.L.W.; Baumgarth, N.; Yu, D.; Barry, P.A. Human Cytomegalovirus-Encoded Interleukin-10 Homolog Inhibits Maturation of Dendritic Cells and Alters Their Functionality. J. Virol. 2004, 78, 8720–8731. [Google Scholar] [CrossRef]
- Weekes, M.P.; Tomasec, P.; Huttlin, E.L.; Fielding, C.A.; Nusinow, D.; Stanton, R.J.; Wang, E.C.Y.; Aicheler, R.; Murrell, I.; Wilkinson, G.W.G.; et al. Quantitative Temporal Viromics: An Approach to Investigate Host-Pathogen Interaction. Cell 2014, 157, 1460–1472. [Google Scholar] [CrossRef]
- Lockridge, K.M.; Zhou, S.S.; Kravitz, R.H.; Johnson, J.L.; Sawai, E.T.; Blewett, E.L.; Barry, P.A. Primate Cytomegaloviruses Encode and Express an IL-10-like Protein. Virology 2000, 268, 272–280. [Google Scholar] [CrossRef]
- Jenkins, C.; Abendroth, A.; Slobedman, B. A Novel Viral Transcript with Homology to Human Interleukin-10 Is Expressed during Latent Human Cytomegalovirus Infection. J. Virol. 2004, 78, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.; Garcia, W.; Abendroth, A.; Slobedman, B. Expression of a Human Cytomegalovirus Latency-Associated Homolog of Interleukin-10 during the Productive Phase of Infection. Virology 2008, 370, 285–294. [Google Scholar] [CrossRef]
- Jenkins, C.; Garcia, W.; Godwin, M.J.; Spencer, J.V.; Stern, J.L.; Abendroth, A.; Slobedman, B. Immunomodulatory Properties of a Viral Homolog of Human Interleukin-10 Expressed by Human Cytomegalovirus during the Latent Phase of Infection. J. Virol. 2008, 82, 3736–3750. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Chang, P.C.; Wang, Y.; Li, M. Identification of Novel Viral Interleukin-10 Isoforms of Human Cytomegalovirus AD169. Virus Res. 2008, 131, 213–223. [Google Scholar] [CrossRef]
- Pantaleão, S.Q.; de Moraes Bomediano Camillo, L.; Neves, T.C.; de Godoy Menezes, I.; Stangherlin, L.M.; de Carvalho Ruthner Batista, H.B.; Poole, E.; Nevels, M.; Philot, E.A.; Scott, A.L.; et al. Molecular Modelling of the HCMV IL-10 Protein Isoforms and Analysis of Their Interaction with the Human IL-10 Receptor. PLoS ONE 2022, 17, e0277953. [Google Scholar] [CrossRef]
- Sinzger, C.; Hahn, G.; Digel, M.; Katona, R.; Sampaio, K.L.; Messerle, M.; Hengel, H.; Koszinowski, U.; Brune, W.; Adler, B. Cloning and Sequencing of a Highly Productive, Endotheliotropic Virus Strain Derived from Human Cytomegalovirus TB40/E. J. Gen. Virol. 2008, 89, 359–368. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.M.; Murphy, E.A. A Myeloid Progenitor Cell Line Capable of Supporting Human Cytomegalovirus Latency and Reactivation, Resulting in Infectious Progeny. J. Virol. 2012, 86, 9854–9865. [Google Scholar] [CrossRef]
- Poole, E.; Carlan da Silva, M.C.; Huang, C.; Perera, M.; Wills, M.; Rana, A.; Sinclair, J.; Jackson, S.; Groves, I.J. A Bmpr2/Yy1 Signaling Axis Is Required for Human Cytomegalovirus Latency in Undifferentiated Myeloid Cells. MBio 2021, 12, e0022721. [Google Scholar] [CrossRef]
- Nakamura, M.R.; Requião-Moura, L.R.; Gallo, R.M.; Botelho, C.; Taddeo, J.; Viana, L.A.; Felipe, C.R.; Medina-Pestana, J.; Tedesco-Silva, H. Transition from antigenemia to quantitative nucleic acid amplification testing in cytomegalovirus-seropositive kidney transplant recipients receiving preemptive therapy for cytomegalovirus infection. Sci. Rep. 2022, 12, 12783. [Google Scholar] [CrossRef]
- Requião-Moura, L.R.; deMatos, A.C.; Pacheco-Silva, A. Cytomegalovirus infection in renal transplantation: Clinical aspects, management and the perspectives. Einstein 2015, 13, 142–148. [Google Scholar] [CrossRef]
- Poole, E.; Groves, I.; Jackson, S.; Wills, M.; Sinclair, J. Using Primary Human Cells to Analyze Human Cytomegalovirus Biology. In Human Cytomegaloviruses: Methods and Protocols; Yurochko, A.D., Ed.; Springer: New York, NY, USA, 2021; pp. 51–81. ISBN 978-1-0716-1111-1. [Google Scholar]
- Yang, D.; Le, J. Targeted Amplification of Alternatively Spliced Transcripts of Major Histocompatibility Complex Class I Heavy Chain. J. Immunol. Methods 1994, 176, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Poole, E.; Lau, J.C.H.; Sinclair, J. Latent Infection of Myeloid Progenitors by Human Cytomegalovirus Protects Cells from FAS-Mediated Apoptosis through the Cellular IL-10/PEA-15 Pathway. J. Gen. Virol. 2015, 96, 2355–2359. [Google Scholar] [CrossRef]
- Poole, E.; Sinclair, J. Sleepless Latency of Human Cytomegalovirus. Med. Microbiol. Immunol. 2015, 204, 421–429. [Google Scholar] [PubMed]
- Poole, E.; Neves, T.C.; Oliveira, M.T.; Sinclair, J.; da Silva, M.C.C. Human Cytomegalovirus Interleukin 10 Homologs: Facing the Immune System. Front. Cell. Infect. Microbiol. 2020, 10, 245. [Google Scholar] [CrossRef]
- Cheung, A.K.L.; Gottlieb, D.J.; Plachter, B.; Pepperl-Klindworth, S.; Avdic, S.; Cunningham, A.L.; Abendroth, A.; Slobedman, B. The Role of the Human Cytomegalovirus UL111A Gene in Down-Regulating CD4+T-Cell Recognition of Latently Infected Cells: Implications for Virus Elimination during Latency. Blood 2009, 114, 4128–4137. [Google Scholar] [CrossRef]
- Rossetto, C.C.; Tarrant-Elorza, M.; Pari, G.S. Cis and Trans Acting Factors Involved in Human Cytomegalovirus Experimental and Natural Latent Infection of CD14 (+) Monocytes and CD34 (+) Cells. PLoS Pathog. 2013, 9, e1003366. [Google Scholar] [CrossRef]
- Goodrum, F.D.; Jordan, C.T.; High, K.; Shenk, T. Human Cytomegalovirus Gene Expression during Infection of Primary Hematopoietic Progenitor Cells: A Model for Latency. Proc. Natl. Acad. Sci. USA 2002, 99, 16255–16260. [Google Scholar] [CrossRef]
- Huang, S.F.; Huang, Y.C.; Lee, C.T.; Chou, K.T.; Chen, H.P.; Huang, C.C.; Ji, D.D.; Chan, Y.J.; Yang, Y.Y. Cytomegalovirus Viral Interleukin-10 in Patients with Aspergillus Infection and Effects on Clinical Outcome. Mycoses 2022, 65, 760–769. [Google Scholar] [CrossRef]
- Young, V.P.; Mariano, M.C.; Tu, C.C.; Allaire, K.M.; Avdic, S.; Slobedman, B.; Spencer, J.V. Modulation of the Host Environment by Human Cytomegalovirus with Viral Interleukin 10 in Peripheral Blood. J. Infect. Dis. 2017, 215, 874–882. [Google Scholar] [CrossRef]
- Veld, L.f.; Waters, S.; Irish, A.; Price, P.; Lee, S. An IL-10 Homologue Encoded by Human Cytomegalovirus Is Linked with the Viral “Footprint” in Clinical Samples. Cytokine 2024, 180, 156654. [Google Scholar] [CrossRef]
- Sinclair, J. Human Cytomegalovirus: Latency and Reactivation in the Myeloid Lineage. J. Clin. Virol. 2008, 41, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Wiedeman, J.; Sissons, J.G.P.; Borysiewicz, L.K.; Sinclair, J.H. Monocytes Are a Major Site of Persistence of Human Cytomegalovirus in Peripheral Blood Mononuclear Cells. J. Gen. Virol. 1991, 72, 2059–2064. [Google Scholar] [CrossRef] [PubMed]
- Slobedman, B.; Mocarski, E.S. Quantitative Analysis of Latent Human Cytomegalovirus. J. Virol. 1999, 73, 4806–4812. [Google Scholar] [CrossRef] [PubMed]
- Bernshtein, B.; Nachshon, A.; Shnayder, M.; Stern, L.; Avdic, S.; Blyth, E.; Gottlieb, D.; Abendroth, A.; Slobedman, B.; Stern-Ginossar, N.; et al. Profiling the Blood Compartment of Hematopoietic Stem Cell Transplant Patients During Human Cytomegalovirus Reactivation. Front. Cell. Infect. Microbiol. 2021, 10, 607470. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, G.W.C.; Oliveira, M.T.; Martines, I.G.L.; Fiori, G.C.; Nevels, M.M.; Groves, I.J.; Sinclair, J.; Medina-Pestana, J.; da Silva, R.S.; Nakamura, M.; et al. Expression Profile of Human Cytomegalovirus UL111A cmvIL-10 and LAcmvIL-10 Transcripts in Primary Cells and Cells from Renal Transplant Recipients. Viruses 2025, 17, 501. https://doi.org/10.3390/v17040501
Almeida GWC, Oliveira MT, Martines IGL, Fiori GC, Nevels MM, Groves IJ, Sinclair J, Medina-Pestana J, da Silva RS, Nakamura M, et al. Expression Profile of Human Cytomegalovirus UL111A cmvIL-10 and LAcmvIL-10 Transcripts in Primary Cells and Cells from Renal Transplant Recipients. Viruses. 2025; 17(4):501. https://doi.org/10.3390/v17040501
Chicago/Turabian StyleAlmeida, Giovana W. C., Martha T. Oliveira, Isabella G. L. Martines, Giuliano C. Fiori, Michael M. Nevels, Ian J. Groves, John Sinclair, José Medina-Pestana, Rayra Sampaio da Silva, Monica Nakamura, and et al. 2025. "Expression Profile of Human Cytomegalovirus UL111A cmvIL-10 and LAcmvIL-10 Transcripts in Primary Cells and Cells from Renal Transplant Recipients" Viruses 17, no. 4: 501. https://doi.org/10.3390/v17040501
APA StyleAlmeida, G. W. C., Oliveira, M. T., Martines, I. G. L., Fiori, G. C., Nevels, M. M., Groves, I. J., Sinclair, J., Medina-Pestana, J., da Silva, R. S., Nakamura, M., Requião-Moura, L., Poole, E., & Silva, M. C. C. d. (2025). Expression Profile of Human Cytomegalovirus UL111A cmvIL-10 and LAcmvIL-10 Transcripts in Primary Cells and Cells from Renal Transplant Recipients. Viruses, 17(4), 501. https://doi.org/10.3390/v17040501





