The Effect of Immunosuppressive Treatment on Torque Teno Virus Load in Lung Transplant Recipients: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Immunosuppression Regimen and Sampling Methods
2.3. TTV Measurement and Immunosuppression Assessment
3. Results
3.1. Study Participants and Clinical Profiles
3.2. Strong Positive Correlation Between Time Since Transplantation and log10 TTV Levels
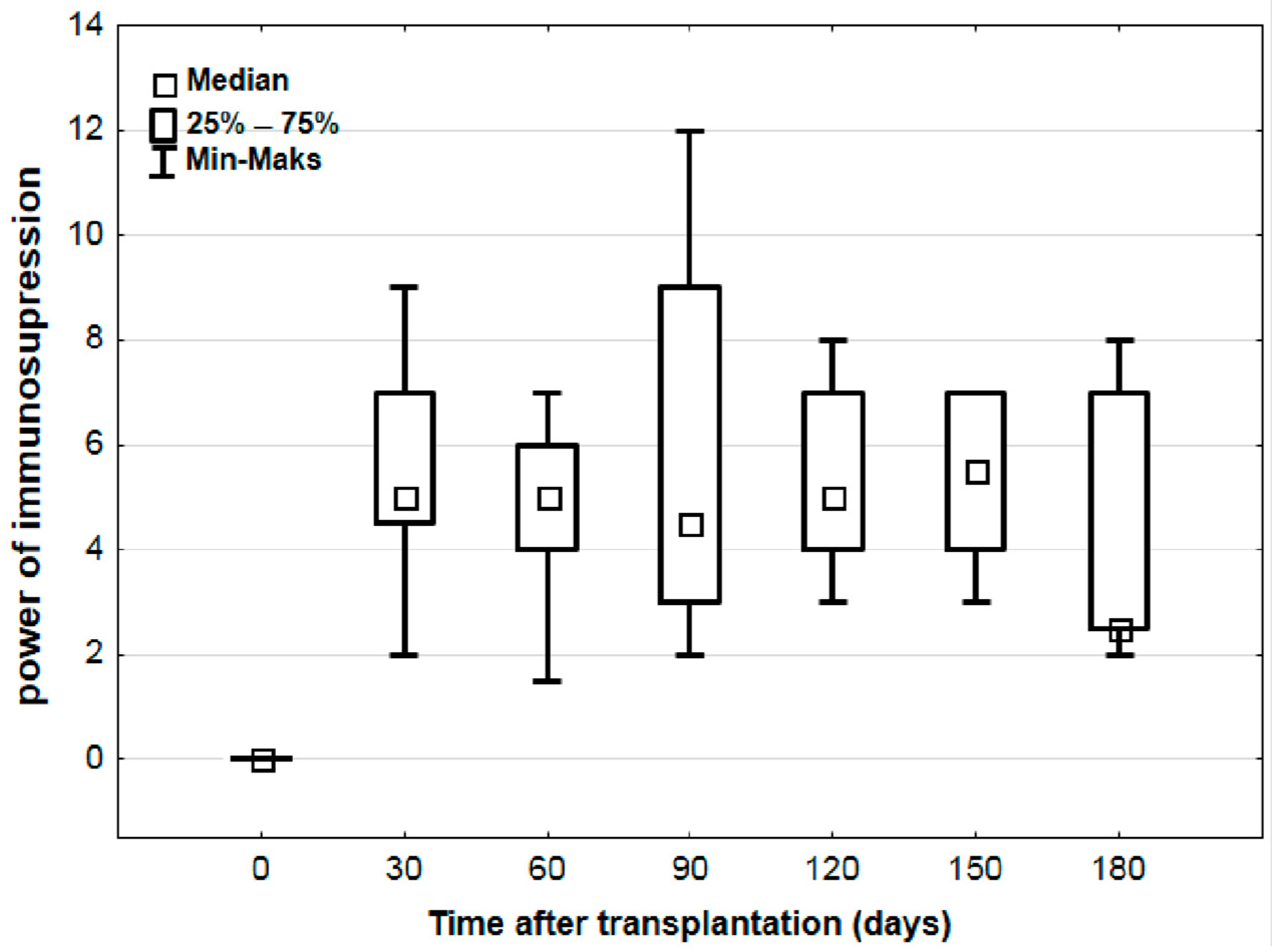
3.3. Systematic Increase in log10 TTV Without Corresponding Changes in Immunosuppression Strength
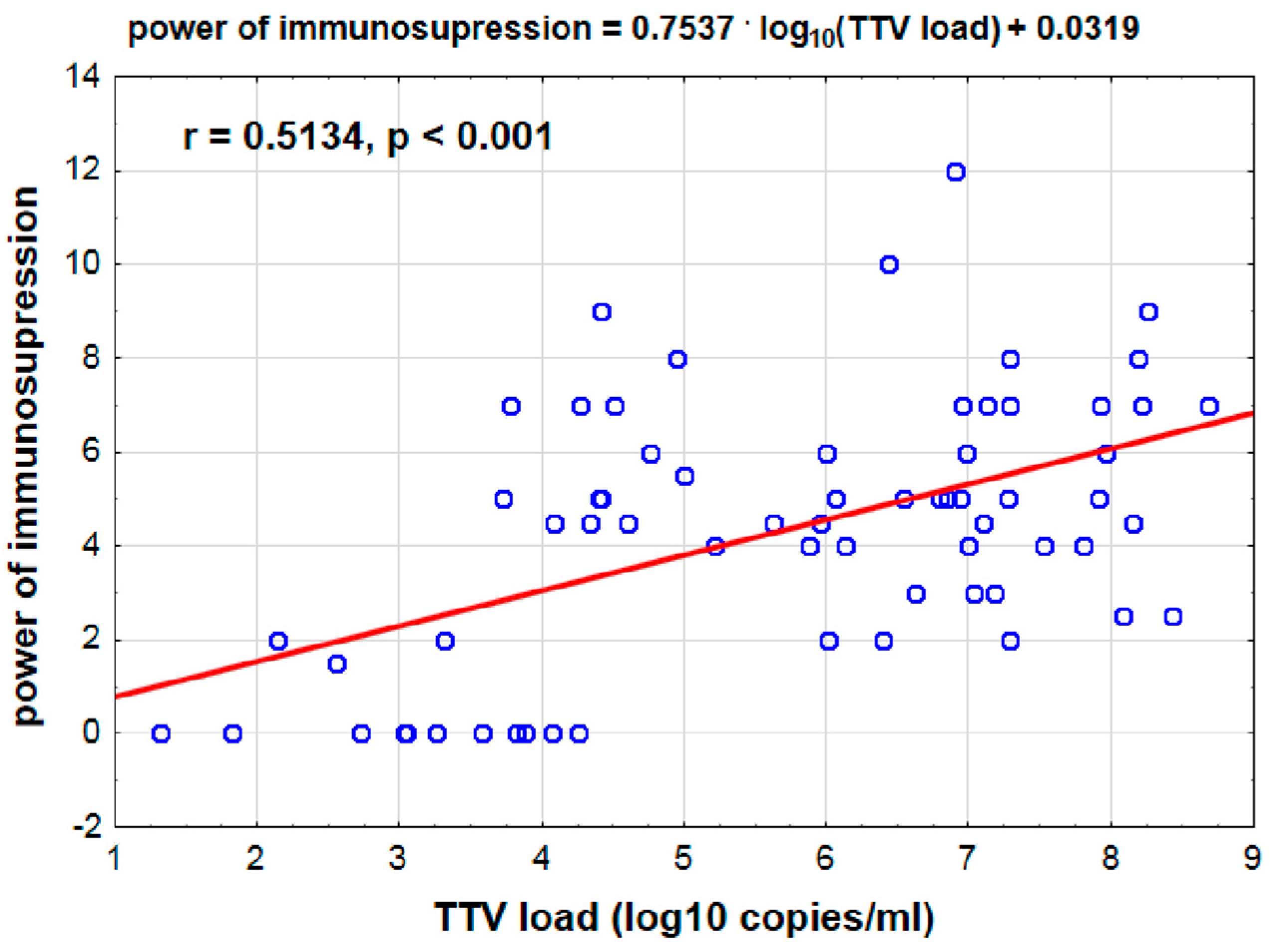
3.4. Positive Correlation Between log10 TTV Levels and Immunosuppression Strength
3.5. No Correlation Between TTV Levels and Donor-Specific Antibodies or CMV Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaksch, P.; Kundi, M.; Görzer, I.; Muraközy, G.; Lambers, C.; Benazzo, A.; Hoetzenecker, K.; Klepetko, W.; Puchhammer-Stöckl, E. Torque Teno Virus as a Novel Biomarker Targeting the Efficacy of Immunosuppression After Lung Transplantation. J. Infect. Dis. 2018, 218, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; Alvey, N.; Bowman, L.; Schulte, J.; Segovia, M.C.; McDermott, J.; Te, H.S.; Kapila, N.; Levine, D.J.; Gottlieb, R.L.; et al. Consensus recommendations for use of maintenance immunosuppression in solid organ transplantation: Endorsed by the American College of Clinical Pharmacy, American Society of Transplantation, and the International Society for Heart and Lung Transplantation. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2022, 42, 599–633. [Google Scholar] [CrossRef] [PubMed]
- Schuurmans, M.M.; Raeber, M.E.; Roeder, M.; Hage, R. Adaptive Immunosuppression in Lung Transplant Recipients Applying Complementary Biomarkers: The Zurich Protocol. Medicina 2023, 59, 488. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.; Khan, H. Immunosuppressive Drugs. Encycl. Infect. Immun. 2022, 4, 726–740. [Google Scholar] [CrossRef]
- Keller, M.; Agbor-Enoh, S. Cell-free DNA in lung transplantation: Research tool or clinical workhorse? Curr. Opin. Organ Transpl. 2022, 27, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Deng, X.; Da Costa, A.C.; Bruhn, R.; Deeks, S.G.; Delwart, E. Virome analysis of antiretroviral-treated HIV patients shows no correlation between T-cell activation and anelloviruses levels. J. Clin. Virol. 2015, 72, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Yeo, W.; Tang, M.W.; Lin, X.; Mo, F.; Ho, W.M.; Hui, P.; Johnson, P.J. Gross Elevation of TT Virus Genome Load in the Peripheral Blood Mononuclear Cells of Cancer Patients. Ann. N. Y. Acad. Sci. 2001, 945, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Görzer, I.; Haloschan, M.; Jaksch, P.; Klepetko, W.; Puchhammer-Stöckl, E. Plasma DNA levels of Torque teno virus and immunosuppression after lung transplantation. J. Heart Lung Transpl. 2014, 33, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.; Clemmensen, T.S.; Petersen, M.S.; Mogensen, L.J.; Christiansen, M.; Rolid, K.; Nytrøen, K.; Møller, B.K.; Gullestad, L.; Eiskjær, H.; et al. Kinetics of Torque Teno virus in heart transplant patients. Hum. Immunol. 2023, 84, 110720. [Google Scholar] [CrossRef] [PubMed]
- Solis, M.; Velay, A.; Gantner, P.; Bausson, J.; Filipputtu, A.; Freitag, R.; Moulin, B.; Caillard, S.; Fafi-Kremer, S. Torquetenovirus viremia for early prediction of graft rejection after kidney transplantation. J. Infect. 2019, 79, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Schiemann, M.; Puchhammer-Stöckl, E.; Eskandary, F.; Kohlbeck, P.; Rasoul-Rockenschaub, S.; Heilos, A.; Kozakowski, N.; Görzer, I.; Kikić, Ž.; Herkner, H.; et al. Torque Teno Virus Load—Inverse Association with Antibody-Mediated Rejection After Kidney Transplantation. Transplantation 2017, 101, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Frye, B.C.; Bierbaum, S.; Falcone, V.; Köhler, T.C.; Gasplmayr, M.; Hettich, I.; Dürk, T.; Idzko, M.; Zissel, G.; Hengel, H.; et al. Kinetics of Torque Teno Virus-DNA Plasma Load Predict Rejection in Lung Transplant Recipients. Transplantation 2019, 103, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Macera, L.; Pistello, M.; Maggi, F. Torque Teno Virus Viremia Correlates with Intensity of Maintenance Immunosuppression in Adult Orthotopic Liver Transplant. J. Infect. Dis. 2014, 210, 667–668. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochman, M.; Galle, D.; Kowal, A.; Królikowska, M.; Zawadzki, F.; Stanjek-Cichoracka, A.; Łaszewska, A.; Chełmecka, E.; Hrapkowicz, T. The Effect of Immunosuppressive Treatment on Torque Teno Virus Load in Lung Transplant Recipients: A Preliminary Study. Viruses 2025, 17, 438. https://doi.org/10.3390/v17030438
Ochman M, Galle D, Kowal A, Królikowska M, Zawadzki F, Stanjek-Cichoracka A, Łaszewska A, Chełmecka E, Hrapkowicz T. The Effect of Immunosuppressive Treatment on Torque Teno Virus Load in Lung Transplant Recipients: A Preliminary Study. Viruses. 2025; 17(3):438. https://doi.org/10.3390/v17030438
Chicago/Turabian StyleOchman, Marek, Dagmara Galle, Anna Kowal, Magdalena Królikowska, Fryderyk Zawadzki, Anita Stanjek-Cichoracka, Anna Łaszewska, Elżbieta Chełmecka, and Tomasz Hrapkowicz. 2025. "The Effect of Immunosuppressive Treatment on Torque Teno Virus Load in Lung Transplant Recipients: A Preliminary Study" Viruses 17, no. 3: 438. https://doi.org/10.3390/v17030438
APA StyleOchman, M., Galle, D., Kowal, A., Królikowska, M., Zawadzki, F., Stanjek-Cichoracka, A., Łaszewska, A., Chełmecka, E., & Hrapkowicz, T. (2025). The Effect of Immunosuppressive Treatment on Torque Teno Virus Load in Lung Transplant Recipients: A Preliminary Study. Viruses, 17(3), 438. https://doi.org/10.3390/v17030438





