Biochemical Screening of Phytochemicals and Identification of Scopoletin as a Potential Inhibitor of SARS-CoV-2 Mpro, Revealing Its Biophysical Impact on Structural Stability
Abstract
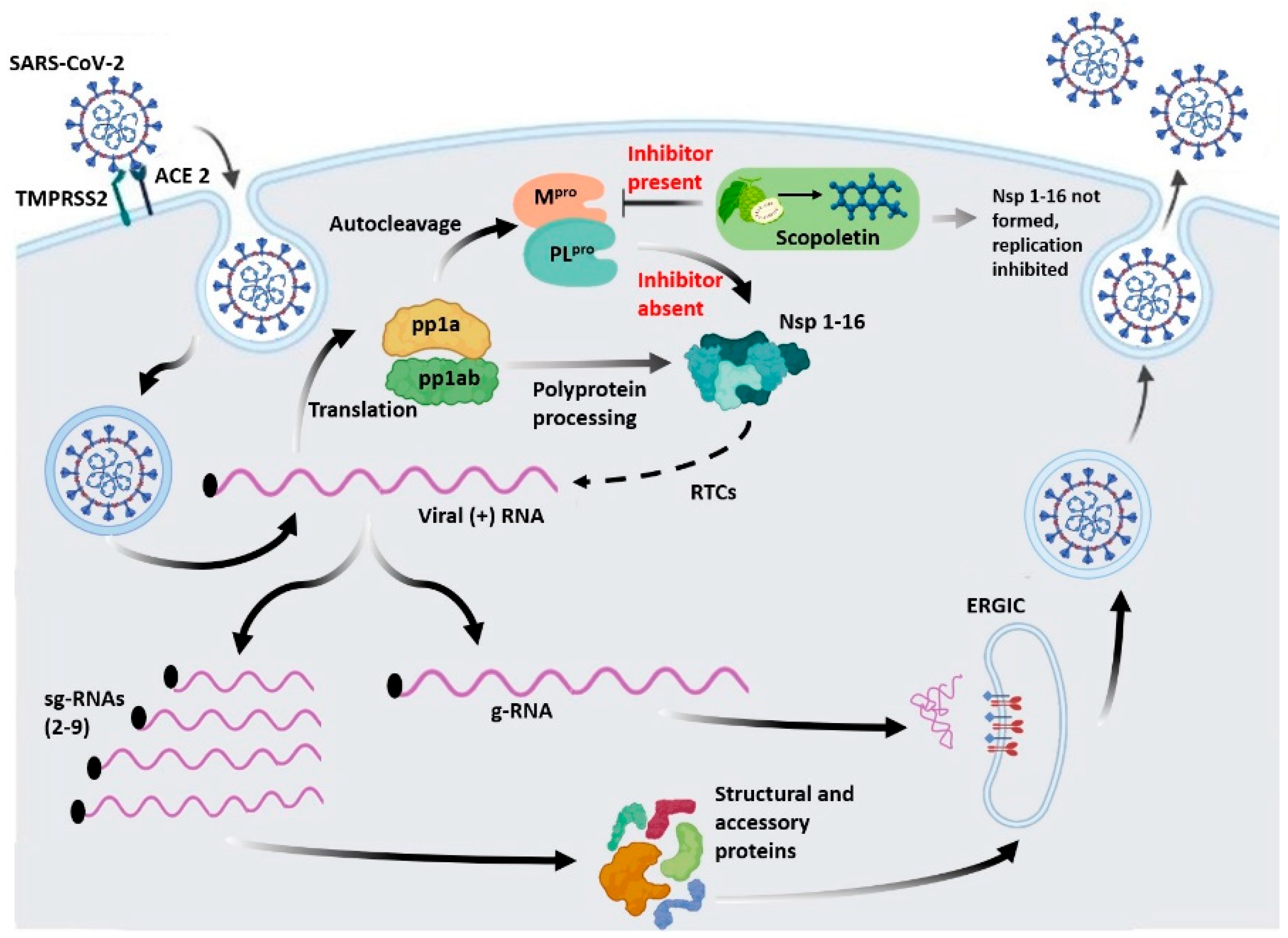
:1. Introduction
2. Materials and Methods
2.1. Virtual Screening of Selected Phytochemicals
Analysis of Molecular Interactions
2.2. Protein Expression
2.3. Protein Purification
2.4. Western Blot Analysis
2.5. FRET-Based Enzymatic Activity Assay
2.6. Fluorescence Spectroscopy
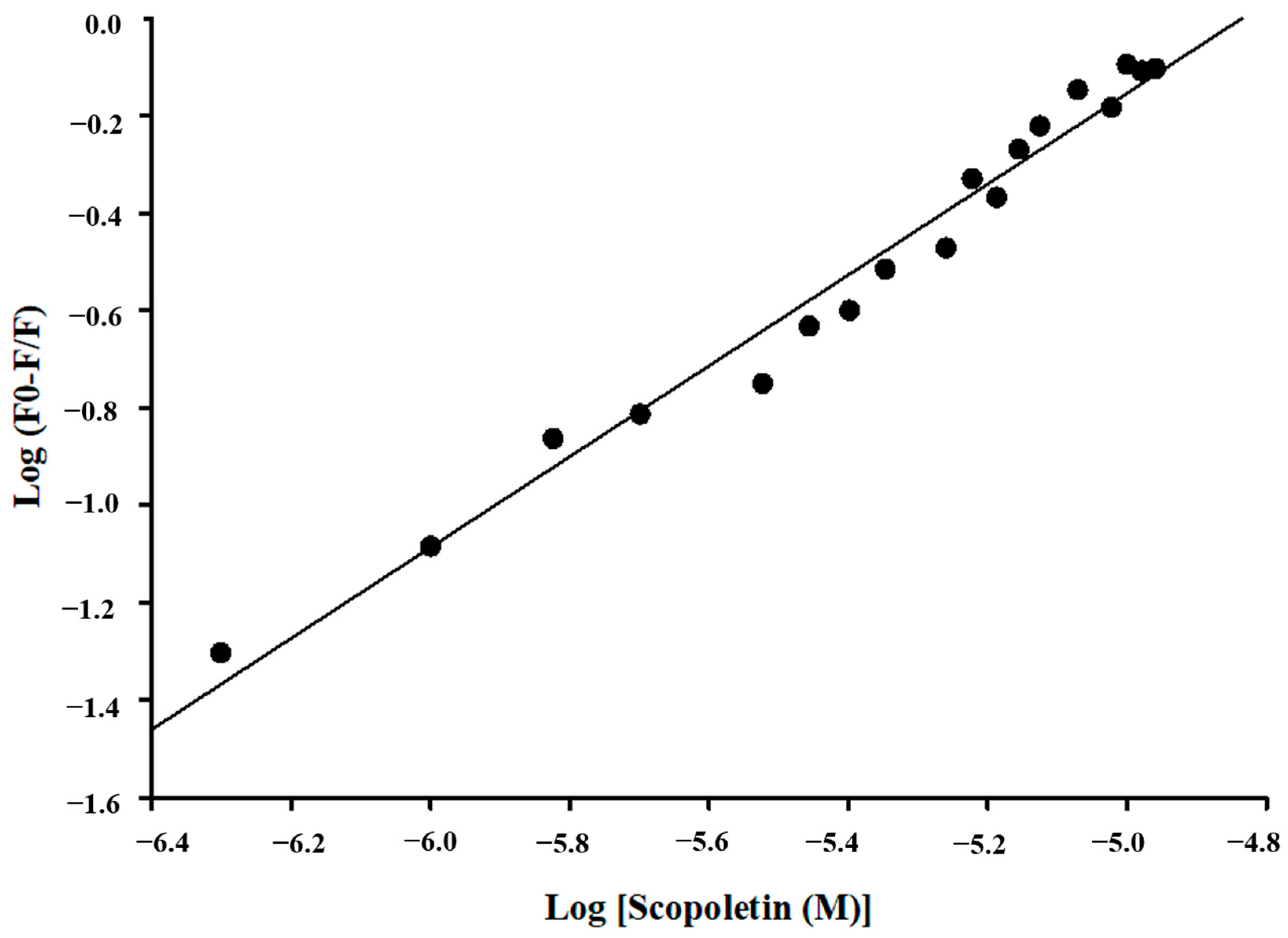
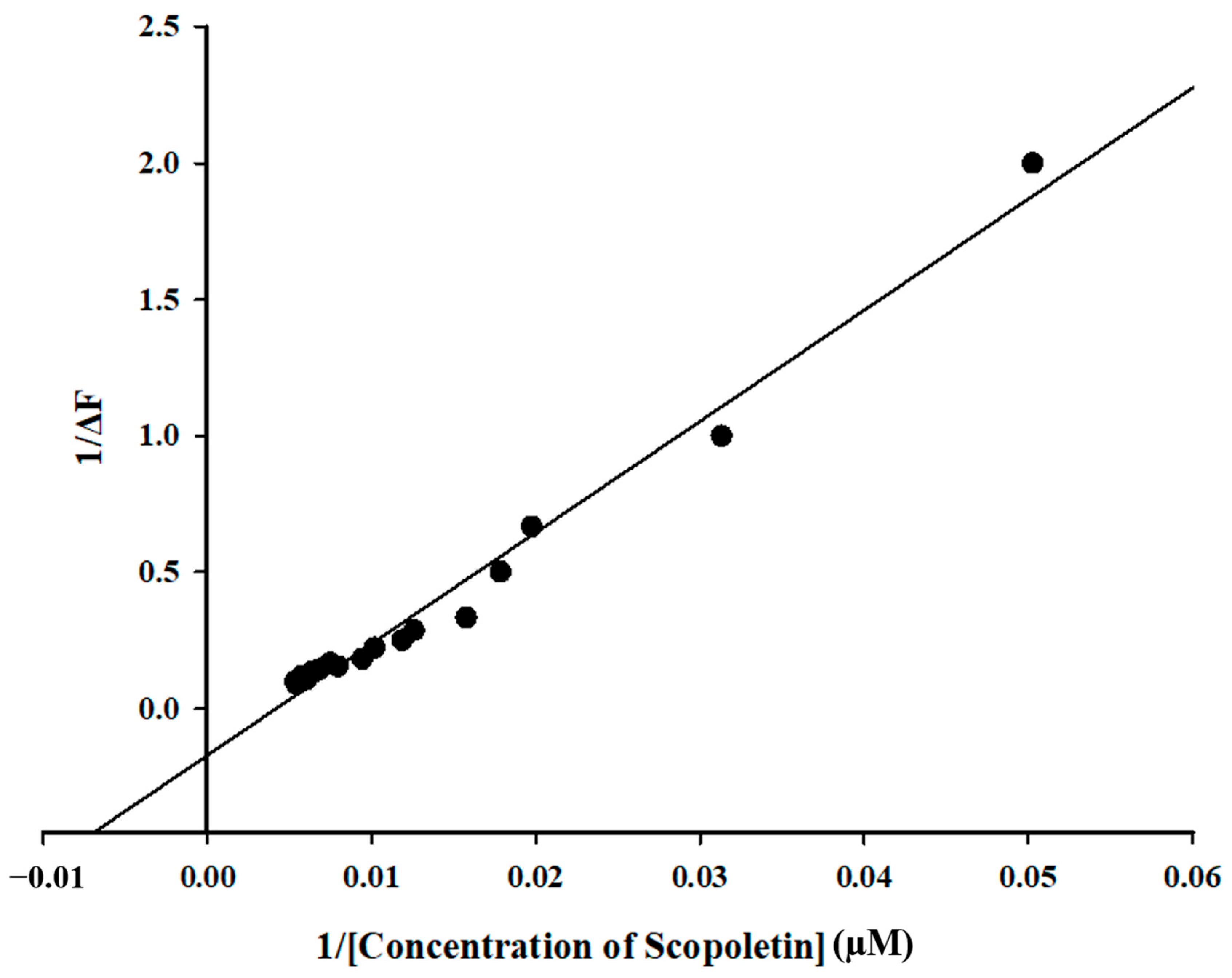
Calculation of Binding Parameters
2.7. Thermodynamic Investigation Using Isothermal Titration Calorimetry (ITC)
2.8. ADMET Analysis of Scopoletin
2.9. MD Simulation
2.10. Evaluation of Cytotoxicity by Scopoletin Using MTT Assay
3. Results
3.1. Selection of Best Phytochemical, Scopoletin, Based on Virtual Screening
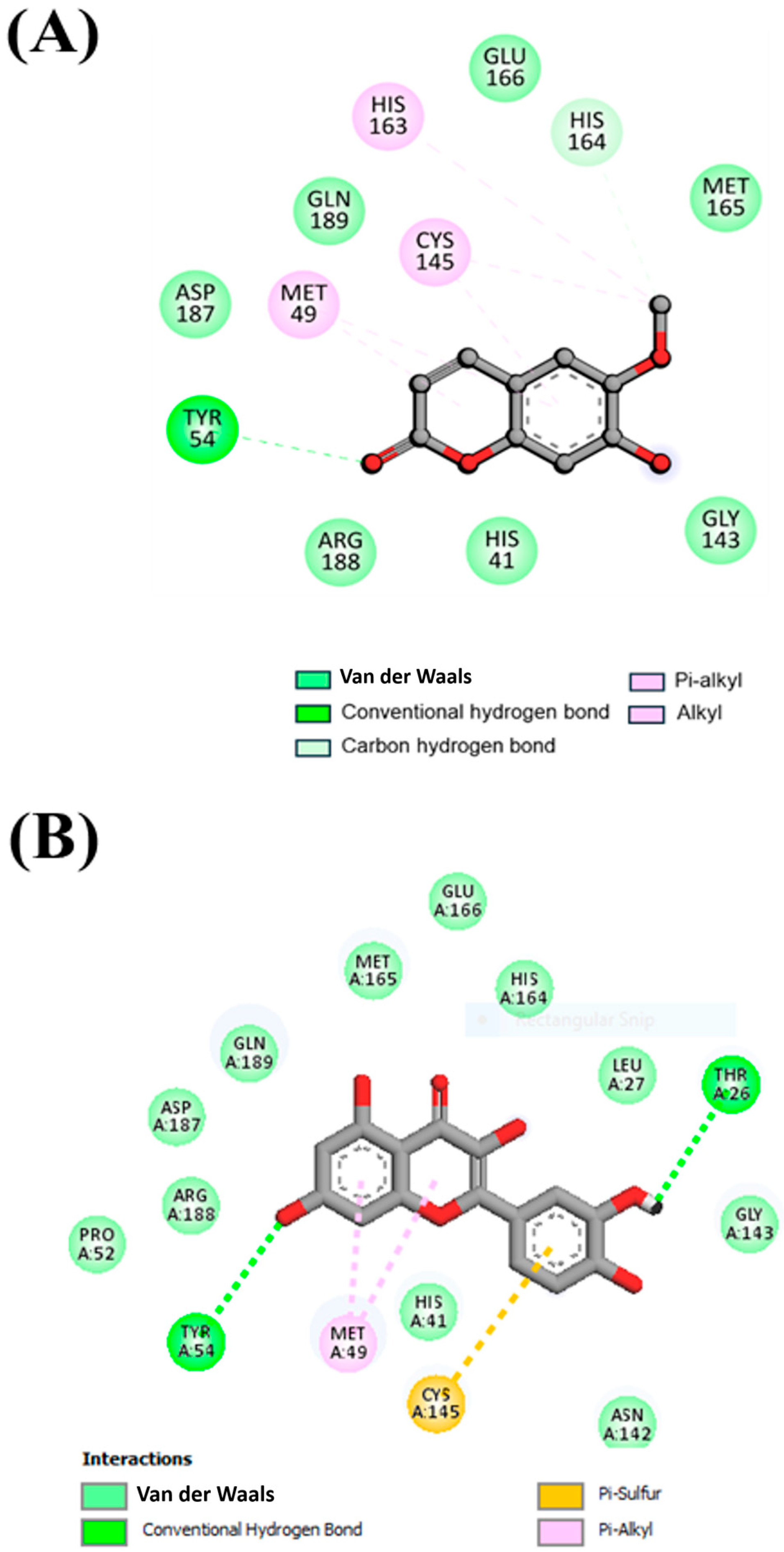
3.2. Interaction Analysis
3.3. Mpro Could Be Expressed and Purified Successfully
3.4. FRET-Based Enzymatic Activity Assay Deciphers the IC50 of Scopoletin as 15.75 uM
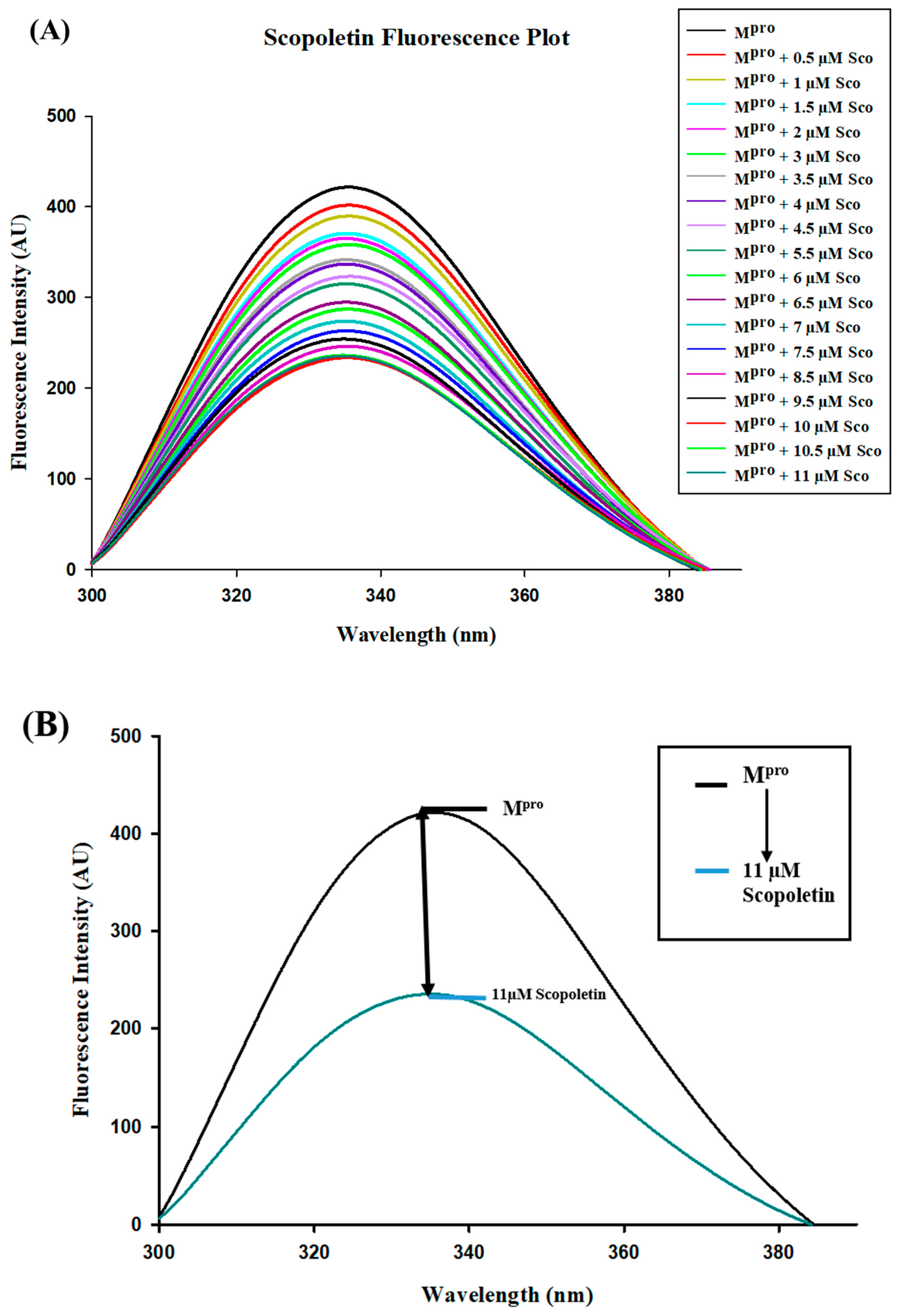
3.5. Fluorescence Quenching of Mpro with Inhibitor Scopoletin Indicates High Affinity
3.6. CD Spectroscopy Confirmed Changes in Secondary Structure of Mpro upon Binding of Scopoletin
3.7. Isothermal Titration Calorimetry (ITC)
3.8. ADMET Analysis
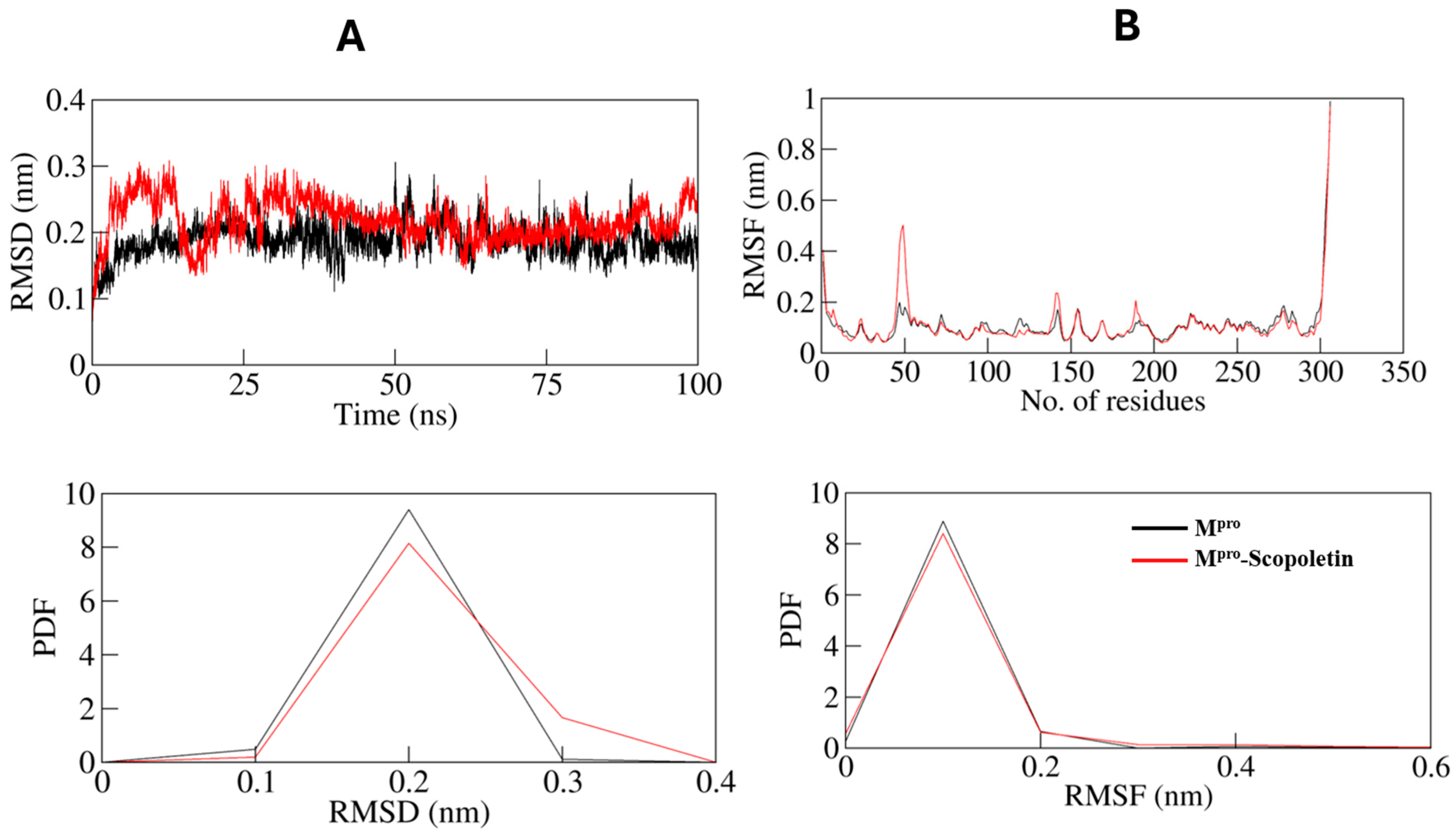
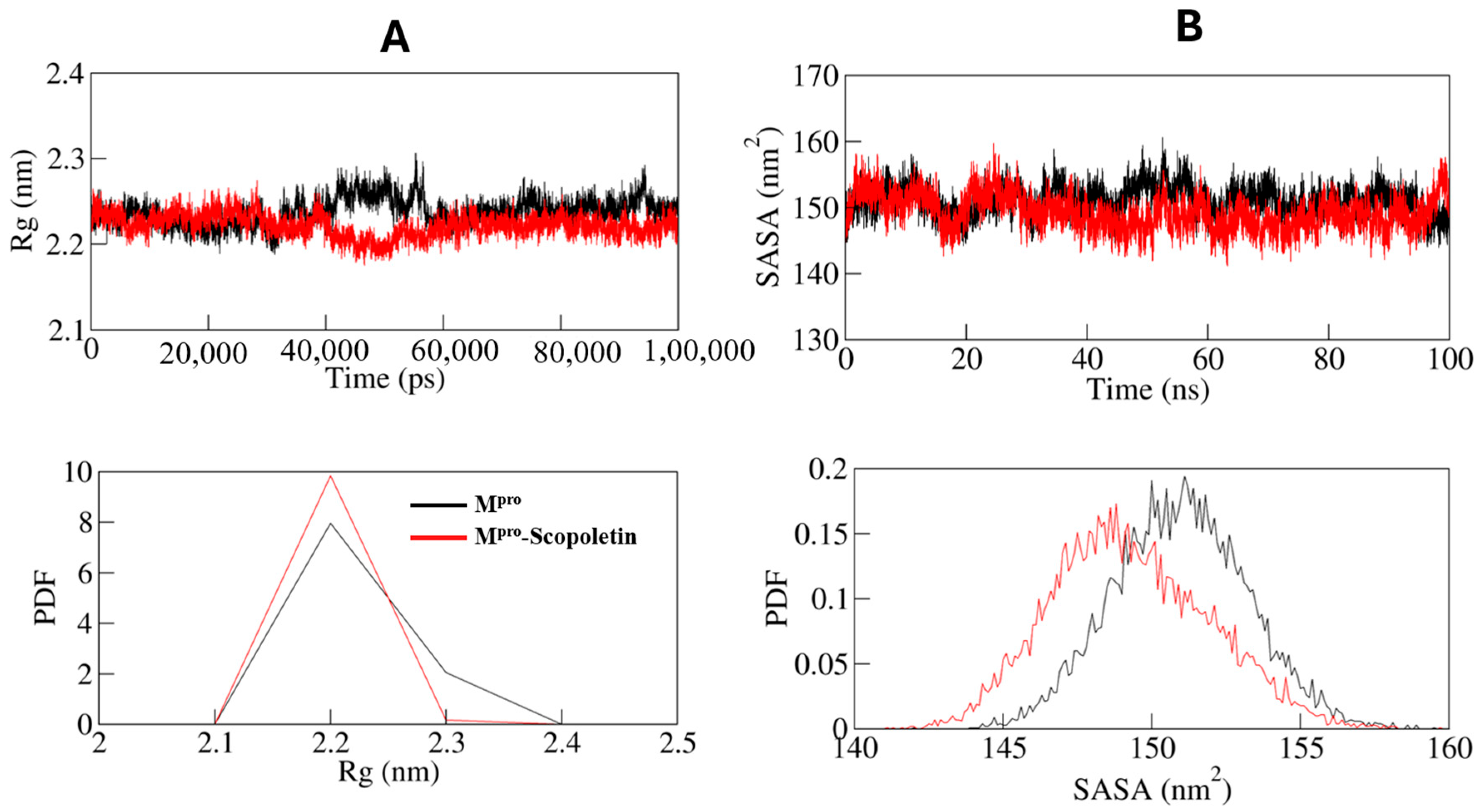
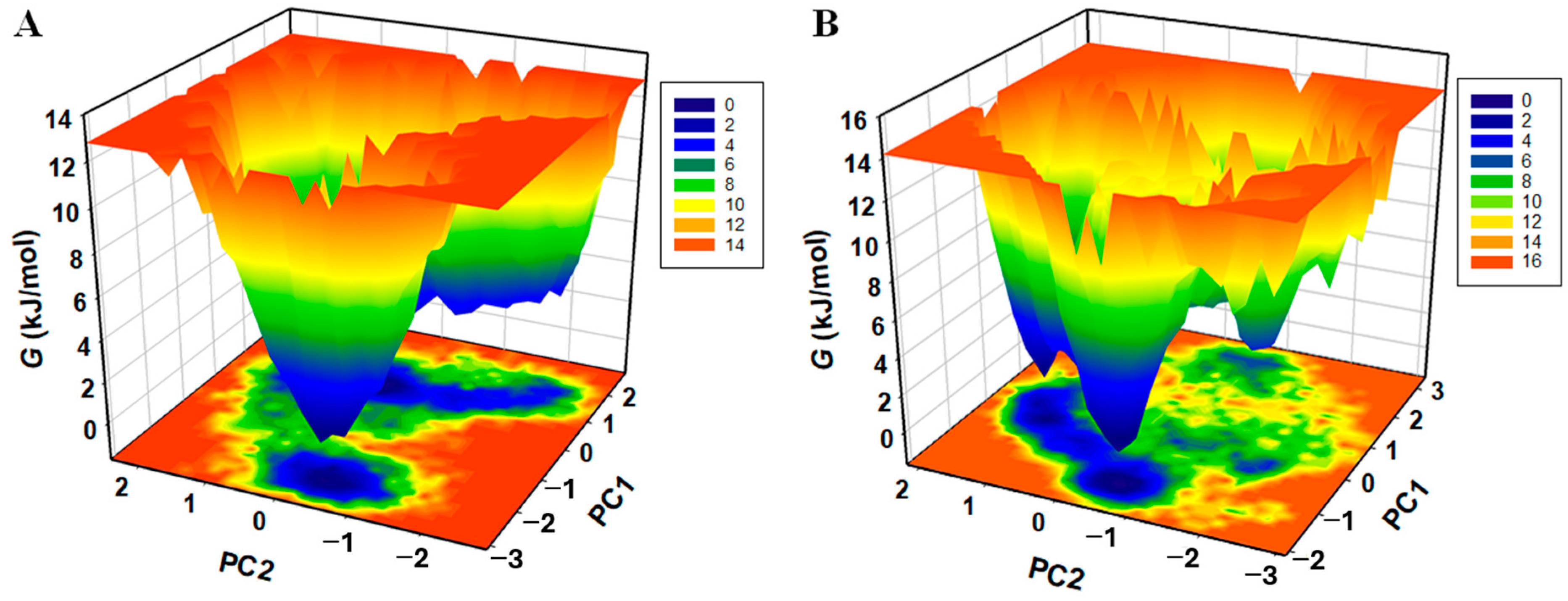
3.9. MD Simulations Supported the Spectral Data at Dynamic Level
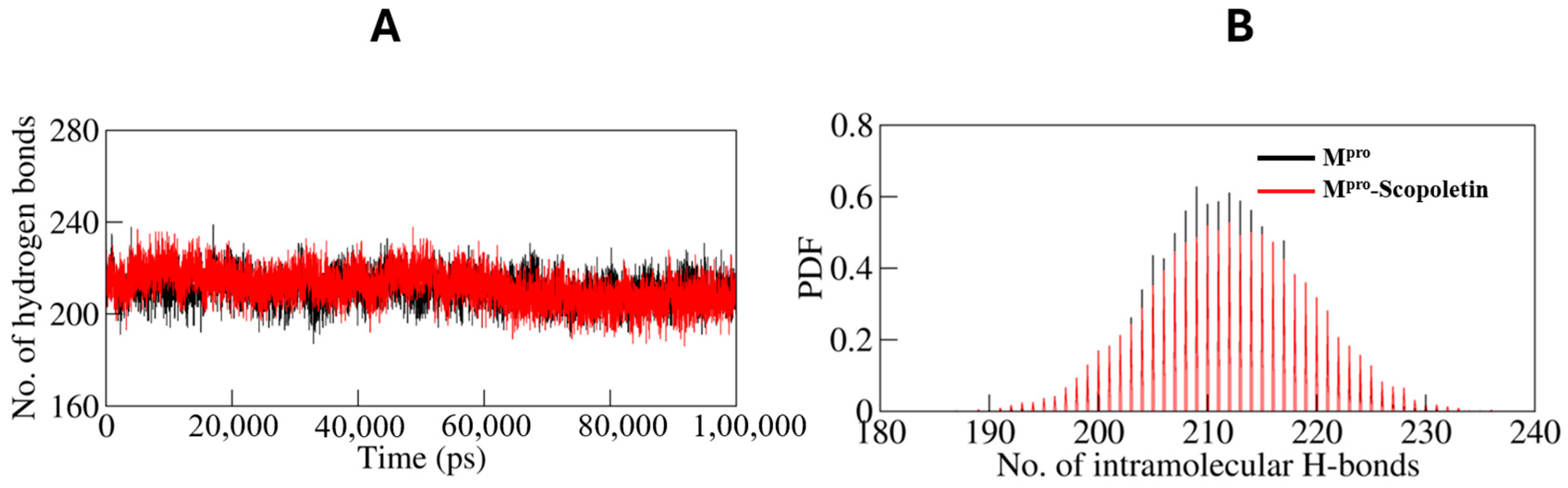
3.9.1. Dynamics of Hydrogen Bonds
3.9.2. Principal Component and Free Energy Landscape Analyses
3.10. MTT Assay of Scopoletin to Evaluate Its Cytotoxicity in HEK293 Cells
4. Discussion
Scopoletin, a Phytochemical, Is a Potential Inhibitor of Mpro and Suitable for SARS-CoV-2 Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pang, X.; Xu, W.; Liu, Y.; Li, H.; Chen, L. The research progress of SARS-CoV-2 main protease inhibitors from 2020 to 2022. Eur. J. Med. Chem. 2023, 257, 115491. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, Z.; Li, M.; Wang, X. Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Front. Immunol. 2020, 11, 552909. [Google Scholar] [CrossRef] [PubMed]
- Dey, J.K.; Dey, S.K. SARS-CoV-2 pandemic, COVID-19 case fatality rates and deaths per million population in India. J. Bioinform. Comput. Syst. Biol. 2020, 2, 5000110. [Google Scholar]
- Yang, H.; Rao, Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat. Rev. Microbiol. 2021, 19, 685–700. [Google Scholar] [CrossRef]
- Tao, X.; Zhang, L.; Du, L.; Liao, R.; Cai, H.; Lu, K.; Zhao, Z.; Xie, Y.; Wang, P.-H.; Pan, J.-A.; et al. Allosteric inhibition of SARS-CoV-2 3CL protease by colloidal bismuth subcitrate. Chem. Sci. 2021, 12, 14098–14102. [Google Scholar] [CrossRef]
- Yadav, R.; Courouble, V.V.; Dey, S.K.; Harrison, J.; Timm, J.; Hopkins, J.B.; Slack, R.L.; Sarafianos, S.G.; Ruiz, F.X.; Griffin, P.R.; et al. Biochemical and structural insights into SARS-CoV-2 polyprotein processing by Mpro. Sci. Adv. 2022, 8, eadd2191. [Google Scholar] [CrossRef]
- Courouble, V.V.; Dey, S.K.; Yadav, R.; Timm, J.; Harrison, J.J.E.K.; Ruiz, F.X.; Arnold, E.; Griffin, P.R. Revealing the Structural Plasticity of SARS-CoV-2 nsp7 and nsp8 Using Structural Proteomics. J. Am. Soc. Mass Spectrom. 2021, 32, 1618–1630. [Google Scholar] [CrossRef]
- Chhetri, A.; Chettri, S.; Rai, P.; Mishra, D.K.; Sinha, B.; Brahman, D. Synthesis, characterization and computational study on potential inhibitory action of novel azo imidazole derivatives against COVID-19 main protease (M(pro): 6LU7). J. Mol. Struct. 2021, 1225, 129230. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiang, R.; Huo, S.; Zhou, Y.; Jiang, S.; Wang, Q.; Yu, F. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct. Target. Ther. 2021, 6, 233. [Google Scholar] [CrossRef]
- Faddis, R.; Du, S.; Stewart, J.; Hasan, M.M.; Lewit, N.; Ali, M.A.; White, C.B.; Okoto, P.; Thallapuranam, S.; Halim, M.A. Molecular Modelling, Synthesis, and In-Vitro Assay to Identify Potential Antiviral Peptides Targeting the 3-Chymotrypsin-Like Protease of SARS-CoV-2. Int. J. Pept. Res. Ther. 2023, 29, 89. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Mengist, H.M.; Dilnessa, T.; Jin, T. Structural Basis of Potential Inhibitors Targeting SARS-CoV-2 Main Protease. Front. Chem. 2021, 9, 622898. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Bravo, C.; Torres-Carranza, D.; Sanchez-Trujillo, L.; Gómez-Lahoz, A.M.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; Bujan, J.; et al. An Updated Review of SARS-CoV-2 Vaccines and the Importance of Effective Vaccination Programs in Pandemic Times. Vaccines 2021, 9, 433. [Google Scholar] [CrossRef] [PubMed]
- Faksova, K.; Walsh, D.; Jiang, Y.; Griffin, J.; Phillips, A.; Gentile, A.; Kwong, J.C.; Macartney, K.; Naus, M.; Grange, Z.; et al. COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine 2024, 42, 2200–2211. [Google Scholar] [CrossRef]
- Behera, P.; Singh, A.K.; Subba, S.H.; Mc, A.; Sahu, D.P.; Chandanshive, P.D.; Pradhan, S.K.; Parida, S.P.; Mishra, A.; Patro, B.K.; et al. Effectiveness of COVID-19 vaccine (Covaxin) against breakthrough SARS-CoV-2 infection in India. Hum. Vaccines Immunother. 2022, 18, 2034456. [Google Scholar] [CrossRef]
- Das, S.; Nath, S.; Shahjahan; Dey, S.K. Plausible mechanism of drug resistance and side-effects of COVID-19 therapeutics: A bottleneck for its eradication. DARU J. Pharm. Sci. 2024, 32, 801–823. [Google Scholar] [CrossRef]
- Li, Z.; Liu, S.; Li, F.; Li, Y.; Li, Y.; Peng, P.; Li, S.; He, L.; Liu, T. Efficacy, immunogenicity and safety of COVID-19 vaccines in older adults: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 965971. [Google Scholar] [CrossRef]
- Teo, S.P. Review of COVID-19 Vaccines and Their Evidence in Older Adults. Ann. Geriatr. Med. Res. 2021, 25, 4–9. [Google Scholar] [CrossRef]
- Nanishi, E.; Levy, O.; Ozonoff, A. Waning effectiveness of SARS-CoV-2 mRNA vaccines in older adults: A rapid review. Hum. Vaccines Immunother. 2022, 18, 2045857. [Google Scholar] [CrossRef]
- Ng, T.I.; Correia, I.; Seagal, J.; DeGoey, D.A.; Schrimpf, M.R.; Hardee, D.J.; Noey, E.L.; Kati, W.M. Antiviral Drug Discovery for the Treatment of COVID-19 Infections. Viruses 2022, 14, 961. [Google Scholar] [CrossRef]
- Majerová, T.; Konvalinka, J. Viral proteases as therapeutic targets. Mol. Asp. Med. 2022, 88, 101159. [Google Scholar] [CrossRef] [PubMed]
- Teli, D.; Balar, P.; Patel, K.; Sharma, A.; Chavda, V.; Vora, L. Molnupiravir: A Versatile Prodrug against SARS-CoV-2 Variants. Metabolites 2023, 13, 309. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.I.; Kang, T.; Ali, H.; Lai, D. Remdesivir Strongly Binds to RNA-Dependent RNA Polymerase, Membrane Protein, and Main Protease of SARS-CoV-2: Indication From Molecular Modeling and Simulations. Front. Pharmacol. 2021, 12, 710778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef]
- Wilkinson, D.A.; Joffrin, L.; Lebarbenchon, C.; Mavingui, P. Partial RdRp sequences offer a robust method for Coronavirus subgenus classification. bioRxiv 2020. [Google Scholar] [CrossRef]
- Majchrzak, M.; Madej, Ł.; Łysek-Gładysińska, M.; Zarębska-Michaluk, D.; Zegadło, K.; Dziuba, A.; Nogal-Nowak, K.; Kondziołka, W.; Sufin, I.; Myszona-Tarnowska, M.; et al. The RdRp genotyping of SARS-CoV-2 isolated from patients with different clinical spectrum of COVID-19. BMC Infect. Dis. 2024, 24, 281. [Google Scholar] [CrossRef]
- Bano, S.; Ahmedi, S.; Manzoor, N.; Dey, S.K. Toxicology of Antifungal and Antiviral Drugs. In Advances in Antifungal Drug Development: Natural Products with Antifungal Potential; Manzoor, N., Ed.; Springer Nature Singapore: Singapore, 2024; pp. 633–652. [Google Scholar]
- Zagórska, A.; Czopek, A.; Fryc, M.; Jończyk, J. Inhibitors of SARS-CoV-2 Main Protease (Mpro) as Anti-Coronavirus Agents. Biomolecules 2024, 14, 797. [Google Scholar] [CrossRef]
- Singh, J.; Singhal, A.; Pandey, A.; Amit; Mallik, S.; Dubey, M. Phytochemical Investigation on Tinospora cordifolia and Alstonia scholaris. Res. J. Pharm. Technol. 2024, 17, 2689–2693. [Google Scholar] [CrossRef]
- Gao, L.; Du, L.D.; Qin, X.M.; Wang, J.H.; Du, G.H. Strychnine. In Natural Small Molecule Drugs from Plants; Springer: Singapore, 2018; pp. 331–336. [Google Scholar]
- Promdam, N.; Panichayupakaranant, P. [6]-Gingerol: A narrative review of its beneficial effect on human health. Food Chem. Adv. 2022, 1, 100043. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Chen, X.-F.; Ding, Y.-Y.; Guan, H.-R.; Zhou, C.-J.; He, X.; Shao, Y.-T.; Wang, Y.-B.; Wang, N.; Li, B.; Lv, G.-Y.; et al. The Pharmacological Effects and Potential Applications of Limonene from Citrus Plants: A Review. Nat. Prod. Commun. 2024, 19, 1–12. [Google Scholar] [CrossRef]
- Torres Neto, L.; Monteiro, M.L.G.; Fernández-Romero, J.; Teleshova, N.; Sailer, J.; Conte Junior, C.A. Essential oils block cellular entry of SARS-CoV-2 delta variant. Sci. Rep. 2022, 12, 20639. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Statti, G.A.; Menichini, F.; Lampronti, I.; Gambari, R.; Cinatl, J.; Doerr, H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodivers. 2008, 5, 461–470. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene-What Are the Potential Health Benefits of This Flavouring and Aroma Agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef] [PubMed]
- Ikanovic, T.; Šeherčehajić, E.; Saric Medic, B.; Tomic, N.; Hadžiselimović, R. In Silico Analysis of Scopoletin Interaction with Potential SARS-CoV-2 Target. In International Conference “New Technologies, Development and Applications”; Springer International Publishing: Cham, Switzerland, 2021; pp. 897–903. [Google Scholar]
- Baggieri, M.; Gioacchini, S.; Borgonovo, G.; Catinella, G.; Marchi, A.; Picone, P.; Vasto, S.; Fioravanti, R.; Bucci, P.; Kojouri, M.; et al. Antiviral, virucidal and antioxidant properties of Artemisia annua against SARS-CoV-2. Biomed. Pharmacother. 2023, 168, 115682. [Google Scholar] [CrossRef]
- Alarabei, A.A.; Abd Aziz, N.A.L.; Ab Razak, N.I.; Abas, R.; Bahari, H.; Abdullah, M.A.; Hussain, M.K.; Abdul Majid, A.M.S.; Basir, R. Immunomodulating Phytochemicals: An Insight Into Their Potential Use in Cytokine Storm Situations. Adv. Pharm. Bull. 2024, 14, 105–119. [Google Scholar] [CrossRef]
- Yadav, H.; Mahalvar, A.; Pradhan, M.; Yadav, K.; Kumar Sahu, K.; Yadav, R. Exploring the potential of phytochemicals and nanomaterial: A boon to antimicrobial treatment. Med. Drug Discov. 2023, 17, 100151. [Google Scholar] [CrossRef]
- Gao, X.Y.; Li, X.Y.; Zhang, C.Y.; Bai, C.Y. Scopoletin: A review of its pharmacology, pharmacokinetics, and toxicity. Front. Pharmacol. 2024, 15, 1268464. [Google Scholar] [CrossRef]
- Panikar, S.; Shoba, G.; Arun, M.; Sahayarayan, J.J.; Usha Raja Nanthini, A.; Chinnathambi, A.; Alharbi, S.A.; Nasif, O.; Kim, H.-J. Essential oils as an effective alternative for the treatment of COVID-19: Molecular interaction analysis of protease (Mpro) with pharmacokinetics and toxicological properties. J. Infect. Public Health 2021, 14, 601–610. [Google Scholar] [CrossRef]
- Elsebai, M.F.; Albalawi, M.A. Essential Oils and COVID-19. Molecules 2022, 27, 7893. [Google Scholar] [CrossRef]
- Adegbola, P.I.; Fadahunsi, O.S.; Ogunjinmi, O.E.; Adegbola, A.E.; Ojeniyi, F.D.; Adesanya, A.; Olagoke, E.; Adisa, A.D.; Ehigie, A.F.; Adetutu, A.; et al. Potential inhibitory properties of structurally modified quercetin/isohamnetin glucosides against SARS-CoV-2 Mpro; molecular docking and dynamics simulation strategies. Inform. Med. Unlocked 2023, 37, 101167. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Mujawdiya, P.K.; Lysiuk, R.; Shanaida, M.; Peana, M.; Gasmi Benahmed, A.; Beley, N.; Kovalska, N.; Bjørklund, G. Quercetin in the Prevention and Treatment of Coronavirus Infections: A Focus on SARS-CoV-2. Pharmaceuticals 2022, 15, 1049. [Google Scholar] [CrossRef] [PubMed]
- Abian, O.; Ortega-Alarcon, D.; Jimenez-Alesanco, A.; Ceballos-Laita, L.; Vega, S.; Reyburn, H.T.; Rizzuti, B.; Velazquez-Campoy, A. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int. J. Biol. Macromol. 2020, 164, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, T.; Mathur, Y.; Hassan, M.I. InstaDock: A single-click graphical user interface for molecular docking-based virtual high-throughput screening. Brief. Bioinform. 2021, 22, bbaa279. [Google Scholar] [CrossRef]
- Rut, W.; Groborz, K.; Zhang, L.; Sun, X.; Zmudzinski, M.; Hilgenfeld, R.; Drag, M. Substrate specificity profiling of SARS-CoV-2 Mpro protease provides basis for anti-COVID-19 drug design. BioRxiv 2020. [Google Scholar] [CrossRef]
- Rut, W.; Groborz, K.; Zhang, L.; Sun, X.; Zmudzinski, M.; Pawlik, B.; Wang, X.; Jochmans, D.; Neyts, J.; Młynarski, W.; et al. SARS-CoV-2 Mpro inhibitors and activity-based probes for patient-sample imaging. Nat. Chem. Biol. 2021, 17, 222–228. [Google Scholar] [CrossRef]
- Ihssen, J.; Faccio, G.; Yao, C.; Sirec, T.; Spitz, U. Fluorogenic in vitro activity assay for the main protease Mpro from SARS-CoV-2 and its adaptation to the identification of inhibitors. STAR Protoc. 2021, 2, 100793. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, Y.; Yang, J.; Fu, L.; Shi, Y.; Huang, L.; Gao, G.F. Reply to Yan et al.: Quercetin possesses a fluorescence quenching effect but is a weak inhibitor against SARS-CoV-2 main protease. Proc. Natl. Acad. Sci. USA 2023, 120, e2309870120. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, R.; Liu, X.; Wang, Y.; Chen, Y. Reframing quercetin as a promiscuous inhibitor against SARS-CoV-2 main protease. Proc. Natl. Acad. Sci. USA 2023, 120, e2309289120. [Google Scholar] [CrossRef]
- Kaul, R.; Paul, P.; Kumar, S.; Büsselberg, D.; Dwivedi, V.D.; Chaari, A. Promising Antiviral Activities of Natural Flavonoids against SARS-CoV-2 Targets: Systematic Review. Int. J. Mol. Sci. 2021, 22, 11069. [Google Scholar] [CrossRef]
- Rizwan, T.; Kothidar, A.; Meghwani, H.; Sharma, V.; Shobhawat, R.; Saini, R.; Vaishnav, H.K.; Singh, V.; Pratap, M.; Sihag, H. Comparative analysis of SARS-CoV-2 envelope viroporin mutations from COVID-19 deceased and surviving patients revealed implications on its ion-channel activities and correlation with patient mortality. J. Biomol. Struct. Dyn. 2022, 40, 10454–10469. [Google Scholar] [CrossRef] [PubMed]
- Farasati Far, B.; Bokov, D.; Widjaja, G.; Setia Budi, H.; Kamal Abdelbasset, W.; Javanshir, S.; Seif, F.; Pazoki-Toroudi, H.; Dey, S.K. Metronidazole, acyclovir and tetrahydrobiopterin may be promising to treat COVID-19 patients, through interaction with interleukin-12. J. Biomol. Struct. Dyn. 2023, 41, 4253–4271. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.K.; Saini, M.; Dhembla, C.; Bhatt, S.; Rajesh, A.S.; Anand, V.; Das, H.K.; Kundu, S. Suramin, penciclovir, and anidulafungin exhibit potential in the treatment of COVID-19 via binding to nsp12 of SARS-CoV-2. J. Biomol. Struct. Dyn. 2022, 40, 14067–14083. [Google Scholar] [CrossRef]
- Waseem, R.; Shamsi, A.; Khan, T.; Hassan, M.I.; Kazim, S.N.; Shahid, M.; Islam, A. Unraveling the Binding Mechanism of Alzheimer’s Drugs with Irisin: Spectroscopic, Calorimetric, and Computational Approaches. Int. J. Mol. Sci. 2022, 23, 5965. [Google Scholar] [CrossRef]
- Waseem, R.; Anwar, S.; Khan, S.; Shamsi, A.; Hassan, M.I.; Anjum, F.; Shafie, A.; Islam, A.; Yadav, D.K. MAP/Microtubule Affinity Regulating Kinase 4 Inhibitory Potential of Irisin: A New Therapeutic Strategy to Combat Cancer and Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 10986. [Google Scholar] [CrossRef]
- Gehlen, M.H. The centenary of the Stern-Volmer equation of fluorescence quenching: From the single line plot to the SV quenching map. J. Photochem. Photobiol. C Photochem. Rev. 2020, 42, 100338. [Google Scholar] [CrossRef]
- Shamsi, A.; Al Shahwan, M.; Ahamad, S.; Hassan, M.I.; Ahmad, F.; Islam, A. Spectroscopic, calorimetric and molecular docking insight into the interaction of Alzheimer’s drug donepezil with human transferrin: Implications of Alzheimer’s drug. J. Biomol. Struct. Dyn. 2020, 38, 1094–1102. [Google Scholar] [CrossRef]
- Pascetta, V.G. Investigating the Main Protease (MPro) of SARS-CoV-2 as a Potential Drug Target. Bachelor’s Thesis, University of New Hampshire, Durham, NH, USA, 2022. [Google Scholar]
- Yammine, A.; Gao, J.; Kwan, A.H. Tryptophan Fluorescence Quenching Assays for Measuring Protein-ligand Binding Affinities: Principles and a Practical Guide. Bio-Protoc. 2019, 9, e3253. [Google Scholar] [CrossRef]
- Mátyus, L.; Szöllősi, J.; Jenei, A. Steady-state fluorescence quenching applications for studying protein structure and dynamics. J. Photochem. Photobiol. B Biol. 2006, 83, 223–236. [Google Scholar] [CrossRef]
- Forman, D. Ames, the Ames test, and the causes of cancer. BMJ 1991, 303, 428–429. [Google Scholar] [CrossRef]
- Jangir, D.K.; Dey, S.K.; Kundu, S.; Mehrotra, R. Assessment of amsacrine binding with DNA using UV–visible, circular dichroism and Raman spectroscopic techniques. J. Photochem. Photobiol. B Biol. 2012, 114, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.; Thumar, R.; Patel, B.; Athar, M.; Jha, P.C.; Patel, D. Structural differences in 3C-like protease (Mpro) from SARS-CoV and SARS-CoV-2: Molecular insights revealed by Molecular Dynamics Simulations. Struct. Chem. 2023, 34, 1309–1326. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, I.; Sapountzaki, E.; Rova, U.; Christakopoulos, P. Inhibition of the main protease of SARS-CoV-2 (M(pro)) by repurposing/designing drug-like substances and utilizing nature’s toolbox of bioactive compounds. Comput. Struct. Biotechnol. J. 2022, 20, 1306–1344. [Google Scholar] [CrossRef] [PubMed]
- Riveiro, M.E.; Kimpe, N.D.; Moglioni, A.; Vazquez, R.; Monczor, F.; Shayo, C.; Davio, C. Coumarins: Old Compounds with Novel Promising Therapeutic Perspectives. Curr. Med. Chem. 2010, 17, 1325–1338. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, M.-H.; Wang, F.; Chen, P.-Y.; Ke, X.-G.; Yu, B.; Yang, Y.-F.; You, P.-T.; Wu, H.-Z. Network pharmacology and molecular docking reveal the mechanism of Scopoletin against non-small cell lung cancer. Life Sci. 2021, 270, 119105. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Zhao, Q.; Kuete, V. 20-Harmful and Protective Effects of Phenolic Compounds from African Medicinal Plants. In Toxicological Survey of African Medicinal Plants; Kuete, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 577–609. [Google Scholar]
- Lemos, A.S.O.; Florêncio, J.R.; Pinto, N.C.C.; Campos, L.M.; Silva, T.P.; Grazul, R.M.; Pinto, P.F.; Tavares, G.D.; Scio, E.; Apolônio, A.C.M.; et al. Antifungal Activity of the Natural Coumarin Scopoletin Against Planktonic Cells and Biofilms From a Multidrug-Resistant Candida tropicalis Strain. Front. Microbiol. 2020, 11, 1525. [Google Scholar] [CrossRef]




 boxes indicate change in heat energy in kJ mol−1 per injection of ligand to the protein.
boxes indicate change in heat energy in kJ mol−1 per injection of ligand to the protein.
 boxes indicate change in heat energy in kJ mol−1 per injection of ligand to the protein.
boxes indicate change in heat energy in kJ mol−1 per injection of ligand to the protein.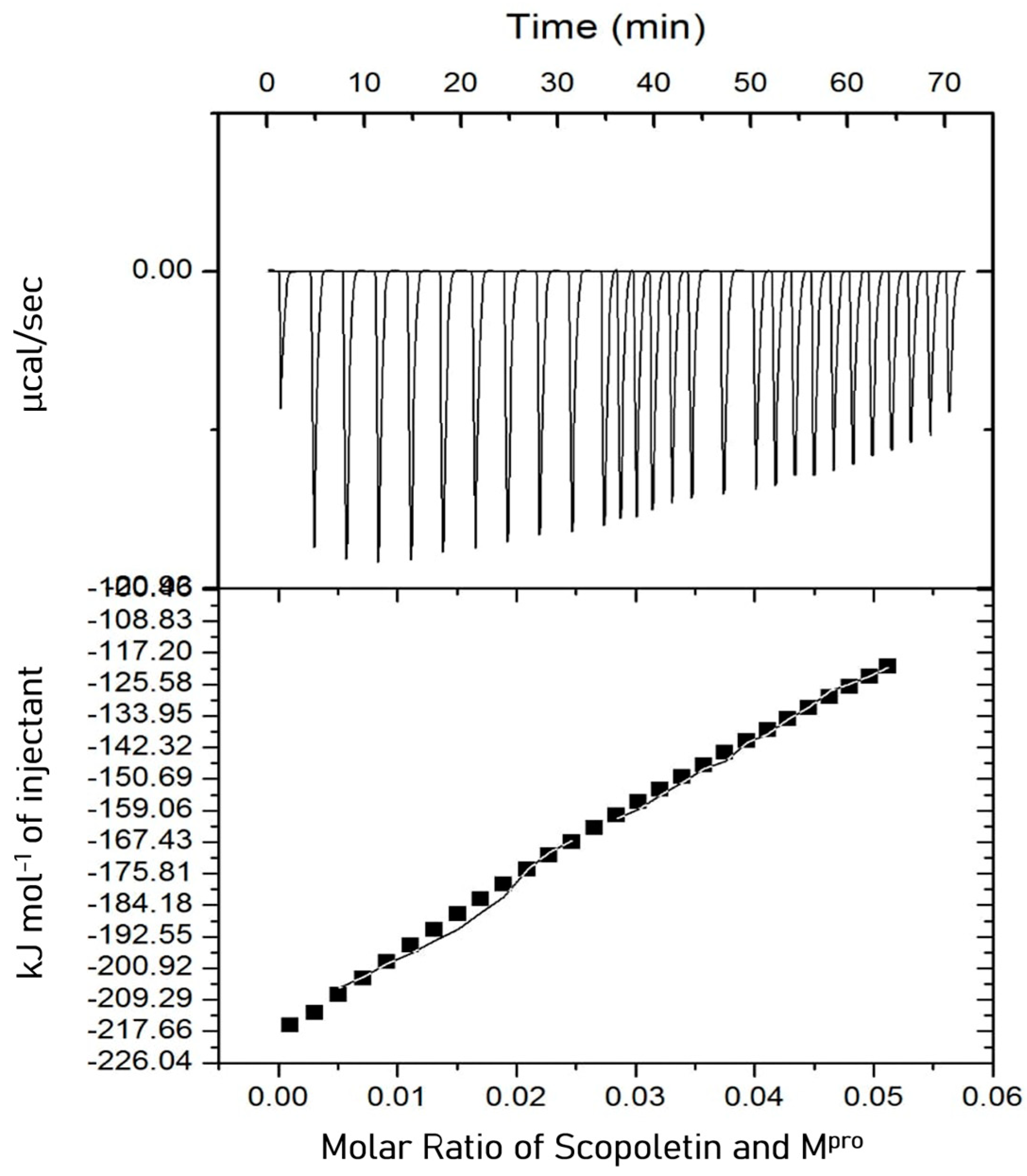


| Ligand Name | Binding Free Energy (kcal/mol) | pKi | Ligand Efficiency (kcal/mol/non-H Atom) | Torsional Energy (kcal/mol) |
|---|---|---|---|---|
| Scopoletin | −5.5 | 4.03 | 0.3929 | 0.6226 |
| Gingerol | −4.6 | 3.37 | 0.219 | 3.7356 |
| Pinine | −4.9 | 3.59 | 0.49 | 0 |
| Limonene | −4.9 | 3.59 | 0.49 | 0.3113 |
| Myrcene | −3.9 | 2.86 | 0.39 | 1.2452 |
| Quercetin | −7.1 | 3.89 | 0.3786 | 0.6226 |
| Compound and Its Chemical Structure | Conventional Hydrogen Bonds with Mpro | Carbon Hydrogen Bonds with Mpro | Van der Waals Force/Interaction/Bonds with Mpro | P-alkyl/Alkyl Bonds with Mpro |
|---|---|---|---|---|
Scopoletin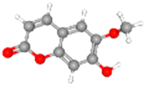 | TYR54 | HIS164 | HIS41, GLY143, ARG188, ASP187, GLN189, GLU166, MET165 | HIS163, CYS145, MET49 |
Quercetin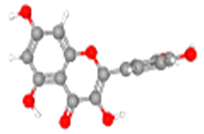 | TYR54, THR26 | Nil | ASN142, HIS41, PRO52, ARG188, ASP187, GLN189, MET165, GLU166, HIS164, LEU27, GLY143 | MET49, CYS145 |
| Compound ID | Absorption | Distribution | Metabolism | Excretion | Toxicity | |
|---|---|---|---|---|---|---|
| GI Absorption | Water Solubility | BBB permeation | CYP2D6Inh/Subs | OCT2 substrate | AMES | |
| Scopoletin | High | Yes | Yes | No | No | No |
| Name of the Ligand | Plant Source | Ligand Structure | Chemical Name | Biological Activities | References |
|---|---|---|---|---|---|
| Scopoletin | Plants from Artemisia, Scopolia, Viburnum, and Mitracarpus genus. |  | 6-methoxy-7-hydrocoumarian | Antiviral Anti-cancer Antimicrobial Immunomodulatory Anti-angiogenesis Anti-oxidation Neuroprotective Anti-diabetic Antihypertensive Hepatoprotective Anti-inflammatory | [41], [70,71], PubChem CID: 5,280,460 [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bano, S.; Singh, J.; Zehra, Z.; Sulaimani, M.N.; Mohammad, T.; Yumlembam, S.; Hassan, M.I.; Islam, A.; Dey, S.K. Biochemical Screening of Phytochemicals and Identification of Scopoletin as a Potential Inhibitor of SARS-CoV-2 Mpro, Revealing Its Biophysical Impact on Structural Stability. Viruses 2025, 17, 402. https://doi.org/10.3390/v17030402
Bano S, Singh J, Zehra Z, Sulaimani MN, Mohammad T, Yumlembam S, Hassan MI, Islam A, Dey SK. Biochemical Screening of Phytochemicals and Identification of Scopoletin as a Potential Inhibitor of SARS-CoV-2 Mpro, Revealing Its Biophysical Impact on Structural Stability. Viruses. 2025; 17(3):402. https://doi.org/10.3390/v17030402
Chicago/Turabian StyleBano, Sarika, Jyotishna Singh, Zainy Zehra, Md Nayab Sulaimani, Taj Mohammad, Seemasundari Yumlembam, Md Imtaiyaz Hassan, Asimul Islam, and Sanjay Kumar Dey. 2025. "Biochemical Screening of Phytochemicals and Identification of Scopoletin as a Potential Inhibitor of SARS-CoV-2 Mpro, Revealing Its Biophysical Impact on Structural Stability" Viruses 17, no. 3: 402. https://doi.org/10.3390/v17030402
APA StyleBano, S., Singh, J., Zehra, Z., Sulaimani, M. N., Mohammad, T., Yumlembam, S., Hassan, M. I., Islam, A., & Dey, S. K. (2025). Biochemical Screening of Phytochemicals and Identification of Scopoletin as a Potential Inhibitor of SARS-CoV-2 Mpro, Revealing Its Biophysical Impact on Structural Stability. Viruses, 17(3), 402. https://doi.org/10.3390/v17030402











