Deciphering Host–Virus Interactions and Advancing Therapeutics for Chronic Viral Infection
Abstract
1. Mechanisms of Host–Virus Interaction in Chronic Viral Infections
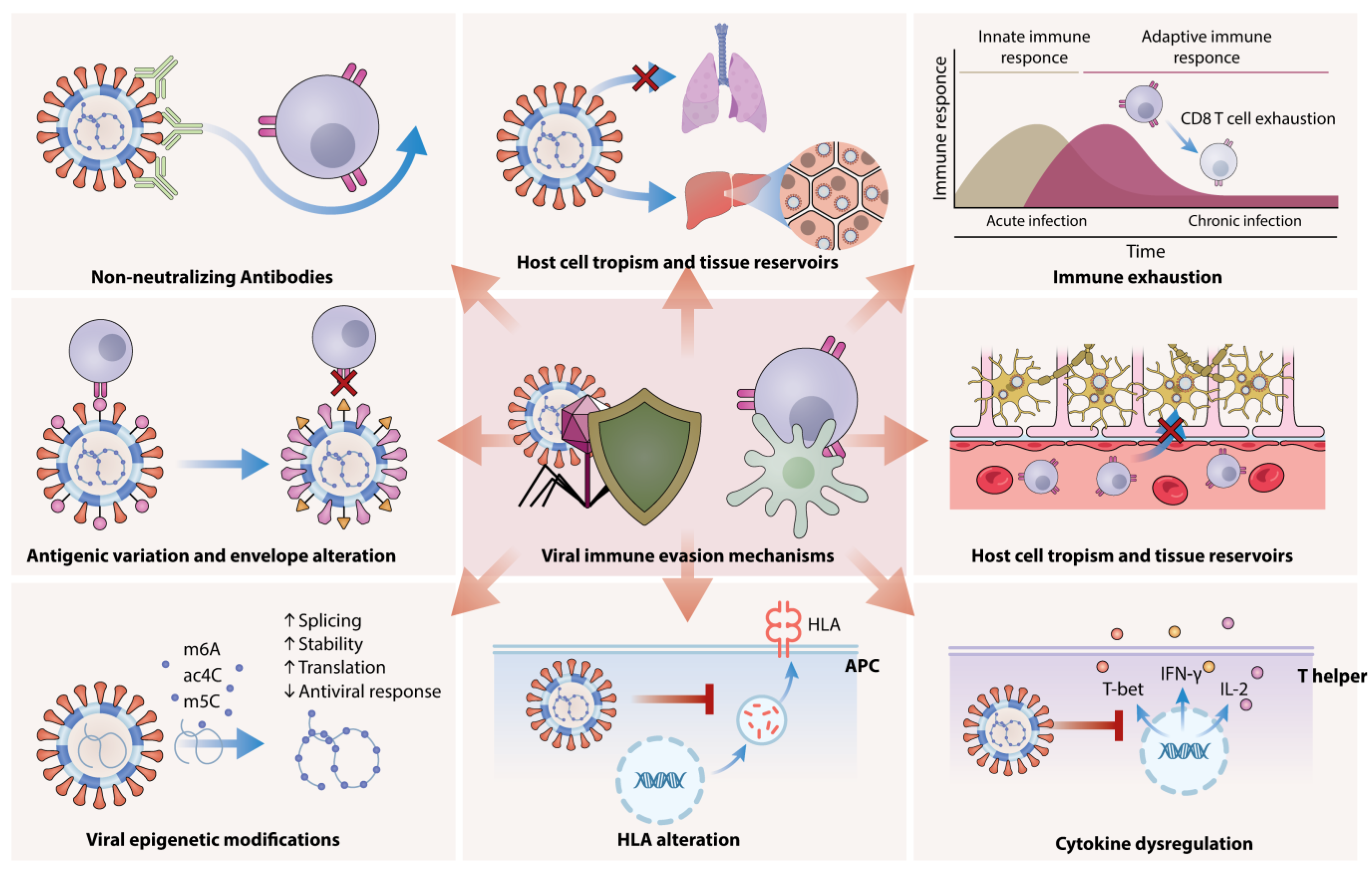
2. Molecular Pathways of Viral Evasion and Adaptation
2.1. Antigenic Variation and Envelope Alteration
2.2. Viral Epigenetic Modifications
2.3. Non-Neutralizing Antibodies
2.4. Host Cell Tropism and Tissue Reservoirs
2.5. Latency and Integration
3. Viral Evasion of Host Immunity Through HLA Alteration, Immune Exhaustion, and Cytokine Dysregulation
4. Host Genetic Factors Influencing Infection Outcomes
4.1. Human Leukocyte Antigen (HLA) Diversity
4.2. Immune Regulators
4.3. Ethnic and Genetic Variation
5. Targeting Host–Virus Interactions for Chronic Infection Therapies
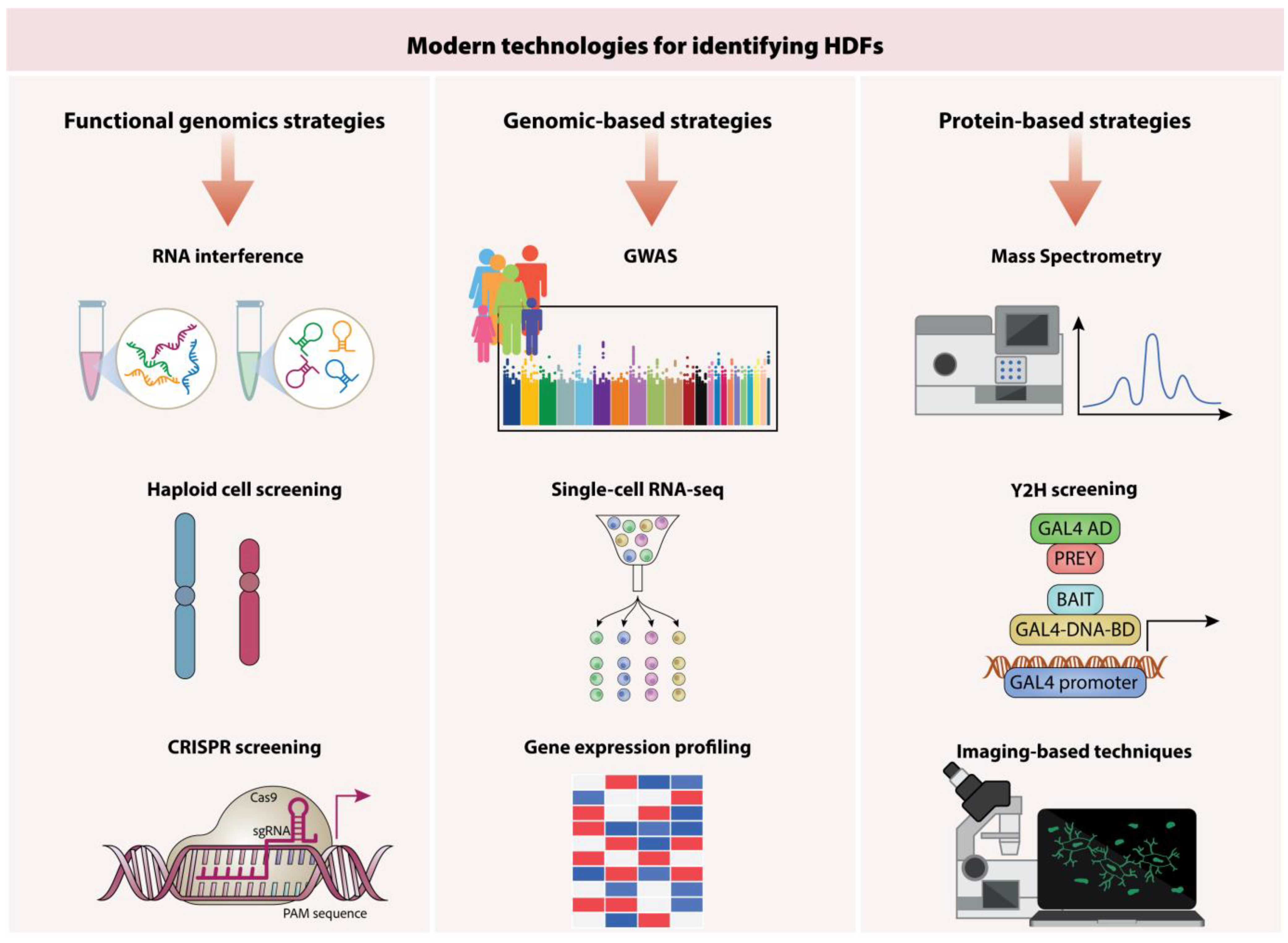
6. Identifying Key Host Targets for Therapeutic Intervention
6.1. Functional Genomics Approaches
6.2. RNA Interference
6.3. Haploid Cell Screening
6.4. CRISPR Screening Technologies
6.5. Genomics-Based Strategies
6.6. Proteomics-Based Strategies
6.7. Strategies for Modulating Host Immune Responses
6.8. Cytokine-Based Therapies
6.9. Checkpoint Inhibition Therapy
6.10. Adoptive Cell Therapy
6.11. Therapeutic Vaccines
7. Role of Host–Virus Interactions in Therapeutic Innovations Potential of Immunotherapy in Chronic Viral Diseases
7.1. Antiviral Host Factors in Some Viral Diseases
7.2. Insight into the Antiviral Responses Elicited by Type-1 Interferons (IFN-I)
7.3. How Viruses Undermine the Immune System’s Response to Interferon
8. Exploring Host–Virus Pathways for Enhanced Antiviral Treatments
8.1. Viral Transcription, Translation, and Host Factors
8.2. Block Assembly and Viral Release by Host Factors
9. Novel Strategies in Managing Chronic Viral Infections
9.1. Focusing on Interactions Between Viral and Host Proteins for Treatment Options
9.2. A Novel Family of Host Factors That Combat Viruses Is Histone Deacetylases
9.3. Conventional Functions of Histone Deacetylases
9.4. Post-Translational Modifications and HDACs
9.5. Gene Transcription Control and HDACs
9.6. Connection of Health and Disease with HDACs
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Virgin, H.W.; Wherry, E.J.; Ahmed, R. Redefining chronic viral infection. Cell 2009, 138, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, E.I.; Macal, M.; Lewis, G.M.; Harker, J.A. Innate and Adaptive Immune Regulation During Chronic Viral Infections. Annu. Rev. Virol. 2015, 2, 573–597. [Google Scholar] [CrossRef]
- Heim, M.H.; Thimme, R. Innate and adaptive immune responses in HCV infections. J. Hepatol. 2014, 61, S14–S25. [Google Scholar] [CrossRef]
- Yin, D.; Zhong, Y.; Ling, S.; Lu, S.; Wang, X.; Jiang, Z.; Wang, J.; Dai, Y.; Tian, X.; Huang, Q. Dendritic-cell-targeting virus-like particles as potent mRNA vaccine carriers. Nat. Biomed. Eng. 2024, 9, 185–200. [Google Scholar] [CrossRef]
- Upasani, V.; Rodenhuis-Zybert, I.; Cantaert, T. Antibody-independent functions of B cells during viral infections. PLoS Pathog. 2021, 17, e1009708. [Google Scholar] [CrossRef] [PubMed]
- Khanam, A.; Chua, J.V.; Kottilil, S. Immunopathology of chronic hepatitis B infection: Role of innate and adaptive immune response in disease progression. Int. J. Mol. Sci. 2021, 22, 5497. [Google Scholar] [CrossRef] [PubMed]
- Kahan, S.M.; Zajac, A.J. Immune exhaustion: Past lessons and new insights from lymphocytic choriomeningitis virus. Viruses 2019, 11, 156. [Google Scholar] [CrossRef]
- Ortega-Prieto, A.M.; Dorner, M. Immune Evasion Strategies during Chronic Hepatitis B and C Virus Infection. Vaccines 2017, 5, 24. [Google Scholar] [CrossRef]
- German Advisory Committee Blood (Arbeitskreis Blut), Subgroup ‘Assessment of Pathogens Transmissible by Blood’. Human immunodeficiency virus (HIV). Transfus. Med. Hemotherapy 2016, 43, 203–222. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, X.; Wang, Z.; Fang, L.; Liu, J. Recent advances in understanding T cell activation and exhaustion during HBV infection. Virol. Sin. 2023, 38, 851–859. [Google Scholar] [CrossRef]
- Masenga, S.K.; Mweene, B.C.; Luwaya, E.; Muchaili, L.; Chona, M.; Kirabo, A. HIV–Host Cell Interactions. Cells 2023, 12, 1351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.J.; Hu, Y.F.; Han, Q.J.; Zhang, J. Innate and adaptive immune escape mechanisms of hepatitis B virus. World J. Gastroenterol. 2022, 28, 881–896. [Google Scholar] [CrossRef]
- Dandri, M.; Bertoletti, A.; Lütgehetmann, M. Innate Immunity in Hepatitis B and D Virus Infection: Consequences for Viral Persistence, Inflammation, and T Cell Recognition. In Seminars in Immunopathology; Springer Nature: London, UK, 2021; pp. 535–548. [Google Scholar]
- Herrscher, C.; Roingeard, P.; Blanchard, E. Hepatitis B Virus Entry into Cells. Cells 2020, 9, 1486. [Google Scholar] [CrossRef] [PubMed]
- Rehman, U.U.; Ghafoor, D.; Ullah, A.; Ahmad, R.; Hanif, S. Epigenetics regulation during virus-host interaction and their effects on the virus and host cell. Microb. Pathog. 2023, 182, 106271. [Google Scholar] [CrossRef]
- Ding, S.; Liu, H.; Liu, L.; Ma, L.; Chen, Z.; Zhu, M.; Liu, L.; Zhang, X.; Hao, H.; Zuo, L.; et al. Epigenetic addition of m5C to HBV transcripts promotes viral replication and evasion of innate antiviral responses. Cell Death Dis. 2024, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Chandler, T.L.; Yang, A.; Otero, C.E.; Permar, S.R.; Caddy, S.L. Protective mechanisms of nonneutralizing antiviral antibodies. PLoS Pathog. 2023, 19, e1011670. [Google Scholar] [CrossRef]
- Mader, K.; Dustin, L.B. Beyond bNAbs: Uses, risks, and opportunities for therapeutic application of non-neutralising antibodies in viral infection. Antibodies 2024, 13, 28. [Google Scholar] [CrossRef]
- Garg, S.; Ochetto, A.; Hu, J.; Wang, J.C.-Y. Unveiling the Molecular Architecture of HBV Spherical Subviral Particles: Structure, Symmetry, and Lipid Dynamics. Viruses 2024, 17, 48. [Google Scholar] [CrossRef]
- Bhiman, J.N. Defining Virus-Antibody Interplay During the Development of HIV-1 Neutralization Breadth to Inform Vaccine Design. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2016. [Google Scholar]
- Ferrari, C.; Barili, V.; Varchetta, S.; Mondelli, M.U. Immune Mechanisms of Viral Clearance and Disease Pathogenesis During viral hepatitis. In The Liver: Biology and Pathobiology; Wiley: Hoboken, NJ, USA, 2020; pp. 821–850. [Google Scholar]
- Liu, Y.; Yan, X.; Zhang, F.; Zhang, X.; Tang, F.; Han, Z.; Li, Y. TCR-T immunotherapy: The challenges and solutions. Front. Oncol. 2022, 11, 794183. [Google Scholar] [CrossRef]
- Naz, S.S.; Aslam, A.; Malik, T. An overview of immune evasion strategies of DNA and RNA viruses. Infect. Disord.-Drug Targets (Former. Curr. Drug Targets-Infect. Disord.) 2021, 21, 1–16. [Google Scholar] [CrossRef]
- Strumillo, S.T.; Kartavykh, D.; de Carvalho, F.F., Jr.; Cruz, N.C.; de Souza Teodoro, A.C.; Sobhie Diaz, R.; Curcio, M.F. Host-virus interaction and viral evasion. Cell Biol. Int. 2021, 45, 1124–1147. [Google Scholar] [CrossRef]
- Forrester, J.V.; Mölzer, C.; Kuffova, L. Immune privilege furnishes a niche for latent infection. Front. Ophthalmol. 2022, 2, 869046. [Google Scholar] [CrossRef]
- Shang, Z.; Li, X. Human cytomegalovirus: Pathogenesis, prevention, and treatment. Mol. Biomed. 2024, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef] [PubMed]
- Schwanke, H.; Stempel, M.; Brinkmann, M.M. Of keeping and tipping the balance: Host regulation and viral modulation of IRF3-dependent IFNB1 expression. Viruses 2020, 12, 733. [Google Scholar] [CrossRef]
- Leonhards, J.R. Antigen-Specific Immune Responses Mediated by NK Cells in HIV and Influenza; Staats-und Universitätsbibliothek Hamburg Carl von Ossietzky: Hamburg, Germany, 2020. [Google Scholar]
- Jiang, J.; Tang, H. Mechanism of inhibiting type I interferon induction by hepatitis B virus X protein. Protein Cell 2010, 1, 1106–1117. [Google Scholar] [CrossRef]
- Fenwick, C.; Joo, V.; Jacquier, P.; Noto, A.; Banga, R.; Perreau, M.; Pantaleo, G. T-cell exhaustion in HIV infection. Immunol. Rev. 2019, 292, 149–163. [Google Scholar] [CrossRef]
- Kurachi, M. CD8(+) T cell exhaustion. Semin. Immunopathol. 2019, 41, 327–337. [Google Scholar] [CrossRef]
- Dell’Oste, V.; Biolatti, M.; Galitska, G.; Griffante, G.; Gugliesi, F.; Pasquero, S.; Zingoni, A.; Cerboni, C.; De Andrea, M. Tuning the orchestra: HCMV vs. innate immunity. Front. Microbiol. 2020, 11, 661. [Google Scholar] [CrossRef]
- Kachuri, L.; Francis, S.S.; Morrison, M.L.; Wendt, G.A.; Bossé, Y.; Cavazos, T.B.; Rashkin, S.R.; Ziv, E.; Witte, J.S. The landscape of host genetic factors involved in immune response to common viral infections. Genome Med. 2020, 12, 93. [Google Scholar] [CrossRef]
- Akcay, I.M.; Katrinli, S.; Ozdil, K.; Doganay, G.D.; Doganay, L. Host genetic factors affecting hepatitis B infection outcomes: Insights from genome-wide association studies. World J. Gastroenterol. 2018, 24, 3347–3360. [Google Scholar] [CrossRef]
- Aksak-Wąs, B.; Parczewski, M. Genetic factors influencing HIV infection: A review. HIV AIDS Rev. Int. J. HIV-Relat. Probl. 2023, 22, 1. [Google Scholar] [CrossRef]
- Herráiz-Nicuesa, L.; Hernández-Flórez, D.C.; Valor, L.; García-Consuegra, S.; Navarro-Valdivieso, J.P.; Fernández-Cruz, E.; Rodríguez-Sainz, C. Impact of the Polymorphism rs9264942 near the HLA-C Gene on HIV-1 DNA Reservoirs in Asymptomatic Chronically Infected Patients Initiating Antiviral Therapy. J. Immunol. Res. 2017, 2017, 8689313. [Google Scholar] [CrossRef]
- Singh, R.; Kaul, R.; Kaul, A.; Khan, K. A comparative review of HLA associations with hepatitis B and C viral infections across global populations. World J. Gastroenterol. 2007, 13, 1770–1787. [Google Scholar] [CrossRef]
- Naidoo, L.; Arumugam, T.; Ramsuran, V. Host Genetic Impact on Infectious Diseases among Different Ethnic Groups. Adv. Genet. 2023, 4, 2300181. [Google Scholar] [CrossRef]
- Young, C.; Walzl, G.; Du Plessis, N. Therapeutic host-directed strategies to improve outcome in tuberculosis. Mucosal Immunol. 2020, 13, 190–204. [Google Scholar] [CrossRef]
- Andrei, G. Vaccines and antivirals: Grand challenges and great opportunities. Front. Virol. 2021, 1, 666548. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-directed therapies for bacterial and viral infections. Nat. Rev. Drug Discov. 2018, 17, 35–56. [Google Scholar] [CrossRef]
- Gholap, A.D.; Khuspe, P.R.; Pardeshi, S.R.; Uddin, M.J.; Das, U.; Hatvate, N.T.; Rojekar, S.; Giram, P.; Khalid, M.; Choonara, Y.E. Achieving Optimal Health With Host—Directed Therapies (HDTs) in Infectious Diseases—A New Horizon. Adv. Ther. 2024, volume, 2400169. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, S.; Kumar, R.; Tripathi, B.N.; Barua, S.; Ly, H.; Rouse, B.T. Host-directed antiviral therapy. Clin. Microbiol. Rev. 2020, 33, 3. [Google Scholar] [CrossRef] [PubMed]
- Badia, R.; Garcia-Vidal, E.; Ballana, E. Viral-host dependency factors as therapeutic targets to overcome antiviral drug-resistance: A focus on innate immune modulation. Front. Virol. 2022, 2, 935933. [Google Scholar] [CrossRef]
- Ligat, G.; Verrier, E.R.; Nassal, M.; Baumert, T.F. Hepatitis B virus-host interactions and novel targets for viral cure. Curr. Opin. Virol. 2021, 49, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Dasaradhi, P.; Mohmmed, A.; Malhotra, P.; Bhatnagar, R.K.; Mukherjee, S.K. RNA interference: Biology, mechanism, and applications. Microbiol. Mol. Biol. Rev. 2003, 67, 657–685. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.N.; Ng, A.; Sukumaran, B.; Gilfoy, F.D.; Uchil, P.D.; Sultana, H.; Brass, A.L.; Adametz, R.; Tsui, M.; Qian, F. RNA interference screen for human genes associated with West Nile virus infection. Nature 2008, 455, 242–245. [Google Scholar] [CrossRef]
- Khaitov, M.; Nikonova, A.; Shilovskiy, I.; Kozhikhova, K.; Kofiadi, I.; Vishnyakova, L.; Nikolskii, A.; Gattinger, P.; Kovchina, V.; Barvinskaia, E. Silencing of SARS-CoV-2 with modified siRNA-peptide dendrimer formulation. Allergy 2021, 76, 2840–2854. [Google Scholar] [CrossRef]
- Fessler, E.; Jae, L.T. Haploid Screening for the Identification of Host Factors in Virus Infection. Methods Mol. Biol. 2018, 1836, 121–137. [Google Scholar] [CrossRef]
- Carette, J.E.; Raaben, M.; Wong, A.C.; Herbert, A.S.; Obernosterer, G.; Mulherkar, N.; Kuehne, A.I.; Kranzusch, P.J.; Griffin, A.M.; Ruthel, G. Ebola virus entry requires the cholesterol transporter Niemann–Pick C1. Nature 2011, 477, 340–343. [Google Scholar] [CrossRef]
- Röling, M.; Mollapour Sisakht, M.; Ne, E.; Moulos, P.; Crespo, R.; Stoszko, M.; De Crignis, E.; Bodmer, H.; Kan, T.W.; Akbarzadeh, M. A two-color haploid genetic screen identifies novel host factors involved in HIV-1 latency. Mbio 2021, 12, 6. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Lin, G.; Li, J. CRISPR-Cas system: A promising tool for rapid detection of SARS-CoV-2 variants. J. Med. Virol. 2024, 96, e29356. [Google Scholar] [CrossRef]
- Israeli, M.a.; Finkel, Y.; Yahalom-Ronen, Y.; Paran, N.; Chitlaru, T.; Israeli, O.; Cohen-Gihon, I.; Aftalion, M.; Falach, R.; Rotem, S. Genome-wide CRISPR screens identify GATA6 as a proviral host factor for SARS-CoV-2 via modulation of ACE2. Nat. Commun. 2022, 13, 2237. [Google Scholar] [CrossRef] [PubMed]
- Kwok, A.J.; Mentzer, A.; Knight, J.C. Host genetics and infectious disease: New tools, insights and translational opportunities. Nat. Rev. Genet. 2021, 22, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.J.; Hill, A.V. Human genetic susceptibility to infectious disease. Nat. Rev. Genet. 2012, 13, 175–188. [Google Scholar] [CrossRef]
- Nahon, P.; Cobat, A. Human genetics of HCV infection phenotypes in the era of direct-acting antivirals. Hum. Genet. 2020, 139, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bastard, P.; Cobat, A.; Casanova, J.-L. Human genetic and immunological determinants of critical COVID-19 pneumonia. Nature 2022, 603, 587–598. [Google Scholar] [CrossRef]
- Sun, J.; Ye, F.; Wu, A.; Yang, R.; Pan, M.; Sheng, J.; Zhu, W.; Mao, L.; Wang, M.; Xia, Z. Comparative transcriptome analysis reveals the intensive early stage responses of host cells to SARS-CoV-2 infection. Front. Microbiol. 2020, 11, 593857. [Google Scholar] [CrossRef]
- Luo, G.; Gao, Q.; Zhang, S.; Yan, B. Probing infectious disease by single-cell RNA sequencing: Progresses and perspectives. Comput. Struct. Biotechnol. J. 2020, 18, 2962–2971. [Google Scholar] [CrossRef]
- Lian, X.; Yang, X.; Yang, S.; Zhang, Z. Current status and future perspectives of computational studies on human–virus protein–protein interactions. Brief. Bioinform. 2021, 22, bbab029. [Google Scholar] [CrossRef]
- Lin, W.; Shen, C.; Li, M.; Ma, S.; Liu, C.; Huang, J.; Ren, Z.; Yang, Y.; Zhao, M.; Xie, Q.; et al. Programmable Macrophage Vesicle Based Bionic Self-Adjuvanting Vaccine for Immunization against Monkeypox Virus. Adv. Sci. 2025, 12, e2408608. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Hu, Z.; Yan, X.; Gao, Q.; Li, X.; Zheng, J.; Li, B.; Wu, Y.; Liao, Y. Advancing Aggregation-Induced Emission-Derived Biomaterials in Viral, Tuberculosis, and Fungal Infectious Diseases. Aggregate 2024, e715. [Google Scholar] [CrossRef]
- Mehla, J.; Caufield, J.H.; Uetz, P. Making the Right Choice: Critical Parameters of the Y2H Systems. In Two-Hybrid Systems: Methods and Protocols; Springer Nature: London, UK, 2018; pp. 17–28. [Google Scholar]
- Zhang, M.; Liu, J.; Xia, Q. Role of gut microbiome in cancer immunotherapy: From predictive biomarker to therapeutic target. Exp. Hematol. Oncol. 2023, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Quirino, A.; Marascio, N.; Branda, F.; Ciccozzi, A.; Romano, C.; Locci, C.; Azzena, I.; Pascale, N.; Pavia, G.; Matera, G. Viral Hepatitis: Host Immune Interaction, Pathogenesis and New Therapeutic Strategies. Pathogens 2024, 13, 766. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.; Zhang, Y.; Zhu, X.; Zhao, C.; Li, X.; Lü, H.; Lv, K. Multitargeted Immunomodulatory Therapy for Viral Myocarditis by Engineered Extracellular Vesicles. ACS Nano 2024, 18, 2782–2799. [Google Scholar] [CrossRef]
- Aung, T.; Grubbe, W.S.; Nusbaum, R.J.; Mendoza, J.L. Recent and future perspectives on engineering interferons and other cytokines as therapeutics. Trends Biochem. Sci. 2023, 48, 259–273. [Google Scholar] [CrossRef]
- Gunst, J.D.; Goonetilleke, N.; Rasmussen, T.A.; Søgaard, O.S. Immunomodulation with IL-7 and IL-15 in HIV-1. J. Virus Erad. 2023, 9, 100347. [Google Scholar] [CrossRef]
- Li, X.-F.; Zhang, Y.-J.; Yao, Y.-L.; Chen, M.-X.; Wang, L.-L.; Wang, M.-D.; Hu, X.-Y.; Tang, X.-J.; Zhong, Z.-H.; Fu, L.-J. The association of post–embryo transfer SARS-CoV-2 infection with early pregnancy outcomes in in vitro fertilization: A prospective cohort study. Am. J. Obstet. Gynecol. 2024, 230, 436.e1–436.e12. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, R.; Zhang, W. CTLA-4 interferes with the HBV-specific T cell immune response. Int. J. Mol. Med. 2018, 42, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Pharaon, R.R.; Xing, Y.; Agulnik, M.; Villaflor, V.M. The role of immunotherapy to overcome resistance in viral-associated head and neck cancer. Front. Oncol. 2021, 11, 649963. [Google Scholar] [CrossRef]
- Mancini, N.; Marrone, L.; Clementi, N.; Sautto, G.A.; Clementi, M.; Burioni, R. Adoptive T-cell therapy in the treatment of viral and opportunistic fungal infections. Future Microbiol. 2015, 10, 665–682. [Google Scholar] [CrossRef]
- Seif, M.; Einsele, H.; Löffler, J. CAR T cells beyond cancer: Hope for immunomodulatory therapy of infectious diseases. Front. Immunol. 2019, 10, 2711. [Google Scholar] [CrossRef]
- Zmievskaya, E.; Valiullina, A.; Ganeeva, I.; Petukhov, A.; Rizvanov, A.; Bulatov, E. Application of CAR-T cell therapy beyond oncology: Autoimmune diseases and viral infections. Biomedicines 2021, 9, 59. [Google Scholar] [CrossRef]
- Bobrowski, T.; Melo-Filho, C.C.; Korn, D.; Alves, V.M.; Popov, K.I.; Auerbach, S.; Schmitt, C.; Moorman, N.J.; Muratov, E.N.; Tropsha, A. Learning from history: Do not flatten the curve of antiviral research! Drug Discov. Today 2020, 25, 1604–1613. [Google Scholar] [CrossRef]
- Kayesh, M.E.H.; Kohara, M.; Tsukiyama-Kohara, K. TLR agonists as vaccine adjuvants in the prevention of viral infections: An overview. Front. Microbiol. 2023, 14, 1249718. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.-Y.; Lin, M.-Y.; Coffman, R.L.; Campbell, J.D.; Traquina, P.; Lin, Y.-J.; Liu, L.T.-C.; Cheng, J.; Wu, Y.-C.; Wu, C.-C. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci. Rep. 2020, 10, 20085. [Google Scholar] [CrossRef]
- Gilliet, M.; Cao, W.; Liu, Y.J. Plasmacytoid dendritic cells: Sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008, 8, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.M.; Rahal, E.A. The role of viral infections in the development of autoimmune diseases. Crit. Rev. Microbiol. 2019, 45, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Navratil, V.; de Chassey, B.; Combe, C.R.; Lotteau, V. When the human viral infectome and diseasome networks collide: Towards a systems biology platform for the aetiology of human diseases. BMC Syst. Biol. 2011, 5, 13. [Google Scholar] [CrossRef]
- Ospel’nikova, T.P. The role of interferons in the socially important human viral diseases. Vopr. Virusol. 2013, 58, 4–10. [Google Scholar]
- Qi, W.H.; Tang, N.; Zhao, Z.J.; Li, X.Q. Transient receptor potential channels in viral infectious diseases: Biological characteristics and regulatory mechanisms. J. Adv. Res. 2024. [Google Scholar] [CrossRef]
- Mahajan, S.; Choudhary, S.; Kumar, P.; Tomar, S. Antiviral strategies targeting host factors and mechanisms obliging +ssRNA viral pathogens. Bioorg. Med. Chem. 2021, 46, 116356. [Google Scholar] [CrossRef]
- Sarikonda, G.; von Herrath, M.G. Immunosuppressive mechanisms during viral infectious diseases. Methods Mol. Biol. 2011, 677, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Subudhi, B.B.; Swain, R.P. Quercetin against Emerging RNA Viral Diseases: Potential and Challenges for Translation. Curr. Mol. Med. 2023, 23, 849–862. [Google Scholar] [CrossRef]
- Jimenez-Guardeño, J.M.; Menéndez-Arias, L.; Betancor, G. Editorial: Host factors involved in viral infection. Front. Microbiol. 2024, 15, 1382503. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, C.; Ren, C.; Zhang, S.; Gao, X.; Jin, M.; Chen, H.; Ma, W.; Zhou, H. Eukaryotic Translation Elongation Factor 1 Delta Inhibits the Nuclear Import of the Nucleoprotein and PA-PB1 Heterodimer of Influenza A Virus. J. Virol. 2020, 95, 2. [Google Scholar] [CrossRef] [PubMed]
- Gales, J.P.; Kubina, J.; Geldreich, A.; Dimitrova, M. Strength in Diversity: Nuclear Export of Viral RNAs. Viruses 2020, 12, 1014. [Google Scholar] [CrossRef]
- Rahim, M.N.; Klewes, L.; Zahedi-Amiri, A.; Mai, S.; Coombs, K.M. Global Interactomics Connect Nuclear Mitotic Apparatus Protein NUMA1 to Influenza Virus Maturation. Viruses 2018, 10, 731. [Google Scholar] [CrossRef]
- Fan, Y.; Mok, C.K.; Chan, M.C.; Zhang, Y.; Nal, B.; Kien, F.; Bruzzone, R.; Sanyal, S. Cell Cycle-independent Role of Cyclin D3 in Host Restriction of Influenza Virus Infection. J. Biol. Chem. 2017, 292, 5070–5088. [Google Scholar] [CrossRef]
- Kuss-Duerkop, S.K.; Wang, J.; Mena, I.; White, K.; Metreveli, G.; Sakthivel, R.; Mata, M.A.; Munoz-Moreno, R.; Chen, X.; Krammer, F.; et al. Influenza virus differentially activates mTORC1 and mTORC2 signaling to maximize late stage replication. PLoS Pathog. 2017, 13, e1006635. [Google Scholar] [CrossRef]
- Wang, S.; Chi, X.; Wei, H.; Chen, Y.; Chen, Z.; Huang, S.; Chen, J.L. Influenza A virus-induced degradation of eukaryotic translation initiation factor 4B contributes to viral replication by suppressing IFITM3 protein expression. J. Virol. 2014, 88, 8375–8385. [Google Scholar] [CrossRef]
- Gillen, J.; Nita-Lazar, A. Experimental Analysis of Viral–Host Interactions. Front. Physiol. 2019, 10, 425. [Google Scholar] [CrossRef]
- Hu, S.; Jiang, S.; Qi, X.; Bai, R.; Ye, X.Y.; Xie, T. Races of small molecule clinical trials for the treatment of COVID-19: An up-to-date comprehensive review. Drug Dev. Res. 2022, 83, 16–54. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Chen, Z.; Qu, X.; Zhang, J.; Liu, L.; Zhong, Z.; Zhang, W.; Fan, Y. Comprehensive Characterization of HATs and HDACs in Human Cancers Reveals Their Role in Immune Checkpoint Blockade. Crit. Rev. Eukaryot. Gene Expr. 2024, 34, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zang, C.; Cui, K.; Schones, D.E.; Barski, A.; Peng, W.; Zhao, K. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell 2009, 138, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Kramer, O.H.; Schmid, R.M.; Saur, D. Acetylation as a transcriptional control mechanism-HDACs and HATs in pancreatic ductal adenocarcinoma. J. Gastrointest. Cancer 2011, 42, 85–92. [Google Scholar] [CrossRef]
- Grabiec, A.M.; Tak, P.P.; Reedquist, K.A. Targeting histone deacetylase activity in rheumatoid arthritis and asthma as prototypes of inflammatory disease: Should we keep our HATs on? Arthritis Res. Ther. 2008, 10, 226. [Google Scholar] [CrossRef][Green Version]
- Yang, X.J.; Seto, E. HATs and HDACs: From structure, function and regulation to novel strategies for therapy and prevention. Oncogene 2007, 26, 5310–5318. [Google Scholar] [CrossRef]
- Hamamori, Y.; Schneider, M.D. HATs off to Hop: Recruitment of a class I histone deacetylase incriminates a novel transcriptional pathway that opposes cardiac hypertrophy. J. Clin. Investig. 2003, 112, 824–826. [Google Scholar] [CrossRef]

| Stage | HBV | HCV | HIV |
|---|---|---|---|
| Entry | NTCP (Sodium taurocholate cotransporting polypeptide) | CD81, Claudin-1, Occludin, SR-BI | CD4, CCR5, CXCR4 |
| Replication | Host polymerases, m6A methylation | miR-122, lipid metabolism factors | NF-κB, Sp1, Tat protein interaction with host factors |
| Secretion | Endoplasmic reticulum (ER), Golgi apparatus | ER, Golgi, exosomal pathways | ESCRT (Endosomal Sorting Complex Required for Transport) |
| Immune Evasion | Downregulation of HLA-DP, HLA-DQ, IL-10 modulation | IFN pathway inhibition, suppression of ISG responses | Nef-mediated MHC-I downregulation, PD-1 upregulation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eslami, M.; Arjmand, N.; Mahmoudian, F.; Babaeizad, A.; Tahmasebi, H.; Fattahi, F.; Oksenych, V. Deciphering Host–Virus Interactions and Advancing Therapeutics for Chronic Viral Infection. Viruses 2025, 17, 390. https://doi.org/10.3390/v17030390
Eslami M, Arjmand N, Mahmoudian F, Babaeizad A, Tahmasebi H, Fattahi F, Oksenych V. Deciphering Host–Virus Interactions and Advancing Therapeutics for Chronic Viral Infection. Viruses. 2025; 17(3):390. https://doi.org/10.3390/v17030390
Chicago/Turabian StyleEslami, Majid, Neda Arjmand, Fatemeh Mahmoudian, Ali Babaeizad, Hamed Tahmasebi, Fahimeh Fattahi, and Valentyn Oksenych. 2025. "Deciphering Host–Virus Interactions and Advancing Therapeutics for Chronic Viral Infection" Viruses 17, no. 3: 390. https://doi.org/10.3390/v17030390
APA StyleEslami, M., Arjmand, N., Mahmoudian, F., Babaeizad, A., Tahmasebi, H., Fattahi, F., & Oksenych, V. (2025). Deciphering Host–Virus Interactions and Advancing Therapeutics for Chronic Viral Infection. Viruses, 17(3), 390. https://doi.org/10.3390/v17030390







